
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 21 February 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1119617
This article is part of the Research TopicAdvances in Proctology and Colorectal SurgeryView all 45 articles
Background: Rectal hyposensitivity (RH) is not uncommon in patients with functional defecation disorder (FDD). FDD patients with RH are usually unsatisfied with their treatment.
Aims: The aim of this study was to find the significance of RH in patients with FDD and the related factors of RH.
Methods: Patients with FDD first completed clinical questionnaires regarding constipation symptoms, mental state, and quality of life. Then anorectal physiologic tests (anorectal manometry and balloon expulsion test) were performed. Rectal sensory testing (assessing rectal response to balloon distension using anorectal manometry) was applied to obtain three sensory thresholds. Patients were separated into three groups (non-RH, borderline RH, and RH) based on the London Classification. The associations between RH and clinical symptoms, mental state, quality of life, and rectal/anal motility were investigated.
Results: Of 331 included patients with FDD, 87 patients (26.3%) had at least one abnormally elevated rectal sensory threshold and 50 patients (15.1%) were diagnosed with RH. Patients with RH were older and mostly men. Defecation symptoms were more severe (p = 0.013), and hard stool (p < 0.001) and manual maneuver (p = 0.003) were more frequently seen in the RH group. No difference in rectal/anal pressure was found among the three groups. Elevated defecatory desire volume (DDV) existed in all patients with RH. With the number of elevated sensory thresholds increasing, defecation symptoms got more severe (r = 0.35, p = 0.001). Gender (male) (6.78 [3.07–15.00], p < 0.001) and hard stool (5.92 [2.28–15.33], p < 0.001) were main related factors of RH.
Conclusion: Rectal hyposensitivity plays an important role in the occurrence of FDD and is associated with defecation symptom severity. Older male FDD patients with hard stool are prone to suffer from RH and need more care.
Approximately 50% of patients with functional constipation have difficulty in defecating (1) and may have the functional defecatory disorder (FDD) (2). FDD significantly affects productivity, mental health, and quality of life (QOL) (3).
Intact rectal sensation and motility are critical to normal bowel movement and defecation. The presence of sufficient stool and intact sensation will trigger the perception of rectal fullness through rectal afferent pathways (4). Rectal hyposensitivity (RH) refers to a blunted sensation of mechanical distension, which is indicated by the elevation of sensory thresholds beyond the normal range (5). As sensation and motility are inextricably linked, alteration in one domain can affect the other. RH, rectal motor dysfunction, and altered recto-anal reflex activity are particularly associated with FDD (6).
Patients with RH commonly present with constipation (48%) (7), and about 18%–68% of constipated patients have RH (8). It is reported that RH is more common in patients with functional disorders (i.e., dyssynergic defecation) rather than structural diseases (i.e., rectocele and intussusception) (9). Our team has found that RH is associated with defecation symptoms and specifies an eventual diagnosis of FDD over delayed gut transit (10).
Rectal hyposensitivity is associated with constipation, but its clinical importance remains unclear. In addition, little is known about the characteristics of FDD patients with RH and the related factors of RH in these individuals. Given the above deficiencies, we carried out this study to explore the influence of RH on constipation symptoms, mental state as well as QOL, and related factors of RH in an FDD population.
This is a cross-sectional study. We enrolled patients with FDD (Rome IV core criteria defined) who were referred to our gastrointestinal motility clinic between January 2014 and May 2021. Patients with pregnancy, drug-induced constipation, secondary constipation due to other diseases, a history of the prior bowl or anorectal surgery, or an abuse history were excluded. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital with Nanjing Medical University (2022-SR-210).
All of our target patients underwent high-resolution anorectal manometry (HR-ARM) and balloon expulsion test (BET) and completed the required questionnaires.
A high-resolution solid-state anorectal manometry device (Manoscan AR 360; Given Imaging, Yokneam, Israel) with 12 sensors was adopted to evaluate patients’ defecation function. The absolute parameters were assessed as follows: anal resting pressure (20–30 s), anal sphincter length, duration of the sustained squeeze, anal pressure during squeeze (three attempts for a maximum duration of 20–30 s), rectal pressure, and anal residual pressure during attempted defecation (typically 20–30 s, three times, with a 2-min rest interval). Comprehensive parameters were also collected for analysis including manometric defecation index (MDI), recto-anal pressure gradient (RAG), and anal relaxation rate during attempted defecation (11).
The rectal sensation was evaluated by incrementally distending the rectal balloon by 10 mL from 0 to 400 mL, and the thresholds for first constant sensation volume (FCSV), defecatory desire volume (DDV), and maximum tolerable volume (MTV) were recorded.
A previously published dataset of 54 healthy individuals (35 women) assessed by our motility center (Table 1) was used to define the upper limits of normal (95%) for three sensory thresholds (men and women have different upper limits of normal) (10). The healthy individuals did not have any surgical history related to constipation and they all had normal bowel movements.
According to the London Classification published in Jan 2020, RH is defined as an abnormal elevation of ≥2 sensory thresholds while borderline RH refers to one of the three sensory thresholds exceeding the upper limit of the normal range (12).
A 4-cm long balloon filled with 50 mL of warm water was placed in the patient’s rectum while the patient was seated on a commode and was asked to expel the balloon, in privacy. If the subject could not expel the balloon after 1 min of straining, it was deflated and removed and the result was identified as abnormal (13).
Patients who were suspected to suffer from rectal structural diseases such as rectocele or intussusception underwent defecography. The presence of poor opening of the anorectal angle, poor relaxation of the anal canal, or poor expulsive effort generated which is related to retention of more than 50% contrast was defined as abnormal (14).
Patients with FDD were asked about their spontaneous bowel movements (SBMs) (times per week), defecation duration, and stool consistency evaluated by Bristol Stool Formation Scale (BSFS). In addition, Rome IV core criteria for functional constipation were adopted to evaluate symptoms including fewer bowel movements (<3 times per week), straining, feeling incomplete defecation, anal blockage, lumpy or hard stool, and manual maneuvers during the last 6 months (15). In addition to collecting the typical symptoms, we used Patient Assessment of Constipation Symptoms (PAC-SYM) (16) to measure patients’ subjective feelings about constipation, with higher scores indicating more severe symptoms.
General Anxiety Disorder 7-item (GAD-7) (17) and Patient Health Questionnaire-9 (PHQ-9) (18) were adopted to measure anxiety and depression symptoms, respectively. Higher scores suggested more severe symptoms and a score of >5 indicated anxiety or depression state.
The Patient Assessment of Constipation Quality of Life (PAC-QOL) questionnaire specifically assesses constipated patients’ QOL (19). It contains 28 items divided into four subscales (physical discomfort, psychosocial discomfort, worry/anxiety, and satisfaction with treatment). Higher scores showed poorer constipation-related QOL.
Statistical analyses were conducted with SPSS version 26.0. Continuous variables were presented as the mean ± SD or median (interquartile range). Categorical variables were given as relative frequencies. A one-way ANOVA test was used to compare normally distributed variables while a rank-sum test was used to compare non-normally distributed variables. Fisher’s exact test was adopted to analyze categorical variables. The Spearman correlation analysis was applied to find associations between clinical manifestations and three rectal sensory thresholds. And logistic regression was applied to explore related factors of RH in patients with FDD. p-values were corrected for multiple tests with the Bonferroni procedure. p-values less than 0.05 were considered statistically significant.
We enrolled 331 patients with FDD in total, of which 87 (26.3%) had at least one abnormally elevated rectal sensory threshold and 50 (15.1%) had two or three thresholds above the 95% normal upper limit. According to the latest published London Classification of anorectal function, these patients were divided into three groups (non-RH: n = 244 [73.7%]; borderline RH: n = 37 [11.2%]; and RH: n = 50 [15.1%]). Patients in the three groups were similar in BMI and constipation duration. Patients in RH and borderline RH groups were significantly older than those in the non-RH group (p = 0.005 and p = 0.036, respectively). In addition, more male patients were found in the RH group (male/female: 38/12, p < 0.001) compared to those in the non-RH group and borderline RH group. Detailed data are listed in Table 2.
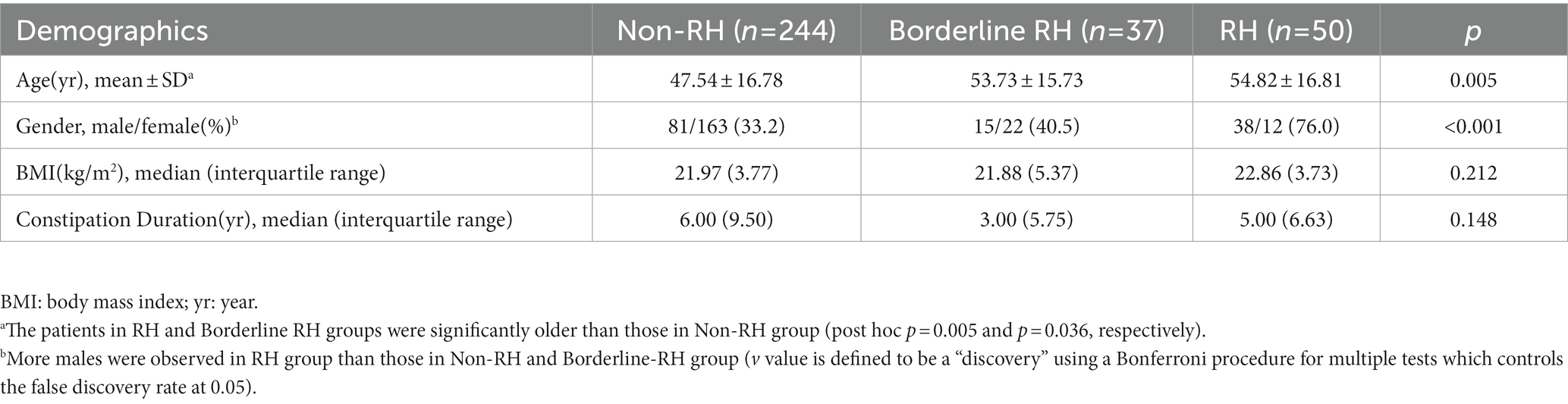
Table 2. Demographic characteristics of patients stratified by rectal sensation in 311 patients with FDD.
Analysis of grouped data suggested that all three rectal sensory thresholds were significantly high in the RH group (Ps < 0.001) but no difference was observed between borderline RH and RH groups (Table 3). Patients with RH showed the lowest anal relaxation rate (p = 0.017), especially lower than those in the non-RH group (p = 0.013). However, the parameters regarding anorectal pressure and pelvic coordination did not differ among the three groups (all Ps > 0.05), which could be referred to in Table 3. As regards to BET, FDD patients with more abnormally elevated sensory thresholds were more likely to fail it, but no significant difference was seen (p = 0.073).
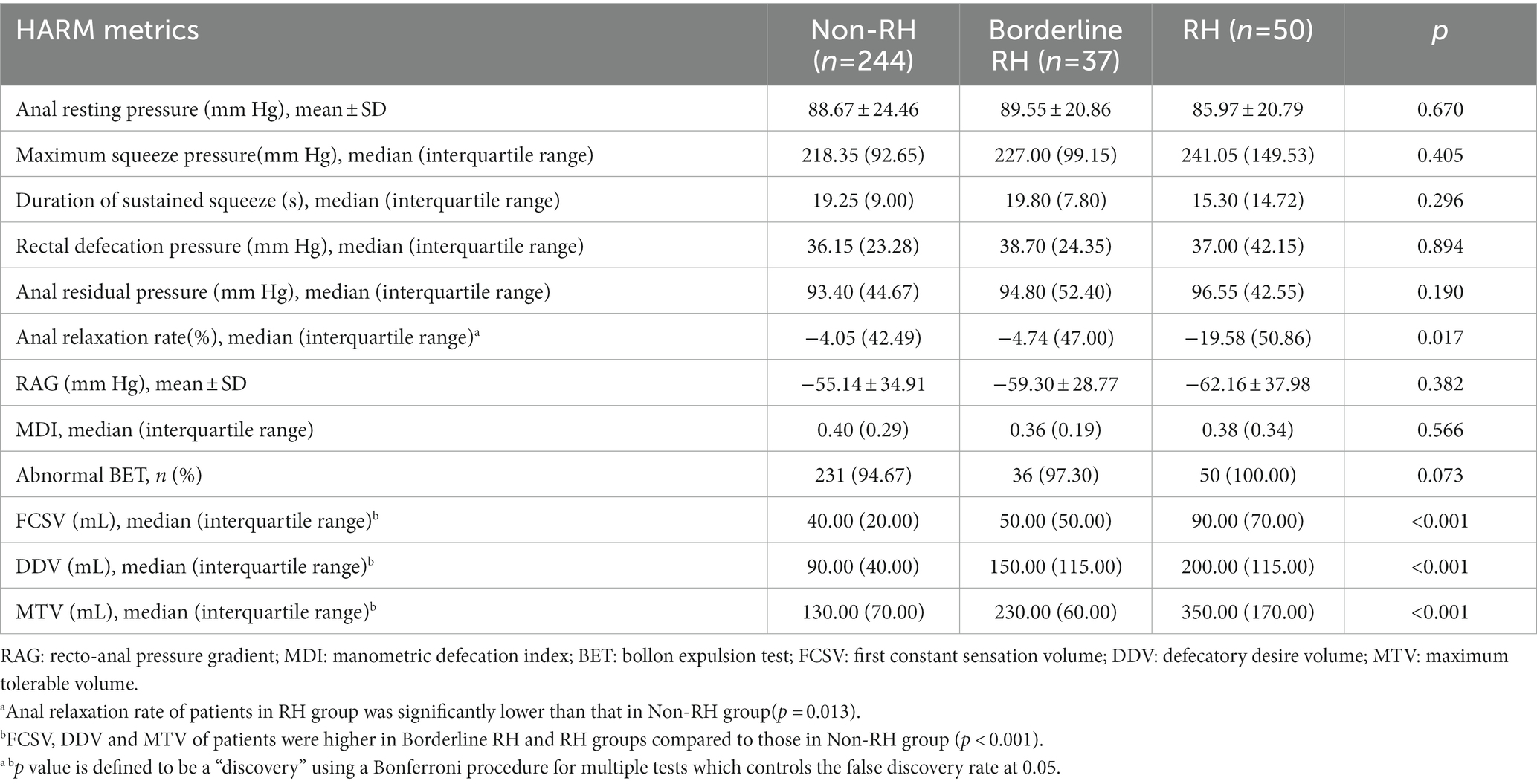
Table 3. Comparisons of rectal/anal pressure, pelvic coordination and rectal sensory thresholds of patients stratified by rectal sensation in 311 patients with FDD.
As shown in Table 4, FDD patients with RH were more likely to suffer abnormally elevated FCSV (p = 0.008), DDV, and MTV compared to patients with borderline RH, especially in DDV and MTV (both Ps < 0.001), which indicated that most patients with FDD tended to have abnormally elevated DDV (45.9% in borderline RH group and 100% in RH group) rather than FCSV and MTV. In the RH group, nearly one-third (n = 16, 32%) of patients with FDD had three elevated rectal sensory thresholds.
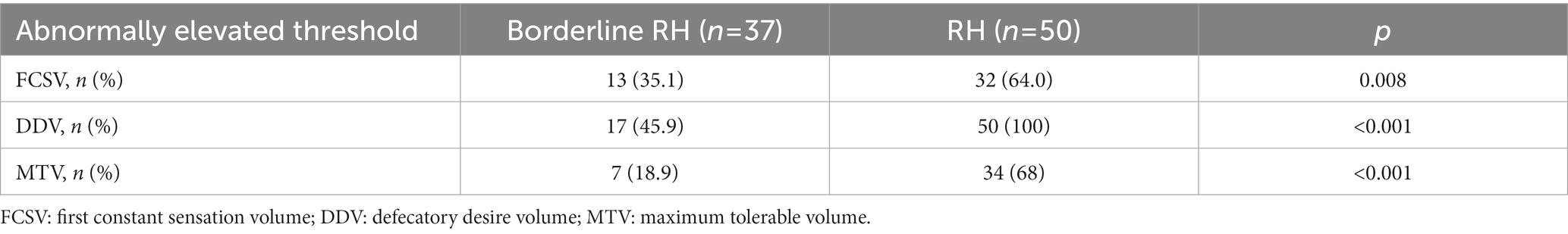
Table 4. Comparison of the occurrence of each abnormally elevated rectal sensory threshold between Borderline RH and RH groups.
There were only weak links between FCSV and anal resting pressure (r = −0.155, p = 0.005), maximum squeeze pressure (r = −0.109, p = 0.047), as well as anal residual pressure (r = −0.148, p = 0.007). No other links between rectal sensory thresholds and motility parameters were found.
Higher score for defecation symptoms (including straining, incomplete or failed defecation, and low stool weights) in PAC-SYM (p = 0.013), lower score for BSFS (p = 0.019), greater proportion of assistance for defecation (p = 0.003), and higher presence of hard stool (p < 0.001) were reported by patients with RH (Table 5). However, no difference in GAD-7, PHQ-9, or PAC-QOL scores was shown in the three groups (all Ps > 0.05). A weak correlation was found between defecation symptoms and mental state (GAD-7: r = 0.329, p = 0.002; PHQ-9: r = 0.371, p < 0.001). In addition, the score for defecation symptom was moderately related to PAC-QOL score (r = 0.570, p < 0.001) as well as scores for sub-scales in PAC-QOL (Physical Discomfort: r = 0.434, p < 0.001; Psychosocial Discomfort: r = 0.50, p < 0.001; and Worry/Anxiety: r = 0.499, p < 0.001).
According to our findings that some variables (age, gender, hard stool, manual maneuvers, feeling incomplete evacuation, feeling anal obstruction) made statistically significant changes at the 10% level and anxiety/depression could also interact with rectal sensation (20), we included them in the logistic regression model. Logistic regression revealed that gender (male) and hard stool were closely related to the occurrence of RH (Figure 1). Furthermore, spearman correlation analysis of clinical manifestations and rectal sensory thresholds suggested that FDD patients with older age and lower BSFS score (indicating hard/lumpy stool) were more likely to suffer abnormally elevated FCSV and DDV. In addition, older patients with higher PHQ-9 scores were prone to have abnormally elevated MTV. No correlation was observed among the PAC-SYM score, SBMs, GAD-7 score, and three rectal sensory thresholds (Table 6). An increasing number of abnormally elevated thresholds suggested a linear relationship with more severe defecation symptoms in PAC-SYM (r = 0.35, p = 0.001).
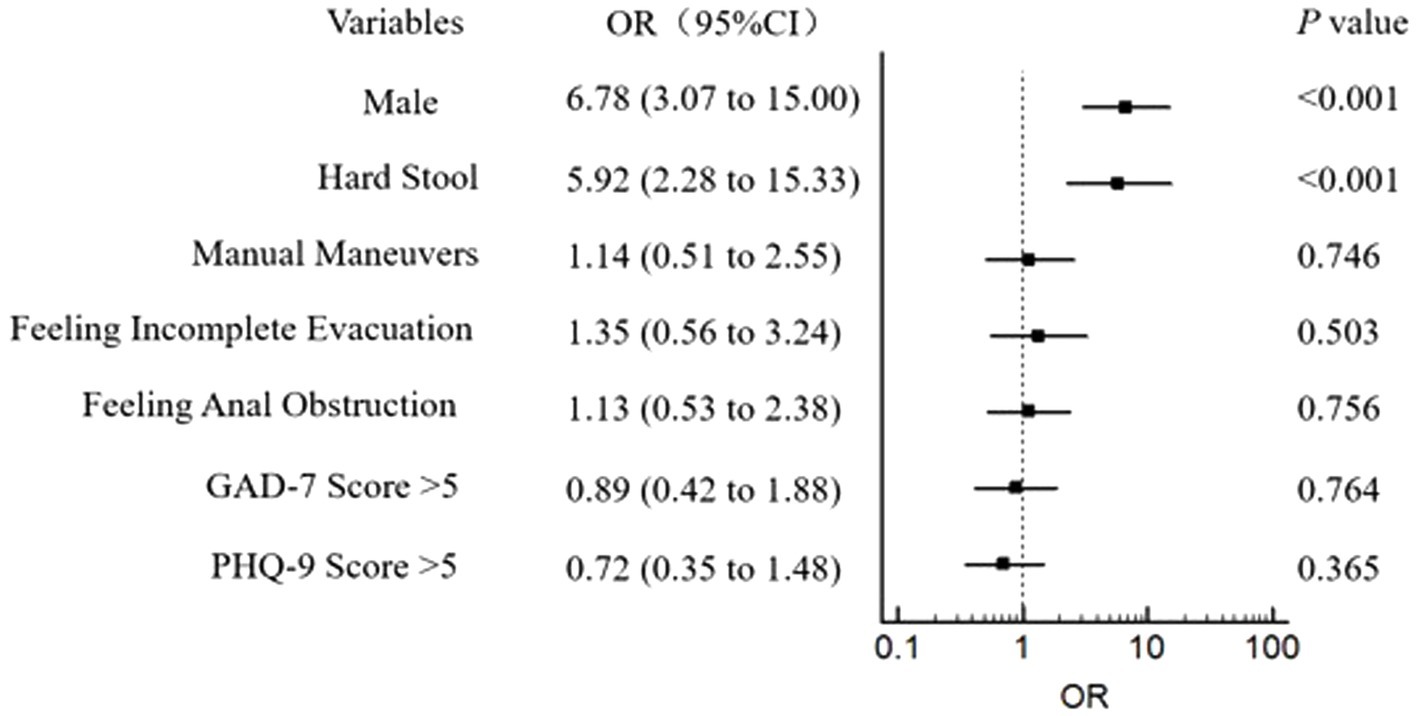
Figure 1. Assocaitions between RH and gender, symptoms of constipation, and mental state in 331 patients of FDD. RH: Rectal hyposensitivity; FDD: Functional defecation disorder; GAD-7: General anxiety disorder 7-item; PHQ-9: Patient health questionnaire-9. The outcome was adjusted for the potential confounding factors: age (years), BMI, and constipation duration.
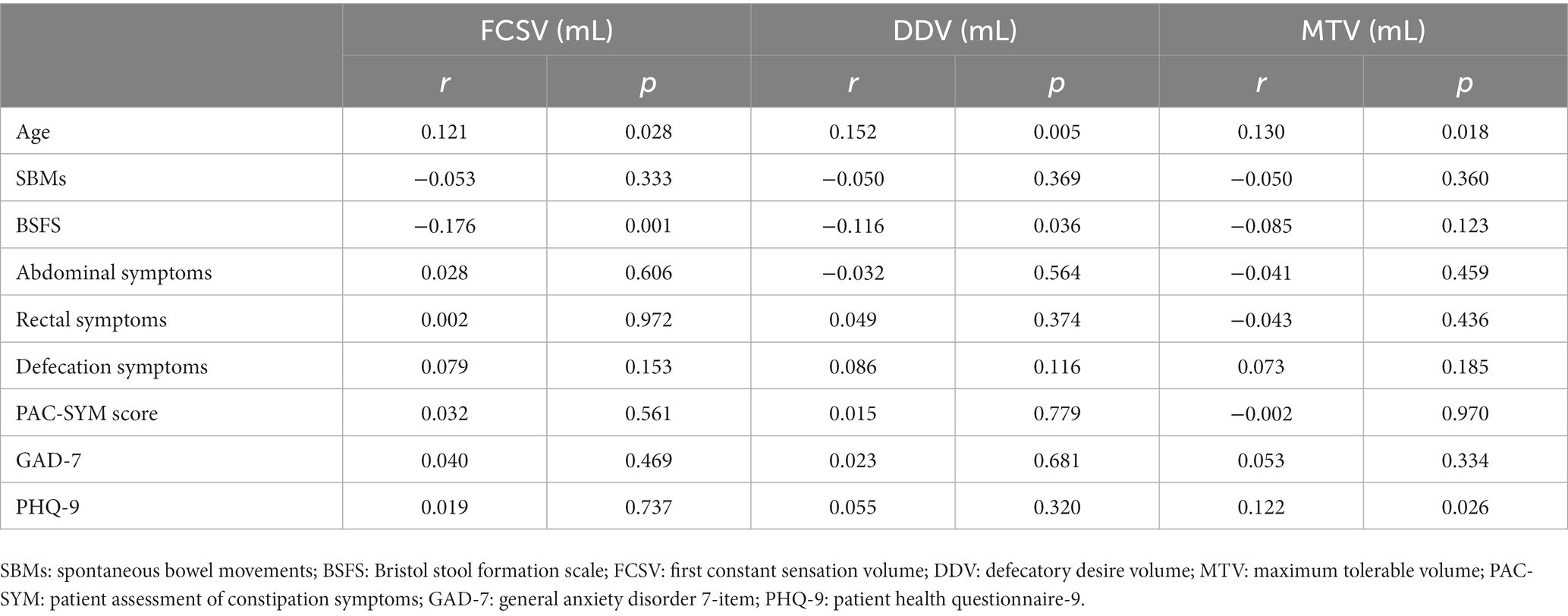
Table 6. Relationship between clinical manifestations and 3 rectal sensory thresholds of 311 patients with FDD.
Rectal hyposensitivity was reported in almost 25% of adult patients with chronic idiopathic constipation (21, 22). RH was associated strongly with pelvic floor dysfunction other than abnormal motility. A recently published study revealed that patients with three abnormally elevated sensory thresholds suffered almost two times as frequent defecation disorder as patients with normal rectal sensation (44.3% vs. 23.2%) (23). However, in a study that enrolled 107 patients with FC (37.4% had RH), no significant difference in RH was observed between the non-FDD and FDD groups (24). The impaired rectal sensation may be negatively associated with abnormal rectal/anal pressure and paradoxical pelvic contraction. Biofeedback therapy (BFT) is the first-line treatment for FDD but patients with RH poorly respond to it (10). Our study might help physicians identify patients with both FDD and RH timely in order to manage them more individually.
We detected RH in 50/311 (15.11%) and borderline RH in 37/331 (11.18%) of patients with FDD. More than a quarter of patients with FDD had one or more abnormally elevated sensory thresholds. This finding is consistent with an observational study where 163 of 667 constipated patients (24.4%) had one or more elevated thresholds (5). It is also suggested that there is a smaller proportion of constipated patients with ≥2 elevated sensory thresholds (13–17%) (23, 25). However, we did not find that patients with FDD suffered more RH than generalized constipated patients.
The underlying mechanism of how RH causes anorectal disorders is still unknown. The intact rectal sensation is fundamental to recto-anal and pelvic floor coordination (26). Some scholars hypothesize that individuals with RH have altered recto-anal reflexes and/or sensorimotor response, and the balloon volumes for inducing their rectoanal inhibitory reflex and contractile reflex were higher (27). Another study showed that patients with RH have reduced rectal wall contractility in response to distension, which likely contributes to failed defecation (28). However, we only found that the anal relaxation rate was lowest in the RH group but no difference in anorectal pressure or presence of pelvic floor disorder was observed among the three groups. As to comparisons of three rectal sensory thresholds, the presence of abnormally elevated FCSV, DDV, and MTV were all higher in patients with RH compared to those with borderline RH and non-RH, especially for DDV which was elevated in all patients with RH. We speculated that DDV might be a useful indicator for impaired rectal sensation. Only weak correlations were seen between FCSV and anal resting pressure, maximum squeeze pressure, and residual pressures, which is of limited clinical significance.
It is demonstrated that an increasing number of elevated sensory thresholds was associated with a more severe constipation phenotype (23). In our study, FDD patients with RH had more severe defecation symptoms. Meanwhile, the BSFS score was lower in these patients, indicating that they suffered from the lumpy or hard stool. The hard stool is closely related to RH in patients with FDD. Thus, more patients with RH needed manual maneuvers to help defecate. The conscious withdrawal of attention from rectal sensations or habitual suppression of the desire to defecate may contribute to impaired call to stool, which could cause rectal impaction and secondary dilatation of the rectum, leading to RH (29–31). The longer stool stays in the colon and rectum, the harder it may become, which could explain the lumpy or hard stool which patients with RH frequently have. Thus, these patients with FDD experience more severe defecation symptoms and need to use digital assistance or enema. Defecation symptom severity was correlated to QOL in these patients, which needs more attention.
We found that patients with RH were older and age was positively correlated to three rectal sensory thresholds, suggesting the decreased rectal sensation might be related to aging. Age-related impairment in the mechanoreceptors of the rectal wall and the pelvic afferent nerves might play a role in this relationship (32). A previous study by our team had a similar finding in a general functional constipation population (10). In addition, the proportion of male patients was high in the RH group. It is known that female patients are prone to constipation but most of them suffer slow transit constipation compared with male patients. A previous study found that constipated male patients were significantly more likely to suffer from defecation disorder than female patients (33). Additionally, our team has found that male patients tended to have much more paradoxical anal sphincter contraction and impaired anal sphincter relaxation (34). We speculated that male patients might have higher stress and various pressure than female patients and they are inclined to suppress the stool calling, which could lead to RH and FDD. Based on the analysis of a large patient cohort, older age and male sex were associated with higher rectal sensory thresholds (35), which agrees with our findings. However, the pathophysiological mechanism is still unknown and warranted to be explored in future studies.
The visceral sensation may be influenced by personality profile, autonomic nervous system function, and psychological phenotype (36, 37). However, little evidence has yet to be found directly in patients with RH. In our study, depression symptom was positively related to MTV, though the link is weak. But anxiety symptom was not related to any sensory threshold. The concept of the brain-gut axis is well recognized and peptide hormones (neuropeptide Y, peptide YY, glucagon-like peptide 1, etc.) released from the gut play a critical part in the interaction between the brain and digestive system (38, 39). Our results revealed that GAD-7 and PHQ-9 scores were positively related to defecation symptom severity. Patients with irritable bowel disease (IBS) (40) mostly have acute rectal feelings and psychological distress may aggravate IBS symptoms (41). It is established that anxiety can enhance visceral feelings (42, 43) but the effect of depression on sensation is controversial. According to our findings, we speculate that depression rather than anxiety plays an important role in blunt rectal sensation. However, logistic regression revealed that anxiety or depression was not related to the occurrence of RH. The association between mental state and RH in patients with FDD has been rarely studied and needs to be explored in future research.
We acknowledged that there are some limitations regarding our study. First, our study focused on patients with FDD in a single tertiary center, which unavoidably ended up with a highly selected population so the results could not be generalized to a wider primary care population. Second, volumetric balloon distension instead of barostat was used to test patients’ rectal sensory thresholds. Constipated patients with RH usually have persistent dilatation of the rectum and greater volumes will be required to stimulate the rectum (44). Thus, constipated patients with RH who have elevated volume thresholds might not have impaired rectal sensation actually. Recording pressure thresholds with barostat rather than volume thresholds is of more physiological significance (45). Nevertheless, in routine clinical practice, volumetric balloon distension is well accepted and often used (12, 46). In the future, testing rectal pressure thresholds with barostat may be a better and more rigorous method to identify RH. Finally, the link between constipation and RH is well established, but the cause–effect relationship in observational studies is still unclear. RH could lead to harder stool and more difficult defecation and long duration or severe constipation may result in a dilated rectum and abnormal rectal wall compliance which impairs rectal sensation and vice versa. Advanced prospective researches and cohort studies are in need.
This study has summarized the characteristics of FDD patients with RH by investigating symptomology, mental state, QOL, and functional tests. It is shown that patients with RH are older, more male patients, and vulnerable to suffering more severe defecation symptoms. Elevated DDV is most frequently seen in FDD patients with RH. Although abnormal motility and sensation may interact with each other and induce defecation disorder, we did not find specific links between them. Older age, gender (male), and lumpy or hard stool are related factors of RH in FDD and depression is associated with elevated MTV. The above findings may help physicians identify high-risk patients more efficiently. Thus, FDD patients with RH could get much better management in time.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YJ and YT designed the study. YJ, YW, and MW collected and analyzed the data. YJ wrote the manuscript. YJ, YT, and LL revised the manuscript. All authors contributed to the article and approved the submitted version.
This study is supported by National Natural Science Foundation of China (No. 81870378 and 82170556) for YT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
RH, Rectal hyposensitivity; FDD, Functional defecation disorder; QOL, Quality of life; HR-ARM, High-resolution anorectal manometry; BET, Balloon expulsion test; MDI, Manometric defecation index; RAG, Recto-anal pressure gradient; FCSV, First constant sensation volume; DDV, Defecatory desire volume; MTV, Maximum tolerable volume; SBMs, Spontaneous bowel movements; BSFS, Bristol Stool Formation Scale; PAC-SYM, Patient assessment of constipation symptoms; GAD-7, General anxiety disorder 7-item; PHQ-9, Patient health questionnaire-9; PAC-QOL, Patient assessment of constipation quality of life.
1. Bharucha, AE, and Rao, SS. An update on anorectal disorders for gastroenterologists. Gastroenterology. (2014) 146:37–45.e2. doi: 10.1053/j.gastro.2013.10.062
2. Longstreth, GF, Thompson, WG, Chey, WD, Houghton, LA, Mearin, F, and Spiller, RC. Functional bowel disorders. Gastroenterology. (2006) 130:1480–91. doi: 10.1053/j.gastro.2005.11.061
3. Rao, SS, Tuteja, AK, Vellema, T, Kempf, J, and Stessman, M. Dyssynergic defecation: demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol. (2004) 38:680–5. doi: 10.1097/01.mcg.0000135929.78074.8c
4. Palit, S, Lunniss, PJ, and Scott, SM. The physiology of human defecation. Dig Dis Sci. (2012) 57:1445–64. doi: 10.1007/s10620-012-2071-1
5. Gladman, MA, Scott, SM, Chan, CL, Williams, NS, and Lunniss, PJ. Rectal hyposensitivity: prevalence and clinical impact in patients with intractable constipation and fecal incontinence. Dis Colon Rectum. (2003) 46:238–46. doi: 10.1007/s10350-004-6529-x
6. Scott, SM, van den Berg, MM, and Benninga, MA. Rectal sensorimotor dysfunction in constipation. Best Pract Res Clin Gastroenterol. (2011) 25:103–18. doi: 10.1016/j.bpg.2011.01.001
7. Gladman, MA, Scott, SM, Williams, NS, and Lunniss, PJ. Clinical and physiological findings, and possible aetiological factors of rectal hyposensitivity. Br J Surg. (2003) 90:860–6. doi: 10.1002/bjs.4103
8. Gladman, MA, Lunniss, PJ, Scott, SM, and Swash, M. Rectal hyposensitivity. Am J Gastroenterol. (2006) 101:1140–51. doi: 10.1111/j.1572-0241.2006.00604.x
9. Burgell, RE, and Scott, SM. Rectal hyposensitivity. J Neurogastroenterol Motil. (2012) 18:373–84. doi: 10.5056/jnm.2012.18.4.373
10. Yu, T, Qian, D, Zheng, Y, Jiang, Y, Wu, P, and Lin, L. Rectal hyposensitivity is associated with a Defecatory disorder but not delayed colon transit time in a functional constipation population. Medicine. (2016) 95:e3667. doi: 10.1097/MD.0000000000003667
11. Seong, MK. Assessment of functional defecation disorders using anorectal manometry. Ann Surg Treat Res. (2018) 94:330–6. doi: 10.4174/astr.2018.94.6.330
12. Carrington, EV, and Heinrich, H. The international anorectal physiology working group (IAPWG) recommendations: standardized testing protocol and the London classification for disorders of anorectal function. J Gastrointest Motil. (2020) 32:e13679. doi: 10.1111/nmo.13679
13. Woo, M, Pandey, A, Gill, H, Li, D, Buresi, M, Nasser, Y, et al. Manometric parameters, when measured with the 3-dimensional high-definition anorectal manometry probe, poorly predict prolonged balloon expulsion time. Neurogastroenterol Motil. (2022) 34:e14180. doi: 10.1111/nmo.14180
14. Bharucha, AE. Update of tests of colon and rectal structure and function. J Clin Gastroenterol. (2006) 40:96–103. doi: 10.1097/01.mcg.0000196190.42296.a9
15. Mearin, F, Lacy, BE, Chang, L, Chey, WD, Lembo, AJ, Simren, M, et al. Bowel disorders. Gastroenterology. (2016). doi: 10.1053/j.gastro.2016.02.031
16. Neri, L, Conway, PM, and Basilisco, G. Confirmatory factor analysis of the patient assessment of constipation-symptoms (PAC-SYM) among patients with chronic constipation. Qual Life Res Int J Qual Life Asp Treat Care Rehab. (2015) 24:1597–605. doi: 10.1007/s11136-014-0886-2
17. Kroenke, K, Spitzer, RL, Williams, JB, and Löwe, B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. (2010) 32:345–59. doi: 10.1016/j.genhosppsych.2010.03.006
18. Wang, W, Bian, Q, Zhao, Y, Li, X, Wang, W, du, J, et al. Reliability and validity of the Chinese version of the patient health questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry. (2014) 36:539–44. doi: 10.1016/j.genhosppsych.2014.05.021
19. Marquis, P, de la Loge, C, Dubois, D, McDermott, A, and Chassany, O. Development and validation of the patient assessment of constipation quality of life questionnaire. Scand J Gastroenterol. (2005) 40:540–51. doi: 10.1080/00365520510012208
20. Josefsson, A, Rosendahl, A, Jerlstad, P, Näslin, G, Törnblom, H, and Simrén, M. Visceral sensitivity remains stable over time in patients with irritable bowel syndrome, but with individual fluctuations. Neurogastroenterol Motil. (2019) 31:e13603. doi: 10.1111/nmo.13603
21. Shouler, P, and Keighley, MR. Changes in colorectal function in severe idiopathic chronic constipation. Gastroenterology. (1986) 90:414–20. doi: 10.1016/0016-5085(86)90941-8
22. Gladman, MA, Aziz, Q, Scott, SM, Williams, NS, and Lunniss, PJ. Rectal hyposensitivity: pathophysiological mechanisms. Neurogastroenterology and motility: the official journal of the European gastrointestinal motility. Society. (2009) 21:e4-5:508–16. doi: 10.1111/j.1365-2982.2008.01216.x
23. Vollebregt, PF, Burgell, RE, Hooper, RL, Knowles, CH, and Scott, SM. Clinical impact of rectal hyposensitivity: a cross-sectional study of 2,876 patients with refractory functional constipation. Am J Gastroenterol. (2021) 116:758–68. doi: 10.14309/ajg.0000000000001039
24. Lee, TH, Lee, JS, Hong, SJ, Jeon, SR, Kwon, SH, Kim, WJ, et al. Rectal hyposensitivity and functional anorectal outlet obstruction are common entities in patients with functional constipation but are not significantly associated. Korean J Intern Med. (2013) 28:54–61. doi: 10.3904/kjim.2013.28.1.54
25. Vollebregt, PF, Hooper, RL, Farmer, AD, Miller, J, Knowles, CH, and Scott, SM. Association between opioid usage and rectal dysfunction in constipation: a cross‐sectional study of 2754 patients. J Gastrointest Motil. (2020) 32:e13839. doi: 10.1111/nmo.13839
26. Scott, SM, and Lunniss, PJ. Rectal hyposensitivity and functional hindgut disorders: cause and effect or an epiphenomenon? J Pediatr Gastroenterol Nutr. (2011) 53:S47–9.
27. Remes-Troche, JM, de-Ocampo, S, Valestin, J, and Rao, SSC. Rectoanal reflexes and sensorimotor response in rectal hyposensitivity. Dis Colon Rectum. (2010) 53:1047–54. doi: 10.1007/DCR.0b013e3181dcb2d6
28. Schouten, WR, Gosselink, MJ, Boerma, MO, and Ginai, AZ. Rectal wall contractility in response to an evoked urge to defecate in patients with obstructed defecation. Dis Colon Rectum. (1998) 41:473–9. doi: 10.1007/BF02235762
29. lunniss,, gladman,, benninga,, and rao,. Pathophysiology of evacuation disorders. Neurogastroenterol Motil. (2009) 21:31–40. doi: 10.1111/j.1365-2982.2009.01402.x
30. Harraf, F, Schmulson, M, Saba, L, Niazi, N, Fass, R, Munakata, J, et al. Subtypes of constipation predominant irritable bowel syndrome based on rectal perception. Gut. (1998) 43:388–94. doi: 10.1136/gut.43.3.388
31. Mimura, T, Nicholls, T, Storrie, JB, and Kamm, MA. Treatment of constipation in adults associated with idiopathic megarectum by behavioural retraining including biofeedback. Colorectal Dis. (2002) 4:477–82. doi: 10.1046/j.1463-1318.2002.00372.x
32. Sloots, CE, Felt-Bersma, RJ, Cuesta, MA, and Meuwissen, SG. Rectal visceral sensitivity in healthy volunteers: influences of gender, age and methods. Neurogastroenterol Motil. (2000) 12:361–8. doi: 10.1046/j.1365-2982.2000.00210.x
33. Zhao, Y, Ren, X, Qiao, W, Dong, L, He, S, and Yin, Y. High-resolution anorectal manometry in the diagnosis of functional defecation disorder in patients with functional constipation: a retrospective cohort study. J Neurogastroenterol Motil. (2019) 25:250–7. doi: 10.5056/jnm18032
34. Jiang, Y, Wang, Y, Tang, Y, and Lin, L. Clinical value of positive BET and pelvic floor dyssynergia in Chinese patients with functional defecation disorder. Scand J Gastroenterol. (2022) 57:775–82. doi: 10.1080/00365521.2022.2039282
35. Mazor, Y, Trieu, RQ, Prott, G, Jones, M, Ejova, A, Kellow, J, et al. Volumetric rectal perception testing: is it clinically relevant? Results from a large patient cohort. Am J Gastroenterol. (2021) 116:2419–29. doi: 10.14309/ajg.0000000000001526
36. Paine, P, Worthen, SF, Gregory, LJ, Thompson, DG, and Aziz, Q. Personality differences affect brainstem autonomic responses to visceral pain. Neurogastroenterol Motil. (2009) 21:1155–e98. doi: 10.1111/j.1365-2982.2009.01348.x
37. Coen, SJ, Kano, M, Farmer, AD, Kumari, V, Giampietro, V, Brammer, M, et al. Neuroticism influences brain activity during the experience of visceral pain. Gastroenterology. (2011) 141:909–17.e1. doi: 10.1053/j.gastro.2011.06.008
38. Lach, G, Schellekens, H, Dinan, TG, and Cryan, JF. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. (2018) 15:36–59. doi: 10.1007/s13311-017-0585-0
39. Sun, LJ, Li, JN, and Nie, YZ. Gut hormones in microbiota-gut-brain cross-talk. Chin Med J. (2020) 133:826–33. doi: 10.1097/CM9.0000000000000706
40. Midenfjord, I, and Polster, A. Anxiety and depression in irritable bowel syndrome: exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol Motil. (2019) 31:e13619. doi: 10.1111/nmo.13619
41. Fond, G, Loundou, A, Hamdani, N, Boukouaci, W, Dargel, A, Oliveira, J, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. (2014) 264:651–60. doi: 10.1007/s00406-014-0502-z
42. Pellissier, S, and Bonaz, B. The place of stress and emotions in the irritable bowel syndrome. Vitam Horm. (2017) 103:327–54. doi: 10.1016/bs.vh.2016.09.005
43. Gunter, WD, Shepard, JD, Foreman, RD, Myers, DA, and Beverley,. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav. (2000) 69:379–82. doi: 10.1016/S0031-9384(99)00254-1
44. Gattuso, JM, and Kamm, MA. Clinical features of idiopathic megarectum and idiopathic megacolon. Gut. (1997) 41:93–9. doi: 10.1136/gut.41.1.93
45. Kamal, AN, Garcia, P, and Clarke, JO. Under pressure: do volume-based measurements define rectal hyposensitivity in clinical practice? Dig Dis Sci. (2019) 64:1062–3. doi: 10.1007/s10620-019-05613-7
Keywords: functional defecatory disorder, rectal hyposensitivity, Bristol stool formation scale, age, male
Citation: Jiang Y, Wang Y, Wang M, Lin L and Tang Y (2023) Clinical significance and related factors of rectal hyposensitivity in patients with functional defecation disorder. Front. Med. 10:1119617. doi: 10.3389/fmed.2023.1119617
Received: 09 December 2022; Accepted: 26 January 2023;
Published: 21 February 2023.
Edited by:
Amir Mari, Nazareth Hospital EMMS, IsraelReviewed by:
Zhen Li, Qilu Hospital, Shandong University, ChinaCopyright © 2023 Jiang, Wang, Wang, Lin and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yurong Tang, ✉ c3RvcnlfdHlyQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.