- 1Graduate School, Tianjin Medical University, Tianjin, China
- 2Department of Nuclear Medicine, The First Central Clinical School, Tianjin Medical University, Tianjin, China
- 3Department of Ultrasound, Shandong Provincial Maternal and Child Health Care Hospital, Jinan, China
Objective: Prenatal ultrasound features, associated anomalies and genetic abnormalities of microtia cases were analyzed to explore the feasibility and value of prenatal ultrasound for the diagnosis of microtia.
Methods: The ultrasonographic features, associated anomalies, chromosome examination results and follow-up results of 81 fetuses with congenital microtia were analyzed retrospectively.
Results: Among the 81 fetuses with microtia diagnosed after birth, 2 cases were missed diagnosis on prenatal ultrasound, and 1 case was diagnosed as unilateral microtia by prenatal ultrasound but was found to be bilateral microtia after birth. Microtia was accompanied by an accessory auricle in 4 cases (4.94%) and low-set ears in 7 cases (8.64%). 22 cases (27.16%) were complicated with other structural anomalies, including 11 cases (13.58%) of cardiac anomalies, 7 cases (8.64%) of ultrasonographic soft marker anomalies, 6 cases (7.41%) of facial anomalies, 6 cases (7.41%) of nervous system anomalies, 3 cases (3.70%) of urogenital system anomalies, 3 cases (3.70%) of digestive tract anomalies and 2 cases (2.47%) of limb anomalies. Chromosome karyotype analysis and gene detection were performed in 44 cases. Trisomy 18, trisomy 13, trisomy 21, pericentric inversion of chromosome 9, partial loss of heterozygosity on chromosome 14, 22q11 microdeletion and a normal karyotype were found in 2 cases, 2 cases, 3 cases, 1 case, 1 case, 1 case, and 34 cases, respectively.
Conclusion: In summary, microtia is often accompanied by congenital defects of other organs and structures, especially the heart and face, and prenatal ultrasound diagnosis of microtia and associated anomalies is of important clinical significance.
1. Introduction
Congenital microtia is a common congenital malformation presenting with external birth defects, which are also often accompanied by atresia and stricture of the external acoustic meatus and middle ear deformity, leading to conductive deafness and affecting the development of hearing and language. An external auricle length less than twice the standard deviation of the mean fetal auricle length at the same gestational age indicates microtia (1, 2).
Fetal ear examination is not a routine item in prenatal ultrasound screening, but microtia and associated anomalies can be detected by prenatal ultrasound, thereby guiding patients to receive targeted screening and providing a basis for prognostic evaluations of fetuses with microtia.
In this study, fetuses with microtia in our study site from July 2017 to July 2022 were retrospectively analyzed, and ultrasound features, common associated anomalies, and genetic features are summarized.
2. Materials and methods
2.1. Patients
Fetuses diagnosed with microtia by postnatal diagnosis in our hospital from July 2017 to July 2022 were selected.
The inclusion criteria were as follows: (1) fetuses whose mothers had a definite gestational age (determined by the last menstrual period and ultrasound in the first trimester), (2) those diagnosed with microtia that was confirmed after birth or induced labor, and (3) those with good-quality images that could be retrospectively analyzed. 81 fetuses meeting the diagnostic criteria for microtia by prenatal ultrasound were included in the study. Two cases were missed by prenatal ultrasound. One case was one of monochorionic diamniotic twin pregnancies, and the other 80 cases were singleton pregnancies. The median age of the pregnant women was 30 years (19 ~ 39 years), and the median gestational age at diagnosis was 24+1 weeks (15+1 ~ 33+3 weeks).
2.2. Image acquisition
Ultrasonography was performed using a Voluson E10 ultrasonography machine (GE Healthcare, Zipf, Austria), with a RAB2-5 (2.5 ~ 5 MHz) transabdominal volume convex probe.
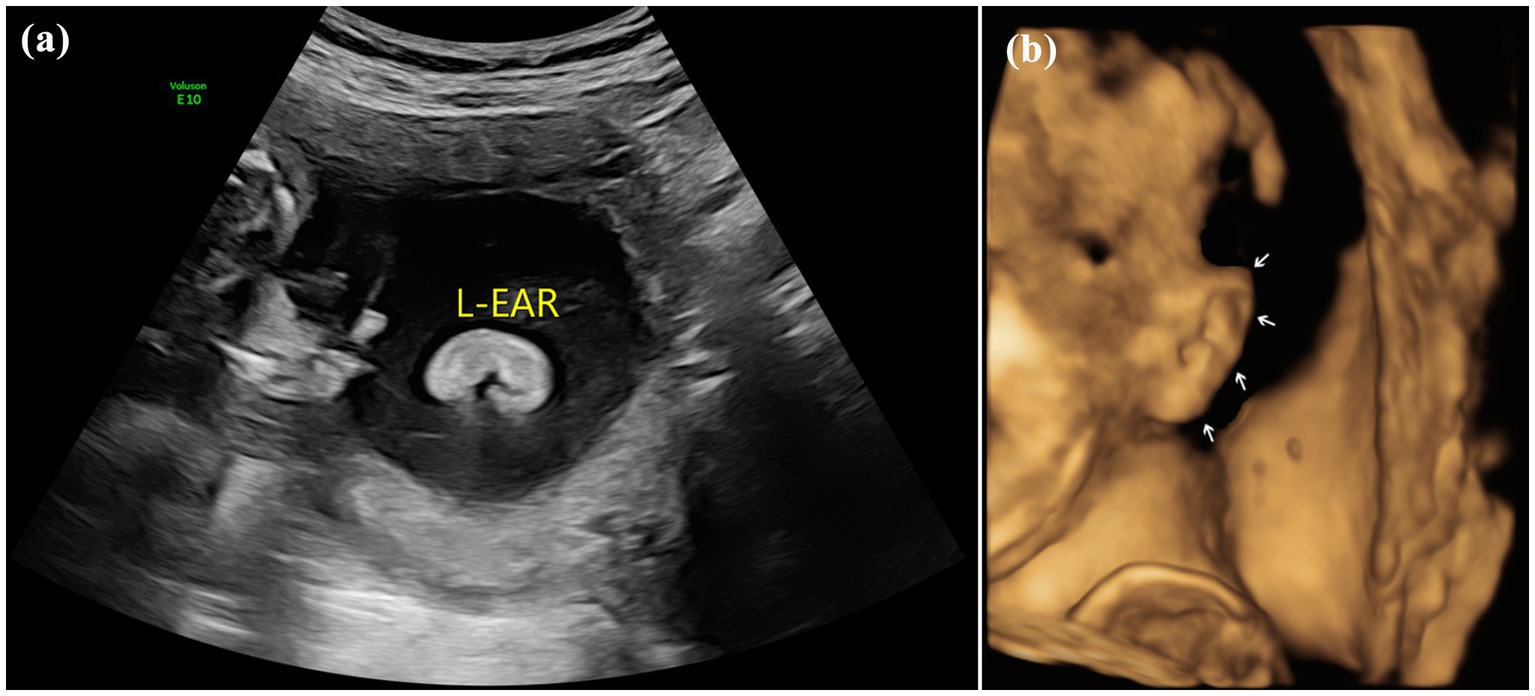
Pregnant women were placed in the supine position under obstetric conditions. The structural development of the fetuses was systematically screened by ultrasound, and biological diameters were measured. In our study site, fetal auricles were generally observed on the parasagittal plane of the temporal bone, and the probe was deflected left and right on the sagittal plane of the fetal brain or face to display the bilateral auricles. Multiplane scans (including the coronal plane and cervical posterior transverse oblique plane) and 3D imaging were further performed in cases with suspected auricle abnormality (Figure 1).
2.3. Methods
The position, symmetry, size and shape of the bilateral auricles were observed. If the above structures of the fetus failed to be observed due to fetal position, the pregnant woman was instructed to change her position or wait for the fetus to turn to an observable position. In cases of suspected microtia, the length of the auricle was measured 3 times and averaged. The principle of minimum energy for prenatal ultrasound diagnosis was followed.
The cases meeting the inclusion criteria were screened, as summarized in Table 1 and their ultrasound images were retrospectively analyzed. The following variables were analyzed and summarized: maternal age, gestational age at diagnosis, ultrasound features, associated anomalies, and genetic features.
3. Results
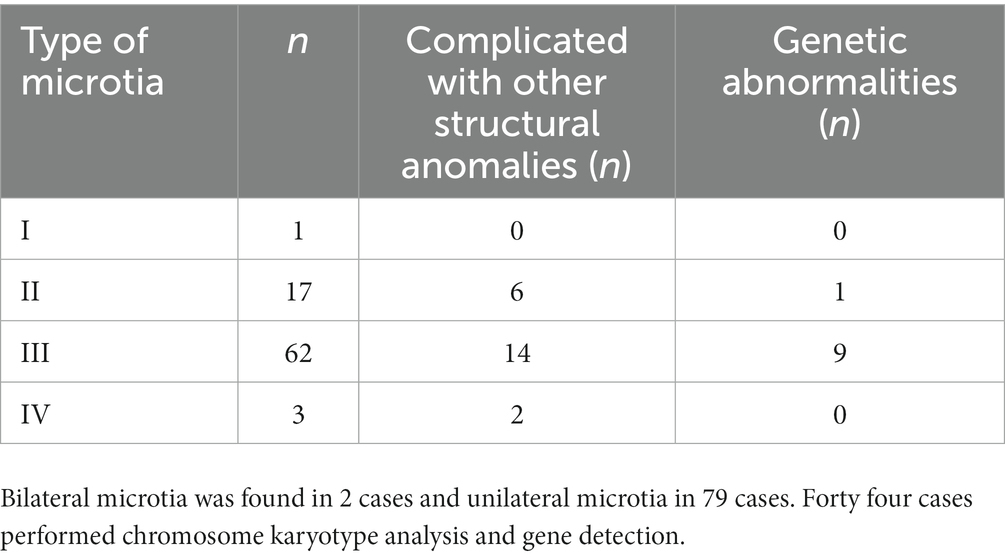
As indicated in research literature on the standard for the normal diameter of the fetal auricle, an external auricle length less than twice the standard deviation of the mean fetal auricle length at the same gestational age indicates microtia (1). In this study, all 81 cases met the diagnostic criteria for microtia and were accompanied by varying degrees of morphological abnormalities. Microtia can be morphologically classified into 4 types from mild to severe: type I: mild deformity and a slightly small auricle with a clear structure; type II: moderate deformity and a small auricle with a partially preserved structure (Figure 2); type III: severe deformity with only partial auricular cartilage and earlobe preserved, accompanied by atresia of external acoustic meatus (Figure 3); and type IV: anotia with atresia of the external acoustic meatus.
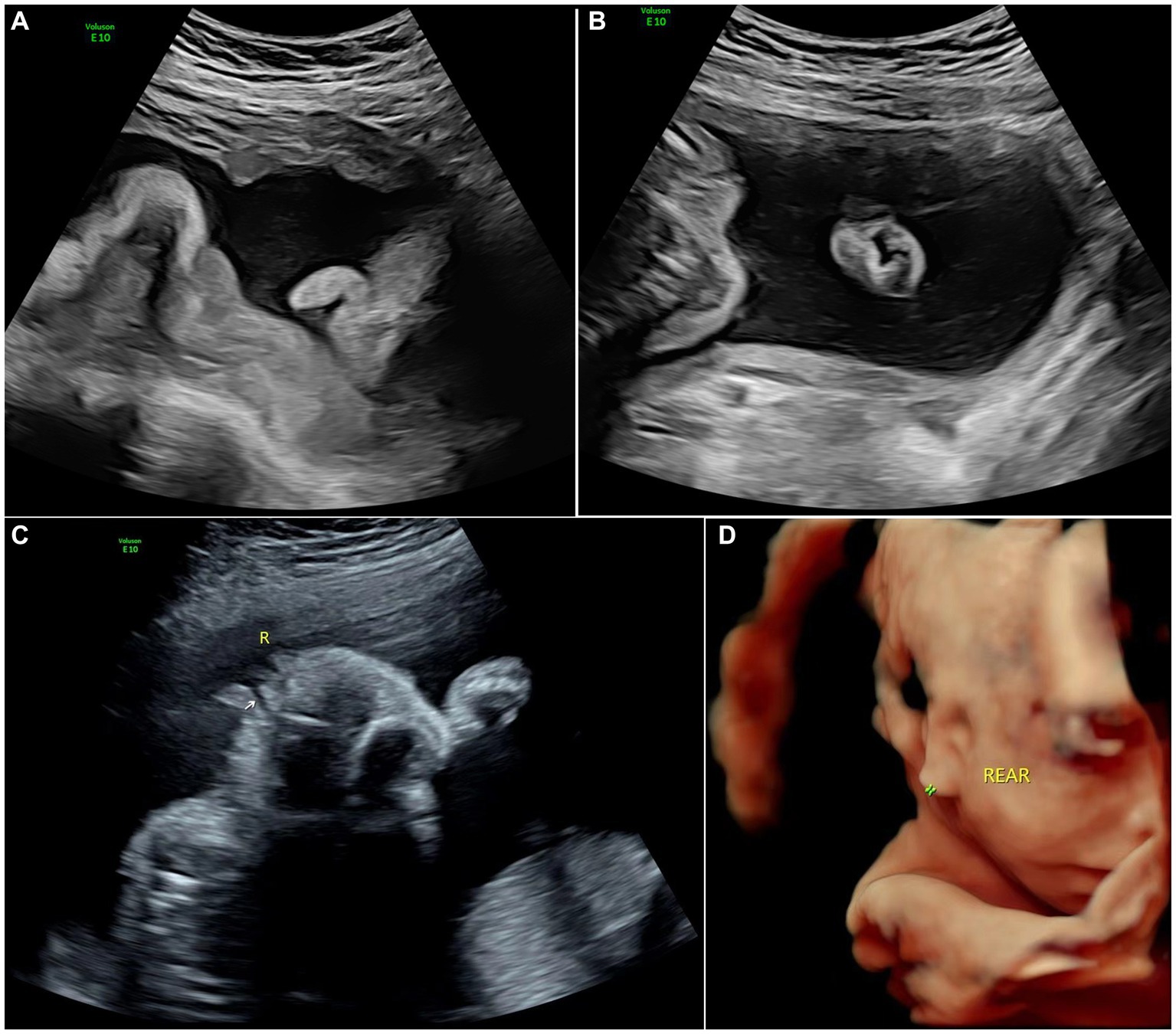
Figure 2. Images of type II of microtia: (A) 2D sonography of microtia; (B) 2D sonography of contralateral ear(normal); (C) 2D sonography of unilateral microtia type II with normal external acoustic meatus; and (D) 3D sonography of Unilateral microtia type II.
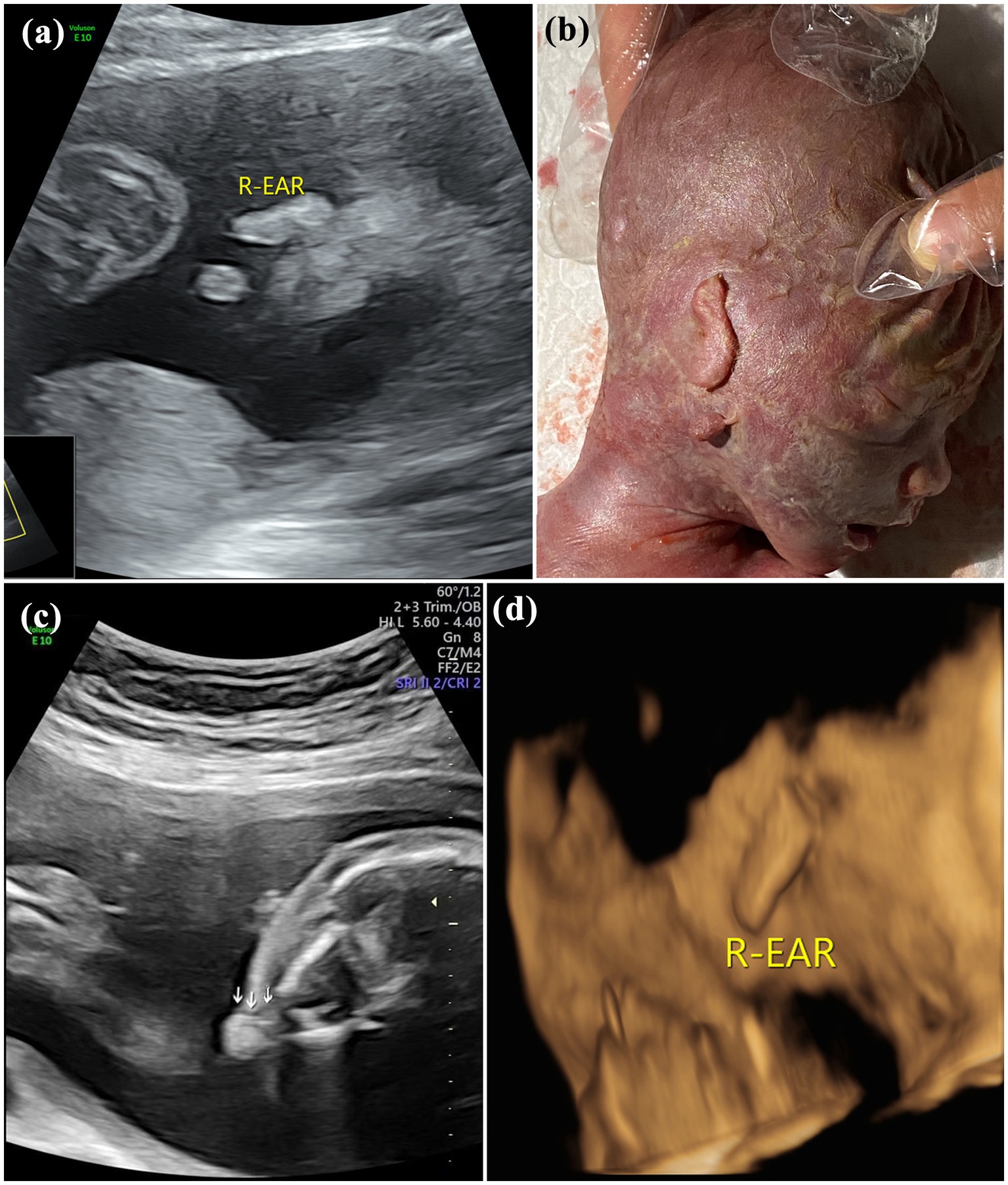
Figure 3. Images of type III of microtia: (A) 2D sonography and (B) photo (induced labor) of microtia type III accompanied by an accessory auricle; (C) 2D sonography of unilateral microtia type III accompanied by atresia of external acoustic meatus; and (D) 3D sonography of Unilateral microtia type III.
Among the 81 fetuses with microtia diagnosed after birth or induced labor, 2 cases were missed diagnosis on prenatal ultrasound, and 1 case was diagnosed as unilateral microtia by prenatal ultrasound but was found to be bilateral microtia after birth. The concordance rate of prenatal ultrasound diagnosis was 96.3%. Bilateral microtia was found in 2 cases and unilateral microtia in 79 cases, including 1 case of grade I, 17 cases of grade II, 62 cases of grade III and 3 cases of grade IV. Microtia was accompanied by an accessory auricle in 4 cases and low-set ears in 7 cases.
Twenty-two cases (27.16%) were complicated with other structural anomalies, including 11 cases (13.58%) of cardiac anomalies, 7 cases (8.64%) of ultrasonographic soft marker anomalies, 6 cases (7.41%) of facial anomalies, 6 cases (7.41%) of nervous system anomalies, 3 cases (3.70%) of urogenital system anomalies, 3 cases (3.70%) of digestive tract anomalies, and 2 cases (2.47%) of limb anomalies, as shown in Table 2.
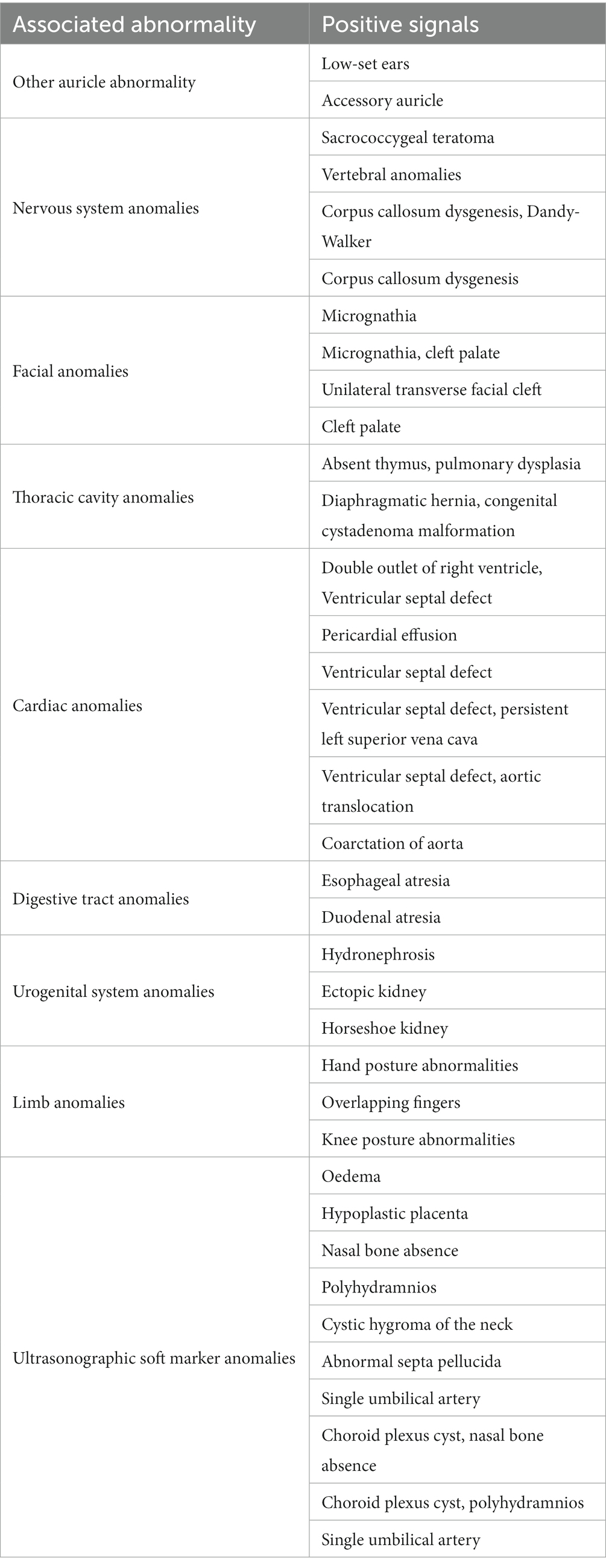
Table 2. Prenatal ultrasound features, associated anomalies and genetic abnormalities of 81 fetuses with congenital microtia.
Chromosome karyotype analysis and gene detection were performed in 44 cases. Trisomy 18, trisomy 13, trisomy 21, pericentric inversion of chromosome 9, partial loss of heterozygosity on chromosome 14, 22q11 microdeletion and a normal karyotype were found in 2 cases, 2 cases, 3 cases, 1 case, 1 case, 1 case, and 34 cases, respectively.
4. Discussion
The external ear consists of the auricle and the external acoustic meatus. As important auditory organs, the auricle and the external acoustic meatus are mainly responsible for sound collection and aesthetics. The auricle is formed in the first branchial groove and the adjacent first and second branchial arches at the 5th–6th weeks of embryo development (3, 4). At the 20th week of embryo development, the anatomical morphology of the auricle is similar to that of adults. Blockage of this developmental process in the embryo can result in microtia characterized by a range of developmental anomalies of the auricle, including partial structural defects of the auricle and complete structural defects of the external ear, which are often accompanied by atresia of the external acoustic meatus, low-set ears and middle ear deformity along with varying degrees of conductive hearing impairment (5).
The incidence of congenital microtia in fetuses is 1‱–3‱, and the number of unilateral microtia cases is 3–5 times that of bilateral microtia cases, with unilateral microtia being more common in the right ear and in males. The incidence of microtia is positively correlated with maternal age, with an older age corresponding to a higher incidence. Due to ethnic differences, the Asian population is more susceptible to microtia (6–9).
2D ultrasound is the preferred method for fetal auricle observation. As reported in the literature, the second trimester is the best time to observe the fetal ear morphology (10). In this study, the median gestational age at diagnosis was 24+1 weeks, with a display rate of 97.53%, which is consistent with the conclusion in the above literature. Ultrasonically, the normal fetal auricle shows a clear and bright “C”- or “S”-type medium echo (4). The left and right auricles are symmetrical and almost equal in size. The normal auricle length measured on the parasagittal plane is linearly correlated with gestational age. The main prenatal ultrasound features of microtia include an obviously smaller length diameter of the auricle on the affected side than that on the unaffected side, disappearance of the normal auricle morphology on the affected side, which is replaced by punctate, lumpy or abnormal soft tissue echoes, and obvious asymmetry in the size and morphology of the bilateral fetal auricles on the horizontal transverse plane and coronal plane. In addition to the length of the auricles, the morphology and position of the auricles should also be observed on fetal auricle examination. In cases of fetal auricle abnormalities, the contralateral auricle should be examined carefully, and whether it is accompanied by other developmental abnormalities should be assessed. In this study, 1 case was diagnosed with unilateral microtia by prenatal ultrasound but with bilateral microtia of different degrees after birth and induced labor, and mild deformity occurred on the side with the missed diagnosis. Therefore, using the contralateral auricle as a reference for diagnosis is not recommended. Additionally, 2 cases of missed diagnosis on prenatal ultrasound were noted, but they were diagnosed after birth. According to the retrospective analysis of the images, only the unilateral auricle was shown in 1 case in the first ultrasound examination due to fetal position limitation, and the pregnant woman was instructed to undergo the examination again after a half-hour. However, the ipsilateral auricle was again mistaken for the contralateral auricle in the image. In another case, an image of the auricle was not obtained. 3D images have advantages in stereoscopic visual display of auricle deformity. However, due to the limitation of acquisition conditions including fetal position, amniotic fluid volume and other factors, 3D images acquisition was only performed in some cases.
Microtia is often accompanied by other systemic deformities. As described by Ye et al. (11) in a study involving 672 microtia patients, 1 or multiple associated abnormalities were found in 293 patients, including ear-face-neck abnormalities (40% of all associated abnormalities) and musculoskeletal system and cardiovascular system abnormalities. Research also suggests that microtia is often associated with renal dysplasia (12), with poorer auricle development corresponding to a higher risk of associated anomalies (13). Guo et al. (14) conducted a study on microtia in fetuses after birth and found that the prevalence of congenital heart disease among microtia patients is higher than that among the general population. Among the associated anomalies in this study, cardiac anomalies had the highest incidence, followed by ultrasonographic soft marker anomalies, facial anomalies and nervous system anomalies. The incidence of fetal microtia with cardiac anomalies remains high.
The pathogenesis of congenital microtia is still unclear but may involve multiple genetic and environmental factors. Microtia can be caused by many environmental risk factors, such as maternal anemia during pregnancy, diabetes mellitus, elderly age, multiple pregnancy and race. Some studies have also shown that tretinoin, thalidomide and mycophenolate mofetil are closely associated with microtia (15, 16).
Fetal auricle examination is not covered in prenatal ultrasound screening, but many recent studies have verified that microtia may indicate chromosomal abnormalities, especially triploidy (1). Chromosomal variations are considered to be associated with the occurrence of congenital microtia, especially in cases with multiple deformities or syndromic microtia. However, chromosomal variations have a lower incidence in nonsyndromic, isolated microtia (8).
Mortier et al. (7) performed gene detection on 44 microtia patients using single nucleotide polymorphism microarray technology. They found no pathological copy number variations that can explain the phenotype by genome-wide deletion repeat analysis using the microarray and argued that grade III microtia is the most common. Si et al. (17) found 2 cases of microtia associated with 22q11 deletion syndrome. In this study, one case of 22q11 microdeletion was found by genetic testing.
In this study, the pregnant women and their families had a less positive attitude toward genetic testing, and fetal chromosome karyotype analysis and gene detection were conducted on only a few cases; therefore, statistical analysis was performed. In addition, some studies suggest that fetal auricle abnormalities may be a new clinical indicator for intrauterine growth restriction, but more studies are required (18).
5. Conclusion
The results of this study demonstrated that prenatal ultrasound is reliable for diagnosing fetal microtia, and the second trimester is the best time to observe the fetal auricle. Unilateral fetal microtia is more common and often accompanied by congenital defects of other organs and structures, with frequent involvement of the heart and face. Soft marker anomalies are also of clinical significance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of Tianjin Medical University Ethics committee of Tianjin First Central Hospital Ethics committee of Shandong Provincial Maternal and Child Health Care Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JQ: conceptualization, writing–original draft, data collection and processing, and literature search. YR: conceptualization, writing–original draft, genetic analysis, and discussion. YG: data collection. JS: supervision, review, and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Innovation Research Special Fund Project of Shandong Provincial Maternal and Child Health Association.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1119191/full#supplementary-material
References
1. Chitkara, U, Lee, L, Oehlert, JW, Bloch, DA, Holbrook, RH Jr, El-sayed, YY, et al. Fetal ear length measurement: a useful predictor of aneuploidy? Ultrasound Obstet Gynecol. (2002) 19:131–5. doi: 10.1046/j.0960-7692.2001.00558.x
2. Kösling, S, Omenzetter, M, and Bartel-Friedrich, S. Congenital malformations of the external and middle ear. Eur J Radiol. (2009) 69:269–79. doi: 10.1016/j.ejrad.2007.10.019
3. Lim, R, and Brichta, AM. Anatomical and physiological development of the human inner ear. Hear Res. (2016) 338:9–21. doi: 10.1016/j.heares.2016.02.004
4. Suutarla, S, Rautio, J, and Klockars, T. Cleft lip and/or palate and auricular malformations. Cleft Palate Craniofac J. (2015) 52:62–5. doi: 10.1597/13-069
5. Luquetti, DV, Heike, CL, Hing, AV, Cunningham, ML, and Cox, TC. Microtia: epidemiology and genetics. Am J Med Genet A. (2012) 158A:124–39. doi: 10.1002/ajmg.a.34352
6. Hoyt, AT, Canfield, MA, Shaw, GM, Waller, DK, Polen, KND, Ramadhani, T, et al. Sociodemographic and hispanic acculturation factors and isolated anotia/microtia. Birth Defects Res A Clin Mol Teratol. (2014) 100:852–62. doi: 10.1002/bdra.23282
7. Mortier, J, van den Ende, J, Declau, F, Vercruysse, H, Wuyts, W, Van Camp, G, et al. Search for a genetic cause in children with unilateral isolated microtia and congenital aural atresia. Eur Arch Otorhinolaryngol. (2022) 280:623–31. doi: 10.1007/s00405-022-07522-4
8. Paul, A, Achard, S, Simon, F, Garcelon, N, Garabedian, EN, Couloigner, V, et al. Congenital abnormalities associated with microtia: a 10-YEARS retrospective study. Int J Pediatr Otorhinolaryngol. (2021) 146:110764. doi: 10.1016/j.ijporl.2021.110764
9. Sun, P, Lin, Q, Zhang, M, Liu, Z, and Zhu, L. Epidemiological study of neonatal congenital Microtia in Shandong Province, China, 2011–2020. J Craniofac Surg. (2022) 33:e828–31. doi: 10.1097/SCS.0000000000008761
10. Anderka, MT, Lin, AE, Abuelo, DN, Mitchell, AA, and Rasmussen, SA. Reviewing the evidence for mycophenolate mofetil as a new teratogen: case report and review of the literature. Am J Med Genet A. (2009) 149A:1241–8. doi: 10.1002/ajmg.a.32685
11. Zhang, Y, Jiang, H, Yang, Q, He, L, Yu, X, Huang, X, et al. Microtia in a Chinese specialty clinic population: clinical heterogeneity and associated congenital anomalies. Plast Reconstr Surg. (2018) 142:892e–903e. doi: 10.1097/PRS.0000000000005066
12. Kini, S, Barton, GW, and Carol Liu, Y-C. Renal anomalies and microtia: determining the clinical utility of screening affected children. Int J Pediatr Otorhinolaryngol. (2020) 133:109957. doi: 10.1016/j.ijporl.2020.109957
13. Özdemir, ME, Karahasanoğlu, A, Aygün, M, Hale, A, Yazicioğlu, F, and İşil, U. Sonographic measurement of fetal ear length in Turkish women with a normal pregnancy. Balkan Med J. (2014) 31:302–6. doi: 10.5152/balkanmedj.2014.19784
14. Guo, F, Lin, L, Yu, X, Song, Y, Yang, Q, He, L, et al. Congenital heart defects in patients with isolated microtia: evaluation using colour Doppler echocardiographic image. Cardiol Young. (2020) 31:260–3. doi: 10.1017/S104795112000387X
15. Forrester, MB, and Merz, RD. Descriptive epidemiology of anotia and microtia, Hawaii, 1986–2002. Congenit Anom. (2005) 45:119–24. doi: 10.1111/j.1741-4520.2005.00080.x
16. Wei, J, Ran, S, Yang, Z, Lin, Y, Tang, J, and Ran, H. Prenatal ultrasound screening for external ear abnormality in the fetuses. Biomed Res Int. (2014) 2014:357564. doi: 10.1155/2014/357564
17. Si, N, Zhang, Z, Huang, X, Wang, C, Guo, P, Pan, B, et al. De novo 22q11.2 deletions and auricular findings in two Chinese patients with microtia. Mol Genet Genomic Med. (2022) 10:e1862. doi: 10.1002/mgg3.1862
Keywords: microtia, ultrasonic soft index, prenatal ultrasound, associated anomalies, genetic abnormalities
Citation: Qiu J, Ru Y, Gao Y and Shen J (2023) Experience in prenatal ultrasound diagnosis of fetal microtia and associated abnormalities. Front. Med. 10:1119191. doi: 10.3389/fmed.2023.1119191
Edited by:
Zaleha Abdullah Mahdy, National University of Malaysia, MalaysiaReviewed by:
Chenlong Li, Fudan University, ChinaVallikannu Narayanan, University of Malaya, Malaysia
Copyright © 2023 Qiu, Ru, Gao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Shen, c2hlbmppZV92aXBAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jing Qiu
Jing Qiu Yanhui Ru
Yanhui Ru Yang Gao3
Yang Gao3