- 1Department of Rheumatology, Nelson R Mandela School of Medicine, University of KwaZulu-Natal and Inkosi Albert Luthuli Central Hospital, Durban, South Africa
- 2Division of Internal Medicine, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
Objective:: Infections are common in systemic lupus erythematosus (SLE), with tuberculosis (TB) being important in an endemic environment. We studied the prevalence and spectrum of TB in SLE in Durban, South Africa.
Methods:: A medical records review of SLE patients seen over 13-year period, and the demographic data, clinical manifestations, laboratory findings, treatment and outcome were noted.
Results:: There were 512 SLE patients and 72 (14.1%) had TB. Thirty (41.7%) had pulmonary TB (PTB) and 42 (58.3%) had extra-pulmonary TB (EPTB). The prevalence of TB among the different ethnic groups was 36/282 (12.8%) for Indian people, 29/184 (15.8%) Black African people, 7/26 (26.9%) admixed African people and none among the 18 White people. Comparison of the 72 SLE-TB patients with 72 SLE controls showed no difference in gender, age at SLE diagnosis and disease duration. The SLE-TB patients had a significant increase in the clinical and laboratory features of disease activity (arthritis, mucocutaneous lesions, renal involvement, vasculitis, low complement, raised ds-DNA antibodies), and cumulative prednisone use over the preceding 3 months.
Compared to PTB, the EPTB patients were significantly younger, developed TB earlier after SLE diagnosis, and had higher disease activity. The EPTB patients also had increase in features of disease activity (renal, thrombocytopenia, ds–DNA antibodies), and increase in ever use of intravenous methylprednisolone (IV-MP) and mycophenolate mofetil (MMF). On multivariate analysis, the independent risk factors for EPTB were ever use of MMF (p = 0.003) and IV-MP (p = 0.027). Analysis of the cumulative SLE criteria showed renal involvement was an independent risk factor for EPTB. The outcome was similar in both groups.
Conclusion:: We show an increased prevalence of TB (14.1%) and EPTB (58.3%) in SLE in an endemic area and confirm that features of disease activity and use of immunosuppressive therapy are the major risk factors. Renal involvement (as a cumulative criterion) is an independent risk factor for EPTB.
Introduction
Although there is marked improvement in the outcome and survival in SLE, infections remain an important cause of morbidity and mortality (1). They may occur in the early or late stages of SLE, and account for about 14–50% of the hospitalizations (2, 3). In the Euro-lupus cohort of 1,000 patients, followed up for 10 years, infections accounted for 25% of the mortality (4). The increased risk of infections is attributed to disease related factors which lead to dysregulation of the immune system, and the use of immunosuppressive drugs (5).
A recent systematic review and meta-analysis on the global incidence and prevalence of tuberculosis (TB) in SLE noted an increased prevalence in Africa and countries with a high TB burden, and a lower prevalence in Europe and North America. (6). Based on a review of 46,327 patients in 35 studies, Wu et al. (6) reported an incidence of TB in SLE of 1.16 [95% confidence interval (CI) 0.69–1.93] per 100 person years and the prevalence was 3.59% (95% CI 2.57–5.02%). In clinical studies, there was a wide variation in the prevalence of TB in SLE with a lower prevalence of 0.66% in Taiwan and 1.3% in Mexico, and a higher prevalence of 17.1% in South Africa and 25% in Colombia (7–10). A review of studies with more than 50 patients, showed increased extrapulmonary TB (EPTB) in Colombia (54%), Hong Kong (67%) and Philippines (67%) (10–12).
Risk factors for the development of TB include the use of intravenous and oral corticosteroids (mean daily doses prior to the diagnosis of TB, the cumulative dose and duration of treatment), use of immunosuppressive therapy, disease activity and manifestations such as nephritis and vasculitis (8–15).
The 2022 WHO Global report on TB estimated 10.6 million new TB cases in 2021, being most common in South- East Asia (45%), Africa (23%), Western Pacific (18%), and Eastern Mediterranean (8.3%) (16). There were a smaller proportion in the Americas (2.9%) and Europe (2.2%) (16). The estimated number of global TB deaths is about 1.6 million, of whom about 187,000 were in HIV positive patients (16). South Africa has a very high burden of TB with an estimated incidence of 513 per 100,000 population (17). The high burden increases the risk of TB in patients who are immunocompromised or have autoimmune diseases and require intensive immunosuppressive therapy. Thus, this study was undertaken to determine the prevalence, clinical spectrum, risk factors and outcome of TB in SLE patients in a multi-ethnic population in a single academic center in a high TB burden environment in Durban, South Africa. We also studied the risk factors for TB in SLE patients compared to controls, risk factors for PTB vs. EPTB, and compared our findings with observations in other parts of the world.
Methods
We reviewed the medical records of all the patients with SLE seen in the Department of Rheumatology at, Inkosi Albert Luthuli Central Hospital (IALCH) in Durban, South Africa from June 2003 to March 2016. Patients who fulfilled the Systemic Lupus International Collaborating Clinics (SLICC) criteria for SLE were selected for inclusion in the study (18). Patients with incomplete SLE, concomitant HIV infection, mixed connective tissue disease or overlapping features of another connective tissue disease such as scleroderma or inflammatory myositis, were excluded from the study.
Patients were classified as having TB if: (a) acid fast bacilli were identified on microscopy of sputum or other tissue/specimen; (b) a positive GeneXpert test result was obtained on the sputum or other specimen; (c) Mycobacterium tuberculosis was cultured from the sputum or other appropriate specimen; (d) the presence of histological evidence of caseating granuloma or (e) clinical diagnosis: patients in whom a diagnosis of TB was made on the basis of a combination of symptoms, clinical findings, imaging studies or the results of laboratory investigations and were treated for TB by the attending physician.
The records of the patients with SLE and TB were analyzed further. The ethnicity/racial background was defined according to the guidance by Flanagin et al. (19). The demographic data recorded included gender, age at diagnosis of SLE and the interval from SLE diagnosis to TB diagnosis. The clinical manifestations, results of laboratory tests and imaging studies, and the SLEDAI – 2 K (Systemic Lupus Erythematosus Disease activity index -2 K) at TB diagnosis were also recorded (20). The treatment recorded included whether patients received chloroquine, corticosteroids, or immunosuppressive therapy such as cyclophosphamide, methotrexate, mycophenolate mofetil (MMF) or azathioprine at the time of TB diagnosis, in the preceding 3 months or in the past. For corticosteroids, the mode of administration, current dose, and cumulative dose at the time of TB diagnosis were recorded. The outcome was recorded as continuing follow-up, lost to follow-up, or died. We calculated the interval from the time of diagnosis of SLE to TB diagnosis in patients who developed TB. The control group comprised a similar number of patients with SLE but without TB, who were matched for disease duration, and their disease activity and treatment at the time of their last visit were recorded.
Ethical approval for the study was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (BE 223/16), the management of IALCH and the KwaZulu-Natal Department of Health.
Statistical analysis
The data collected was analyzed using SPSS version 23 (IBM Corp, Armonk, NY, United States). Categorical variables are expressed as numbers (percentages). Depending on the normality test, numerical variables are represented as either means ± standard deviations (S.D.) or medians and interquartile range (IQR). The Pearson’s Chi-squared or Fisher’s exact test was used to test for association between categorical variables. The independent samples t-test or the Mann–Whitney U-test was used to test for equality of means or median values. Multiple logistic regression analysis was performed to identify independent risk factors for TB when comparing patients with SLE-TB and SLE controls, and for EPTB when comparing patients with EPTB vs. PTB. We also analyzed the cumulative SLE criteria to identify any independent risk factors for TB compared to controls, and for EPTB compared to PTB. The significance level was set at p ≤ 0.05 for all tests.
Results
Demographic data and prevalence of TB
There were 512 patients who fulfilled the SLICC criteria for SLE and did not have HIV infection or features of any other connective tissue diseases. The ethnicity of the patients was 282 (55.1%) Indian people, 184 (35.9%) Black African people, 28 (5.5%) admixed African people and 18 (3.5%) White people (19). The mean age at the diagnosis of SLE was 32.09 ± 14.13 years and 466 (91.0%) were females. There were 72 patients who were diagnosed with TB, representing a prevalence of 14.1% of our SLE patients. The prevalence of TB among the different ethnic groups was 12.8% (36/282) for Indian people, 15.8% (29/184) Black African people, 26.9% (7/26) admixed African people and none among the 18 White people. Among the patients with TB, 30/72 (41.7%) had PTB while 42/72 (58.3%) had EPTB.
Comparison of the SLE patients with TB and SLE controls
Demographic data
The gender, ethnicity, age at diagnosis of SLE, duration of SLE, duration of follow up, the cumulative SLICC criteria, the SLEDAI 2 K score and outcome in our TB patients and controls are shown in Table 1. In the TB patients, the age at diagnosis of TB, interval from diagnosis of SLE to the TB, number of SLICC criteria at diagnosis of SLE and the SLEDAI 2-K score at diagnosis of TB are also shown in Table 1.
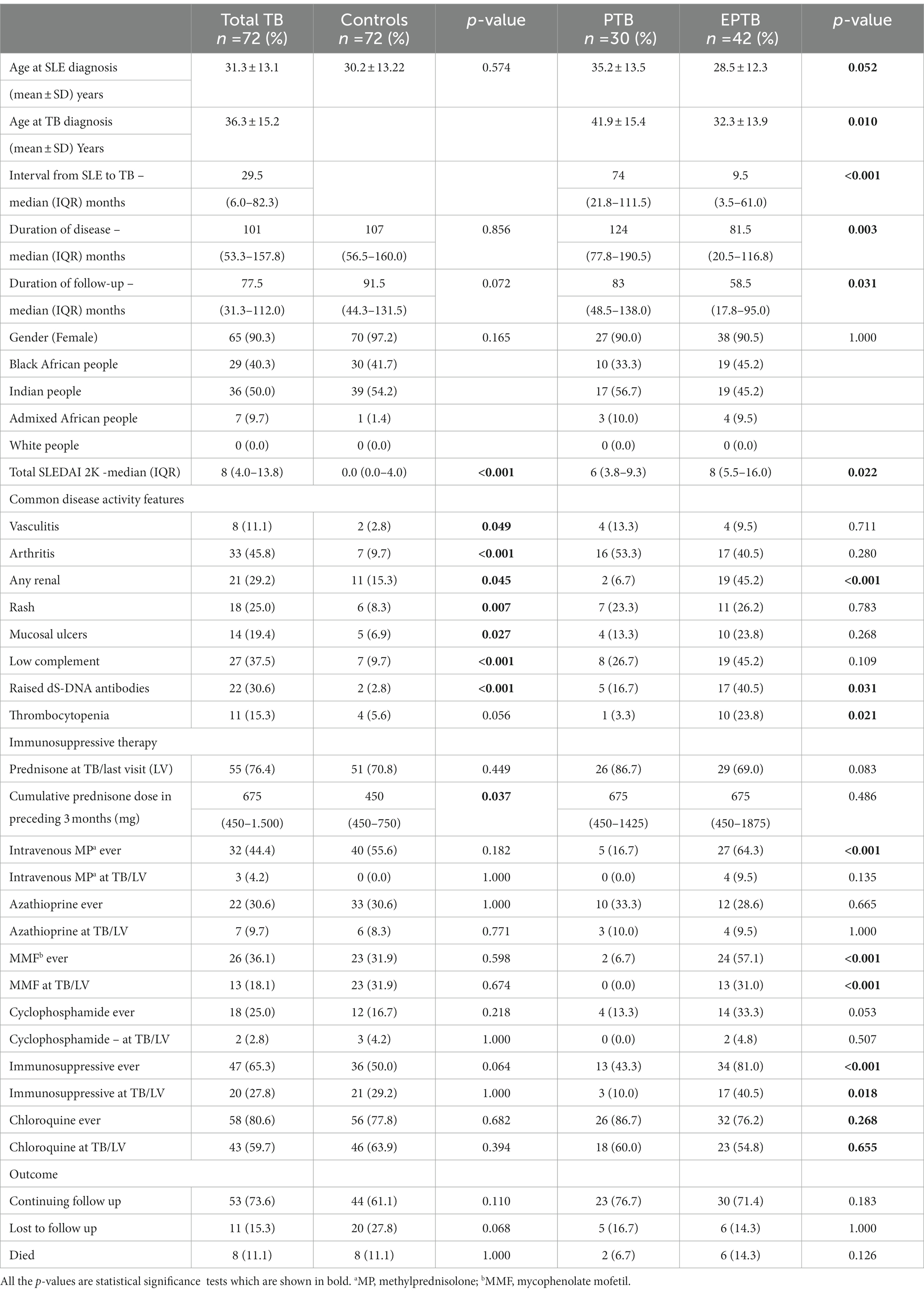
Table 1. Comparison of the demographic data, disease activity, treatment and outcome in SLE-TB patients and controls and patients with PTB and EPTB.
A comparison of the TB patients and controls showed that there was no difference in the mean age at the diagnosis of SLE (p = 0.574), median duration of disease in months (p = 0.856), gender (females 90.3% vs. 97.2%; p = 0.165), and proportion of Indian people (50.0% vs. 54.2%) and Black African people (40.3% vs. 41.7%).
Comparison of the clinical and laboratory features, treatment, and outcome between SLE-TB patients with SLE controls
The SLEDAI-2K scores and the components of the SLEDAI-2K which showed significant differences are shown in Table 1. Patients with TB had a significant increase in disease activity (SLEDAI-2K score) at the time of diagnosis of TB compared to controls at their last visit with a median score of 8 (IQR 4.0–13.75) vs. 0.0 (IQR 0.0–4.0; p < 0.001). The disease activity components which were significantly increased in patients with SLE-TB were arthritis (p < 0.001), skin rashes (p = 0.007), mouth ulcers (p = 0.027), renal involvement (p = 0.045), vasculitis (p = 0.049), and low complement (p < 0.001) and raised dS-DNA antibodies (p < 0.001).
The immunosuppressive treatment which the patients received at the time of diagnosis of TB, or at any stage of the disease is shown in Table 1. The median cumulative dose of prednisolone was higher in patients with TB than in control group (675 mg vs. 450 mg, p = 0.037). There was no significant difference in the use of immunosuppressive medication between the two groups as shown in Table 1.
A review of the outcome of the patients with SLE-TB and controls showed that there was no significant difference in the number of patients who were continuing follow up or had died, but an increased number of controls (27.8% vs. 15.3%; p = 0.068) were lost to follow up but this difference was not significant.
We undertook a multivariate logistic regression analysis using the variables which were significantly different between SLE -TB patients and controls. We found that the only independent predictor for TB among our SLE patients was the presence of arthritis (p = 0.030).
Comparison of patients with PTB and EPTB
Demographic data
The results of the gender, ethnicity, age at diagnosis of SLE, age at diagnosis of TB, interval from diagnosis of SLE to the TB, duration of disease, duration of follow up, the SLEDAI 2-K score at diagnosis of TB and outcome for patients with PTB and EPTB are shown in Table 1.
Comparison of the 42 patients with EPTB and 30 patients with PTB showed that there was no difference in the proportion of females (p = 1.000). Patients with EPTB were younger at SLE diagnosis but the difference was not significant (p = 0.052). When compared to patients with PTB, the EPTB patients were significantly younger at diagnosis of TB (32.3 ± 13.9 vs. 41.9 ± 15.4 p = 0.010), and they had a shorter median interval between diagnosis of SLE and TB (9.5 vs. 74.0 months, p < 0.001). They also had a shorter median duration of disease (81.5 vs. 124.0, p = 0.003) and median duration of follow up (58.5 vs. 83.0, p = 0.031).
Comparison of the clinical and laboratory features, treatment, and outcome between patients with EPTB and PTB
Patients with EPTB had significantly higher disease activity scores compared to patients with PTB (p = 0.022) with SLEDAI scores of 8 (IQR 5.5–16.0) vs. 6 (IQR 3.8–9.3). The disease activity components which were significantly increased were renal disease (p < 0.001), thrombocytopenia (p = 0.021) and raised ds-DNA antibodies (p = 0.031). The EPTB patients also received more intravenous methylprednisolone (IV-MP) (64.3% vs. 16.7%; p < 0.001), MMF at TB diagnosis (31% vs. 0; p < 0.001) or ever received MMF (57.1% vs. 6.7%; p < 0.001).
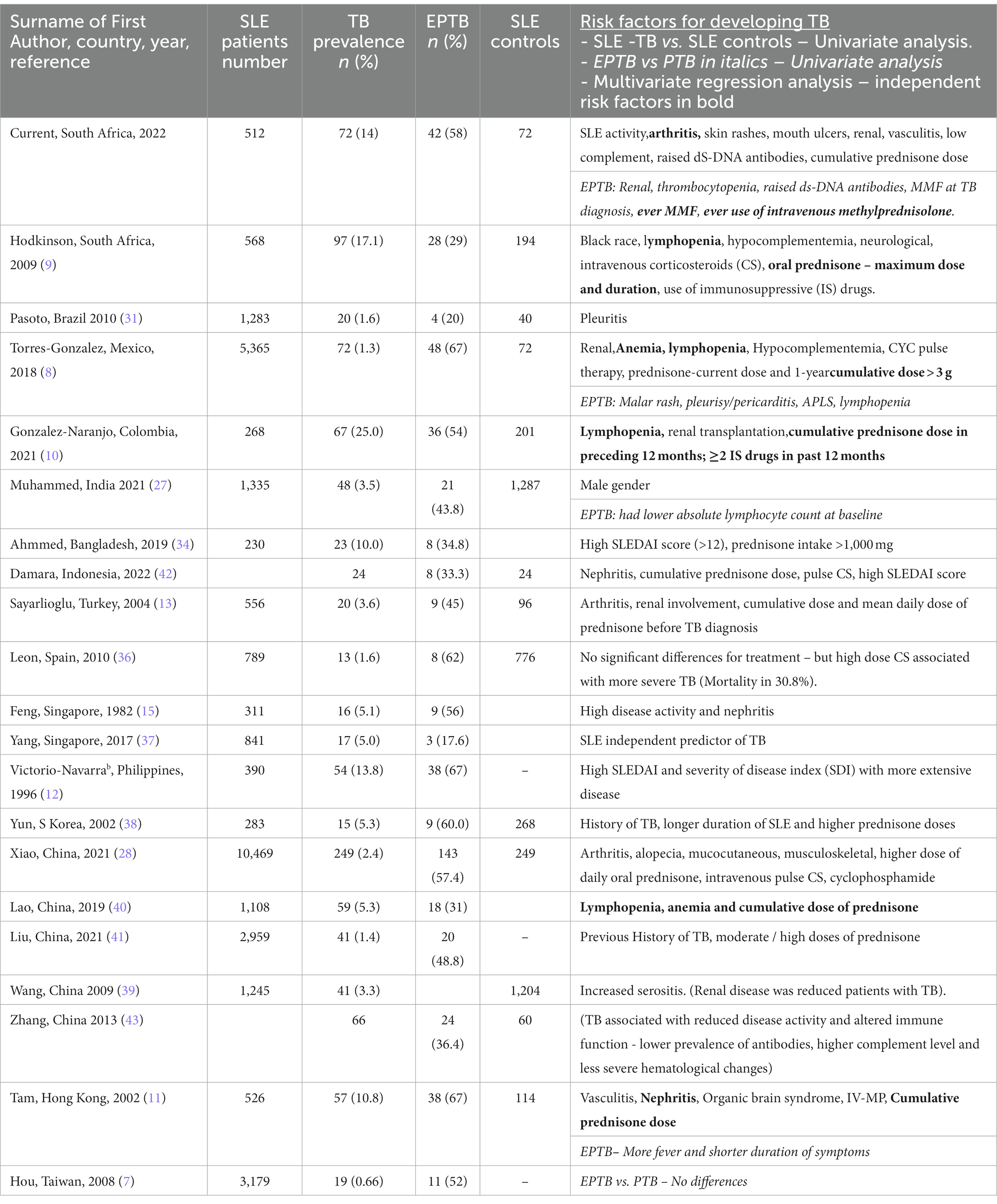
The laboratory findings in patients with PTB and EPTB are shown in Table 2. The only significant abnormality between the groups was a higher median globulin level 46.0 (IQR 37.25–49.75) vs. 36.0 (IQR 31.5–42.0) g/l in patients with PTB (p < 0.001). Comparison of the outcome in Table 1 showed that even though there were more deaths among our EPTB patients, this difference was not significant (14.3% vs. 6.7%; p = 0.126).
A multivariate logistic regression analysis using the significantly different variables on univariate analysis showed that the only independent predictors for EPTB were the ever use of MMF (p = 0.003) and ever use of IV-MP (p = 0.027).
Comparison of the cumulative ACR criteria in SLE-TB patients vs. SLE controls and EPTB vs. PTB
A comparison of the cumulative SLICC criteria among patients with SLE-TB and SLE controls in Table 3 showed that acute cutaneous lesions were more common in controls (88.9% vs. 77.8%; p = 0.074) while antiphospholipid antibodies were more common in patients with TB (25.0% vs. 12.5%; p = 0.055) but these differences were not statistically significant.
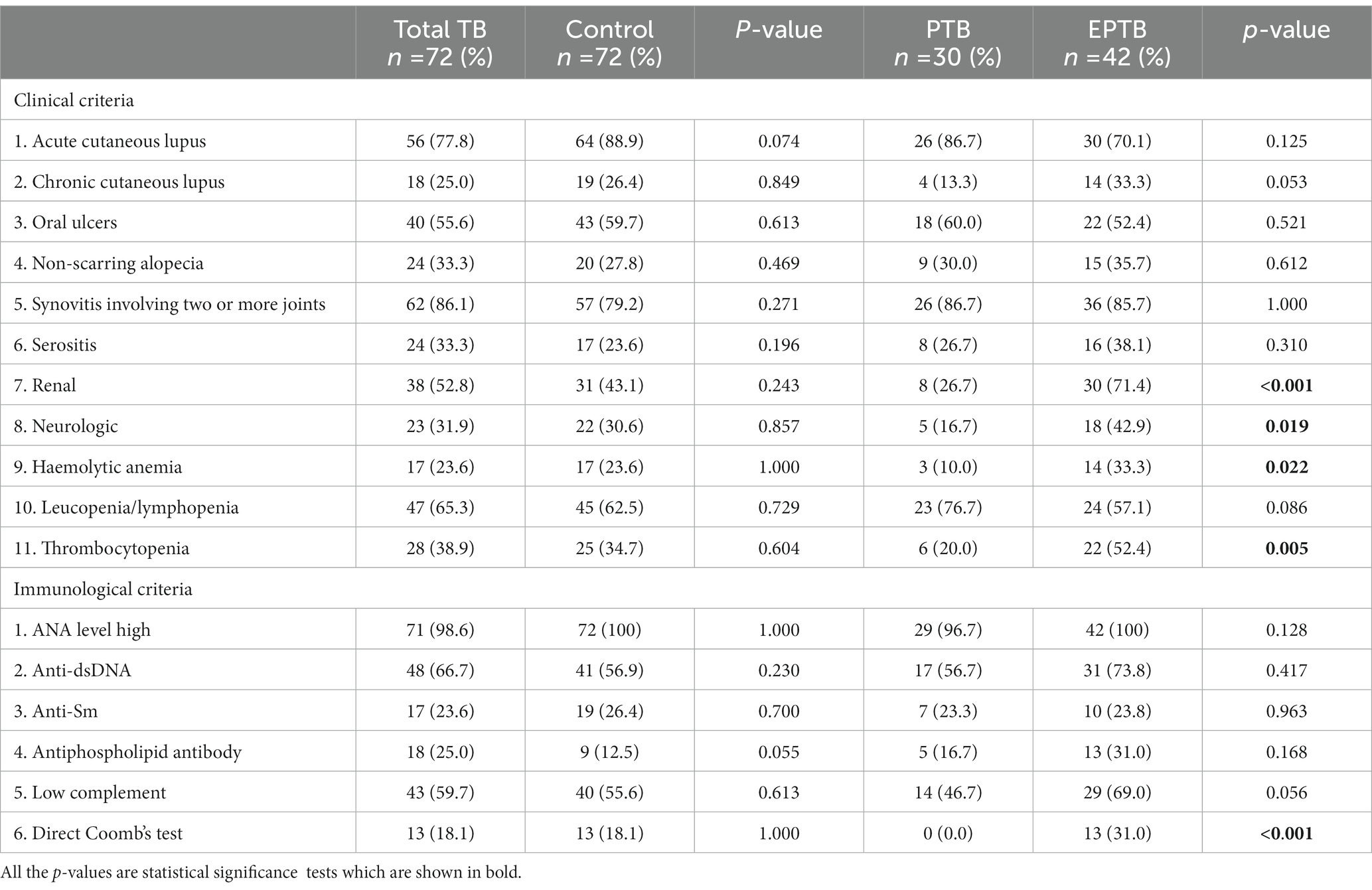
Table 3. Comparison of the cumulative criteria in patients with SLE-TB and controls and patients with PTB and EPTB.
The EPTB patients had a significant increase in renal involvement (p < 0.001), neurologic manifestations (p = 0.019), hemolytic anemia (p = 0.022), positive Coomb’s test (p < 0.001) and thrombocytopenia (p = 0.005) compared to patients with PTB.
The multivariate logistic regression analysis showed that among the SLICC cumulative criteria, the presence of renal disease (p = 0.007) was the only independent risk factor for EPTB.
Mode of diagnosis of tuberculosis and sites of extrapulmonary tuberculosis
The mode of diagnosis in patients with PTB was by sputum examination (microscopy, culture, and or GeneXpert) in 27 (90%) of 30 patients (Supplementary Table S1).
The diagnosis of EPTB was made by analysis of the specimens by microscopy, GeneXpert, culture and or histology in 25 patients. In the remaining 17 patients, diagnosis was based on history, clinical examination, laboratory tests, imaging studies and/or response to treatment.
The most common extra-pulmonary sites were pleural (10 patients), lymph nodes (8), pericardial (5), meningitis (3), and skin (3). Four patients had abscesses, two had joint involvement, two had urogenital involvement and one each had peritoneal, brain, liver, and bone marrow involvement.
Discussion
Most of our patients were Black African people and Indian people and there was no significant difference in the prevalence of TB between them (Table 1). There are no epidemiological data on the prevalence of SLE in South Africa. The reasons for the higher proportion of Indian people in our study are multifactorial and include better access to care as most of them live in urban areas, socioeconomic factors, education, and cultural factors.
Tuberculosis is a common communicable disease and remains one of the leading causes of death worldwide. In 2014 and 2015, the WHO’s End TB Strategy was adopted by member countries of the WHO and United Nations (16). Most of the 30 high burden countries have 150–400 cases per 100,000 population, while South Africa is one of the few countries with an even higher burden of more than 500 cases per 100,000 population (17). In view of the high background prevalence of TB in our environment, we undertook this medical records review study to determine the prevalence of TB, proportion of patients with EPTB, identify risk factors for PTB and EPTB and compare our findings with other centers around the world.
There is a complex interplay between SLE and TB (21, 22). Patients with SLE are at increased risk of developing infections, including TB. In SLE patients, TB is more likely to be extrapulmonary, the pulmonary involvement, is more severe, and relapses occur more frequently (22).
Infections may also trigger autoimmune diseases and contribute to the induction and flares in SLE (21, 23). In Taiwan the prevalence of TB is higher in SLE, and TB is a risk factor for precipitating SLE (24). There was a history of TB in 20% of 70 SLE patients in India (25). A concurrent diagnosis of SLE and TB was made in 12 (16%) of the 76 patients in Hong Kong, 11 (22.9%) of 48 patients in North India, and 32 (12.9%) of 249 patients in China (26–28).
Establishing a diagnosis of TB in patients with SLE is often a challenging task as they share many similar manifestations including fever, weight loss and constitutional disturbances. In addition, manifestations such as serositis, pulmonary and neurological may occur in both SLE and TB. The diagnosis of latent TB is associated with additional challenges in SLE. The tuberculin skin test (TST) produces false negative results on immunosuppressive therapy and are of no value in countries with BCG (Bacillus Calmette-Guerin) vaccination programs. The interferon gamma release assays have improved the detection of latent TB but are less effective in identifying patients at risk of developing active disease (29).
In Table 4, we compare our findings with published reports of some of the larger studies from the different WHO regions around the world (6–15, 26–28, 30–41). We include the number of patients studied, number and percentage of patients with TB (including their age, gender, and interval from diagnosis of SLE to TB, where available), the number of SLE controls, and number and proportion of patients with EPTB. SLE was most common between age 25 and 35 years and majority of the patients were females.
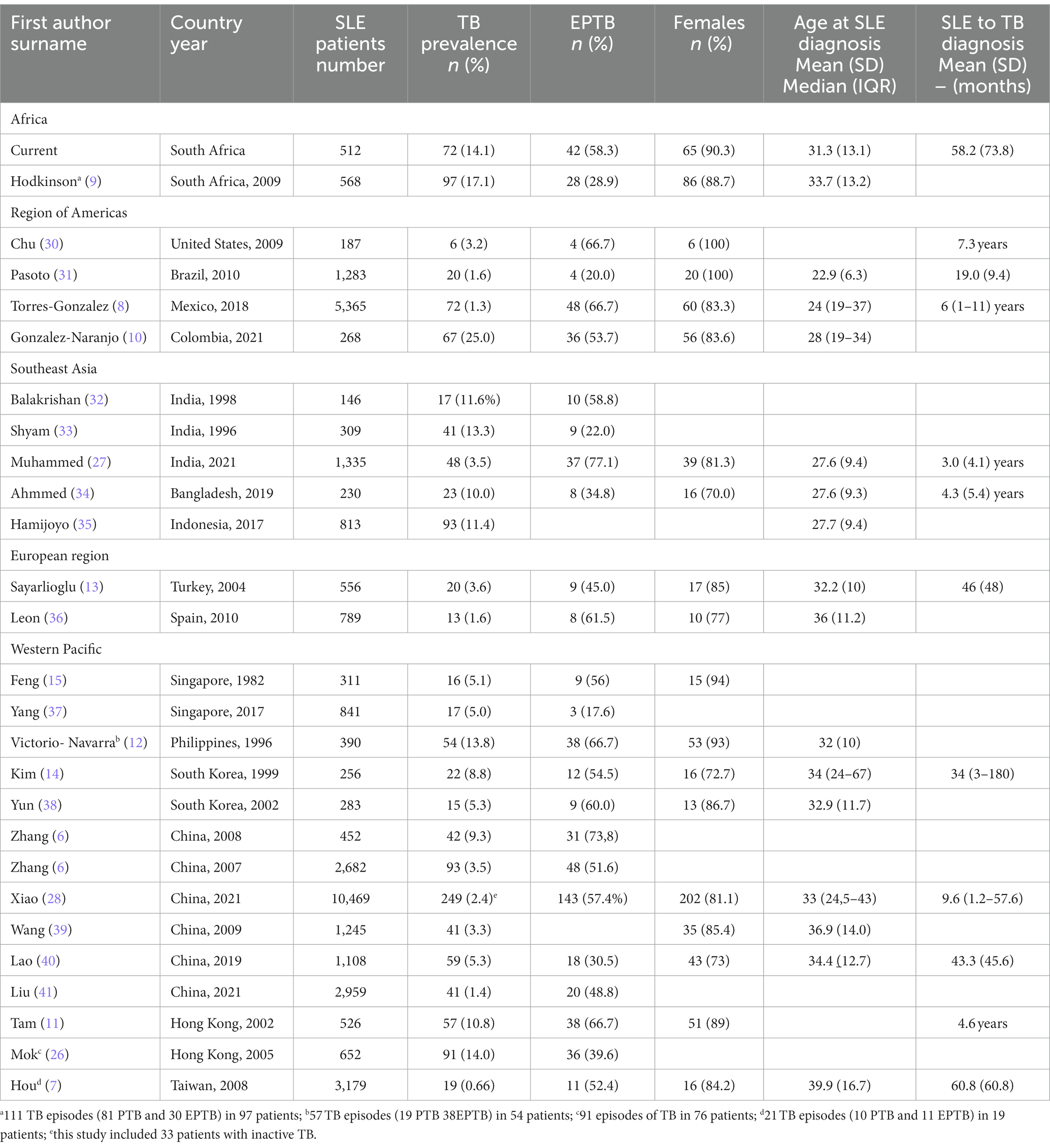
Table 4. Comparison of the demographic data and prevalence of TB (PTB and EPTB) in SLE in different WHO regions.
There is a wide variation in the prevalence of TB in SLE around the world ranging from 0.66% in Taiwan to 25% in Colombia (7, 10). Some of the larger studies which report a low prevalence of TB are from Taiwan (0.66% of 3,179), Mexico (1.3% of 5,365), China (1.4% of 2,959 and 2.4% of 10,469) and Brazil (1.6% of 1,283) as shown in Table 4 (7, 8, 28, 31, 41). A higher prevalence was reported in Indonesia (11.4% of 813), Philippines (13.8% of 390), India (13.3% of 309), South Africa (17.1% of 568) and 14.1% of 512 in the current study (9, 12, 33, 35).
Table 4 shows that the prevalence of EPTB also varies with17.6% of 17 TB patients in Singapore (2017), 20% of 20 patients in Brazil (2010), 22% of 41 patients in India (1996), and 28.9% of 97 patients in Johannesburg (2009), South Africa (9, 31, 33, 37). We found a prevalence of EPTB of 58.2% among our 72 patients with TB and noted a high prevalence in some of the more recent series with 48.8% of 41 patients and 57.4% of 249 patients in two Chinese series (2021), 67% of 72 patients in Mexico (2018) and 54% of 67 patients in Colombia (2021) (8, 10, 28, 41). The prevalence of EPTB was between 50 and 60% in most of the studies in Table 4.
In Table 5 we compare the risk factors for SLE-TB patients compared to SLE controls. and the risk factors for EPTB vs. PTB (7–13, 15, 27, 28, 31, 34, 36–41, 42, 43). There are many variables which are identified on univariate analysis as risk factors for TB in SLE patients compared to SLE controls. The main factors are overall disease activity scores, disease activity of different organs and the cumulative dose and duration of corticosteroid therapy. We found high SLEDAI score as a risk factor for TB, similar to observations in Singapore, Philippines, and Bangladesh (12, 15, 34).The most common clinical feature was nephritis followed by arthritis, serositis, neurological and vasculitis in Table 5 (8, 11, 13, 15, 42). The laboratory findings associated with TB were lymphopenia, hypocomplementemia and raised ds-DNA antibodies. A common risk factor was the use of prednisone – in high cumulative dose, or for prolonged periods, and the use of IV-MP (Table 5).
On multivariate analysis, the most common risk factors in the studies shown in Table 5 were the cumulative dose, and duration of corticosteroids, and lymphopenia, anemia, and nephritis.
There are fewer studies on the risk factors in patients with EPTB compared to PTB. In Table 5 we show the risk factors for EPTB identified in Mexico were malar rash, pleurisy/pericarditis and anti-phospholipid antibody syndrome and lymphopenia, in India absolute lymphopenia, in Hong Kong more fever and a shorter duration of symptoms, while no differences were noted in Taiwan (7, 8, 11, 27). We found that on univariate analysis, the risk factors for EPTB were higher disease activity scores, renal disease, thrombocytopenia, raised ds-DNA antibodies, ever use of IV-MP, and ever use of MMF, and MMF at TB diagnosis as shown in Table 1. However, on multivariate regression analysis, the only independent risk factors for EPTB were the ever use of MMF (p = 0.003) and IV-MP (p = 0.027). We note that while high disease activity and use of corticosteroids increase the risk of TB, it is possible that the extent of the disease activity (significantly higher for EPTB than PTB), and intensity of immunosuppressive therapy (greater IV-MP use for EPTB than PTB) which may contribute to the increased risk of EPTB.
In the earlier SLE studies, TB was diagnosed within 12 months of SLE diagnosis in 43% in Singapore, and 53% in India (15, 32).Our median interval between the diagnosis of SLE and development of TB was 29.5 (IQR 6.0–82.3) months but there was a significantly lower interval in patients with EPTB of 9.5 (IQR 3.5–61.0) compared to 74.0 (IQR 21.8–111.5) (p = 0.010) for PTB. Table 5 shows that the mean or median interval between diagnosis of SLE and the development of TB was between 3 and 5 years for most of the studies except for a median duration of 9.6 (1.2–57.6) months by Xiao in China and a mean of 19.4 ± 9.4 months in Brazil (28, 31). A possible explanation for the early onset of TB after SLE diagnosis could be a delay in referral or diagnosis, resulting in presentation with severe acute multiorgan involvement which requires intensive immunosuppression and increases the risk of infections, including TB.
Even with progress towards the WHO and UN End TB strategy, many exposed people in endemic areas will be at risk of reactivation of TB with autoimmune diseases or immunosuppressive medication (44). A multidisciplinary approach is required to identify patients at increased risk of TB who will benefit from prophylactic therapy.
In conclusion, we report a 14.1% prevalence of TB (with EPTB in 58.3%) in a multi-ethnic cohort of patients with SLE seen in a single center in a TB endemic area in Durban, South Africa. The risk factors in SLE patients who developed TB compared to SLE controls were an increased prevalence of clinical and laboratory measures of disease activity and increase in the cumulative prednisone use over the preceding 3 months. Compared to PTB patients, the EPTB also had an increase in features of disease activity and ever use of IV-MP and MMF, with the ever use of MMF and IV-MP being the only risk factors on multivariate analysis. Renal involvement was the only cumulative criterion that was a risk factor for EPTB. Despite the limitations of a medical records review study with the lack of a standardized protocol and missing data, we believe this study is timely to raise further evidence of the burden of TB in SLE while the global community works towards the WHO and United Nations End TB strategy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Biomedical Research Ethics Committee of the University of KwaZulu-Natal – number BE 223/16. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
KA-a: design of the study, acquisition, analysis and interpretation of the data, preparation of the draft, preparation of the final submission, accountable for all aspects of the study. NM: design of the study, interpretation of the data, revision of the draft, review of the final submission, accountable for all aspects of the study. GM: conception and design of the study, analysis and interpretation of the data, revision of the draft, review of the final submission, and accountable for all aspects of study. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Fundile Habana for data capture, data analysis, and statistical analysis for this project. The authors thank M. Tikly for his review of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1118390/full#supplementary-material
References
1. Moreno-Torres, V, Martínez-Urbistondo, M, Gutiérrez-Rojas, A, Castejón, R, Sánchez, E, Calderón-Parra, J, et al. Impact of severe infections in SLE: an observational study from the Spanish national registry. Lupus Sci Med. (2022) 9:e000711. doi: 10.1136/lupus-2022-000711
2. Barber, C, Gold, WL, and Fortin, PR. Infections in the lupus patient: perspectives on prevention. Curr Opin Rheumatol. (2011) 23:358–65. doi: 10.1097/BOR.0b013e3283476cd8
3. Fessler, BJ. Infectious diseases in systemic lupus erythematosus: risk factors, management and prophylaxis. Best Pract Res Clin Rheumatol. (2002) 16:281–91. doi: 10.1053/berh.2001.0226
4. Cervera, R, Khamashta, MA, Font, J, Sebastiani, GD, Gil, A, Lavilla, P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore). (2003) 82:299–308. doi: 10.1097/01.md.0000091181.93122.55
5. James, JA, Sestak, AL, and Vista, ES. SLE and infection In: DJ Wallace and BH Hahn, editors. DUBOIS' Lupus Erythematosus and Related Syndromes. China: ELSEVIER Saunders (2013). 555–62.
6. Wu, Q, Liu, Y, Wang, W, Zhang, Y, Liu, K, Chen, SH, et al. Incidence and prevalence of tuberculosis in systemic lupus erythematosus patients: a systematic review and meta-analysis. Front Immunol. (2022) 13:938406. doi: 10.3389/fimmu.2022.938406
7. Hou, CL, Tsai, YC, Chen, LC, and Huang, JL. Tuberculosis infection in patients with systemic lupus erythematosus: pulmonary and extra-pulmonary infection compared. Clin Rheumatol. (2008) 27:557–63. doi: 10.1007/s10067-007-0741-8
8. Torres-González, P, Romero-Díaz, J, Cervera-Hernández, ME, Ocampo-Torres, M, Chaires-Garza, LG, Lastiri-González, EA, et al. Tuberculosis and systemic lupus erythematosus: a case-control study in Mexico City. Clin Rheumatol. (2018) 37:2095–102. doi: 10.1007/s10067-018-4109-z
9. Hodkinson, B, Musenge, E, and Tikly, M. Osteoarticular tuberculosis in patients with systemic lupus erythematosus. QJM. (2009) 102:321–8. doi: 10.1093/qjmed/hcp015
10. González-Naranjo, LA, Coral-Enríquez, JA, Restrepo-Escobar, M, Muñoz-Vahos, CH, Jaramillo-Arroyave, D, Vanegas-García, AL, et al. Factors associated with active tuberculosis in Colombian patients with systemic lupus erythematosus: a case-control study. Clin Rheumatol. (2021) 40:181–91. doi: 10.1007/s10067-020-05225-x
11. Tam, LS, Li, EK, Wong, SM, and Szeto, CC. Risk factors and clinical features for tuberculosis among patients with systemic lupus erythematosus in Hong Kong. Scand J Rheumatol. (2002) 31:296–300. doi: 10.1080/030097402760375205
12. Victorio-Navarra, ST, Dy, EE, Arroyo, CG, and Torralba, TP. Tuberculosis among Filipino patients with systemic lupus erythematosus. Semin Arthritis Rheum. (1996) 26:628–34. doi: 10.1016/S0049-0172(96)80013-8
13. Sayarlioglu, M, Inanc, M, Kamali, S, Cefle, A, Karaman, O, Gul, A, et al. Tuberculosis in Turkish patients with systemic lupus erythematosus: increased frequency of extrapulmonary localization. Lupus. (2004) 13:274–8. doi: 10.1191/0961203303lu529xx
14. Kim, HY, Im, JG, Goo, JM, Lee, JK, Song, JW, and Kim, SK. Pulmonary tuberculosis in patients with systematic lupus erythematosus. AJR Am J Roentgenol. (1999) 173:1639–42. doi: 10.2214/ajr.173.6.10584813
15. Feng, PH, and Tan, TH. Tuberculosis in patients with systemic lupus erythematosus. Ann Rheum Dis. (1982) 41:11–4. doi: 10.1136/ard.41.1.11
16. World Health Organization. Global tuberculosis report 2022. Report no.: Geneva: World Health Organization; (2022). Licence: CC BY-NC-SA 3.0 IGO.
17. Tuberculosis profile. Tuberculosis profile: South Africa. (2021). Available at: https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country%22&lan=%22EN%22&iso2=%22ZA%22
18. Petri, M, Orbai, AM, Alarcón, GS, Gordon, C, Merrill, JT, Fortin, PR, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. (2012) 64:2677–86. doi: 10.1002/art.34473
19. Flanagin, A, Frey, T, and Christiansen, SL, Committee AMAMoS. Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. (2021) 326:621–7. doi: 10.1001/jama.2021.13304
20. Gladman, DD, Ibañez, D, and Urowitz, MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. (2002) 29:288–91.
21. Prabu, V, and Agrawal, S. Systemic lupus erythematosus and tuberculosis: a review of complex interactions of complicated diseases. J Postgrad Med. (2010) 56:244–50. doi: 10.4103/0022-3859.68653
22. Balbi, GGM, Machado-Ribeiro, F, Marques, CDL, Signorelli, F, and Levy, RA. The interplay between tuberculosis and systemic lupus erythematosus. Curr Opin Rheumatol. (2018) 30:395–402. doi: 10.1097/BOR.0000000000000493
23. Ribeiro, FM, Szyper-Kravitz, M, Klumb, EM, Lannes, G, Ribeiro, FR, Albuquerque, EM, et al. Can lupus flares be associated with tuberculosis infection? Clin Rev Allergy Immunol. (2010) 38:163–8. doi: 10.1007/s12016-009-8149-7
24. Lin, YC, Liang, SJ, Liu, YH, Hsu, WH, Shih, CM, Sung, FC, et al. Tuberculosis as a risk factor for systemic lupus erythematosus: results of a nationwide study in Taiwan. Rheumatol Int. (2012) 32:1669–73. doi: 10.1007/s00296-011-1847-5
25. Ghosh, K, Patwardhan, M, and Pradhan, V. Mycobacterium tuberculosis infection precipitates SLE in patients from endemic areas. Rheumatol Int. (2009) 29:1047–50. doi: 10.1007/s00296-009-0903-x
26. Mok, MY, Lo, Y, Chan, TM, Wong, WS, and Lau, CS. Tuberculosis in systemic lupus erythematosus in an endemic area and the role of isoniazid prophylaxis during corticosteroid therapy. J Rheumatol. (2005) 32:609–15.
27. Muhammed, H, Jain, A, Pattanaik, SS, Chatterjee, R, Naveen, R, Kabeer, H, et al. Clinical spectrum of active tuberculosis in patients with systemic lupus erythematosus. Rheumatol Int. (2021) 41:2185–93. doi: 10.1007/s00296-021-04933-0
28. Xiao, X, Da, G, Xie, X, Liu, X, Zhang, L, Zhou, B, et al. Tuberculosis in patients with systemic lupus erythematosus-a 37-year longitudinal survey-based study. J Intern Med. (2021) 290:101–15. doi: 10.1111/joim.13218
29. Goletti, D, Delogu, G, Matteelli, A, and Migliori, GB. The role of IGRA in the diagnosis of tuberculosis infection, differentiating from active tuberculosis, and decision making for initiating treatment or preventive therapy of tuberculosis infection. Int J Infect Dis. (2022) 124:S12–9. doi: 10.1016/j.ijid.2022.02.047
30. Chu, AD, Polesky, AH, Bhatia, G, and Bush, TM. Active and latent tuberculosis in patients with systemic lupus erythematosus living in the United States. J Clin Rheumatol. (2009) 15:226–9. doi: 10.1097/RHU.0b013e3181b0c85d
31. Pasoto, SG, Borba, EF, Bonfa, E, and Shinjo, SK. Lupus pleuritis: a relevant risk factor for pulmonary tuberculosis. Lupus. (2010) 19:1585–90. doi: 10.1177/0961203310375269
32. Balakrishnan, C, Mangat, G, Mittal, G, and Joshi, VR. Tuberculosis in patients with systemic lupus erythematosus. J Assoc Physicians India. (1998) 46:682–3.
33. Shyam, C, and Malaviya, A. Infection-related morbidity in systemic lupus erythematosus: a clinico-epidemiological study from northern India. Rheumatol Int. (1996) 16:1–3. doi: 10.1007/BF01419946
34. Ahmmed, M, Islam, M, Ferdous, S, Azad, A, and Ferdous, N. Tuberculosis in systemic lupus Erythematosus patients. Mymensingh Med J. (2019) 28:797–807.
35. Hamijoyo, L, Candrianita, S, Rahmadi, A, Dewi, S, Darmawan, G, Suryajaya, B, et al. The clinical characteristics of systemic lupus erythematosus patients in Indonesia: a cohort registry from an Indonesia-based tertiary referral hospital. Lupus. (2019) 28:1604–9. doi: 10.1177/0961203319878499
36. León, RG, Rasco, RG, Palomares, EC, Hernández, FJG, Palma, MJC, and Roman, JS. Tuberculosis en una cohorte de pacientes con lupus eritematoso sistémico. Reumatol Clín. (2010) 6:256–61. doi: 10.1016/j.reuma.2009.11.002
37. Yang, Y, Thumboo, J, Tan, BH, Tan, TT, Fong, CHJ, Ng, HS, et al. The risk of tuberculosis in SLE patients from an Asian tertiary hospital. Rheumatol Int. (2017) 37:1027–33. doi: 10.1007/s00296-017-3696-3
38. Yun, JE, Lee, SW, Kim, TH, Jun, JB, Jung, S, Bae, SC, et al. The incidence and clinical characteristics of Mycobacterium tuberculosis infection among systemic lupus erythematosus and rheumatoid arthritis patients in Korea. Clin Exp Rheumatol. (2002) 20:127–32.
39. Wang, J, Pan, H-F, Su, H, Li, X-P, Xu, J-H, and Ye, D-Q. Tuberculosis in systemic lupus erythematosus in Chinese patients. Trop Dr. (2009) 39:165–7. doi: 10.1258/td.2008.080379
40. Lao, M, Chen, D, Wu, X, Chen, H, Qiu, Q, Yang, X, et al. Active tuberculosis in patients with systemic lupus erythematosus from southern China: a retrospective study. Clin Rheumatol. (2019) 38:535–43. doi: 10.1007/s10067-018-4303-z
41. Liu, X, Zhang, L, Zhang, F, Zeng, X, Zhao, Y, Wang, Q, et al. Prevalence and risk factors of active tuberculosis in patients with rheumatic diseases: a multi-center, cross-sectional study in China. Emerg Microbes Infect. (2021) 10:2303–12. doi: 10.1080/22221751.2021.2004864
42. Damara, I, Ariane, A, and Winston, K. Predisposing factors of tuberculosis infection in systemic lupus Erythematosus patients: a single-center case-control study. Cureus. (2022) 14:e26410. doi: 10.7759/cureus.26410
43. Zhang, CR, Niu, YY, Lin, JC, Wu, WH, Li, M, and Li, JF. Retrospective analysis on the impact of tuberculosis on patients with systemic lupus erythematosus (SLE). Rheumatol Int. (2013) 33:25–8. doi: 10.1007/s00296-011-2358-0
44. WHO. The end TB strategy. (2015). Available from: https://www.who.int/publications/i/item/WHO-HTM-TB-2015.19
Keywords: tuberculosis, systemic lupus erythematosus, mortality, infection, corticosteroids, Black African people, Indian people
Citation: Al-arbi KMS, Magula NP and Mody GM (2023) Tuberculosis remains a major burden in systemic lupus erythematosus patients in Durban, South Africa. Front. Med. 10:1118390. doi: 10.3389/fmed.2023.1118390
Edited by:
Xiaoming Shu, China-Japan Friendship Hospital, ChinaReviewed by:
Juan C. Rueda, Biosciences Programme, ColombiaGraciela Alarcon, University of Alabama at Birmingham, United States
Copyright © 2023 Al-arbi, Magula and Mody. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Girish M. Mody, bW9keWdAdWt6bi5hYy56YQ==
†Present address: Khaled Mohamed Sefow Al-arbi, Department of Internal Medicine, Hamad General Hospital, Hamad Medical Corporation, Doha, Qatar
 Khaled Mohamed Sefow Al-arbi1†
Khaled Mohamed Sefow Al-arbi1† Girish M. Mody
Girish M. Mody