- 1Department of Medicine, Luminis Health Anne Arundel Medical Center, Annapolis, MD, United States
- 2Division of Rheumatology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
The aim of this review is to examine and evaluate published literature associated with idiopathic inflammatory myopathies (IIM) and interstitial lung disease (ILD) based on myositis specific autoantibodies (MSA) and the potential clinical significance of each autoantibody subtype for the practicing clinician. The review is a comprehensive search of literature published in PubMed from the year 2005 and onward coinciding with the surge in the discovery of new MSAs. Additionally, we comment on recommended multidisciplinary longitudinal care practices for patients with IIM-ILD with regard to imaging and other testing. Treatment is not covered in this review.
Introduction
Idiopathic inflammatory myopathies (IIM) are a diverse group of autoimmune inflammatory conditions with multi-organ system involvement. Historically, our understanding of IIM was limited to two broad classifications, dermatomyositis (DM) and polymyositis (PM). The spectrum of IIM has further evolved since the discovery of myositis specific auto-antibodies, yielding new subsets of IIM with distinct clinical, histopathological, and radiologic features aiding in our understanding of the various clinical phenotypes of disease and helping prognosticate organ involvement. Currently, IIM is broadly delineated into dermatomyositis, immune mediated necrotizing myopathy, inclusion body myositis, and overlap syndrome which are further subcategorized on the basis of individual myositis specific autoantibodies. Although they are termed myopathies (often interchangeable with the term “myositis”), they present with varying clinical manifestations. Extra-muscular involvement, including the lungs, skin, joints, and the gastrointestinal tract are among a few organs involved, exemplifying the systemic nature of the disease. Lung involvement can be catastrophic and may lead to mortality. Interstitial Lung Disease (ILD) has been associated with IIM, but it is also recognized that while associated with myositis-specific autoantibodies ILD may be the predominant or sole phenotypic element of the syndrome, with little to no myopathic symptoms present.
Idiopathic inflammatory myopathies are rare diseases that have an extensive range of estimates in determining the incidence and prevalence of IIM whether within the United States and/or worldwide. The determination of incidence and prevalence is multifactorial and may depend on the presentation of patients to specialty centers for accurate diagnosis and continuous monitoring as well as accurate reporting of these diagnoses via diagnostic International Classification of Diseases (ICD) codes, which may pose as a challenge with the discovery of new subgroups of IIM and new clinical classifications. As a comparison, Furst et al. determined the adjusted annual incidence of IIM to be 5.8–7.9 per 100,000 person-years, and prevalence ranged from 14 to 17.4 per 100,000 in the United States from the years 2003–2008. Furst et al. (1) A Swedish study surveying its national registrar estimated the incidence of IIM to be 11 per 1,000,000 person years and prevalence of IIM to be 14 per 100,000. Svensson et al. (2) A Korean population study estimated the incidence of IIM to be 2.9–5.2 per 1,000,000 person-years and prevalence of IIM to be 2.3–4 per 100,000. Cho et al. (3) The above data exemplifies the vast incidence and prevalence rates of IIM and while we were unable to find recently evaluated rates for global incidence and prevalence of IIM, we suspect that national rates may be underestimations of the true incidence and prevalence of disease given the challenging nature of disease presentation, especially when IIM may manifest with extramuscular manifestations of the disease. The global incidence and prevalence of interstitial lung diseases was recently investigated by Kaul et al. in 2022 and they found that the estimated global incidence of ILD ranged from 1 to 31.5 per 100,000 person-years and prevalence ranged from 6.3 to 71 per 100,000 people (4) whereas the global prevalence of ILD-IIM has risen significantly from an estimated 5% in 1974 (5) to 41% (6) of ILD cases as reported in a meta-analysis examining patients over a course of 20 years (7).
IIMs, particularly dermatomyositis, immune mediated necrotizing myopathy, and overlap syndromes are more prevalent in women whereas inclusion body myositis is seen more commonly in men (2, 8–11). A United States cohort assessment found an increasing incidence of IIM occurring in the fifth and sixth decades of life (1).
The clinical manifestations of IIM are heterogeneous and can present with acute, subacute, or chronic symptoms. Typically, myopathic symptoms such as complaints of symmetrical proximal muscle weakness usually evoke clinicians to suspect IIM, however, the initial presentations of IIM can be clinically amyopathic. Accompanying signs and symptoms may vary between the inflammatory myopathy subtypes, such as the presence of dermatological signs and symptoms in dermatomyositis which may include a heliotrope rash, gottron papules, V-sign rash, lateral thigh or holster rash, mechanic’s hands, alopecia, and calcinosis; presence of dysphagia in immune mediated necrotizing myopathy and inclusion body myositis, or presence of subtle findings such as atrophy of wrist and finger flexors in inclusion body myositis (12).
The extramuscular manifestations and multi-system organ involvement are particularly important for a practicing clinician to remain cognizant of, as IIM can involve the cardiovascular system, gastrointestinal system, skeletal system, and pulmonary system of which interstitial lung disease is a common manifestation. The signs and symptoms of ILD may present with cough, dyspnea, exercise intolerance, digital clubbing, and signs of pulmonary hypertension such as an accentuated closure of the pulmonic valve on cardiac valve auscultation (13). In IIM-ILD, pulmonary involvement may present in tandem to or subsequently after the myocutaneous manifestations of disease, however, it is of importance to note that pulmonary involvement may be the leading presentation of disease without concomitant myopathic or cutaneous manifestations, therefore, necessitating a high index of suspicion for underlying rheumatologic processes when evaluating ILD as a sole presenting manifestation (14, 15).
Diagnostic evaluation of IIM-ILD entails clinical suspicion from patient history and physical examination in combination with serologic testing (of which negative serologies may not exclude disease), radiologic testing, invasive testing through tissue biopsy, and multidisciplinary evaluations to rule out other conditions. We discuss and comment on individual diagnostic evaluations of IIM-ILD by individual myositis specific auto-antibodies in this review.
Management of IIM-ILD is particularly challenging for patients and clinicians due to the varying clinical presentations of disease and the potential of multi-systemic organ involvement coupled with a paucity of standard treatment regimens which generates variable treatment practices among providers. Additionally, most treatment guidance is from retrospective cohort studies, while only a few randomized controlled studies exist (16). The initial treatment approach usually begins with glucocorticoids, which may or may not help patients in attaining functional improvement, and the chronic use of glucocorticoids is also limited due to its adverse effects and long term complications. The data supporting glucocorticoid use is variable and is mostly based on historical precedent with scant prospective evidence supporting its use (16–18). Subsequent therapy options include immunosuppressive therapy and biologic agents, salvage therapy, and even intravenous immunoglobulins and plasma exchange, which all have varying levels of clinical evidence and benefit which can vary with specific myositis specific autoantibodies (14).
Overall, the development of ILD in patients with IIM portends a poorer prognosis with an increased risk for mortality; clinical outcomes are variable in part due to the unknown response to treatment in individual patients and in part to varying prognosis among individual myositis specific autoantibodies (16). The presence of certain autoantibodies such as anti-MDA5, anti-Jo-1, and anti-Ro-52 have been associated with an increased risk of mortality. Interestingly, while malignancy has been associated with a poorer prognosis in myositis, patients who develop malignancy are at decreased risk of developing ILD (14, 16).
Methods
This comprehensive review aims to analyze and review literature by searching the electronic medical database Pubmed for the following keywords that were chosen due to their established association with our topic of interest: (12, 15, 19, 20) idiopathic inflammatory myopathy, dermatomyositis (and individual MSA’s: anti-MDA5, anti-NXP2, anti-Mi-2, anti-TIF1γ, anti-SAE), immune mediated necrotizing myopathy (and individual MSA’s: anti-HMGCR, anti-SRP), inclusion body myositis, and overlap syndrome (and individual MSA’s: anti PM/Scl, anti-Ku, anti-RNP and anti-Ro, anti-synthetase antibodies and its entities) each in combination with interstitial lung disease, nonspecific interstitial pneumonia, usual interstitial pneumonia, and organizing pneumonia, from the year 2005 and onward (Figure 1).

Figure 1. Timeline of Myositis specific auto-antibodies. Adapted from Neil J. McHugh and Sarah L. Tansley.
Idiopathic inflammatory myopathy classification
First described in the literature by Wagner and Unverricht as early as 1863, dermatomyositis (DM) and polymyositis (PM) criteria were not established until 1975 by Bohan and Peter (21–24). Their criteria focused on the presence or absence of clinical manifestations of muscle weakness, elevation of serum markers of skeletal muscle enzymes, characteristic findings of myopathy on electromyography, select muscle biopsy findings, and the presence of typical cutaneous changes to categorize IIM into DM or PM based “definite,” “probable,” or “possible” diagnoses (23, 24). Over the next few decades, the discovery of myositis specific autoantibodies led to remarkable progress in the understanding of the pathophysiologic processes behind IIM and allowed for the creation of entities and subsets within IIM that better represented individual disease manifestations. Thus, the discovery of myositis specific auto-antibodies ultimately necessitated a reconstruction of the current framework on the approach to IIM and led to the 2017 European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) classification criteria (25). For the scope of this review, we categorize IIM into the following categories: Dermatomyositis (DM), Immune Mediated Necrotizing Myopathy (IMNM), Antisynthetase syndrome (ASynS), and Overlap Myositis (OM). The term “polymyositis” has fallen out of favor recently, with the recognition that this term was often comprised of those with overlap myositis, inclusion body myositis, the antisynthetase syndrome without a rash, or a mimic of myositis, such as muscular dystrophy (26, 27). Thus, polymyositis is not included here. Additionally, inclusion body myositis does not have associated interstitial lung disease and is therefore also not included (14). It must be noted that neither Bohan and Peter nor the ACR/EULAR criteria include pulmonary symptoms as part of the formal classification criteria.
Interstitial lung disease
The reported global prevalence of ILD is likely as high as 41% in patients diagnosed with IIM based on a meta-analysis analyzing 34 studies with a cohort of 10,130 patients over a 20-year period (6). High resolution CT scan (HRCT) is a non-invasive diagnostic modality that is often used to assess pulmonary involvement and is considered the gold standard. Idiopathic interstitial pneumonia can be further classified as idiopathic pulmonary fibrosis which presents with a usual interstitial pneumonia (UIP) pattern or as nonspecific interstitial pneumonia (NSIP) which may mimic interstitial pulmonary fibrosis (28). A diagnosis of UIP can be made with the presence of subpleural or basal honeycombing and by identifying a reticular pattern of fine lines. Additionally, the presence of peripheral traction bronchiectasis represents lung fibrosis and can be used as a prognostic indicator (29). NSIP is the second most common presenting pattern of idiopathic interstitial pneumonia after UIP and can be challenging to distinguish due to features that overlap with UIP patterns, however, the absence of honeycombing, subpleural sparing, and presence of ground glass opacities (GGOs) are more consistent with NSIP (28, 29). A meta-analysis by Ebner et al., determining CT patterns and clinical features to distinguish UIP and NSIP found that in the general population, UIP patterns were more prevalent in elderly male patients with a history of smoking whereas NSIP patterns were seen more often in younger female patients who smoke less often (28). A multi-center retrospective study in 2020 sought to assess organizing pneumonia (OP) patterns based on CT scans in patients with COVID-19 and identified GGOs as the predominant manifestation on imaging followed by variations of mixed abnormalities including GGOs and consolidations with the presence or absence of linear opacities (30).
Up to 25% of patients with symptoms and signs concerning for an autoimmune disease do not meet the classification criteria for connective tissue disease (CTD) as per the American College of Rheumatology, and up to 20% of patients with idiopathic interstitial pneumonias have symptoms and clinical findings suggestive of underlying systemic processes (31). In 2015, the European Respiratory Society/American Thoracic Society developed a task force for “Undifferentiated Forms of CTD-associated ILD” and proposed a research classification of idiopathic pneumonia with autoimmune features (IPAF) to help guide further understanding of these patients (32). The current criteria to diagnose IPAF includes radiological or histopathological evidence of interstitial pneumonia and complete clinical evaluation excluding other etiologies for interstitial pneumonia, and incomplete features of a defined connective tissue disease, in addition to features from a clinical domain, serologic domain, and morphologic domain (32, 33).
There are clear clinical phenotypes with regard to certain MSA’s and IIM-ILD manifestations (Figure 2). Similar to previous reviews, our review found all anti-aminoacyl tRNA synthetase (ARS) antibody subtypes and anti-MDA5 antibodies to be associated with an increased risk of ILD relative to other MSA’s (34–36). The clinical course of anti-ARS-associated ILD appears to be generally more indolent and chronic with significantly lower rates of rapidly progressive ILD compared to anti-MDA5 (37). Previously, it has been noted that the HRCT pattern most associated with ARS antibodies was NSIP representing approximately two-thirds of cases (35). The patterns on HRCT and restrictive pattern pulmonary function tests (PFTs) are largely consistent across ARS autoantibody subtypes, and non-Jo-1 ARS autoantibodies are associated with later diagnosis, increased pulmonary fibrosis, and worsened prognosis (35).
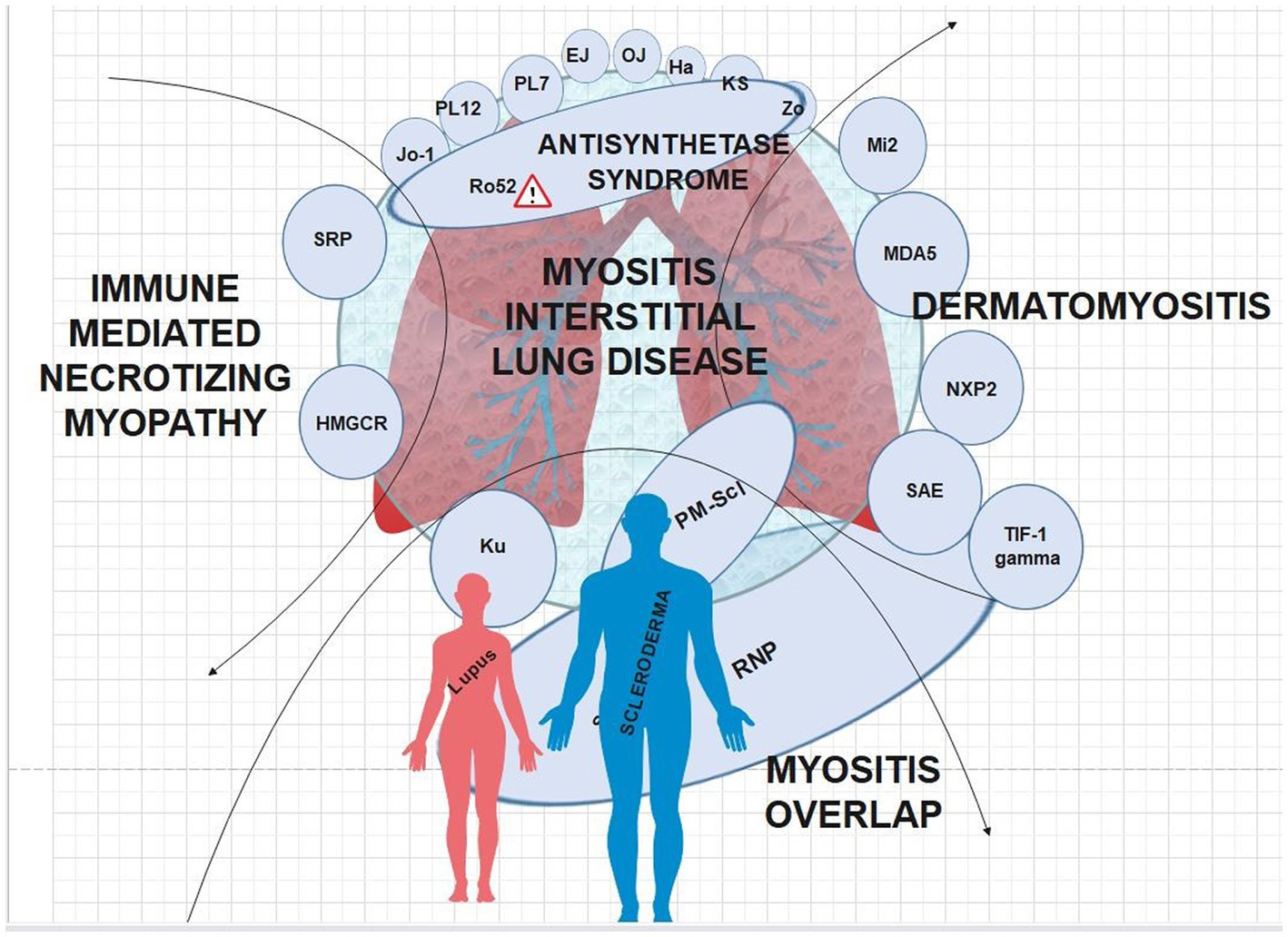
Figure 2. MSA’s interposed onto the IIM’s; caution symbol denotes more severe disease when anti-Ro autoantibodies are present.
Additional imaging modalities to screen for ILD include chest x-rays (CXR) and lung ultrasound. CXR is an easily attainable and economical imaging study that has less exposure to ionizing radiation when compared to a HRCT but has an overall decreased sensitivity in detecting ILD in comparison to the HRCT (38). Ultrasound imaging is also easily attainable and equally economical similar to CXR, moreover, it does not pose a risk to ionizing radiation exposure, and point-of-care ultrasounds can be a quick method for bedside assessment of lung parenchyma; however, it is operator dependent. Assessment of ILD through the combined use of CXR and lung ultrasound may decrease the overall exposure of patients to HRCT, therefore, Vizioli et al., compared the accuracy of combined diagnostic testing through CXR and lung ultrasound in comparison to HRCT in a single center study and concluded that lung ultrasound was highly sensitive (92%) but not specific, (79%) whereas, CXR was highly specific (91%) but not as sensitive (48%) in the detection of ILD (38). Consideration of a step-wise diagnostic approach through the use of CXR, lung ultrasound, and PFTs may be beneficial when screening for ILD in IIM.
It is our common practice to initially screen patients for ILD with HRCT and then follow their course with serial PFTs in the absence of serial imaging if they are in a high risk autoantibody group; it should be noted that serial imaging can expose patients to additional radiation and therefore should be considered when pulmonary function testing continues to show worsening of disease but not as a routine yearly screening tool. IIM-ILD is often cared for in the context of a multidisciplinary care team. If the muscle and/or skin disease is clinically significant, the patient may see only a rheumatologist, neurologist and/or dermatologist. Clinically significant interstitial lung disease requires care by a pulmonologist with specialized training. IIM-interstitial lung disease can be present in the context of dermatomyositis, the anti-synthetase syndrome, overlap myositis, or, less frequently, immune mediated necrotizing myopathy.
Dermatomyositis
Dermatomyositis (DM) has been associated with 5 myositis-specific autoantibodies. They include anti-melanoma differentiation associated protein (anti-MDA5), antinuclear matrix protein (anti-NXP-2), anti-Mi-2, anti-transcription intermediary factor 1-𝛄 (anti-TIF1𝛄), and anti-small ubiquitin like modifier activating enzyme (anti-SAE).
Anti-MDA5
Anti-MDA5 antibodies were first identified in Japanese patients with clinically amyopathic dermatomyositis, per Sato et al. in 2005 (39). Recent literature estimates anti-MDA5 positivity in 10–30% of all DM patients (40). Clinical manifestations of dermatomyositis are variable among certain myositis specific autoantibodies. Cutaneous manifestations can include pathognomonic findings such as Gottron’s papules, Gottron’s sign, and heliotrope rash; characteristic findings such as shawl sign, V sign, holster sign, nailfold changes, and scalp involvement; or less common and unique findings calcinosis cutis, mechanic’s hands, and panniculitis (40).
Some mucocutaneous manifestations are unique to anti-MDA5-associated DM and can present with cutaneous ulcerations in up to 82% of cases with a penchant for developing on existing gottron papules, nail folds, and overlying existing erythematous macules on extensor surfaces. Palmar papules, sometimes referred to as “inverse gottron papules,” and panniculitis are also unique findings, and if seen, should incline clinicians to suspect anti-MDA5 positivity. In addition, oral ulcers and diffuse non-scarring alopecia have an associated high prevalence. Moreover, it is thought that this peculiar constellation of findings may be due to underlying vasculopathy (41). Patients with anti-MDA5 can have muscle weakness, however, most patients have mild muscular involvement and often no muscular involvement at all, characterized as clinically amyopathic dermatomyositis (CADM) (12).
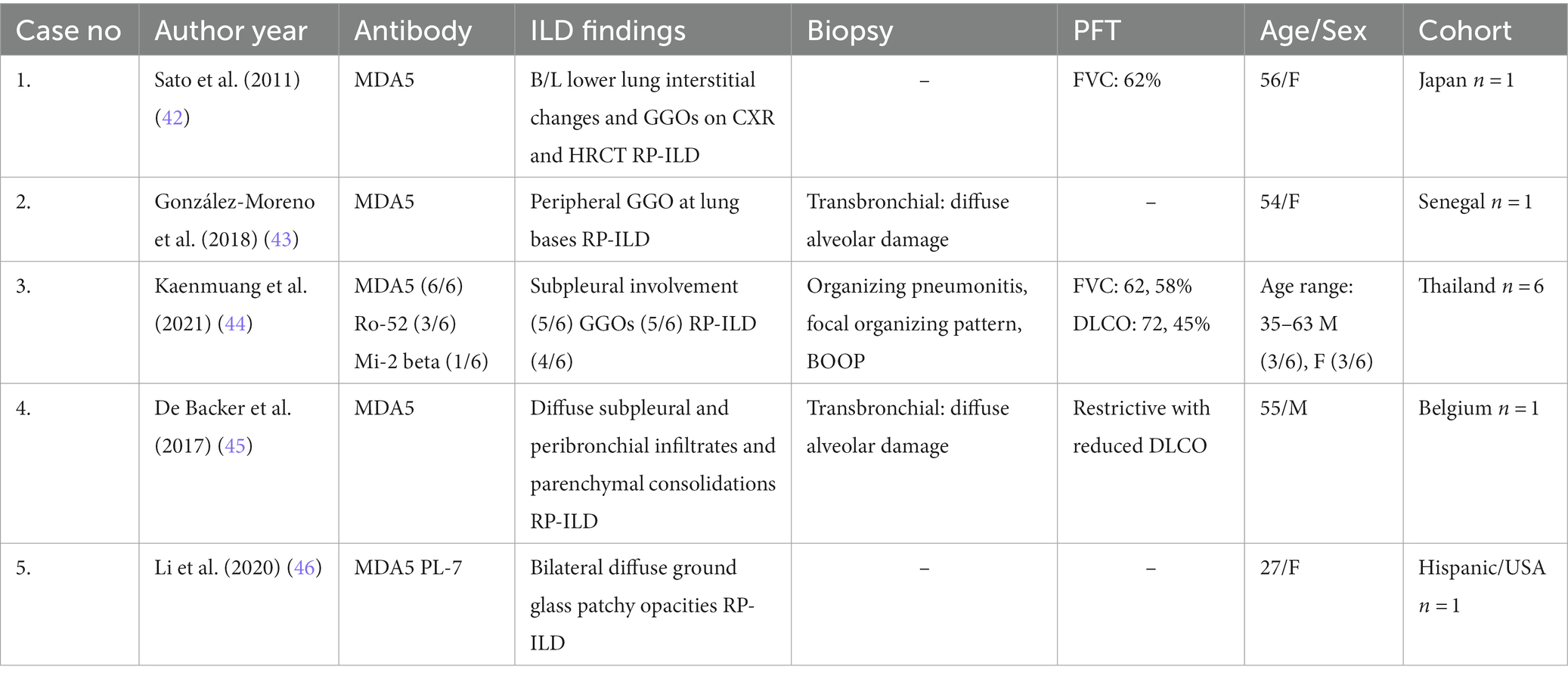
Of the 5 myositis specific autoantibodies, anti-MDA5 has been most strongly associated with ILD which portends an increased risk for mortality (Table 1). Patients with anti-MDA5 may develop features of ILD associated with classic DM or develop the life-threatening rapidly progressive subtype of ILD (RP-ILD). There is an increased prevalence of anti-MDA5 DM in Asian patients in comparison to US and European cohorts (47). The presence of anti-MDA5 is associated with the development of ILD with a reported prevalence ranging from 42 to 100% (41) with an increased predilection for development in Asian cohorts (47).
A review in 2020 compared the features of classic DM with ILD to anti-MDA5 DM with ILD and found that classic DM with ILD is slowly progressive, with a relapsing–remitting course, that clinically manifests with bilateral peribronchovascular ground glass opacities (GGO) or consolidations on CT scan, and nonspecific interstitial pneumonia (NSIP) or organizing pneumonia (OP) on histopathology. In comparison, RP-ILD has an epidemiologic prevalence in Asian countries, is rapidly progressive with a higher mortality rate that clinically manifests with bilateral GGO or consolidation in the posterior and peripheral lungs with the presence of diffuse alveolar damage and microangiopathy on histopathology (48). Intriguingly, the presence of anti-MDA5 antibodies is associated with an increased risk of mortality in Asian patient cohorts. Takada et al. reported a case of a 41-year-old Japanese female with clinically amyopathic dermatomyositis complicated by RP-ILD, and in an effort to increase disease awareness compared the clinical features of DM in Japanese patients with patients in the United States. The study’s findings suggest that greater than 90% of Japanese patients develop complications from ILD (of which approximately 80% of patients develop the rapidly progressive subtype), whereas only 50% of American patients with anti-MDA5 develop ILD, with fewer patients developing RP-ILD (49).
A recent multicenter retrospective cohort study of non-Asian patients from European and American centers assessed 149 patients with MDA5 DM of which 72% of patients developed ILD and only 21.5% of patients developed RP-ILD (50), comparatively, fewer patients developed RP-ILD in this cohort compared to the higher incidence of development in Asian cohorts (49, 51). Clinical manifestations also varied in this cohort with 56% of patients exhibiting muscular involvement, whereas Asian cohorts have a higher predilection for amyopathic disease (50). Consistent with our current knowledge of ILD in MDA5, the non-Asian cohort was predominantly found to have NSIP on HRCT, followed by an OP pattern. Of note13% of patients had a UIP pattern on imaging which is a less common pattern seen with MDA5; however, this reflects the diversity of disease manifestations and should remind clinicians to not anchor on the presence or absence of certain findings and to interpret objective data comprehensively (50).
The presence of certain features has been associated with the development of ILD and furthermore, poor prognosis and increased risk for mortality. There is a strong association between MDA5 and the development of cutaneous ulcerations as discussed previously. Intriguingly, the presence of cutaneous ulcers may be indicative of the presence or development of ILD. A retrospective study of 152 DM patients at Stanford University found that a majority of patients with anti-MDA5 antibodies who developed ILD also had cutaneous ulcers (52). While the presence of anti-MDA5 antibodies is associated with increased risk for the development of ILD and consequently increased risk for mortality, Chen et al. (53) the concomitant presence of anti-Ro-52 antibodies is associated with worse outcomes, increased risk of progression to RP-ILD, and decreased rates of survival as evidenced in recent Asian cohorts studies (54–56).
Anti-MDA5-associated dermatomyositis is one of the deadliest and severe ILD phenotypes in the IIMs when present. It may present with hypomyopathic or amyopathic DM. While out of the scope of this review, early and aggressive treatment is imperative; thus, early recognition is paramount, and knowledge of the unique mucocutaneous disease features may be the clinician’s first clue to diagnosing the disease.
Anti-NXP2
Anti-NXP2 antibodies, previously reported in the literature as anti-MJ antibodies, are more commonly seen in juvenile dermatomyositis with a reported prevalence ranging from 20 to 25% in comparison to adult cohorts where the reported prevalence is 14–25% in the United States adult IIM population and 2–5% in the adult Japanese IIM population (57). Clinical features of dermatomyositis in the presence of anti-NXP2 antibodies can include the development of characteristic cutaneous manifestations, calcinosis cutis (which is prevalent in up to 37% of patients) as well as an increased prevalence of peripheral edema (58). While dermatomyositis is conventionally considered to be a disease to affect proximal muscles and cause proximal muscle weakness, anti-NXP2-associated dermatomyositis has been reported to also affect distal muscles as well causing distal arm and leg weakness. Additionally, these patients can develop symptoms of dysphagia, reflective of significant myopathic involvement (59).
In contrast to anti-MDA5 DM, pulmonary manifestations are relatively scarce, and development of ILD is rare; however, cases do exist (Table 2) (62, 63). A retrospective case series of 7 adult DM patients in France observed pulmonary involvement in 2/7 patients; PFTs of 6/7 patients observed a mean FVC of 90% and a mean DLCO of 56%. HRCT revealed NSIP in one patient, OP in one patient, and normal HRCT in four patients (60). Similarly, in a longitudinal cohort study of anti-NXP2 positive patients in the United States, only 7% of patients developed ILD with a reported mean FVC of 87%, unfortunately, this study did not discuss whether these patients underwent pulmonary imaging (59). The findings reflected in France and the United States are also similarly reflected in a Chinese cohort that identified 17 patients with anti-NXP2 antibodies of which 5 patients developed ILD in a predominantly mixed NSIP + OP pattern on HRCT (64).
Yan et al. performed a retrospective analysis of 33 patients with anti-NXP2 DM over a course of approximately 3 years in which 14/33 individuals developed ILD with 11/14 manifesting features of NSIP and/or OP in lung imaging (65). Interestingly, Kaplan–Meier survival curves did not reveal a statistically significant association between ILD and all-cause mortality (65). In comparison, Li et al. found 21 patients out of 70 patients to have ILD, none developing RP-ILD, in their retrospective 10 year longitudinal cohort study in China (66).
There is an association between anti-NXP2 antibodies and malignancy that was most notably reported in a Japanese cohort of adult patients in which ~37% of patients were found to have malignancy. Their findings were similar to a United States cohort study which found malignancy among ~24% of patients, however, definite associations were not exhibited (67, 68). Moreover, a recent United States cohort study from our cohort at Johns Hopkins and an DM cohort at Stanford determined that patients with anti-NXP2 antibodies are at increased risk of malignancy when compared to the general population (59).
While anti-NXP2 autoantibodies are associated with an increase in malignancy, they do not appear to have an increased association with ILD. This finding supports the observation that malignancy and ILD are inversely proportional to each other and those autoantibodies associated with a higher risk if malignancy have a lower risk of ILD.
Anti-Mi-2
The presence of anti-Mi-2 antibodies in adults ranges from 2 to 38% among dermatomyositis (57). Patients with anti-Mi-2 antibodies predominantly present with the classic cutaneous manifestations of dermatomyositis including heliotrope rash, V sign, shawl sign, gottron papules and gottron sign, additionally, these patients can develop cuticular overgrowths (69). A recent longitudinal cohort study in our center in the United States found that the presence of anti-Mi-2 antibodies is associated with significant and persistent muscle weakness that weakly correlates with elevated creatine kinase levels (70).
Pulmonary involvement is relatively rare in anti-Mi-2 dermatomyositis with multiple cohort studies reporting minuscule lung involvement (71–73). A longitudinal study of anti-Mi-2 patients in the United States found only 3 patients out of 58 developed features of ILD (70). Literature search revealed a case report from the United States of a patient with persistent dry cough and dyspnea who was found to have bibasilar infiltrates on CXR and bilateral patchy ground glass infiltrates on HRCT, with serial imaging revealing of organizing pneumonia, in addition to the development of progressive proximal myopathy in the presence of anti-Mi-2 antibody positivity (74).
While weakness may persist in some Mi-2 + patients, overall, patients who express antibodies to anti-Mi-2 have a favorable prognosis (57, 75). Mi-2 autoantibodies are not generally associated with ILD and thus likely do not require serial PFT and other pulmonary imaging follow-up.
Anti-transcription intermediary factor 1-𝛄
Anti-TIF1𝛄 typically manifests with more prominent cutaneous manifestations of disease and is less frequently associated with ILD. The reported prevalence of anti-TIF1𝛄 antibodies in adult dermatomyositis ranges from 13 to 31% and is more prevalent in Caucasians as compared to Asians (47, 57). Similar to other autoantibodies, cutaneous manifestations of the disease include gottron papules, heliotrope rash, and V sign, however, these patients are highly photosensitive and can present with unique cutaneous features such as ovoid palatal patches, psoriasis-like skin lesions, palmar hyperkeratosis, and hypopigmented patches overlying telangiectasias (40). In contrast, extracutaneous manifestations of the disease are less common and features of Raynaud’s phenomenon, calcinosis, arthritis/arthralgia, and pulmonary involvement are less prevalent (76).
The development of ILD is relatively uncommon with anti-TIF1𝛄 antibodies. A retrospective analysis by Harada et al. analyzed 14 patients with anti-TIF1𝛄 positivity out of a pool of 85 patients with DM over a prolonged 18-year course and identified dermatologic manifestations such as erythema, V neck sign, heliotrope rash, and nail fold telangiectasias more frequently present, whereas no patients developed features of ILD on HRCT (77). Intriguingly, patients with anti-TIF1𝛄 positivity have been found to have an increased incidence of developing malignant tumors (78, 79). Patients with anti-TIF1𝛄 and pulmonary involvement should be followed closely for the development of malignancy. Xie et al. reported a case of initial misdiagnosis of interstitial pneumonia with autoimmune features with NSIP on initial HRCT, which was identified to be right lung squamous carcinoma during a one-year follow-up (80).
Anti-TIF1𝛄 autoantibodies do not have a known association with ILD. Alternate diagnoses should be suspected if lung involvement is found in this subset of patients with DM. Again, the intriguing inverse relationship between cancer (common in this DM subset) and ILD (uncommon in TIF1𝛄 positive patients) is noteworthy.
Anti-small ubiquitin like modifier activating enzyme
The frequency of anti-SAE antibody expression in dermatomyositis is approximately 8% (81). Patients typically present with cutaneous manifestations of the disease that precede muscle involvement (81). Extracutaneous manifestations are common, and the development of dysphagia is a frequent finding (81).
While this phenotype is more strongly associated with the dermatologic manifestations of the disease, there have been reports of mild pulmonary involvement. Gono et al., describe two case reports of Asian patients who presented with predominantly skin-related symptoms, found to have preserved pulmonary function on pulmonary function testing, but evidence of peripheral lower lobe lung involvement with subpleural ground glass opacities, more consistent with NSIP (82). In a North American cohort of 9 patients with anti-SAE positivity at the Johns Hopkins Myositis Center, 7/9 patients developed mild features of ILD, with CT findings of multiple peripheral pulmonary nodules (83). Interestingly, Kishi et al., report a case within the pediatric age group of an 8-year-old Japanese girl who presented with juvenile DM with predominantly cutaneous manifestations complicated by non-rapidly progressive ILD (84).
Overall, ILD is generally mild in these patients and improves with treatment (85). Mild ILD that seems to be clinically less significant may be a feature in patients with dermatomyositis and anti-SAE autoantibodies.
Immune mediated necrotizing myopathy
IMNM has been associated with two prototypic autoantibodies: Anti-HMGCR (3-hydroxy-3-methylglutaryl-CoA reductase) and anti-SRP (signal recognition particle).
Anti-HMGCR antibody myopathy was identified in a US cohort of patients with necrotizing myopathy in 2010 (86) and has a reported frequency of approximately 6% (87). Patients may or may not have had exposure to statin medications and clinical manifestations can include severe proximal muscle weakness and extramuscular manifestations are mostly limited to dysphagia (87). Pulmonary involvement is uncommon with anti-HMGCR and a cohort study in China found the presence of anti-HMGCR to be a protective factor against the development of ILD (88).
Similar to anti-HMGCR antibodies, anti-signal recognition particle (SRP) antibodies are relatively rare with a reported prevalence of 4–6% in European cohorts and a slightly higher prevalence in Asian cohorts, up to approximately 13% (87). Clinical manifestations include severe proximal muscle weakness that can lead to severe debilitation, additionally, patients are at an increased risk of developing dysphagia (47).
While anti-HMGCR autoantibodies are not typically associated with interstitial lung disease, the development of ILD has been reported with anti-SRP IMNM. In a retrospective single-center study, 27 out of 60 individuals with anti-SRP IMNM were diagnosed with extra-muscular manifestations of ILD, of which the radiologic presentation of ILD was NSIP (63%), OP (33.3%), and lymphocytic interstitial pneumonia (3.7%) (89). In their cohort, opacities were primarily distributed in the lower lobes and peribronchovascular sites (89). Patients in this cohort were reported to be mostly asymptomatic with slow disease progression; they were classified as having mild to moderate severity; and none of the patients progressed to RP-ILD. Of note, patients in this cohort did not undergo confirmatory diagnostic testing with bronchoscopy or lung biopsy (89). Anti-SRP IMNM has occasionally been associated with severe forms of ILD. In a case report by Qureshi et al. a 40-year-old African American female developed ventilator-dependent respiratory failure and was found to have mildly elevated CK levels and autoantibody positivity for anti-SRP. Interestingly, the patient did not respond to corticosteroids and immunosuppressants ultimately requiring lung transplantation (90). Additionally, a literature search revealed a case report of a 29-year-old male who presented with progressive exertional dyspnea and was identified to have pulmonary arterial hypertension in addition to findings consistent with ILD. Radiographic imaging was initially consistent with an NSIP pattern with diffuse ground glass opacities but rapidly progressed to a UIP pattern with fibrotic and inflammatory changes within a mere 18 months. Ultimately, the patient underwent lung transplantation and histology from the explanted lung revealed mixed features of UIP and fibrotic NSIP (91).
IMNM is a relatively newly understood and recognized subset of IIM in the last two decades. The prototypic associated autoantibodies, anti HMGCR and anti-SRP have different predilection for ILD, with the former having no clear association and the later having a rare association but one that may be severe in nature and can present in a UIP pattern requiring lung transplantation in the most severe cases.
Myositis overlap: anti PM/Scl, anti-Ku, anti-RNP
Myositis overlap is a heterogeneous entity in which patients can share symptoms of multiple distinct connective tissue diseases. Notable myositis overlap autoantibodies include anti-PM/Scl which generally demonstrates clinical features of overlap between scleroderma (namely Raynaud’s phenomenon, telangiectasias and possible skin thickening) and myositis with or without the skin rash of dermatomyositis.
Lung involvement is common in patients with anti-PM/Scl, ranging from 35 to 87%, and has been reported to have better functional outcomes when compared to other groups (92). In a single center study of anti-PM/Scl antibody patients in China, 30 patients were found to be positive for either anti-PM/Scl-75, anti-PM/Scl-100, or both, of which NSIP, UIP, OP, NSIP/OP overlap, and LIP were identified, respectively, in descending order of frequency through either HRCT or lung biopsy; interestingly, ILD was the sole manifesting feature in ~26% of the cohort (93).
Anti-Ku antibodies are myositis associated auto-antibodies and can be identified in patients with myositis as well as in patients with other systemic autoimmune conditions and can present with features of extramuscular involvement, such as ILD. A retrospective study seeking to identify predictive features of ILD found that within their cohort anti-ku antibodies were present in patients who developed ILD at least 12 months after the onset of their myositis, suggesting anti-ku antibodies could be associated with a slow disease progression (94). A study from the Johns Hopkins myositis cohort looking to further describe the phenotype of anti-ku positive patients found that within the cohort, ILD was the presenting feature in only 19% of patients but 56% of patients ultimately developed pulmonary disease (95).
Anti-RNP (ribonuclear protein) antibodies are prevalent in a myriad of systemic diseases such as myositis, mixed connective tissue disease, systemic lupus erythematosus, rheumatoid arthritis, and systemic sclerosis (96). A study aiming to characterize the pulmonary manifestations among patients with anti-RNP antibodies found that out of a total of 544 patients, ~25% had ILD with NSIP being the predominant radiological finding followed by UIP. Cystic lesions with ground-glass attenuation were identified in a subset of NSIP patients without signs of fibrosis on imaging, identifying an original radiologic pattern termed interstitial cystic lung disease associated with anti-RNP antibody (ICLAR) (97).
Many of the myositis CTD overlap syndromes can present with significant ILD. The practicing clinician must be aware of this potential involvement, and serial monitoring with pulmonary function testing and assessment of patient symptoms including cough and breathlessness should be closely evaluated.
Antisynthetase syndrome
The antisynthetase syndrome is characterized by antibodies directed against an aminoacyl-transfer RNA (tRNA) synthetase (ARAs) and is associated with ILD, myositis, inflammatory arthritis, mechanic’s hands, fever, and Raynaud’s phenomenon (98). It is generally accepted that the presence of a positive antisynthetase antibody in addition to the presence of two of the following features: ILD, inflammatory myopathy, or inflammatory polyarthritis is classified as anti-synthetase syndrome (34). Alternatively, our group has proposed antisynthetase syndrome criteria that includes positive serologic testing for an anti-tRNA synthetase autoantibody in the presence of any one of the protean symptoms (ILD, myositis, inflammatory arthritis, mechanic’s hands, fever, and Raynaud’s phenomenon) (15). In a retrospective cohort of 108 patients with anti-synthetase syndrome and ILD, patients had 5 distinct antibodies, anti-Jo-1, anti-PL-7, anti-PL-12, anti-EJ, and anti-OJ (99). Thirty out of 108 cases received bronchoscopy for transbronchial biopsy to assist in pathological diagnoses, and the remaining cases were diagnosed based on radiological pattern discussions with multi-disciplinary teams (99). Data from this cohort revealed that an OP pattern was seen the most in the EJ + group, NSIP pattern was seen the most in the PL-12 + group, a mixture of OP + NSIP pattern was seen the most in the OJ + group, and UIP was seen the most in the PL-7 + group; all groups had a positive response to steroid therapy (99). In another single-center retrospective study of 84 ILD patients, the NSIP pattern was seen more in the Jo-1, PL-7, and EJ group, OP pattern was seen more in the PL-12 group, and UIP was seen more in the OJ group (100). In a retrospective cohort of 1,194 patients, patients were compared to healthy controls for the presence of anti-Ha, anti-Ks, anti-Zoα, cN1A novel myositis autoantibodies and found that the prevalence of ILD was significantly higher in those with novel myositis antibodies. Radiologic and histologic findings of UIP pattern were less frequently characterized when compared to patients with idiopathic pulmonary fibrosis (101).
Finally, anti-Ro-52 antibodies have been associated with ILD and have been considered to be an independent predictor for complications of ILD. While they may be seen in isolation, they are more often associated with other MSAs, specifically anti-ARS autoantibodies. A prospective observational study in China assessed patients with anti-Ro-52 positivity for the presence of ILD and found that patients with isolated anti-Ro-52 antibodies and non-RP-ILD had an NSIP pattern on radiographic studies whereas patients who developed RP-ILD had an OP pattern on imaging studies (102). Similarly, a retrospective analysis of ILD patients in Italy found that the presence of anti-Ro-52 antibodies could predict the development of ILD. Interestingly, patients in their cohort had statistically significant improvement in DLCO at 5 years from baseline (94).
The antisynthetase antibodies have a strong association with ILD. It must be noted that isolated ILD may be the presenting and sole feature of the illness; thus a high index of suspicion for the antisynthetase syndrome in any patient presenting with an otherwise idiopathic pneumonia, especially in an NSIP pattern, must be present. More often than not, in the antisynthetase syndrome anti-PL12 and anti-PL7 antibodies present with ILD in isolation. The unfortunate nomenclature of “myositis associated autoantibodies” can be confusing to clinicians. Thus attributing these antibodies to a syndromic complex where ILD may be the first or only symptom is an important construct to understand.
Summary
Myositis-specific autoantibodies (MSAs) and Myositis-associated antibodies (MAAs) testing has become commercially available in recent years and is now more accessible worldwide. The diagnostic utility of the MSAs and MAAs in helping to make an accurate diagnosis and assist in the prognosis of myositis-ILD is excellent in the appropriate clinical setting. While anti-MDA5 is associated with the most severe ILD phenotype with respect to rapidly progressive ILD, many other myositis-specific and myositis-associated autoantibodies are found in conjunction with ILD in various frequencies. It is important for the practicing clinician caring for patients with myositis to recognize the significant association with ILD and appropriately triage some patients in higher-risk autoantibody-associated groups to imaging and serial pulmonary function testing for close follow-up.
Author contributions
SC and LC-S contributed to the conception, writing, and critical review and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
LC-S was supported by the Huayi and Siuling Zhang Discovery Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Furst, DE, Amato, AA, Iorga, ŞR, Gajria, K, and Fernandes, AW. Epidemiology of adult idiopathic inflammatory myopathies in a U.S. managed care plan. Muscle Nerve. (2012) 45:676–3. doi: 10.1002/mus.23302
2. Svensson, J, Arkema, EV, Lundberg, IE, and Holmqvist, M. Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: a nationwide population-based study. Rheumatology. (2017) 56:802–11. doi: 10.1093/rheumatology/kew503
3. Cho, SK, Kim, H, Myung, J, Nam, E, Jung, S-Y, Jang, EJ, et al. Incidence and prevalence of idiopathic inflammatory myopathies in Korea: a Nationwide population-based study. J Korean Med Sci. (2019) 34:e55. doi: 10.3346/jkms.2019.34.e55
4. Kaul, B, Cottin, V, Collard, HR, and Valenzuela, C. Variability in global prevalence of interstitial lung disease. Front Med. (2021) 8:751181. doi: 10.3389/fmed.2021.751181
5. Frazier, AR, and Miller, RD. Interstitial pneumonitis in association with Polymyositis and Dermatomyositis. Chest. (1974) 65:403–7. doi: 10.1378/chest.65.4.403
6. Sun, KY, Fan, Y, Wang, YX, Zhong, YJ, and Wang, GF. Prevalence of interstitial lung disease in polymyositis and dermatomyositis: a meta-analysis from 2000 to 2020. Semin Arthritis Rheum. (2021) 51:175–1. doi: 10.1016/j.semarthrit.2020.11.009
7. Basuita, M, and Fidler, LM. Myositis antibodies and interstitial lung disease. J Appl Lab Med. (2022) 7:240–8. doi: 10.1093/jalm/jfab108
8. Lindgren, U, Pullerits, R, Lindberg, C, and Oldfors, A. Epidemiology survival, and clinical characteristics of inclusion body myositis. Ann Neurol. (2022) 92:201–2. doi: 10.1002/ana.26412
9. Bernatsky, S, Joseph, L, Pineau, CA, Boivin, DB, Banerjee, DB, Clarke, AE, et al. Estimating the prevalence of polymyositis and dermatomyositis from administrative data: age, sex and regional differences. Ann Rheum Dis. (2009) 68:1192–6. doi: 10.1136/ard.2008.093161
10. Betteridge, Z, Tansley, S, Shaddick, G, Lilleker, JB, Vencovsky, J, Chazarain, L, et al. Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J Autoimmun. (2019) 101:48–55. doi: 10.1016/j.jaut.2019.04.001
11. Lilleker, JB, Vencovsky, J, Wang, G, Wedderburn, LR, Diederichsen, LP, Schmidt, J, et al. The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann Rheum Dis. (2018) 77:30–9. doi: 10.1136/annrheumdis-2017-211868
12. Lundberg, IE, Fujimoto, M, Vencovsky, J, Aggarwal, R, Holmqvist, M, Christopher-Stine, L, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primer. (2021) 7:1–22. doi: 10.1038/s41572-021-00321-x
14. Lega, JC, Reynaud, Q, Belot, A, Fabien, N, Durieu, I, and Cottin, V. Idiopathic inflammatory myopathies and the lung. Eur Respir Rev. (2015) 24:216–8. doi: 10.1183/16000617.00002015
15. Connors, GR, Christopher-Stine, L, Oddis, CV, and Danoff, SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest. (2010) 138:1464–74. doi: 10.1378/chest.10-0180
16. Long, K, and Danoff, SK. Interstitial lung disease in polymyositis and dermatomyositis. Clin Chest Med. (2019) 40:561–2. doi: 10.1016/j.ccm.2019.05.004
17. Fujisawa, T. Management of myositis-associated interstitial lung disease. Medicina (Mex). (2021) 57:347. doi: 10.3390/medicina57040347
18. Chandra, T, and Aggarwal, R. Clinical trials and novel therapeutics in dermatomyositis. Expert Opin Emerg Drugs. (2020) 25:213–8. doi: 10.1080/14728214.2020.1787985
19. Satoh, M, Tanaka, S, Ceribelli, A, Calise, SJ, and Chan, EKL. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. (2017) 52:1–19. doi: 10.1007/s12016-015-8510-y
20. Halilu, F, and Christopher-Stine, L. Myositis-specific antibodies: overview and clinical utilization. Rheumatol Immunol Res. (2022) 3:1–10. doi: 10.2478/rir-2022-0001
21. Levine, TD. History of Dermatomyositis. Arch Neurol. (2003) 60:780–2. doi: 10.1001/archneur.60.5.780
22. Leclair, V, and Lundberg, IE. New myositis classification criteria—what we have learned since Bohan and Peter. Curr Rheumatol Rep. (2018) 20:18. doi: 10.1007/s11926-018-0726-4
23. Bohan, A, and Peter, JB. Polymyositis and Dermatomyositis. N Engl J Med. (1975a) 292:344–7. doi: 10.1056/NEJM197502132920706
24. Bohan, A, and Peter, JB. Polymyositis and Dermatomyositis. N Engl J Med. (1975b) 292:403–7. doi: 10.1056/NEJM197502202920807
25. Lundberg, IE, Tjärnlund, A, Bottai, M, Werth, VP, Pilkington, C, de Visser, M, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. (2017) 76:1955–64. doi: 10.1136/annrheumdis-2017-211468
26. Ramanathan, RS. Polymyositis mimicking inflammatory dystrophy. ARC J Neurosci. (2017) 2:1–2. doi: 10.20431/2456-057X.0201001
27. van der Meulen, MFG, Bronner, IM, Hoogendijk, JE, Voskuyl, AE, Dinant, HJ, Linssen, WHJP, et al. Polymyositis: an overdiagnosed entity. Neurology. (2003) 61:316–1. doi: 10.1212/WNL.61.3.316
28. Ebner, L, Christodoulidis, S, Stathopoulou, T, Geiser, T, Stalder, O, Limacher, A, et al. Meta-analysis of the radiological and clinical features of usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP). PLoS One. (2020) 15:e0226084. doi: 10.1371/journal.pone.0226084
29. Lynch, DA, Sverzellati, N, Travis, WD, Brown, KK, Colby, TV, Galvin, JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner society white paper. Lancet Respir Med. (2018) 6:138–3. doi: 10.1016/S2213-2600(17)30433-2
30. Wang, Y, Jin, C, Wu, CC, Zhao, H, Liang, T, Liu, Z, et al. Organizing pneumonia of COVID-19: time-dependent evolution and outcome in CT findings. PLoS One. (2020) 15:e0240347. doi: 10.1371/journal.pone.0240347
31. Karjigi, U, and Dharmanand, BG. Interstitial pneumonia with autoimmune features. Indian J Rheumatol. (2021) 16:S39. doi: 10.4103/0973-3698.332977
32. Fernandes, L, Nasser, M, Ahmad, K, and Cottin, V. Interstitial pneumonia with autoimmune features (IPAF). Front Med. (2019) 6:209. doi: 10.3389/fmed.2019.00209
33. Karampeli, M, Thomas, K, Flouda, S, Chavatza, A, Nikolopoulos, D, Pieta, A, et al. Interstitial pneumonia with autoimmune features (IPAF): a single-Centre prospective study. Mediterr J Rheumatol. (2020) 31:330–6. doi: 10.31138/mjr.31.3.330
34. Solomon, J, Swigris, JJ, and Brown, KK. Myositis-related interstitial lung disease and antisynthetase syndrome. Front Med. (2013) 7:16. doi: 10.3389/fmed.2020.609595
35. Gasparotto, M, Gatto, M, Saccon, F, Ghirardello, A, Iaccarino, L, and Doria, A. Pulmonary involvement in antisynthetase syndrome. Curr Opin Rheumatol. (2019) 31:603–11. doi: 10.1097/BOR.0000000000000663
36. Teel, A, Lu, J, Park, J, Singh, N, and Basharat, P. The role of myositis-specific autoantibodies and the Management of Interstitial Lung Disease in idiopathic inflammatory myopathies: a systematic review. Semin Arthritis Rheum. (2022) 57:152088. doi: 10.1016/j.semarthrit.2022.152088
37. Sato, S, Murakami, A, Kuwajima, A, Mishima, M, Suda, T, Seishima, M, et al. Clinical utility of an enzyme-linked Immunosorbent assay for detecting anti-melanoma differentiation-associated gene 5 autoantibodies. PLoS One. (2016) 11:e0154285. doi: 10.1371/journal.pone.0154285
38. Vizioli, L, Ciccarese, F, Forti, P, Chiesa, AM, Giovagnoli, M, Mughetti, M, et al. Integrated use of lung ultrasound and chest X-ray in the detection of interstitial lung disease. Respiration. (2017) 93:15–22. doi: 10.1159/000452225
39. Sato, S, Hirakata, M, Kuwana, M, Suwa, A, Shinichi, M, Tsuneyo, M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. (2005) 52:1571–6. doi: 10.1002/art.21023
40. DeWane, ME, Waldman, R, and Lu, J. Dermatomyositis: clinical features and pathogenesis. J Am Acad Dermatol. (2020) 82:267–1. doi: 10.1016/j.jaad.2019.06.1309
41. Kurtzman, DJB, and Vleugels, RA. Anti-melanoma differentiation–associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. (2018) 78:776–5. doi: 10.1016/j.jaad.2017.12.010
42. Sato, S, Kuwana, M, Fujita, T, and Suzuki, Y. Amyopathic dermatomyositis developing rapidly progressive interstitial lung disease with elevation of anti-CADM-140/MDA5 autoantibodies. Mod Rheumatol. (2012) 22:625–9. doi: 10.3109/s10165-011-0558-9
43. González-Moreno, J, Raya-Cruz, M, Losada-Lopez, I, Cacheda, AP, Oliver, C, and Colom, B. Rapidly progressive interstitial lung disease due to anti-MDA5 antibodies without skin involvement: a case report and literature review. Rheumatol Int. (2018) 38:1293–6. doi: 10.1007/s00296-018-3991-7
44. Kaenmuang, P, and Navasakulpong, A. Clinical characteristics of anti-MDA5 antibody-positive interstitial lung disease. Respirol Case Rep. (2020) 9:e00701. doi: 10.1002/rcr2.701
45. De Backer, E, Gremonprez, F, Brusselle, G, Depuydt, P, Van Dorpe, J, Van Haverbeke, C, et al. Anti-MDA5 positive dermatomyositis complicated with rapidly progressive interstitial lung disease – a case report. Acta Clin Belg. (2018) 73:413–7. doi: 10.1080/17843286.2017.1420521
46. Li, ZY, Gill, E, Mo, F, and Reyes, C. Double anti-PL-7 and anti-MDA-5 positive Amyopathic Dermatomyositis with rapidly progressive interstitial lung disease in a Hispanic patient. BMC Pulm Med. (2020) 20:220. doi: 10.1186/s12890-020-01256-x
47. Betteridge, Z, and McHugh, N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med. (2016) 280:8–23. doi: 10.1111/joim.12451
48. De Lorenzis, E, Natalello, G, Gigante, L, Verardi, L, Bosello, SL, and Gremese, E. What can we learn from rapidly progressive interstitial lung disease related to anti-MDA5 dermatomyositis in the management of COVID-19? Autoimmun Rev. (2020) 19:102666. doi: 10.1016/j.autrev.2020.102666
49. Takada, T, Asakawa, K, and Barrios, R. A Japanese-American female with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Clin Rheumatol. (2021) 40:1159–65. doi: 10.1007/s10067-020-05292-0
50. Cavagna, L, Meloni, F, Meyer, A, Sambataro, G, Belliato, M, De Langhe, E, et al. Clinical spectrum time course in non-Asian patients positive for anti-MDA5 antibodies. Clin Exp Rheumatol. (2022) 40:274–3. doi: 10.55563/clinexprheumatol/di1083
51. Moghadam-Kia, S, Oddis, CV, Sato, S, Kuwana, M, and Aggarwal, R. Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical Spectrum in north American patients with Dermatomyositis. J Rheumatol. (2017) 44:319–5. doi: 10.3899/jrheum.160682
52. Narang, NS, Casciola-Rosen, L, Li, S, Chung, L, and Fiorentino, DF. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res. (2015) 67:667–2. doi: 10.1002/acr.22498
53. Chen, Z, Cao, M, Plana, MN, Liang, J, Cai, H, Kuwana, M, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res. (2013) 65:1316–24. doi: 10.1002/acr.21985
54. Xu, A, Ye, Y, Fu, Q, Lian, X, Chen, S, Guo, Q, et al. Prognostic values of anti-Ro52 antibodies in anti-MDA5-positive clinically amyopathic dermatomyositis associated with interstitial lung disease. Rheumatol Oxf Engl. (2021) 60:3343–51. doi: 10.1093/rheumatology/keaa786
55. Gui, X, Shenyun, S, Ding, H, Wang, R, Tong, J, Yu, M, et al. Anti-Ro52 antibodies are associated with the prognosis of adult idiopathic inflammatory myopathy-associated interstitial lung disease. Rheumatol Oxf Engl. (2022) 61:4570–8. doi: 10.1093/rheumatology/keac090
56. Lv, C, You, H, Xu, L, Wang, L, Yuan, F, Li, J, et al. Coexistence of anti-Ro52 antibodies in anti-MDA5 antibody-positive Dermatomyositis is highly associated with rapidly progressive interstitial lung disease and mortality risk. J Rheumatol. (2023) 50:219–6. doi: 10.3899/jrheum.220139
57. Wolstencroft, PW, and Fiorentino, DF. Dermatomyositis clinical and pathological phenotypes associated with myositis-specific autoantibodies. Curr Rheumatol Rep. (2018) 20:28. doi: 10.1007/s11926-018-0733-5
58. Rogers, A, Chung, L, Li, S, Casciola-Rosen, L, and Fiorentino, DF. Cutaneous and systemic findings associated with nuclear matrix protein 2 antibodies in adult Dermatomyositis patients. Arthritis Care Res. (2017) 69:1909–14. doi: 10.1002/acr.23210
59. Albayda, J, Pinal-Fernandez, I, Huang, W, Parks, C, Paik, J, Casciola-Rosen, L, et al. Antinuclear matrix protein 2 autoantibodies and edema, muscle disease, and malignancy risk in Dermatomyositis patients. Arthritis Care Res. (2017) 69:1771–6. doi: 10.1002/acr.23188
60. Bermudez, J, Heim, X, Bertin, D, et al. Lung involvement associated with anti-NXP2 autoantibodies in inflammatory myopathies: a French monocenter series. Expert Rev Respir Med. (2020) 14:845–11. doi: 10.1080/17476348.2020.1767598
61. Gossez, M, Levesque, M, Khouatra, C, Cottin, V, Garnier, L, and Fabien, N. Interstitial lung disease in an adult patient with dermatomyositis and anti-NXP2 autoantibody. Eur Respir Rev. (2015) 24:370–2. doi: 10.1183/16000617.00006714
62. Li, L, Wang, H, Wang, Q, Wu, C, Liu, C, Zhang, Y, et al. Myositis-specific autoantibodies in dermatomyositis/polymyositis with interstitial lung disease. J Neurol Sci. (2019) 397:123–8. doi: 10.1016/j.jns.2018.12.040
63. Ceribelli, A, Fredi, M, Taraborelli, M, Cavazzana, I, Franceschini, F, Quinzanini, M, et al. Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res Ther. (2012) 14:R97. doi: 10.1186/ar3822
64. Yan, TT, Zhang, X, Yang, HH, Sun, W-J, Liu, L, Du, Y, et al. Association of anti-NXP2 antibody with clinical characteristics and outcomes in adult dermatomyositis: results from clinical applications based on a myositis-specific antibody. Clin Rheumatol. (2021) 40:3695–02. doi: 10.1007/s10067-021-05667-x
65. Yan, T, Du, Y, Sun, W, Chen, X, Wu, Q, Ye, Q, et al. Interstitial lung disease in adult patients with anti-NXP2 antibody positivity: a multicentre 18-month follow-up study. Clin Exp Rheumatol. Published online. (2023) 41:247–253. doi: 10.55563/clinexprheumatol/lqjx4h
66. Li, S, Sun, C, Zhang, L, Han, J, Yang, H, Gao, S, et al. Clinical heterogeneity of patients with antinuclear matrix protein 2 antibody–positive myositis: a retrospective cohort study in China. J Rheumatol. (2022) 49:922–8. doi: 10.3899/jrheum.211234
67. Ichimura, Y, Matsushita, T, Hamaguchi, Y, Kaji, K, Hasegawa, M, Tanino, Y, et al. Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann Rheum Dis. (2012) 71:710–3. doi: 10.1136/annrheumdis-2011-200697
68. Fiorentino, DF, Chung, LS, Christopher-Stine, L, Zaba, L, Li, S, Mamme, AL, et al. Most patients with Cancer-associated Dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1γ. Arthritis Rheum. (2013) 65:2954–62. doi: 10.1002/art.38093
69. Marvi, U, Chung, L, and Fiorentino, DF. Clinical presentation and evaluation of Dermatomyositis. Indian J Dermatol. (2012) 57:375–1. doi: 10.4103/0019-5154.100486
70. Pinal-Fernandez, I, Mecoli, CA, Casal-Dominguez, M, Pak, K, Hosono, Y, Huapaya, J, et al. More prominent muscle involvement in patients with dermatomyositis with anti-Mi2 autoantibodies. Neurology. (2019) 93:e1768–77. doi: 10.1212/WNL.0000000000008443
71. Gómez, GN, Pérez, N, Braillard Poccard, A, Gómez, RA, Costi, AC, Mercedes, A, et al. Myositis-specific antibodies and clinical characteristics in patients with autoimmune inflammatory myopathies: reported by the argentine registry of inflammatory myopathies of the argentine Society of Rheumatology. Clin Rheumatol. (2021) 40:4473–83. doi: 10.1007/s10067-021-05797-2
72. dos Passos Carvalho, MIC, and Shinjo, SK. Frequency and clinical relevance of anti-Mi-2 autoantibody in adult Brazilian patients with dermatomyositis. Adv Rheumatol. (2019) 59:27. doi: 10.1186/s42358-019-0071-y
73. Srivastava, P, Dwivedi, S, and Misra, R. Myositis-specific and myositis-associated autoantibodies in Indian patients with inflammatory myositis. Rheumatol Int. (2016) 36:935–3. doi: 10.1007/s00296-016-3494-3
74. Ahmad, A, Attoti, Y, and Bernstein, KA. A man with recurrent pneumonitis: a rare case of interstitial lung disease associated with anti-Mi-2 Beta-specific Dermatomyositis. Cureus. (2021) 13:e20334. doi: 10.7759/cureus.20334
75. Komura, K, Fujimoto, M, Matsushita, T, Kaji, K, Kondo, M, Hirano, T, et al. Prevalence and clinical characteristics of anti-Mi-2 antibodies in Japanese patients with dermatomyositis. J Dermatol Sci. (2005) 40:215–7. doi: 10.1016/j.jdermsci.2005.09.004
76. Fiorentino, DF, Kuo, K, Chung, L, Zaba, L, Li, S, and Casciola-Rosen, L. Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1γ antibodies in adults with dermatomyositis. J Am Acad Dermatol. (2015) 72:449–5. doi: 10.1016/j.jaad.2014.12.009
77. Harada, Y, Tominaga, M, Iitoh, E, Kaieda, S, Koga, T, Fujimoto, K, et al. Clinical characteristics of anti-TIF-1γ antibody-positive Dermatomyositis associated with malignancy. J Clin Med. (2022) 11:1925. doi: 10.3390/jcm11071925
78. Varedi, D, Frigerio, A, Scaife, C, and Hull, C. A novel case of TIF1 gamma autoantibody positive dermatomyositis associated with a non-functional pancreatic neuroendocrine tumor. Dermatol Online J. (2019) 25:13030/qt4fc9p1bd. doi: 10.5070/D3253043339
79. Czerwinska, P, Wlodarczyk, NA, Jaworska, AM, and Mackiewicz, AA. The association between TIF1 family members and Cancer Stemness in solid tumors. Cancers. (2021) 13:1528. doi: 10.3390/cancers13071528
80. Xie, J, Jiao, LB, Xu, R, Zhi, DX, and Zhi, HJ. Anti-TIF1 gamma-positive IPAF patient developed stage IVB lung squamous carcinoma in 1 year: a case report. BMC Pulm Med. (2021) 21:204. doi: 10.1186/s12890-021-01570-y
81. Betteridge, ZE, Gunawardena, H, Chinoy, H, North, J, Ollier, WER, Cooper, RG, et al. Clinical and human leucocyte antigen class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target, in UK Caucasian adult-onset myositis. Ann Rheum Dis. (2009) 68:1621–5. doi: 10.1136/ard.2008.097162
82. Gono, T, Tanino, Y, Nishikawa, A, Kawamata, T, Hirai, K, Okazaki, Y, et al. Two cases with autoantibodies to small ubiquitin-like modifier activating enzyme: a potential unique subset of dermatomyositis-associated interstitial lung disease. Int J Rheum Dis. (2019) 22:1582–6. doi: 10.1111/1756-185X.13593
83. Albayda, J, Mecoli, C, Casciola-Rosen, L, Danoff, SK, Lin, CT, Hines, D, et al. A north American cohort of anti-SAE Dermatomyositis: clinical phenotype, testing, and review of cases. ACR Open Rheumatol. (2021) 3:287–4. doi: 10.1002/acr2.11247
84. Kishi, T, Tani, Y, Okiyama, N, Mizuochi, K, Ichimura, Y, Harigai, M, et al. Anti-SAE autoantibody-positive Japanese patient with juvenile dermatomyositis complicated with interstitial lung disease - a case report. Pediatr Rheumatol Online J. (2021) 19:34. doi: 10.1186/s12969-021-00532-2
85. Alenzi, FM. Myositis specific autoantibodies: a clinical perspective. Open Access Rheumatol Res Rev. (2020) 12:9–14. doi: 10.2147/OARRR.S231195
86. Christopher-Stine, L, Casciola-Rosen, LA, Hong, G, Chung, T, Corse, AM, and Mammen, AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. (2010) 62:2757–66. doi: 10.1002/art.27572
87. Anquetil, C, Boyer, O, Wesner, N, Benveniste, O, and Allenbach, Y. Myositis-specific autoantibodies, a cornerstone in immune-mediated necrotizing myopathy. Autoimmun Rev. (2019) 18:223–11. doi: 10.1016/j.autrev.2018.09.008
88. Li, S, Ge, Y, Yang, H, Wang, T, Zheng, X, Peng, Q, et al. The spectrum and clinical significance of myositis-specific autoantibodies in Chinese patients with idiopathic inflammatory myopathies. Clin Rheumatol. (2019) 38:2171–9. doi: 10.1007/s10067-019-04503-7
89. Ge, Y, Yang, H, Xiao, X, Liang, L, Lu, X, and Wang, G. Interstitial lung disease is not rare in immune-mediated necrotizing myopathy with anti-signal recognition particle antibodies. BMC Pulm Med. (2022) 22:14. doi: 10.1186/s12890-021-01802-1
90. Qureshi, A, Brown, D, and Brent, L. Anti-signal recognition particle antibody-associated severe interstitial lung disease requiring lung transplantation. Cureus. (2020) 12:e7962. doi: 10.7759/cureus.7962
91. Baah, S, Gorgone, M, and Lachant, D. Asymptomatic necrotizing myositis in a young male with progressive interstitial lung disease. Respir Med Case Rep. (2021) 32:101374. doi: 10.1016/j.rmcr.2021.101374
92. Kuwana, M, Gil-Vila, A, and Selva-O’Callaghan, A. Role of autoantibodies in the diagnosis and prognosis of interstitial lung disease in autoimmune rheumatic disorders. Ther Adv Musculoskelet Dis. (2021) 13:11032457. doi: 10.1177/1759720X211032457
93. Ge, Y, Shu, X, He, L, Li, C, Lu, X, and Wang, G. Interstitial lung disease is a major characteristic of patients who test positive for anti-PM/Scl antibody. Front Med. (2022) 8:778211. doi: 10.3389/fmed.2021.778211
94. Vojinovic, T, Cavazzana, I, Ceruti, P, Fredi, M, Modina, D, Berlendis, M, et al. Predictive features and clinical presentation of interstitial lung disease in inflammatory myositis. Clin Rev Allergy Immunol. (2021) 60:87–94. doi: 10.1007/s12016-020-08814-5
95. Casal-Dominguez, M, Pinal-Fernandez, I, Derfoul, A, Graf, R, Michelle, H, Albayda, J, et al. The phenotype of myositis patients with anti-Ku autoantibodies. Semin Arthritis Rheum. (2021) 51:728–4. doi: 10.1016/j.semarthrit.2021.04.012
96. Elhani, I, Khoy, K, Mariotte, D, Comby, E, Marcelli, C, Le Mauff, B, et al. The diagnostic challenge of patients with anti-U1-RNP antibodies. Rheumatol Int. (2023) 43:509–521. doi: 10.1007/s00296-022-05161-w
97. Lhote, R, Grenier, P, Haroche, J, Miyara, M, Boussouar, S, Mathian, A, et al. Characterization of interstitial lung disease associated with anti-Ribonucleoprotein antibodies. JCR J Clin Rheumatol. (2020) 26:327–3. doi: 10.1097/RHU.0000000000001127
98. Sawal, N, Mukhopadhyay, S, Rayancha, S, Moore, A, Garcha, P, Kumar, A, et al. A narrative review of interstitial lung disease in anti-synthetase syndrome: a clinical approach. J Thorac Dis. (2021) 13:5556–71. doi: 10.21037/jtd-20-3328
99. Zhan, X, Yan, W, Wang, Y, Li, Q, Shi, X, Gao, Y, et al. Clinical features of anti-synthetase syndrome associated interstitial lung disease: a retrospective cohort in China. BMC Pulm Med. (2021) 21:57. doi: 10.1186/s12890-021-01399-5
100. Jiang, M, Dong, X, and Zheng, Y. Clinical characteristics of interstitial lung diseases positive to different anti-synthetase antibodies. Medicine (Baltimore). (2021) 100:e25816. doi: 10.1097/MD.0000000000025816
101. Moll, SA, Platenburg, MGJP, Platteel, ACM, Vorselaars, ADM, Bonàs, MJ, Roodenburg-Benschop, C, et al. Prevalence of novel myositis autoantibodies in a large cohort of patients with interstitial lung disease. J Clin Med. (2020) 9:2944. doi: 10.3390/jcm9092944
Keywords: myositis, interstitial lung disease, myositis specific autoantibodies, pulmonary fibrosis, narrative review
Citation: Chaudhry S and Christopher-Stine L (2023) Myositis interstitial lung disease and autoantibodies. Front. Med. 10:1117071. doi: 10.3389/fmed.2023.1117071
Edited by:
Makon-Sébastien Njock, University of Liège, BelgiumReviewed by:
Takahisa Gono, Nippon Medical School, JapanGiacomo De Luca, Vita-Salute San Raffaele University, Italy
Copyright © 2023 Chaudhry and Christopher-Stine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa Christopher-Stine, TENocmlzdDRAamhtaS5lZHU=
 Shire Chaudhry
Shire Chaudhry Lisa Christopher-Stine
Lisa Christopher-Stine