- Department of Otorhinolaryngology, Head and Neck Surgery, Medical University of Warsaw, Warsaw, Poland
Tumors of the head and neck region form a heterogeneous group of pathologies, including various benign lesions and malignant neoplasms. Endoglin, also known as CD105, is an accessory receptor for transforming growth factor beta (TGF-β), that regulates angiogenesis, both under physiological and pathological conditions. It is highly expressed in proliferating endothelial cells. Therefore, it is considered as a marker of tumor-related angiogenesis. In this review we discuss the role of endoglin as a possible marker of carcinogenesis, as well as a potential target for antibody-based therapies in the neoplasms of the head and neck region.
Introduction
Tumors of the head and neck region form a heterogeneous group of pathologies, including benign lesions, such as hemangiomas, schwannomas or paragangliomas, and malignant neoplasms, among which squamous cell carcinoma occurs the most frequently. Since the head and neck compartment is the most vascularized anatomical region of the human body, a high level of vascularization is a common feature of the majority of tumors growing in this location. The location of the tumor in the head and neck region is very often surgically challenging, even in case of benign lesions. Therefore, apart from surgical resection, adjuvant treatment is often needed for tumor remnants or in case of recurrence. Hence, a lot of research has concentrated on characterizing the potential prognostic markers of those neoplasms and targets for molecular therapies. Endoglin, also known as CD105, has gained popularity as a marker of tumorigenesis, as well as a potential target for antibody-based therapy (1, 2). It is highly expressed in proliferating endothelial cells. Therefore, it is considered as a marker of tumor-related angiogenesis (3). In this review we summarize the role of endoglin in the pathophysiology of various types of benign, as well as malignant head and neck tumors. We also show the usefulness of endoglin as a prognostic marker and discuss the possibility of various diagnostic and therapeutic strategies based on anti-endoglin antibodies in the head and neck region.
The role of endoglin in tumor angiogenesis: Possible diagnostic and therapeutic implications
Endoglin is a type I transmembrane glycoprotein that acts as an accessory receptor for transforming growth factor beta (TGF-β), a pleiotropic cytokine playing an important role in the regulation of cellular proliferation, differentiation, and migration. Human endoglin is a homodimeric protein with the molecular weight of 180 kDa. It contains a large extracellular domain (561 amino acids), a single transmembrane domain and a short cytosolic domain (4). Structurally, endoglin is a member of the ZP (zona pellucida) family of proteins that share a ZP domain in their extracellular region (5). The extracellular domain of endoglin also contains the tripeptide arginine-glycine-aspartic acid (RGD) motif, a sequence that serves as a biding site for many types of adhesive proteins (6). The ectodomain of endoglin may be released through proteolytic cleavage by matrix metalloproteinase 12 and 14 (MMP-12 and MMP-14) and is present in the circulation as a soluble form of endoglin (Sol-eng) (7). Small amounts of Sol-eng are also present in the serum of healthy subjects. However, an elevated level of Sol-eng was reported in the serum of patients with breast, liver and colorectal cancer, as well as non-small cell lung carcinoma (8–10).
Endoglin modulates the activity of TGF-β mostly via activin-like kinase 1 (ALK1) and activin-like kinase 5 (ALK5) receptors that belong to the superfamily of TGF-β type I receptors (TGF-βR1) (11). These type I receptors activate signaling pathways via Smad-1, –5, and –8 (ALK1) or Smad2 and –3 (ALK5), regulating the expression of various genes involved in angiogenesis (6). Studies showed that the balance between the ALK1 and ALK5 signaling pathways in endothelial cells (ECs) plays a crucial role in angiogenesis and vascular remodeling (12). The overexpression of endoglin in ECs counteracts the antiproliferative effect of TGF-β1 (13). Endoglin also has a protective role against apoptosis induced by hypoxia and TGF-β1 in ECs (13, 14). All these findings support the hypothesis that endoglin and ALK1 participate in a common signaling pathway that is crucial for EC response to TGF-β family members (Figure 1).
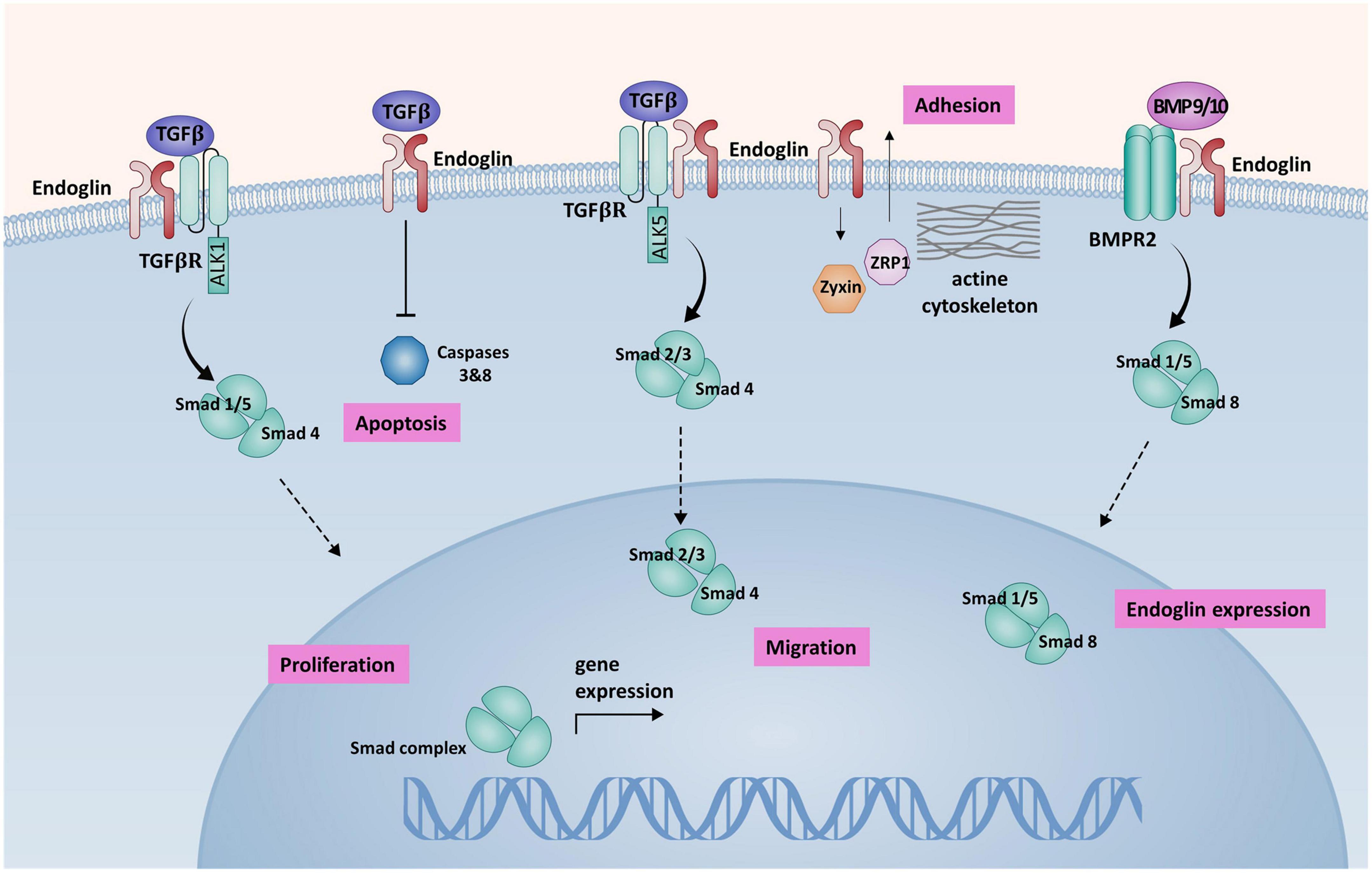
Figure 1. A schematic presentation of endoglin-mediated signaling in endothelial cells (ECs). Endoglin associates with TGF-βRII and ALK1 forming a receptor complex, that binds TGF-β1. Activation of this receptor complex leads to the phosphorylation of Smad1/5 that form a complex with co-Smad (Smad4). Smad complex is then translocated to the nucleus, where it regulates the expression of target genes, promoting cell proliferation. Association of endoglin with the ALK5/TGF-βII receptor complex inhibits Smad2/3 dependent signaling pathway, that has an antiproliferative effect on ECs. Endoglin promotes BMP9/10 signaling via BMPR2 receptor complex. Activation of BMPR2 receptor complex leads to Smad1/5/8 phosphorylation and subsequent increased endoglin expression in a positive feedback loop. Binding of TGF-β by endoglin induces inhibition of apoptosis via caspases 3 and 8 inhibition. Endoglin interaction with ZRP-1 and Zyxin regulates cell migration, inducing the reorganization of actin cytoskeleton and promoting cell adhesion (57).
The main function of endoglin involves the regulation of angiogenesis, both under physiological and pathological conditions. It was demonstrated that the endoglin knockout mice died early in the embryonic development process due to various vascular defects (15). In addition, mutations in the endoglin gene were identified in patients with hereditary hemorrhagic telangiectasia (HHT), an autosomal dominant vascular dysplasia (16, 17). The expression of endoglin is particularly high in actively proliferating ECs. Studies showed, that anti-endoglin antibodies are more specific in staining proliferating ECs than other endothelial markers, such as CD31, CD34, or VEGFR (18). All these data suggest that endoglin might be an excellent marker of tumor vascularization. Anti-endoglin antibodies have been used for microvessel density (MVD) calculation in various types of tumors. It was demonstrated that MVD assessed on the basis of endoglin immunohistochemical expression in the tumor tissue correlated with poor prognosis in colorectal, breast, prostatic and lung cancer patients. Such a correlation was not observed when MVD was calculated using the anti-CD34 or anti-CD31 antibodies, which are traditional markers of angiogenesis (19, 20).
Tumor angiogenesis is an important target in cancer therapy. The first approved antiangiogenic drugs, such as bevacizumab, sorafenib and sunitinib, are anti-VEGF (vascular endothelial growth factor) agents. Nevertheless, the inhibition of non-VEGF angiogenic pathways is an interesting strategy that may address tumor resistance to anti-VEGF therapies. TRC105 (TRACON Pharmaceuticals Inc.) is a chimeric IgG1 anti-endoglin antibody, that induces apoptosis in endoglin-positive tumor cells. Its safety, tolerability and antitumor activity have already been demonstrated in several phase I clinical trials in patients with advanced, refractory solid tumors (21). According to a study where TRC105 was combined with bevacizumab, several patients who had previously progressed on anti-VEGF therapies experienced reductions in tumor volume or remained progression-free for a longer period than on bevacizumab alone. TRC105 in combination with bevacizumab is now tested in randomized phase II trials in glioblastoma and renal cell carcinoma patients (22, 23).
Endoglin as a marker in various malignant and non-malignant head and neck tumors
Head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) includes cancers of the oral cavity, nasopharynx, oropharynx, hypopharynx and larynx. Over the years, significant progress has been made in the diagnosis and treatment of HNSCC, yet the 5-year overall survival rate remains unsatisfactory (24). The basic prognostic factors include the histopathological type of the tumor, its location, size, depth of infiltration, presence of lymph node metastases, tobacco use and human papillomavirus (HPV) infection in case of oropharyngeal cancer, or Epstein-Barr (EBV) virus in nasopharyngeal carcinoma (25). In addition to the described prognostic factors, immunohistochemical markers such as p53, Ki-67, p16, cyclin D1, and vessel density (MVD) calculation play a role in the prognostic evaluation and therapeutic decisions (26). Increased MVD values, recognized as an independent prognostic indicator, reflect the progression of the disease and a shorter disease-free survival rate (DSF). In case of HNSCC, some studies confirmed this correlation with MVD, while others did not find such a correlation. The differences result from the use of different protocols in immunohistochemical staining for endothelial marker studies. Endothelial targets, such as CD31, CD34 and, increasingly, endoglin, which is also involved in angiogenesis, were used as endothelial targets for MDV calculation in HNSCC (27, 28).
Schimming and Marme (29) examined endoglin in the tumor tissue of oral cancer at different TNM stages and in the normal mucosa. TNM is an international classification used for cancer staging. T1-4 describes the tumor size and its local spread to surrounding tissues, N0-3 describes lymph node metastases, whereas N + means any lymph nodes positive for metastases. M0-1 stands for distant metastases. In the reported study endoglin expression was significantly higher in the neoplastic tissue, and it was lower in T1 compared to other T stages, as well as in N0 compared to N + cases. Moreover, higher endoglin expression was observed in moderately differentiated compared to poorly differentiated tumors (29). Nagatsuka et al. (30) investigated endoglin, as well as CD31 and CD34 antibodies in the normal oral mucosa and in 40 cases of HNSCC to study the properties and morphology of blood vessels. Endoglin-positive endothelial cells were found in the neo-vessels of the oral cavity squamous cell carcinomas (SCC) with extensive remodeling and in immature SCC neo-vessels (30).
Chuang et al. (31) demonstrated a high expression of endoglin in biopsy tissues collected from 94 patients with advanced oral SCC and N + cases. In addition, cumulative 5-year disease-free survival (DFS) correlated significantly with low endoglin and VEGF expression (31). Similar results were obtained in other studies on endoglin expression in oral SCC (32, 33).
Endoglin and VEGF testing was performed by Chen et al. (34) in the postoperative specimens of SCC of the hypopharynx. The study showed a high expression of endoglin in N + cases and in clinically advanced tumors. It was reported that the overall 5-year survival rate for patients with low endoglin expression was higher than in patients with high endoglin expression, and high endoglin expression was found to be an independent survival factor (34).
Marioni et al. (35) analyzed MDV by endoglin immunohistochemistry for laryngeal SCC in forty-three patients undergoing partial or total laryngectomy. The study showed that disease recurrence was correlated with endoglin-assessed MVD. Furthermore, it was confirmed that endoglin-assessed MVD might be used as a predictive parameter for patients at an increased risk of local and regional recurrence of laryngeal SCC (35, 36). Zvrko et al. (37) investigated the expression of endoglin in 40 cases of postoperative supraglottic part of larynx carcinoma specimens. The study showed statistical correlation between high endoglin-assessed MVD and advanced clinical stage, pN + and loco-regional recurrence. Moreover, endoglin-assessed MVD was found to be the only independent predictor of recurrence (37). The study was also performed on 40 glottal SCC biopsies. A significant correlation was confirmed between endoglin-assessed MVD and advanced pT stage and clinical stage as well as recurrence. Also, high endoglin-assessed MVD was associated with poorer DFS (38). In Marioni et al. (27) analyzed MVD via endoglin and CD31 in forty-five paired SCC biopsies and surgical specimens of the larynx. The median endoglin-assessed MVD was shown to be higher in N + cases. Statistical analysis revealed that DFS correlated with endoglin and CD31-assessed MVD in both biopsies and surgical specimens. The multivariate Cox regression showed that the pathological grade and endoglin-assessed MVD predicted DFS in SCC of the larynx. The authors emphasized that further research was required to determine the role of endoglin-assessed MVD as a prognostic marker suitable for identifying patients at a higher risk of recurrence requiring more aggressive treatment and clinically N0 patients requiring elective neck dissection (27).
Paragangliomas
Paragangliomas are rare, usually benign tumors, derived from the autonomic nervous system paraganglia. Paragangliomas of the head and neck region most commonly develop in the carotid body. Less common anatomical locations include the middle ear (tympanic paragangliomas), jugular fossa (jugular paragangliomas) and vagus nerve (vagus paragangliomas). Since head and neck paragangliomas arise from parasympathetic ganglia, they very rarely secrete catecholamines, unlike adrenal paragangliomas (pheochromocytomas), that are derived from the sympathetic system (39). Approximately 30% of paragangliomas have a familial occurrence, so wide genetic studies were conducted to discover the genetic background of the tumors (40). Germline and somatic mutations that lead to paraganglioma development may be classified into three clusters: pseudohypoxia-related genes (clusters 1A and 1B), kinase signaling–related genes (cluster 2) and Wnt signaling–related genes (cluster 3). Cluster 1 is divided into A and B subcategories according to the position of the mutation either in the Krebs cycle or hypoxia signaling pathway (41). The most common mutations in the cluster 1 are located in genes coding the succinate dehydrogenase enzyme complex (SDH), which participates in the citric acid cycle and mitochondrial electron transport chain. Mutations in the SDHx genes trigger a state of pseudohypoxia, which leads to the activation of the hypoxia-inducible factor 1-a (HIF-1α) transcription factor pathway. The activation of the HIF-1α signaling pathway leads to the upregulation of endoglin expression (42). Eleno et al. (43) were the first to assess the level of endoglin expression in cervical paragangliomas. They reported a significantly higher expression of endoglin in paraganglioma tissues than in the control lung tissue, whereas VEGF level was similar in both tissues. Endoglin was almost exclusively expressed on endothelial cell surface, without any staining of tumor parenchymal cells. Recently, in our center, we have also investigated the expression of endoglin in various types of head and neck paragangliomas, as well as the level of Sol-eng in the patients’ serum (44). The results of this study showed a high level of endoglin expression in tumor samples. The level of Sol-eng in serum samples was significantly higher in the tumor group than in healthy controls and a positive correlation with the tumor size was observed. In the examined group of patients, a complete surgical resection led to the reduction of Sol-eng level to the values obtained in control group 4 weeks after the operation.
Salivary gland tumors
Malignant salivary gland tumors are characterized by a highly heterogeneous histological structure, with over 20 histopathological types included in the WHO classification. Unpredictable clinical behavior is their unique clinical feature, with distant metastases that may occur even many years after the initial diagnosis and treatment. Several authors assessed angiogenesis in salivary gland tumors, using endoglin as a marker, in order to improve knowledge about the prognosis and adequate management (45–48). Endoglin-positive vessels were absent in the normal salivary gland tissue and rare in pleomorphic adenomas. However, Warthin’s tumors, that are the most frequent benign tumors of parotid gland, presented very high endoglin expression in their lymphoid component (47). A significant increase in endoglin expression was observed in malignant tumors. It was the highest in mucoepidermoid carcinomas, where it reached 83–85% (45, 48) and less common in polymorphous low-grade adenocarcinomas, where 42% increase of endoglin expression was noted (45). The results for adenoid cystic carcinoma vary from 65% in a study by Tadbir et al. (48) to 8% in a study by Cardoso et al. (45). Such differences between studies may result from dissimilar microscopic tumor subtypes. However, no significant difference was found in endoglin expression comparing adenoid cystic carcinoma with and without high-grade transformation (49). Differences in endoglin expression in various types of malignant salivary gland tumors may be explained by their variable myoepithelial differentiation, although no correlation was demonstrated between the degree of angiogenesis and the amount of myoepithelial cells (50). Carcinomas with myoepithelial differentiation, regardless of the amount of myoepithelial cells, were associated with a significantly lower vascular density (51). The highest density of endoglin-positive blood vessels was also observed in tumor stromal areas with marked inflammation (46).
Fonseca et al. (47) did not reveal a significant correlation between endoglin expression and clinicopathological parameters. Moreover, vascular density did not correlate with the survival rates of patients affected by malignant salivary gland tumors (47). The expression of endoglin was also very similar between non-metastasizing and metastasizing primary malignant salivary gland tumors in the study by Cardoso et al. (45) but adenoid cystic carcinomas with endoglin-positive vessels were characterized by an increased risk for metastasis. Gleber-Netto et al. (51) conducted a study concerning minor salivary gland mucoepidermoid carcinoma. They assessed endoglin expression and showed a greater angiogenic activity measured in intratumoral than in peritumoral areas. Contrary to observations in other neoplasms, recurrence and nodal metastasis were associated with low neo formed vessel density, indicating that impaired angiogenesis could lead to an aggressive phenotype (51).
Vascular tumors
Endoglin is also highly expressed in the head and neck tumors of vascular origin, such as capillary hemangiomas and juvenile nasopharyngeal angiofibroma (52, 53). In a study by Matsumoto et al. (52) all capillary hemangioma cases showed moderate-to-strong endoglin staining of blood vessel endothelial cells. The staining score was significantly higher than in normal controls (normal oral mucosa) and cavernous hemangiomas (52). The endothelial cells of capillary hemangiomas have an active proliferative capacity, reflected by significantly elevated Ki-67 expression. Furthermore, the expression of VEGF-a and COX-2 in the stromal fibroblasts and macrophages was stronger in capillary hemangiomas than in the control tissue or cavernous hemangiomas. Wang et al. (53) performed endoglin-based MVD (endoglin/MVD) measurements in juvenile angiofibroma patients. The analysis of the results with clinicopathological features showed a correlation between endoglin/MVD and angiofibroma recurrence. endoglin/MVD was a better predictor of disease recurrence after curative resection than other clinicopathological features (53).
Rhabdomyosarcoma
Endoglin expression is also considered to be promising prognostic marker in rhabdomyosarcoma (54, 55). Radzikowska et al. (54) analyzed microvessel density based on CD31, CD34, and endoglin expression in 49 cases of pediatric rhabdomyosarcoma (RMS). CD31, CD34, and endoglin were expressed in all RMS cases (54). Endoglin/MVD was significantly higher in patients with alveolar RMS and those with metastatic disease. Patients with higher levels of endoglin/MVD were at a higher risk of death. The authors concluded that endoglin was a relevant angiogenesis marker in pediatric RMS, and endoglin/MVD was an independent risk factor of short overall survival in children with RMS. The expression of endoglin was also predictive of aggressive biologic behavior of non-melanoma skin cancers – basal and squamous cell carcinomas located mainly in the head and neck region (55). Most of the examined tumors exhibited negative-to-weak endoglin staining but a statistically significant correlation was found between tumor local recurrence and endoglin expression.
Vestibular schwannomas
Endoglin expression was also studied in a series of NF2-associated vestibular schwannomas (VSs), as compared to a group of sporadic VSs (56). No significant differences were found between NF2-associated VSs and sporadic cases in terms of endoglin expression. A positive correlation was observed between tumor growth rate (measured on contrast-enhanced MRI) and vessel density based on endoglin staining, but only in NF2-associated VSs.
Conclusion
An increasing number of studies point to endoglin as a potential marker in various types of head and neck neoplasms (Table 1). It was shown that MVD calculated using endoglin staining was a reliable marker of more advanced disease, lymph node metastases and poor prognosis in HNSCC and in rhabdomyosarcoma. In addition, several studies demonstrated that a high expression of endoglin in tumor tissues was an independent risk factor of lower 5-year overall survival rate (Table 1). Endoglin is also highly expressed in non-malignant richly vascularized tumors, including paragangliomas and juvenile angiofibromas. All the data considered collectively make endoglin a promising target for biological antibody-based therapy, especially in recurrent and advanced cases. However, even though TRC105, the anti-endoglin antibody was tested as a potential therapeutic agent in various solid tumors, there is a lack of similar studies in head and neck neoplasms. Future research directions in this area should also include studies on a soluble form of endoglin as a potential marker of tumor progression and recurrence.
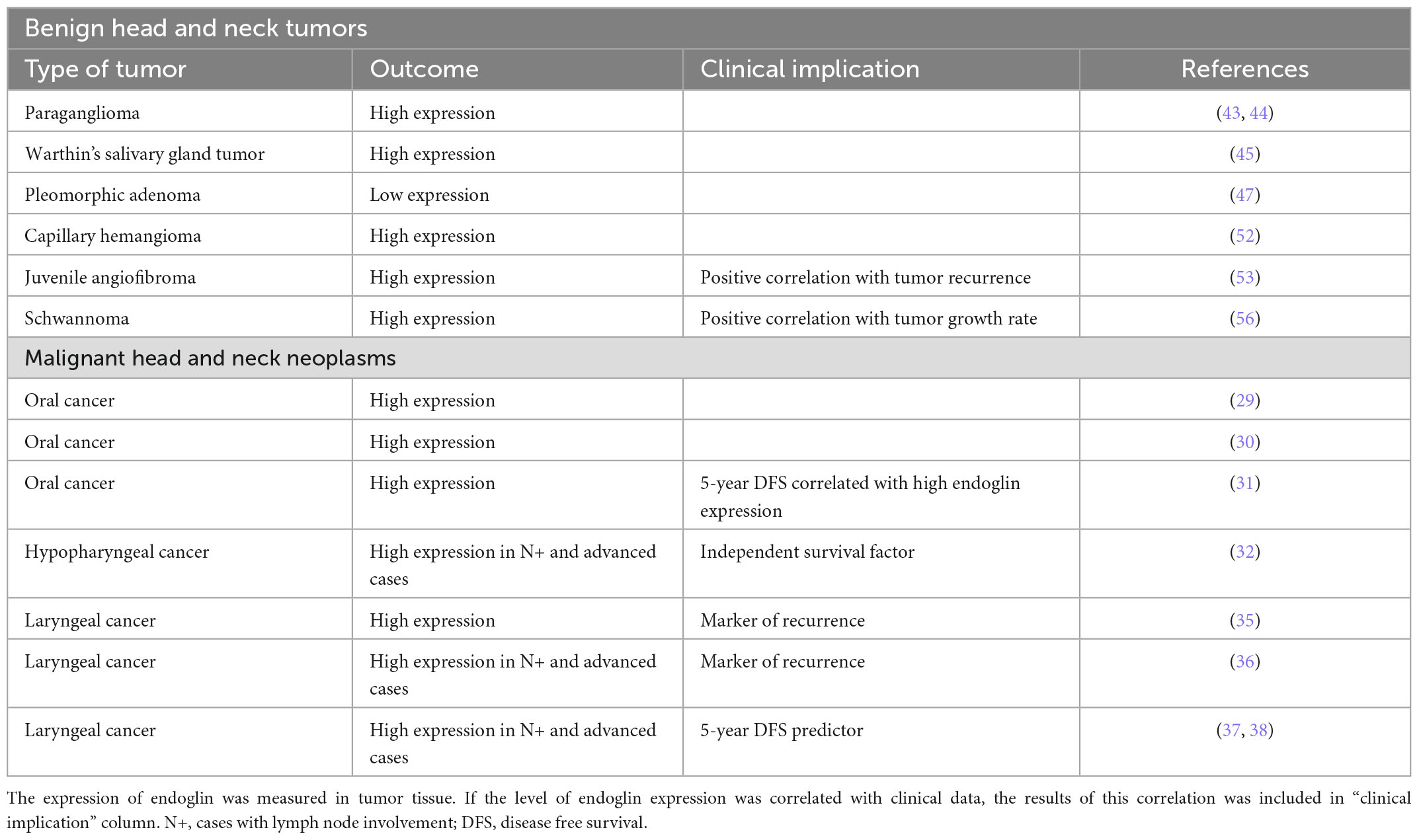
Table 1. The expression of endoglin in various benign and malignant head and neck tumors, reported in different studies.
Author contributions
ML-K contributed to the conception and design of the manuscript and prepared the table and figure. ML-K, MM, and MC wrote the sections of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALK, activin-like kinase; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; DFS, disease free survival; ECs, endothelial cells; EBV, Epstein-Barr virus; HHT, hereditary hemorrhagic telangiectasia; HIF-1α, hypoxia-inducible factor 1-α; HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; MMP, matrix metalloproteinase; MVD, microvessel density; NF2, neurofibromatosis type 2; RMS, rhabdomyosarcoma; SDH, succinate dehydrogenase enzyme complex; SCC, squamous cell carcinoma; Sol-eng, soluble form of endoglin; TGF-β, transforming growth factor beta; TGF-βR, transforming growth factor beta receptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; VS, vestibular schwannoma; ZP, zona pellucida; ZRP-1, zyxin related protein 1.
References
1. Nassiri F, Cusimano M, Scheithauer B, Rotondo F, Fazio A, Yousef G, et al. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. (2011) 31:2283–90.
2. González Muñoz T, Amaral A, Puerto-Camacho P, Peinado H, de Álava E. Endoglin in the Spotlight to Treat Cancer. Int J Mol Sci. (2021) 22:3186. doi: 10.3390/ijms22063186
3. Burrows F, Derbyshire E, Tazzari P, Amlot P, Gazdar A, King S, et al. Up-regulation of endoglin on vascular endothelial cells in human solid tumors: implications for diagnosis and therapy. Clin Cancer Res. (1995) 1:1623–34.
4. Gougos A, Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem. (1990) 265:8361–4. doi: 10.1016/S0021-9258(19)38892-1
5. Llorca O, Trujillo A, Blanco F, Bernabeu C. Structural model of human endoglin, a transmembrane receptor responsible for hereditary hemorrhagic telangiectasia. J Mol Biol (2007) 365:694–705. doi: 10.1016/j.jmb.2006.10.015
6. Rossi E, Bernabeu C, Smadja D. Endoglin as an adhesion molecule in mature and progenitor endothelial cells: a function beyond TGF-β. Front Med. (2019) 30:6–10. doi: 10.3389/fmed.2019.00010
7. Fonsatti E, Altomonte M, Nicotra M, Natali P, Maio M. Endoglin (CD105): a powerful therapeutic target on tumorassociated angiogenetic blood vessels. Oncogene. (2003) 22:6557–63. doi: 10.1038/sj.onc.1206813
8. Kopczyńska E, Dancewicz M, Kowalewski J, Makarewicz R, Kardymowicz H, Kaczmarczyk A, et al. Influence of surgical resection on plasma endoglin (CD105) level in non-small cell lung cancer patients. Exp Oncol. (2012) 34:53–6. doi: 10.5402/2012/638352
9. Takahashi N, Kawanishi-Tabata R, Haba A, Tabata M, Haruta Y, Tsai H, et al. Association of serum endoglin with metastasis in patients with colorectal, breast, and other solid tumors, and suppressive effect of chemotherapy on the serum endoglin. Clin Cancer Res. (2001) 7:524–32.
10. Li C, Guo B, Wilson P, Stewart A, Byrne G, Bundred N, et al. Plasma levels of soluble CD105 correlate with metastasis in patients with breast cancer. Int J Cancer. (2000) 89:122–6. doi: 10.1002/(SICI)1097-0215(20000320)89:2<122::AID-IJC4>3.0.CO;2-M
11. Wong S, Hamel L, Chevalier S, Philip A. Endoglin expression on human microvascular endothelial cells association with betaglycan and formation of higher order complexes with TGF-beta signalling receptors. Eur J Biochem. (2000) 267:5550–60. doi: 10.1046/j.1432-1327.2000.01621.x
12. Ten Dijke P, Goumans M, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. (2008) 11:79–89. doi: 10.1007/s10456-008-9101-9
13. Li C, Hampson I, Hampson L, Kumar P, Bernabeu C, Kumar S. CD105 antagonizes the inhibitory signaling of transforming growth factor beta1 on human vascular endothelial cells. FASEB J. (2000) 14:55–64. doi: 10.1096/fasebj.14.1.55
14. Li C, Issa R, Kumar P, Hampson I, López-Novoa J, Bernabeu C, et al. CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci. (2003) 116:2677–85. doi: 10.1242/jcs.00470
15. Li D, Sorensen L, Brooke B, Urness L, Davis E, Taylor D, et al. Defective angiogenesis in mice lacking endoglin. Science. (1999) 284:1534–7. doi: 10.1126/science.284.5419.1534
16. McAllister K, Baldwin M, Thukkani A, Gallione C, Berg J, Porteous M, et al. Six novel mutations in the endoglin gene in hereditary hemorrhagic telangiectasia type 1 suggest a dominant-negative effect of receptor function. Hum Mol Genet. (1995) 4:1983–5. doi: 10.1093/hmg/4.10.1983
17. Shovlin C, Hughes J, Scott J, Seidman C, Seidman J. Characterization of endoglin and identification of novel mutations in hereditary hemorrhagic telangiectasia. Am J Hum Genet. (1997) 61:68–79. doi: 10.1086/513906
18. Brewer C, Setterdahl J, Li M, Johnston J, McAsey M. Endoglin expression as a measure of microvessel density in cervical cancer. Obstet Gynecol. (2000) 96:224–8. doi: 10.1097/00006250-200008000-00013
19. El-Gohary Y, Silverman J, Olson P, Liu Y, Cohen J, Miller R, et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in prostatic adenocarcinoma. Am J Clin Pathol. (2007) 127:572–9. doi: 10.1309/X6NXYE57DLUE2NQ8
20. Li C, Gardy R, Seon B, Duff S, Abdalla S, Renehan A, et al. Both high intratumoral microvessel density determined using CD105 antibody and elevated plasma levels of CD105 in colorectal cancer patients correlate with poor prognosis. Br J Cancer. (2003) 88:1424–31. doi: 10.1038/sj.bjc.6600874
21. Gordon M, Robert F, Matei D, Mendelson D, Goldman J, Chiorean E, et al. An open-label phase Ib dose-escalation study of TRC105 (anti-endoglin antibody) with bevacizumab in patients with advanced cancer. Clin Cancer Res. (2014) 20:5918–26. doi: 10.1158/1078-0432.CCR-14-1143
22. Galanis E, Anderson S, Twohy E, Butowski N, Hormigo A, Schiff D, et al. Phase I/randomized phase II trial of TRC105 plus bevacizumab versus bevacizumab in recurrent glioblastoma: North Central Cancer Treatment Group N1174 (Alliance). Neurooncol Adv. (2022) 4:vdac041. doi: 10.1093/noajnl/vdac041
23. Dorff T, Longmate J, Pal S, Stadler W, Fishman M, Vaishampayan U, et al. Bevacizumab alone or in combination with TRC105 for patients with refractory metastatic renal cell cancer. Cancer. (2017) 123:4566–73. doi: 10.1002/cncr.30942
24. Bose P, Brockton NT, Dort JC. Head and neck cancer: from anatomy to biology. Int J Cancer. (2013) 133:2013–23. doi: 10.1002/ijc.28112
25. Dong Y, Ma G, Liu Y, Lu S, Liu L. Prognostic value of microvessel density in head and neck squamous cell carcinoma: a meta-analysis. Dis Markers. (2020) 28:8842795. doi: 10.1155/2020/8842795
26. Almangush A, Heikkinen I, Mäkitie A, Coletta R, Läärä E, Leivo I, et al. Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer. (2017) 117:856–66. doi: 10.1038/bjc.2017.244
27. Marioni G, Franz L, Ottaviano G, Contro G, Tealdo G, Carli A, et al. Prognostic Significance of CD105- and CD31-Assessed microvessel density in paired biopsies and surgical samples of laryngeal carcinoma. Cancers. (2020) 12:2059. doi: 10.3390/cancers12082059
28. Marioni G, D’Alessandro E, Giacomelli L, Staffieri A. CD105 is a marker of tumour vasculature and a potential target for the treatment of head and neck squamous cell carcinoma. J Oral Pathol Med. (2010) 39:361–7. doi: 10.1111/j.1600-0714.2010.00888.x
29. Schimming R, Marme D. Endoglin (CD105) expression in squamous cell carcinoma of the oral cavity. Head Neck. (2002) 24:151–6. doi: 10.1002/hed.10040
30. Nagatsuka H, Hibi K, Gunduz M, Tsujigiwa H, Tamamura R, Sugahara T, et al. Various immunostaining patterns of CD31, CD34 and endoglin and their relationship with lymph node metastasis in oral squamous cell carcinomas. J Oral Pathol Med. (2005) 34:70–6. doi: 10.1111/j.1600-0714.2004.00227.x
31. Chuang H, Su C, Huang H, Chien C, Chen C, Huang C. High expression of CD105 as a prognostic predictor of early tongue cancer. Laryngoscope. (2006) 116:1175–9. doi: 10.1097/01.mlg.0000224338.56902.28
32. Chien C, Su C, Hwang C, Chuang H, Chen C, Huang C. High expressions of CD105 and VEGF in early oral cancer predict potential cervical metastasis. J Surg Oncol. (2006) 94:413–7. doi: 10.1002/jso.20546
33. Miyahara M, Tanuma J, Sugihara K, Semba I. Tumor lymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer. (2007) 110:1287–94. doi: 10.1002/cncr.22900
34. Chen C, Su C, Hwang C, Chuang H, Hsiao Y, Wu S, et al. Clinicopathologic significance of CD105 expression in squamous cell carcinoma of the hypopharynx. Head Neck. (2006) 28:441–6. doi: 10.1002/hed.20364
35. Marioni G, Ottaviano G, Giacomelli B, Staffieri C, Casarotti-Todeschini S, Bonandini E, et al. CD105-assessed micro-vessel density is associated with malignancy recurrence in laryngeal squamous cell carcinoma. Eur J Surg Oncol. (2006) 32:1149–53. doi: 10.1016/j.ejso.2006.08.001
36. Marioni G, Giacomelli L, D’Alessandro E, Staffieri C, Guzzardo V, Staffieri A, et al. Laryngeal carcinoma recurrence rate and disease-free interval are related to CD105 expression but not to Vascular Endothelial Growth Factor 2 (Flk-1 /KDR) expression. Anticancer Res. (2008) 28:551–8.
37. Zvrko E, Mikic A, Vuckovic L. CD105 expression as a measure of microvessel density in supraglottic laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol (2009) 266:1971–6. doi: 10.1007/s00405-009-0962-3
38. Zvrko E, Mikic A, Vuckovic L. Clinicopathologic significance of CD105-assessed microvessel density in glottic laryngeal squamous cell carcinoma. Auris Nasus Larynx. (2010) 37:77–83. doi: 10.1016/j.anl.2009.05.005
39. Nölting S, Bechmann N, Taieb D, Beuschlein F, Fassnacht M, Kroiss M, et al. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr Rev. (2022) 43:199–239. doi: 10.1210/endrev/bnab019
40. Hussain I, Husain Q, Baredes S, Eloy J, Jyung R, Liu J. Molecular genetics of paragangliomas of the skull base and head and neck region: implications for medical and surgical management. J Neurosurg. (2014) 120:321–30. doi: 10.3171/2013.10.JNS13659
41. Amorim-Pires D, Peixoto J, Lima J. Hypoxia Pathway Mutations in Pheochromocytomas and Paragangliomas. Cytogenet Genome Res. (2016) 150:227–41. doi: 10.1159/000457479
42. Sánchez-Elsner T, Botella L, Velasco B, Langa C, Bernabéu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J Biol Chem. (2002) 277:43799–808. doi: 10.1074/jbc.M207160200
43. Eleno N, Düwel A, Muñoz A, Paz-Bouza J, López-Novoa J, Lozano F. Endoglin as a marker in cervical paragangliomas. Head Neck. (2010) 32:737–43. doi: 10.1002/hed.21248
44. Litwiniuk M, Niemczyk K, Niderla-Bielińska J, Łukawska-Popieluch I, Grzela T. Soluble Endoglin (CD105) Serum Level as a Potential Marker in the Management of Head and Neck Paragangliomas. Ann Otol Rhinol Laryngol. (2017) 126:717–21. doi: 10.1177/0003489417727548
45. Cardoso SV, Souza K, Faria P, Eisenberg A, Dias F, Loyola A. Assessment of angiogenesis by CD105 antigen in epithelial salivary gland neoplasms with diverse metastatic behavior. BMC Cancer. (2009) 9:391. doi: 10.1186/1471-2407-9-391
46. Gaonkar P, Patankar S, Sridharan G. Assessment of angiogenesis using endoglin in salivary gland tumours – An immunohistochemical study. J Cancer Res Ther. (2022) 18:623–8. doi: 10.4103/jcrt.jcrt_8_21
47. Fonseca F, Bingle L, Santos-Silva A, Lopes M, Coletta R, de Andrade B, et al. Immunoexpression of hoxb7 and hoxb9 in salivary gland tumours. J Oral Pathol Med. (2016) 45:672–81. doi: 10.1111/jop.12438
48. Tadbir A, Pardis S, Ashkavandi Z, Najvani A, Ashraf M, Taheri A, et al. Expression of Ki67 and CD105 as proliferation and angiogenesis markers in salivary gland tumors. Asian Pacific J Cancer Prev. (2012) 13:5155–9. doi: 10.7314/APJCP.2012.13.10.5155
49. Costa A, Tasso M, Mariano FV, Soares A, Chone C, Crespo A, et al. Levels and patterns of expression of hypoxia-inducible factor-1α, vascular endothelial growth factor, glucose transporter-1 and CD105 in adenoid cystic carcinomas with high-grade transformation. Histopathology. (2012) 60:816–25. doi: 10.1111/j.1365-2559.2011.04128.x
50. Costa A, Demasi A, Bonfitto V, Bonfitto J, Furuse C, Araújo V, et al. Angiogenesis in salivary carcinomas with and without myoepithelial differentiation. Virchows Arch. (2008) 453:359–67. doi: 10.1007/s00428-008-0664-z
51. Gleber-Netto F, Florêncio T, de Sousa S, Abreu M, Mendonça E, Aguiar M. Angiogenesis and lymphangiogenesis in mucoepidermoid carcinoma of minor salivary glands. J Oral Pathol Med. (2012) 41:603–9. doi: 10.1111/j.1600-0714.2012.01153.x
52. Matsumoto N, Tsuchiya M, Nomoto S, Matsue Y, Nishikawa Y, Takamura T, et al. CD105 expression in oral capillary hemangiomas and cavernous hemangiomas. J Oral Sci. (2015) 57:45–53. doi: 10.2334/josnusd.57.45
53. Wang J, Sun X, Hu L, Liu Z, Yu H, Li H, et al. Endoglin (CD105) expression on microvessel endothelial cells in juvenile nasopharyngeal angiofibroma: tissue microarray analysis and association with prognostic significance. Head Neck. (2013) 35:1719–25. doi: 10.1002/hed.23210
54. Radzikowska J, Krzeski A, Czarnecka A, Klepacka T, Rychlowska-Pruszynska M, Raciborska A, et al. Endoglin expression and microvessel density as prognostic factors in pediatric rhabdomyosarcoma. J Clin Med. (2021) 10:1–15. doi: 10.3390/jcm10030512
55. Chrisostomidis C, Konofaos P, Karypidis D, Lazaris A, Kostakis A, Papadopoulos O. The impact of Ets-1 oncoprotein and human endoglin (CD105) on the recurrence of non-melanoma skin cancers. Int J Dermatol. (2015) 54:989–95. doi: 10.1111/ijd.12891
56. Marioni G, Nicolè L, Cazzador D, Pavone C, D’Avella D, Martini A, et al. Endoglin (CD105) expression in neurofibromatosis type 2 vestibular schwannoma. Head Neck. (2019) 41:3612–7. doi: 10.1002/hed.25881
Keywords: endoglin, paraganglioma, angiogenesis, head and neck tumors, salivary gland tumors
Citation: Litwiniuk-Kosmala M, Makuszewska M and Czesak M (2023) Endoglin in head and neck neoplasms. Front. Med. 10:1115212. doi: 10.3389/fmed.2023.1115212
Received: 03 December 2022; Accepted: 27 January 2023;
Published: 10 February 2023.
Edited by:
Elisa Rossi, Université de Paris, FranceCopyright © 2023 Litwiniuk-Kosmala, Makuszewska and Czesak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Małgorzata Litwiniuk-Kosmala,  bWxpdHdpbml1a0B3dW0uZWR1LnBs
bWxpdHdpbml1a0B3dW0uZWR1LnBs
 Małgorzata Litwiniuk-Kosmala
Małgorzata Litwiniuk-Kosmala Maria Makuszewska
Maria Makuszewska