
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 21 February 2023
Sec. Obstetrics and Gynecology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1111269
This article is part of the Research Topic Update on Diagnostic and Prognostic Biomarkers for Women's Cancers View all 12 articles
Cervical cancer is the fourth largest malignant tumor among women in the world. Human papillomavirus (HPV) infection can lead to cervical intraepithelial neoplasia (CIN) and cervical cancer. Active papillomavirus infection occurs when the infected basal cells replicate and fill a certain area. Persistent HPV infection can lead to squamous intraepithelial lesions, which are divided into CIN1, CIN2, and CIN3 according to how much epithelium is impacted. Different types of HPV have different possibilities of causing cervical cancer, and high-risk HPV is the main cause of cervical cancer. Research showed that viral load may be an indicator of the progression of cervical precancerous lesions, but this association does not seem to be universal. This article aims to summarize different genotypes, multiple infections, especially viral load, in cervical precancerous lesions, to guide early intervention.
Cervical cancer is a leading cause of mortality among women. In 2020, an estimated 604,000 women were diagnosed with cervical cancer worldwide and about 342,000 women died from the disease (1). According to epidemiological research statistics, in the United States, 75% of people aged 15–50 are infected with human papillomavirus (HPV) in their lifetime, of which 60% are only temporary infections, 10% are persistent infections (the habitual targets of the HPV), 4% have slight cytological changes, and only 1% have clinical cytological damage. Persistent infection with about 15 types of hrHPV is the main risk factor for cervical cancer, of which HPV16 and HPV18 infections account for about 70% of the total cases (2). In the summary analysis of 11 case–control studies (3), HPV 16, 18, 45, 31, 33, 52, 58, and 35 accounted for 95% of HPV DNA-positive squamous cell carcinoma.
Two decades ago, hrHPV testing was proposed as a potential alternative to repeated cytology or immediate colposcopy for the triage of women with Atypical Squamous Cells of Undetermined Significance (ASCUS) cytology (4). In the last few years, the superiority of hrHPV testing compared to cytology to detect high-grade lesions has been demonstrated (5). However, for young women aged 21–24, the specificity of the HPV mRNA test in defining CIN2 lesions in women with ASCUS or Low-grade Squamous Intraepithelial Lesion (LSIL) is much higher than hrHPV DNA test (6). Recently, a Cochrane review (7) pointed out that relevant triage strategies are needed to manage hrHPV-positive women. Biomarkers have been assessed to manage hrHPV-positive women that include HPV genotyping, p16/Ki67 dual-staining, or the methylation status of HPV and some human genes (8–10). According to recent longitudinal studies, hrHPV viral loads can affect cervical diseases to varying degrees. This article summarizes the research progress on the correlation between hrHPV viral load, HPV genotyping, and cervical lesions and provides guidance for the screening of cervical cancer.
Human papillomavirus is a DNA virus in the papillomavirus subgroup. The shell consists of 72 5-polymers, 20 polyhedra, no envelope, 45–55 nm in diameter, and 5 × 106 Da. The HPV genome is an annular double-stranded DNA molecule with about 7,800–7,900 base pairs (bp), whose DNA composition accounts for about 12% of the mass of the virus (11). The complete genome can be divided into 3 coding regions (Figure 1): (1) early region (open reading box): including six genes in total, including E1, E2, E3, E4, E5, and E6, with a total length of about 4,500 bp, which can participate in the replication, transcription, and cell transformation of viral genes; (2) advanced regions (late stage) Coding area: contains a total of 2 genes, L1 and L2, of which L1 is the main capsid protein and L2 is the secondary capsid protein, which can be self-assembled into viral-like particles to induce the body’s immune response and promote the production of protective antibodies. It belongs to the late expression of viral replication; (3) Upstream regulatory area (long control) control area, non-coding area: Located between the L1 gene and the E6 gene, it contains multiple binding sites and can participate in the regulation of virus replication and transcription (12).
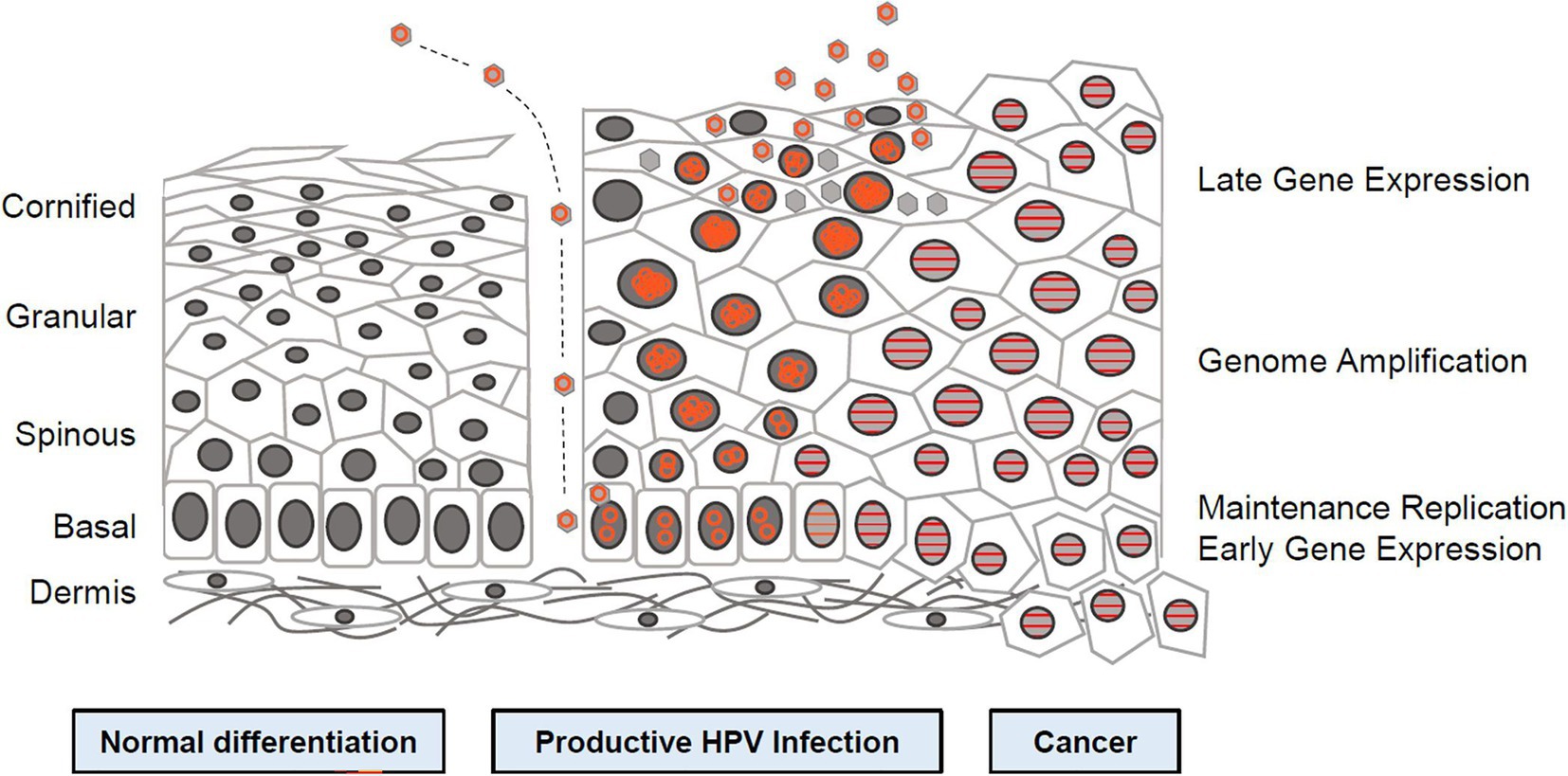
Figure 1. Human papillomavirus (HPV) life cycle and cancer (12). Cartoon depicting normal stratified cervical epithelium (left), HPV infected epithelium (center), and HPV induced cancer (right). Epithelial layers are indicated on the far left and HPV life cycle stages are indicated on the far right. Episomal genomes are shown as orange circles and integrated genomes shown as orange stripes. Left: Normal keratinocyte differentiation. Basal cells divide and daughter cells migrate upward, beginning the differentiation program. As differentiation proceeds, cells exit the cell cycle. Fully keratinized squames slough off from the apical surface. Middle: Productive HPV Infection: HPV virions gain access to basal cells via microwounds. The viral genomes migrate to the nucleus, where they are maintained at approximately100 copies/cell. As daughter cells begin differentiation, viral genomes are amplified. Cell nuclei are retained and chromatin is activated to support viral DNA replication. Right: Cancer. Viral genomes often integrate into the host genome and E6/E7 expression is increased, leading to enhanced proliferation and accumulation of cellular mutations. Cellular differentiation is lost and cancerous cells invade into the dermal layer along with neighboring tissues (12).
Of the approximately 30 types of HPV that infect the anogenital tract, 15 types of HPV, classified as “high-risk” types (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82), are associated with high-grade lesions and invasive cervical cancer (13). On the other hand, 11 different HPV types, classified as “low-risk” HPV types (HPV types 6, 11, 40, 42, 43, 44, 54, 61, 70, and 81), are mainly associated with genital warts and benign cervical lesions (14). In the list of type 1 carcinogens published by the International Agency for Research on Cancer of the World Health Organization, there are types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 of high-risk HPV (11).
The global prevalence of five top hrHPV types among women (15) is reported to be HPV 16: 55.4 (95% CI; 55.0–55.8), HPV 18: 14.6 (95% CI; 14.3–14.9), HPV 45: 4.8 (95% CI; 4.6–5.0), HPV 33: 4.2 (95% CI; 4.1–4.4), HPV 58: 3.8 (95% CI; 3.7–4.0), and HPV 31: 3.5 (95% CI; 3.4–3.7). A global study on HPV genotypes (16), published in the Lancet in 2010, found that genotypes HPV 16, HPV 18, HPV 31, HPV 33, HPV 35, HPV 45, HPV 52, and HPV 58 are easily capable of causing moisturizing cervical cancer. As previously stated, HPV 16/18 is the primary virus of cervical intraepithelial neoplasia (CIN). However, Ma L (17), on the other hand, found that CIN2+ accounted for 31.02% of 648 HPV-positive histopathological data, with HPV16 having the highest infection rate and HPV18 having only 3.75%, but HPV18 can play an important role in severe cervical lesions, with CIN3 and cervical adenocarcinoma being closely related to HPV18 single infection. A recent systematic synthesis (18) showed that individual HPV genotypes carry distinct risk values for high-grade cervical disease. HPV16 consistently carries the highest risk for CIN 3 or worse, HPV31, 18, and 33 carry intermediate-high CIN 3 or worse risk. Beyond HPV 16, 31, 18, and 33, HPV 52, 58, and 45 carry moderate risks, with 35, 39, 51, 56, 59, 66, and 68 consistently having the lowest CIN 3 or worse risks.
Women may be infected with multiple HPV infections with different genotypes throughout their lives (3). It is reported that among HPV-positive women, the prevalence rate of multiple HPV infections is between 18.5 and 46% (19–21). A variety of HR-HPV types infect synergistically in the occurrence of cervical cancer, according to prospective research (22). Fife et al. (23) reported that infection with multiple HR-HPV types tends to increase the severity of cervical diseases. However, other reports provided controversial conclusions. Muñoz et al. (3) found that there is no significant difference in the risk of cervical cancer among women with multiple and single HPV infections. Herrero et al. (24) have shown that multiple infections may be related to the persistence of HPV and increase the duration of infection and the risk of cervical disease, but some studies (25–27) still showed no impact. Recently, Iacobone et al. (28) confirmed that multiple HPV infections are significantly associated with reduced CIN2+ risk, while cervical cancer and changes in precancerous diseases may occur in single infections. Another hrHPV test through Cobas4800 showed that the risk of HPV16 co-infection with other types of CIN3 seems to be lower than that of a single HPV16 infection (29). A study (30) from Beijing, China, also indicated that the incidence of CIN2+ in patients with a single HPV 16 infection (62.2%) is higher than that of patients mixed with other HPV genotypes (52.4%). At the same time, the incidence of CIN2+ in patients infected with HPV 16 may be higher than that of patients with a single HPV 52 and other genotypes. Recently, a study published by Song et al. (31) suggested that co-infection with lower-grade HPV types has little impact on the CIN2 + risk associated with a single hrHPV infection, which confirms the above conclusion.
Most scholars (32–41) believe that there is a clear correlation between HPV viral load and the degree of cervical lesion, that is, as the viral load increases, the risk of cervical lesions increases. A large Chinese retrospective study (34), compared the viral load of ≤ CIN1 and CIN2 + patients in eight high-risk HPV genotypes (HPV16/18/31/33/45/52/58/82). The results showed that statistical significance was only found in HPV16 genotypes; there was no such difference in the other seven genotypes. Zhao et al. (36) conducted a 15-year prospective cohort study in China and found a significant correlation between the change in HPV viral load over time and the probability of CIN2+ in patients. Women with an increased viral load (15.3%) had a 38-fold higher risk of CIN2+ than HPV-negative women (0.4%). We summarized some longitudinal studies (Table 1) published in the past 5 years and found that the HPV viral load is indeed related to the CIN level of cervical lesions, but this law is not universal. It needs to be reflected in a specific genotype. For example, in the cohort of French women (42), only the viral load of HPV16 can predict CIN2+, and this association has not been found in HPV18 and other genotypes. This rule has also been found in the Chinese female cohort (34). More interestingly, a cohort study from Canada (33) showed that the HPV 16/18/31 viral load is related to higher levels of cervical lesions. In the Mexican women’s cohort (35), we found that women in LSIL and HSIL have a higher HPV 16 viral load. In contrast, Del Río-Ospina L et al. (44) study in Colombia has documented that a higher level of cervical lesions in women corresponds to a lower HPV 16 viral load. Wang W et al. (39) have shown that the HPV16 viral load can gradually increase with the development of the lesion, and there is no obvious correlation between the HPV18 viral load and histopathology. At the same time, the viral load of subtypes close to HPV16 (HPV52, HPV58, etc.) can increase with the development of the disease, while the viral load of subtypes close to HPV18 (HPV45, HPV59, etc.) does not change significantly. Unlike the general description of viral load two decades ago, the description of viral loads of different HPV genotypes in recent years has helped us better understand the relationship between viral load and CIN classification.
However, some experts (45–47) do not think that HPV viral load is associated with the degree of cervical lesions (48). They said that CIN1 was in the acute stage of HPV infection, and HPV’s self-replication ability was significantly more prominent in other stages. When CIN1 developed into CIN2-3, there was a significant downward trend in HPV viral load because HPV’s self-replication ability was relatively stable. When it developed from CIN2-3 to cervical cancer stage I again, the HPV gene was integrated into the DNA of the host cell and had the ability to transform cells, causing the cervical lesion to gradually transform into cervical in situ cancer and even infiltrating cancer (48, 49). From this perspective, the viral load may underestimate the severity of the disease, thus delaying treatment.
When we reviewed these studies, we also found that the viral load is also related to age. Compared with women under the age of 30, the relationship between viral load and cervical lesions in women older than 30 years old is stronger (33).
At present, many countries around the world use HPV testing for primary cervical cancer screening. Clinical trials (50) showed that HPV detection and screening of high-level lesions are more sensitive than cytological testing. The randomized clinical trial published in JAMA Oncology (51) indicated that genotyping for hrHPV with cytology triage significantly reduced the colposcopy referral rate compared with cytology for urban women. However, many HPV-positive women have no potential cervical lesions. To avoid overburdening colposcopy services and reduce the harm caused by over-referral, HPV-positive women must undergo a second test. Whether the HPV viral load can be used as a biomarker for triage is a question worth discussing at present. Luo et al.’s (52) research used the viral load as a triage indicator for cervical cancer screening. It is recommended that colposcopy be performed immediately when the viral load is > 10 RLU/CO, and cytological testing should be carried out at > 1 RLU/CO or < 10 RLU/CO to optimize sensitivity, specificity, and the referral rate. This proved that the viral load can be used as a triage indicator. However, The HPV viral load can only predict the risk of cervical lesions under specific HPV typing. This may not apply to all HPV-positive women.
In December 2021, the World Health Organization issued guidelines for the screening and treatment of cervical precancerous lesions, proposing the use of mRNA testing for cervical cancer screening. Even if the viral load cannot be used as a method for secondary triage, it can be used as an indicator of virus replication to predict condition tracking and subsequent treatment.
Due to the close relationship between HPV and cervical cancer, cervical cancer becomes a unique type of cancers. Its etiology is clear, and early prevention and diagnosis can achieve complete eradication. In May 2018, the Director-General of WHO called on countries to take action to jointly achieve the global goal of eliminating cervical cancer. Vaccinating adolescents against HPV is now the primary cervical cancer prevention strategy (53). However, HPV vaccination is not recommended instead of cervical cancer screening (54), although the vaccine can greatly reduce the risk of cervical cancer (55). Regardless of the relationship between HPV viral load and cervical lesions, viral load must be combined with other methods to maximize sensitivity and specificity in cervical cancer screening. Therefore, we need to further study the many factors that may influence the occurrence and development of cervical lesions, to achieve the combined application of multiple methodological detections in the early screening of cervical cancer, which is conducive to the early detection, early diagnosis, and early treatment of cervical cancer, so as to achieve the elimination of cervical cancer by the World Health Organization by 2030.
LZ and YZ conceived the review study. YZ drafted the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by grants from The Maternal and Child Health Research Project in Jiangsu Province (F202166) and The Changzhou Health Commission Guidance Project (WZ202217).
We thank our supervisor for her help in the initial stage of the review study, we gratefully acknowledge the financial supports by The Maternal and Child Health Research Project in Jiangsu Province number F202166 and The Changzhou Health Commission Guidance Project number WZ202217.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Sammarco, ML, Del Riccio, I, Tamburro, M, Grasso, GM, and Ripabelli, G. Type-specific persistence and associated risk factors of human papillomavirus infections in women living in Central Italy. Eur J Obstet Gynecol Reprod Biol. (2013) 168:222–6. doi: 10.1016/j.ejogrb.2013.01.012
3. Muñoz, N, Bosch, FX, de Sanjosé, S, Herrero, R, Castellsagué, X, Shah, KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. (2003) 348:518–27. doi: 10.1056/NEJMoa021641
4. Group, A.L.T.S.A. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. (2003) 188:1383–92. doi: 10.1067/mob.2003.457
5. Ronco, G, and Giorgi, RP. Role of hpv dna testing in modern gynaecological practice. Best Pract Res Clin Obstet Gynaecol. (2018) 47:107–18. doi: 10.1016/j.bpobgyn.2017.08.002
6. Frega, A, Pavone, M, Sesti, F, Leone, C, Bianchi, P, Cozza, G, et al. Sensitivity and specificity values of high-risk hpv dna, p16/ki-67 and hpv mrna in young women with atypical squamous cells of undetermined significance (ascus) or low-grade squamous intraepithelial lesion (lsil). Eur Rev Med Pharmacol Sci. (2019) 23:10672–7. doi: 10.26355/eurrev_201912_19765
7. Koliopoulos, G, Nyaga, VN, Santesso, N, Bryant, A, Martin-Hirsch, PP, Mustafa, RA, et al. Cytology versus hpv testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. (2017) 8:CD008587. doi: 10.1002/14651858.CD008587.pub2
8. Schiffman, M, Burk, RD, Boyle, S, Raine-Bennett, T, Katki, HA, Gage, JC, et al. A study of genotyping for management of human papillomavirus-positive, cytology-negative cervical screening results. J Clin Microbiol. (2015) 53:52–9. doi: 10.1128/JCM.02116-14
9. Stoler, MH, Baker, E, Boyle, S, Aslam, S, Ridder, R, Huh, WK, et al. Approaches to triage optimization in hpv primary screening: extended genotyping and p16/ki-67 dual-stained cytology-retrospective insights from athena. Int J Cancer. (2020) 146:2599–607. doi: 10.1002/ijc.32669
10. Verhoef, VMJ, Bosgraaf, RP, van Kemenade, FJ, Rozendaal, L, Heideman, DAM, Hesselink, AT, et al. Triage by methylation-marker testing versus cytology in women who test hpv-positive on self-collected cervicovaginal specimens (prohtect-3): a randomised controlled non-inferiority trial. Lancet Oncol. (2014) 15:315–22. doi: 10.1016/S1470-2045(14)70019-1
11. Wang, X, Huang, X, and Zhang, Y. Involvement of human papillomaviruses in cervical cancer. Front Microbiol. (2018) 9:2896. doi: 10.3389/fmicb.2018.02896
12. Langsfeld, E, and Laimins, LA. Human papillomaviruses: research priorities for the next decade. Trends cancer. (2016) 2:234–40. doi: 10.1016/j.trecan.2016.04.001
13. Shukla, S, Bharti, AC, Mahata, S, Hussain, S, Kumar, R, Hedau, S, et al. Infection of human papillomaviruses in cancers of different human organ sites. Indian J Med Res. (2009) 130:222–33.
14. Castellsagué, X, Díaz, M, de Sanjosé, S, Muñoz, N, Herrero, R, Franceschi, S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. (2006) 98:303–15. doi: 10.1093/jnci/djj067
15. Pimple, S, and Mishra, G. Cancer cervix: epidemiology and disease burden. Cytojournal. (2022) 19:21. doi: 10.25259/CMAS_03_02_2021
16. de Sanjose, S, Quint, WG, Alemany, L, Geraets, DT, Klaustermeier, JE, Lloveras, B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. (2010) 11:1048–56. doi: 10.1016/S1470-2045(10)70230-8
17. Ma, L, Cong, X, Shi, M, Wang, X, Liu, H, and Bian, M. Distribution of human papillomavirus genotypes in cervical lesions. Exp Ther Med. (2017) 13:535–41. doi: 10.3892/etm.2016.4000
18. Bonde, JH, Sandri, M, Gary, DS, and Andrews, JC. Clinical utility of human papillomavirus genotyping in cervical cancer screening: a systematic review. J Low Genit Tract Dis. (2020) 24:1–13. doi: 10.1097/LGT.0000000000000494
19. Vaccarella, S, Franceschi, S, Snijders, PJF, Herrero, R, Meijer, CJLM, Plummer, M, et al. Concurrent infection with multiple human papillomavirus types: pooled analysis of the iarc hpv prevalence surveys. Cancer Epidemiol Biomarks Prev. (2010) 19:503–10. doi: 10.1158/1055-9965.EPI-09-0983
20. Chaturvedi, AK, Katki, HA, Hildesheim, A, Rodríguez, AC, Quint, W, Schiffman, M, et al. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect Dis. (2011) 203:910–20. doi: 10.1093/infdis/jiq139
21. Dickson, EL, Vogel, RI, Bliss, RL, and Downs, LSJ. Multiple-type human papillomavirus (hpv) infections: a cross-sectional analysis of the prevalence of specific types in 309,000 women referred for hpv testing at the time of cervical cytology. Int J Gynecol Cancer. (2013) 23:1295–302. doi: 10.1097/IGC.0b013e31829e9fb4
22. Trottier, H, Mahmud, S, Costa, MC, Sobrinho, JP, Duarte-Franco, E, Rohan, TE, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarks Prev. (2006) 15:1274–80. doi: 10.1158/1055-9965.EPI-06-0129
23. Fife, KH, Cramer, HM, Schroeder, JM, and Brown, DR. Detection of multiple human papillomavirus types in the lower genital tract correlates with cervical dysplasia. J Med Virol. (2001) 64:550–9. doi: 10.1002/jmv.1085
24. Herrero, R, Castle, PE, Schiffman, M, Bratti, MC, Hildesheim, A, Morales, J, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. (2005) 191:1796–807. doi: 10.1086/428850
25. Wentzensen, N, Schiffman, M, Dunn, T, Zuna, RE, Gold, MA, Allen, RA, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. (2009) 125:2151–8. doi: 10.1002/ijc.24528
26. Adcock, R, Cuzick, J, Hunt, WC, McDonald, RM, and Wheeler, CM. Role of hpv genotype, multiple infections, and viral load on the risk of high-grade cervical neoplasia. Cancer Epidemiol Biomarkers Prev. (2019) 28:1816–24. doi: 10.1158/1055-9965.EPI-19-0239
27. Schmitt, M, Depuydt, C, Benoy, I, Bogers, J, Antoine, J, Arbyn, M, et al. Multiple human papillomavirus infections with high viral loads are associated with cervical lesions but do not differentiate grades of cervical abnormalities. J Clin Microbiol. (2013) 51:1458–64. doi: 10.1128/JCM.00087-13
28. Iacobone, AD, Bottari, F, Radice, D, Preti, EP, Franchi, D, Vidal Urbinati, AM, et al. Distribution of high-risk human papillomavirus genotypes and multiple infections in preneoplastic and neoplastic cervical lesions of unvaccinated women: a cross-sectional study. J Low Genit Tract Dis. (2019) 23:259–64. doi: 10.1097/LGT.0000000000000487
29. Wu, P, Xiong, H, Yang, M, Li, L, Wu, P, Lazare, C, et al. Co-infections of hpv16/18 with other high-risk hpv types and the risk of cervical carcinogenesis: a large population-based study. Gynecol Oncol. (2019) 155:436–43. doi: 10.1016/j.ygyno.2019.10.003
30. Li, M, du, X, Lu, M, Zhang, W, Sun, Z, Li, L, et al. Prevalence characteristics of single and multiple hpv infections in women with cervical cancer and precancerous lesions in Beijing, China. J Med Virol. (2019) 91:473–81. doi: 10.1002/jmv.25331
31. Song, F, Yan, P, Huang, X, Wang, C, Du, H, Qu, X, et al. Roles of extended human papillomavirus genotyping and multiple infections in early detection of cervical precancer and cancer and hpv vaccination. BMC Cancer. (2022) 22:42. doi: 10.1186/s12885-021-09126-3
32. Liu, Y, Xu, C, Pan, J, Sun, C, Zhou, H, and Meng, Y. Significance of the viral load of high-risk hpv in the diagnosis and prediction of cervical lesions: a retrospective study. BMC Womens Health. (2021) 21:353. doi: 10.1186/s12905-021-01493-0
33. Malagón, T, Louvanto, K, Ramanakumar, AV, Koushik, A, Coutlée, F, Franco, EL, et al. Viral load of human papillomavirus types 16/18/31/33/45 as a predictor of cervical intraepithelial neoplasia and cancer by age. Gynecol Oncol. (2019) 155:245–53. doi: 10.1016/j.ygyno.2019.09.010
34. Tao, X, Austin, RM, Yu, T, Zhong, F, Zhou, X, Cong, Q, et al. Risk stratification for cervical neoplasia using extended high-risk hpv genotyping in women with asc-us cytology: a large retrospective study from China. Cancer Cytopathol. (2022) 130:248–58. doi: 10.1002/cncy.22536
35. Oyervides-Muñoz, MA, Pérez-Maya, AA, Sánchez-Domínguez, CN, Berlanga-Garza, A, Antonio-Macedo, M, Valdéz-Chapa, LD, et al. Multiple hpv infections and viral load association in persistent cervical lesions in mexican women. Viruses. (2020) 12:12. doi: 10.3390/v12040380
36. Zhao, X, Zhao, S, Hu, S, Zhao, K, Zhang, Q, Zhang, X, et al. Role of human papillomavirus dna load in predicting the long-term risk of cervical cancer: a 15-year prospective cohort study in China. J Infect Dis. (2019) 219:215–22. doi: 10.1093/infdis/jiy507
37. Basu, P, Muwonge, R, Mittal, S, Banerjee, D, Ghosh, I, Panda, C, et al. Implications of semi-quantitative hpv viral load estimation by hybrid capture 2 in colposcopy practice. J Med Screen. (2016) 23:104–10. doi: 10.1177/0969141315606483
38. Wang, SM, Colombara, D, Shi, JF, Zhao, FH, Li, J, Chen, F, et al. Six-year regression and progression of cervical lesions of different human papillomavirus viral loads in varied histological diagnoses. Int J Gynecol Cancer. (2013) 23:716–23. doi: 10.1097/IGC.0b013e318286a95d
39. Wang, W, Zhang, XH, Li, M, Hao, CH, Zhao, ZM, and Liang, HP. Association between viral loads of different oncogenic human papillomavirus types and the degree of cervical lesions in the progression of cervical cancer. Clin Chim Acta. (2018) 483:249–55. doi: 10.1016/j.cca.2018.05.016
40. Mittal, S, Basu, P, Muwonge, R, Banerjee, D, Ghosh, I, Sengupta, MM, et al. Risk of high-grade precancerous lesions and invasive cancers in high-risk hpv-positive women with normal cervix or cin 1 at baseline-a population-based cohort study. Int J Cancer. (2017) 140:1850–9. doi: 10.1002/ijc.30609
41. Manawapat-Klopfer, A, Wang, L, Haedicke-Jarboui, J, Stubenrauch, F, Munk, C, Thomsen, LT, et al. Hpv16 viral load and physical state measurement as a potential immediate triage strategy for hr-HPV-infected women: a study in 644 women with single hpv16 infections. Am J Cancer Res. (2018) 8:715–22.
42. Baumann, A, Henriques, J, Selmani, Z, Meurisse, A, Lepiller, Q, Vernerey, D, et al. Hpv16 load is a potential biomarker to predict risk of high-grade cervical lesions in high-risk HPV-infected women: a large longitudinal french hospital-based cohort study. Cancers. (2021) 13:4149. doi: 10.3390/cancers13164149
43. Fu, XL, Schiffman, M, Ke, Y, Hughes, JP, Galloway, DA, He, Z, et al. Type-dependent association between risk of cervical intraepithelial neoplasia and viral load of oncogenic human papillomavirus types other than types 16 and 18. Int J Cancer. (2017) 140:1747–56. doi: 10.1002/ijc.30594
44. del Río-Ospina, L, Soto-de León, SC, Camargo, M, Moreno-Pérez, DA, Sánchez, R, Pérez-Prados, A, et al. The dna load of six high-risk human papillomavirus types and its association with cervical lesions. BMC Cancer. (2015) 15:100. doi: 10.1186/s12885-015-1126-z
45. Andersson, S, Safari, H, Mints, M, Lewensohn-Fuchs, I, Gyllensten, U, and Johansson, B. Type distribution, viral load and integration status of high-risk human papillomaviruses in pre-stages of cervical cancer (cin). Br J Cancer. (2005) 92:2195–200. doi: 10.1038/sj.bjc.6602648
46. Lorincz, AT, Castle, PE, Sherman, ME, Scott, DR, Glass, AG, Wacholder, S, et al. Viral load of human papillomavirus and risk of cin3 or cervical cancer. Lancet. (2002) 360:228–9. doi: 10.1016/S0140-6736(02)09463-1
47. Castle, PE, Schiffman, M, and Wheeler, CM. Hybrid capture 2 viral load and the 2-year cumulative risk of cervical intraepithelial neoplasia grade 3 or cancer. Am J Obstet Gynecol. (2004) 191:1590–7. doi: 10.1016/j.ajog.2004.05.018
48. Constandinou-Williams, C, Collins, SI, Roberts, S, Young, LS, Woodman, CBJ, and Murray, PG. Is human papillomavirus viral load a clinically useful predictive marker? A longitudinal study. Cancer Epidemiol Biomarks Prev. (2010) 19:832–7. doi: 10.1158/1055-9965.EPI-09-0838
49. Pett, M, and Coleman, N. Integration of high-risk human papillomavirus: a key event in cervical carcinogenesis? J Pathol. (2007) 212:356–67. doi: 10.1002/path.2192
50. Bottari, F, Iacobone, AD, Radice, D, Preti, EP, Preti, M, Franchi, D, et al. Hpv tests comparison in the detection and follow-up after surgical treatment of cin2+ lesions. Diagnostics. (2022) 12:2359. doi: 10.3390/diagnostics12102359
51. Zhang, J, Zhao, Y, Dai, Y, Dang, L, Ma, L, Yang, C, et al. Effectiveness of high-risk human papillomavirus testing for cervical cancer screening in China: a multicenter, open-label, randomized clinical trial. JAMA Oncol. (2021) 7:263–70. doi: 10.1001/jamaoncol.2020.6575
52. Luo, H, Belinson, JL, Du, H, Liu, Z, Zhang, L, Wang, C, et al. Evaluation of viral load as a triage strategy with primary high-risk human papillomavirus cervical cancer screening. J Low Genit Tract Dis. (2017) 21:12–6. doi: 10.1097/LGT.0000000000000277
53. Human Papillomavirus Vaccination. Committee opinion no. 704: human papillomavirus vaccination. Obstet Gynecol. (2017) 129:1. doi: 10.1097/AOG.0000000000002052
54. Okunade, KS, Sunmonu, O, Osanyin, GE, and Oluwole, AA. Knowledge and acceptability of human papillomavirus vaccination among women attending the gynaecological outpatient clinics of a university teaching hospital in Lagos, Nigeria. J Trop Med. (2017) 2017:8586459. doi: 10.1155/2017/8586459
Keywords: human papillomavirus, viral load, HPV genotyping, multiple infections, cervical lesions
Citation: Zhou Y, Shi X, Liu J and Zhang L (2023) Correlation between human papillomavirus viral load and cervical lesions classification: A review of current research. Front. Med. 10:1111269. doi: 10.3389/fmed.2023.1111269
Received: 29 November 2022; Accepted: 26 January 2023;
Published: 21 February 2023.
Edited by:
Ming Yi, Zhejiang University, ChinaReviewed by:
Manuela Tamburro, University of Molise, ItalyCopyright © 2023 Zhou, Shi, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lina Zhang, ✉ bGluYXpoYW5nQG5qbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.