- 1Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
- 2Division of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
- 3Department of Statistics and Actuarial Science, Soongsil University, Seoul, Republic of Korea
- 4Department of Neurology, Inha University Hospital, Incheon, Republic of Korea
Objective: Patients with type 2 diabetes (T2DM) are at a high risk of developing depression and anxiety. To better stratify the risk, we aimed to assess whether the presence of immune-mediated inflammatory diseases (IMIDs) confers a higher risk of depression and anxiety in these patients.
Methods: Patients with T2DM without prior depression or anxiety who underwent national health examination between 2009 and 2012 (n = 1,612,705) were enrolled from the nationwide health check-up data from Korean National Health Insurance Service. The outcome events were incident depression and anxiety, defined as International Classification of Diseases, 10th Revision codes F32–F33 and F40–F41, respectively. Multivariable Cox proportional hazard regression analyses were conducted to estimate the adjusted hazard ratio (aHR) and 95% confidence interval (CI) according to the existence of IMIDs.
Results: Over an average follow-up time of 6.4 years, existence of gut IMIDs was associated with a higher risk of depression (aHR: 1.28 [95% CI: 1.08–1.53]) and anxiety (1.22 [1.06–1.42]). Existence of joint IMIDs was associated with a higher risk of depression (1.34 [1.31–1.37]) and anxiety (1.31 [1.29–1.34]). Existence of skin IMID was associated with a higher risk of depression (1.18 [1.14–1.23]) and anxiety (1.13 [1.09–1.16]). The effect sizes of IMIDs on depression and anxiety were larger in those with ≥ 2 IMIDs (1.42 [1.19–1.69] and 1.49 [1.29–1.72], respectively) than in those with one IMID (1.30 [1.27–1.32] and 1.26 [1.24–1.28], respectively).
Conclusion: In patients with T2DM, presence of IMIDs was associated with a higher risk of depression and anxiety. More stringent attention and screening for anxiety and depression should be encouraged in patients with T2DM and comorbid IMIDs due to clinical implications of psychological distress on patient-reported outcomes and prognosis.
Introduction
Patients with type 2 diabetes (T2DM) are at higher risk of having depression and anxiety, compared with those without T2DM, as reflected by odds ratios of 1.67 and 1.73, respectively (1, 2). Depression and anxiety are important factors that adversely affect treatment outcomes and quality of life in patients with T2DM (3). Therefore, analyzing the risk of depression and anxiety in patients with T2DM, and closer screening for depression and anxiety in high risk patients are crucial.
Immune-mediated inflammatory diseases (IMIDs) are a group of heterogeneous diseases causing chronic inflammation and organ damage (4). IMIDs can be classified into three groups based on the affected organs. Inflammatory bowel diseases (IBD: Crohn’s disease and ulcerative colitis), inflammatory arthritides (rheumatoid arthritis and ankylosing spondylitis), and psoriasis are IMIDs that mainly affect the gut, joints, and skin, respectively (5–15). Studies have shown that each IMID is associated with a higher risk of depression and anxiety in the general population with effect sizes similar to that of T2DM (16–19). A population-based study reported a 2-fold higher risk of depression and a 1.6-fold higher risk of anxiety in patients with IBD (16). With regard to IMIDs that predominantly affect the joints, patients with rheumatoid arthritis had a 1.5-fold and 1.2-fold higher risk of depression and anxiety, respectively, compared to the controls (17), and patients with ankylosing spondylitis had a 1.7-fold and 1.8-fold higher risk of depression and anxiety, respectively (18). A higher risk of depression and anxiety has also been reported in patients with psoriasis compared with controls (1.4-fold and 1.3-fold higher risk of depression and anxiety, respectively) (19).
However, as the previous studies showing association between IMIDs and risk of depression and anxiety used general population as a control (16–19), it is unclear if the existence of IMIDs also confer an additional risk of depression and anxiety when limited to the patients with T2DM. Considering that low grade inflammation is present in patients with T2DM (20), it is of particular interest to analyze the possible role of IMIDs for depression and anxiety in T2DM. It is important to elucidate the effect of the existence of IMIDs on the risk of depression and anxiety in patients with T2DM, which can lead to a better stratification of the risk in these patients. Herein, we conducted a nationwide, population-based study to assess the association between the presence of IMIDs and the risk of depression and anxiety in patients with T2DM.
Patients and methods
Data source
We used nationwide, population-based data from the Korean National Health Insurance Service (NHIS) claims database. Comprehensive data including the socioeconomic status, demographics, medical procedures and treatments, diagnosis according to the International Classification of Diseases, 10th Revision (ICD-10), and rare intractable disease (RID) registration information (21) are provided by the NHIS. In the RID registration system, a diagnosis is thoroughly reviewed by the NHI and the corresponding healthcare institution. The data source have been described in detail previously (22). All individuals registered in the NHIS database are recommended to get their national health check-ups every 2 years. The health examination data include: blood pressure (BP); anthropometric data; and laboratory data, such as fasting serum glucose levels, creatinine levels, and cholesterol levels. Data on previous medical history and lifestyle factors, including smoking status, alcohol consumption, and physical activity, all of which were based on standardized self-reporting questionnaires, were also available.
Study cohort
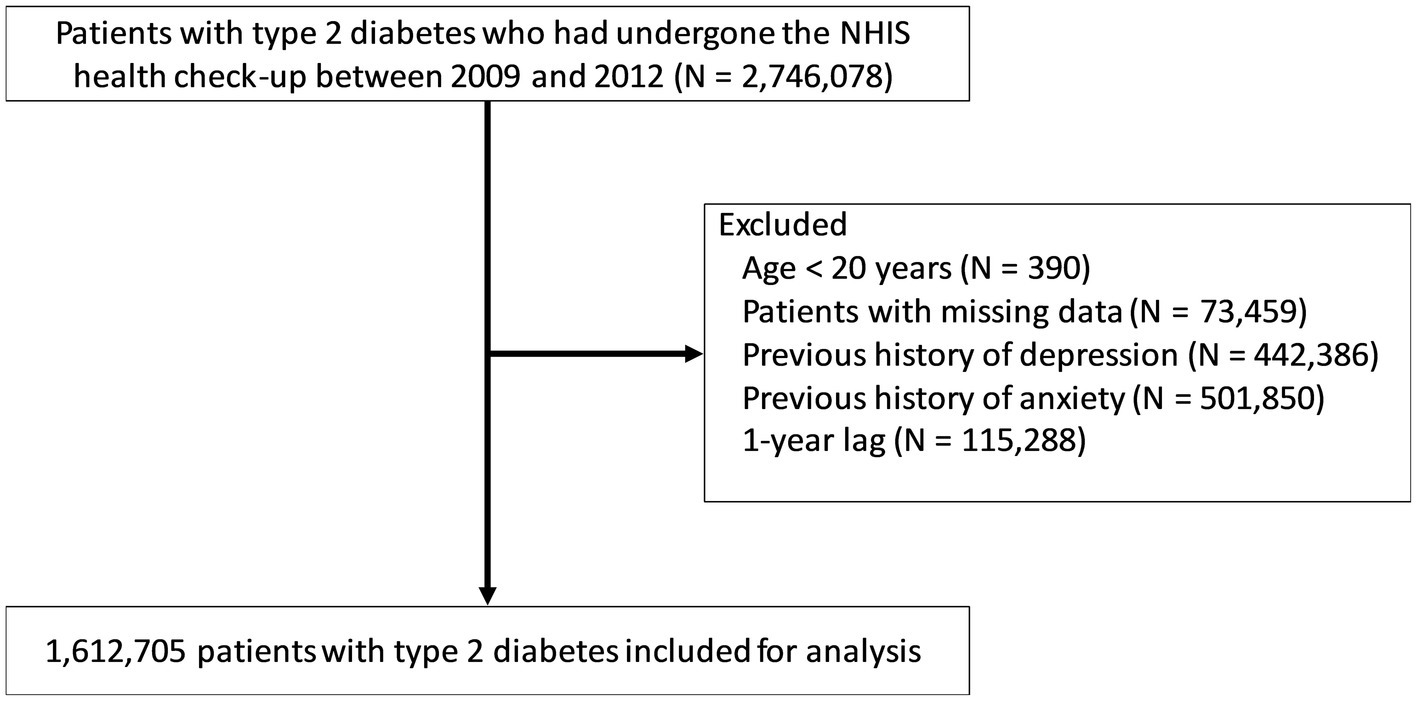
Initially, 2,746,078 patients with T2DM who had underwent the national health check-up between January 2009 and December 2012 were selected. Patients with T2DM were defined as those with ICD-10 codes of E11–14 with prescriptions for anti-diabetic medications or as those with a fasting plasma glucose ≥ 126 mg/dl (23, 24). The following subjects were excluded (Figure 1): (a) age < 20 years (n = 390); (b) missing data (n = 73,459); (c) previous history of depression (individuals with ICD-10 codes F32–F33 prior to the index date; n = 442,386); (d) previous history of anxiety (individuals with ICD-10 codes F40–F41 prior to the index date; n = 501,850); and (e) depression or anxiety or death that occurred within a year from the first health check-up date between January 2009 and December 2012 (n = 115,288). The remaining 1,612,705 patients with T2DM were included in the analysis. They were followed up from the baseline (the first health check-up date between January 2009 and December 2012) to the date of incident depression or anxiety, or December 2018, whichever came first.
Definition of covariates and outcomes
Covariates were defined as previously reported: Crohn’s disease, ICD-10 code K50 + RID code V130 (24–27); ulcerative colitis, ICD-10 code K51 + RID code V131 (24–27); rheumatoid arthritis, ICD-10 code M05 or M06 + prescriptions for disease-modifying anti-rheumatic drugs (24, 28, 29); ankylosing spondylitis, ICD-10 code M45 + RID code V140 (24, 30); and psoriasis, ICD-10 code L40 (24, 31). A multicenter validation study of diagnostic algorithms for Crohn’s disease and ulcerative colitis showed that sensitivity, specificity, positive predictive and negative predictive values for the identification of patients with IBD were 92.1, 97.2, 96.4, and 93.7%, respectively (32). The definition of rheumatoid arthritis identified patients with definite rheumatoid arthritis with 96.5% for sensitivity, 92.4% for positive predictive value, and 58.7% for specificity (33). Diagnostic sensitivity, specificity, and positive predictive value of the definition for psoriasis were 96.2, 91.0, and 78.0%, respectively (34). As there was no nationwide validation study conducted for the definition of ankylosing spondylitis, we evaluated the diagnostic sensitivity and specificity of the definition for ankylosing spondylitis, by reviewing medical records of 229 patients with ankylosing spondylitis at Gangnam Severance Hospital, a tertiary referral hospital in Korea. The diagnostic sensitivity and specificity were 93.4 and 96.4%, respectively. Baseline comorbidities were defined as follows: hypertension, ICD-10 codes I10–I13 and I15 + prescriptions for anti-hypertensive medications or systolic or diastolic BP ≥ 140 mmHg or ≥ 90 mmHg, respectively; dyslipidemia, ICD-10 code E78 + prescriptions for lipid-lowering medications or serum total cholesterol ≥ 240 mg/dl; stroke, ICD-10 codes I63 or I64 during hospitalization with claims for brain computed tomography or magnetic resonance imaging; chronic kidney disease, estimated glomerular filtration rate (calculated using the Modification of Diet in Renal Disease equation) of < 60 ml/min/1.73m2; ischemic heart disease, ICD-10 codes I20–I25; congestive heart failure, ICD-10 code I50; chronic obstructive pulmonary disease (COPD), ICD-10 codes J41–J44; liver cirrhosis, ICD-10 codes K70 or K74; and malignancy, ICD-10 codes C00–C96 (23, 35–40). The outcomes were newly diagnosed depression or anxiety, defined as ICD-10 codes F32–F33 or F40–F41, respectively (41).
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and categorical variables are expressed as numbers (%). Continuous variables were compared using independent Student’s t test and categorical variables were compared using χ2 test. The incidences of outcomes were expressed as event numbers per 1,000 person-years. The cumulative incidences of events according to the existence of IMIDs were visualized using the Kaplan–Meier curve analysis and compared using log-rank test. Six Cox proportional hazard models were conducted to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the outcomes according to the (i) existence of organ-based IMIDs [gut IMIDs (Crohn’s disease or ulcerative colitis), joint IMIDs (rheumatoid arthritis or ankylosing spondylitis), and skin IMID (psoriasis)]; and (ii) number of IMIDs present (0, 1, and ≥ 2). Model 1 was a univariable model. Model 2 was adjusted for age and sex. Additional adjustment for other potential confounders were made in the subsequent models. The final model (model 6) was adjusted for age, sex, smoking status, alcohol consumption, regular physical activity, income, residence, hypertension, dyslipidemia, body mass index, use of insulin, number of oral hypoglycemic agent ≥ 3, duration of T2DM of ≥ 5 years, chronic kidney disease, stroke, ischemic heart disease, congestive heart failure, COPD, liver cirrhosis, and malignancy. Subgroup analyses were performed by stratifying patients according to several risk factors. All value of ps were two-sided, and a value of p < 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, United States).
Results
Baseline characteristics
The baseline characteristics of the 1,612,705 patients with T2DM, and the comparison of the characteristics according to the existence of each IMID are reported in Table 1. The mean age was 54.86 ± 12.28 years, and 1,128,324 (69.96%) patients were male. Of the 1,612,705 patients with T2DM, Crohn’s disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, and psoriasis were present in 93 (0.006%), 549 (0.034%), 30,976 (1.921%), 518 (0.032%), and 13,100 (0.812%) patients, respectively. In comparison with their respective controls, patients with Crohn’s disease and ankylosing spondylitis were younger, whereas those with rheumatoid arthritis and psoriasis were older. The proportion of men was higher in patients with Crohn’s disease, ulcerative colitis, ankylosing spondylitis, and psoriasis, while that of women was higher in patients with rheumatoid arthritis, compared with their respective controls. The proportion of current smokers was lower in patients with ulcerative colitis and rheumatoid arthritis, but higher in those with ankylosing spondylitis and psoriasis, compared with their respective controls. Compared with their respective cohort, the proportion of heavy alcohol drinkers was lower in patients with ulcerative colitis and rheumatoid arthritis, but higher in those with ankylosing spondylitis. With regards to income, the proportion of patients with low income (lowest 25 percentile) was lower in patients with ulcerative colitis and ankylosing spondylitis, but higher in those with rheumatoid arthritis and psoriasis, compared with their respective controls. The existence of baseline comorbidity varied among patients with each IMID. Briefly, compared with their respective controls, hypertension and dyslipidemia were less common, while liver cirrhosis was more common in patients with Crohn’s disease; hypertension and dyslipidemia were less common, while COPD was more common in patients with ulcerative colitis; all comorbidities were more common in patients with rheumatoid arthritis; chronic kidney disease was less common in patients with ankylosing spondylitis; and all comorbidities except chronic kidney disease were more common in patients with psoriasis.
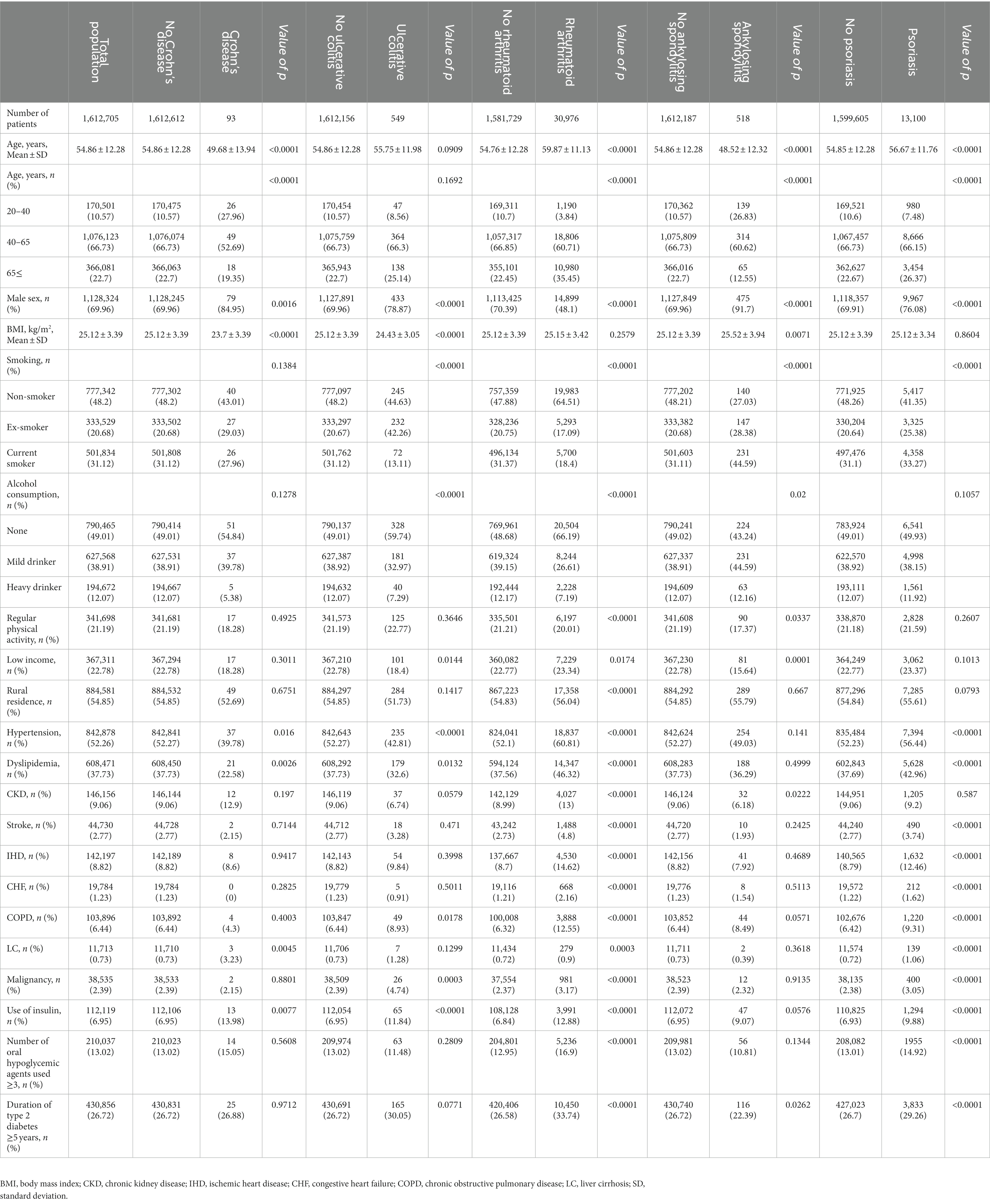
Table 1. Baseline characteristics of the patients with type 2 diabetes according to the presence of immune-mediated inflammatory diseases.
Incidence and risk of depression according to the presence of IMIDs
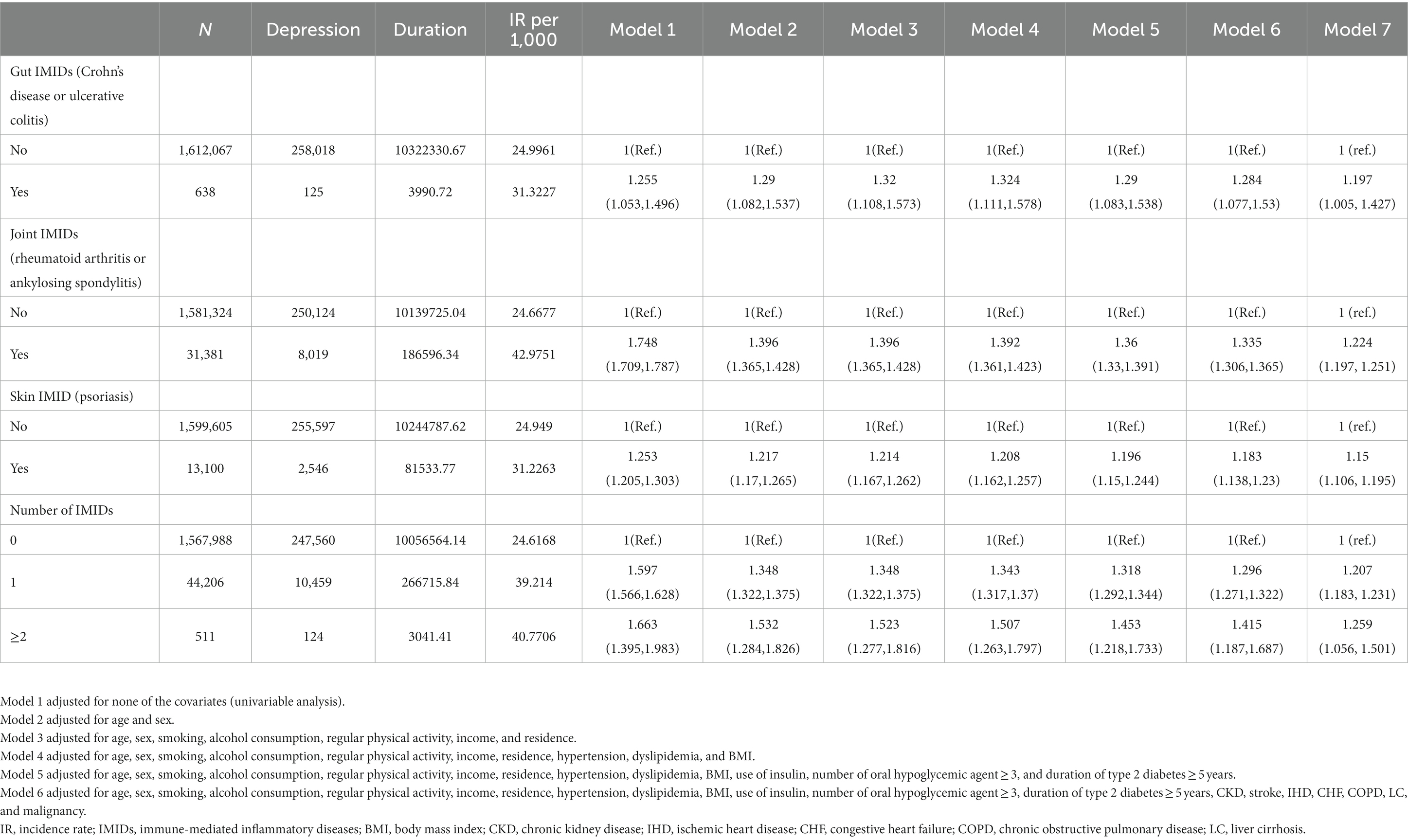
Table 2 shows the incidence rates and HRs of depression, according to the existence of IMIDs. The incidence rates of depression in patients with T2DM with gut IMIDs, joint IMIDs, and skin IMID were 31.32, 42.98, and 31.23 per 1,000 person-years, respectively. The incidence rates of depression stratified by specific diagnoses are summarized in Supplementary Table 1. The cumulative incidence of depression according to the existence of organ-based IMIDs is shown in Figures 2A–C. The univariable Cox proportional hazard model (model 1) revealed that existence of gut IMIDs, joint IMIDs, and skin IMID, respectively, was significantly associated with a higher risk of depression. After adjusting for potential confounders in model 6, existence of gut IMIDs (adjusted HR: 1.284 [95% CI: 1.077–1.530]), joint IMIDs (adjusted HR: 1.335 [95% CI: 1.306–1.365]), and skin IMID (adjusted HR: 1.183 [95% CI: 1.138–1.230]) was significantly associated with a higher risk of depression, respectively. According to the number of IMIDs present, patients with one IMID (adjusted HR: 1.296 [95% CI: 1.271–1.322]) had a significantly higher risk of depression than those without any IMIDs, and patients with ≥ 2 IMIDs (adjusted HR: 1.415 [95% CI: 1.187–1.687]) also had a significantly higher risk of depression than those without any IMIDs, with a larger effect size. When incident anxiety was additionally adjusted (model 7), the associations observed in model 6 remained statistically significant. The cumulative incidence and HR of depression, according to the existence of individual IMIDs, are shown in Figures 3A–E and Table 3.
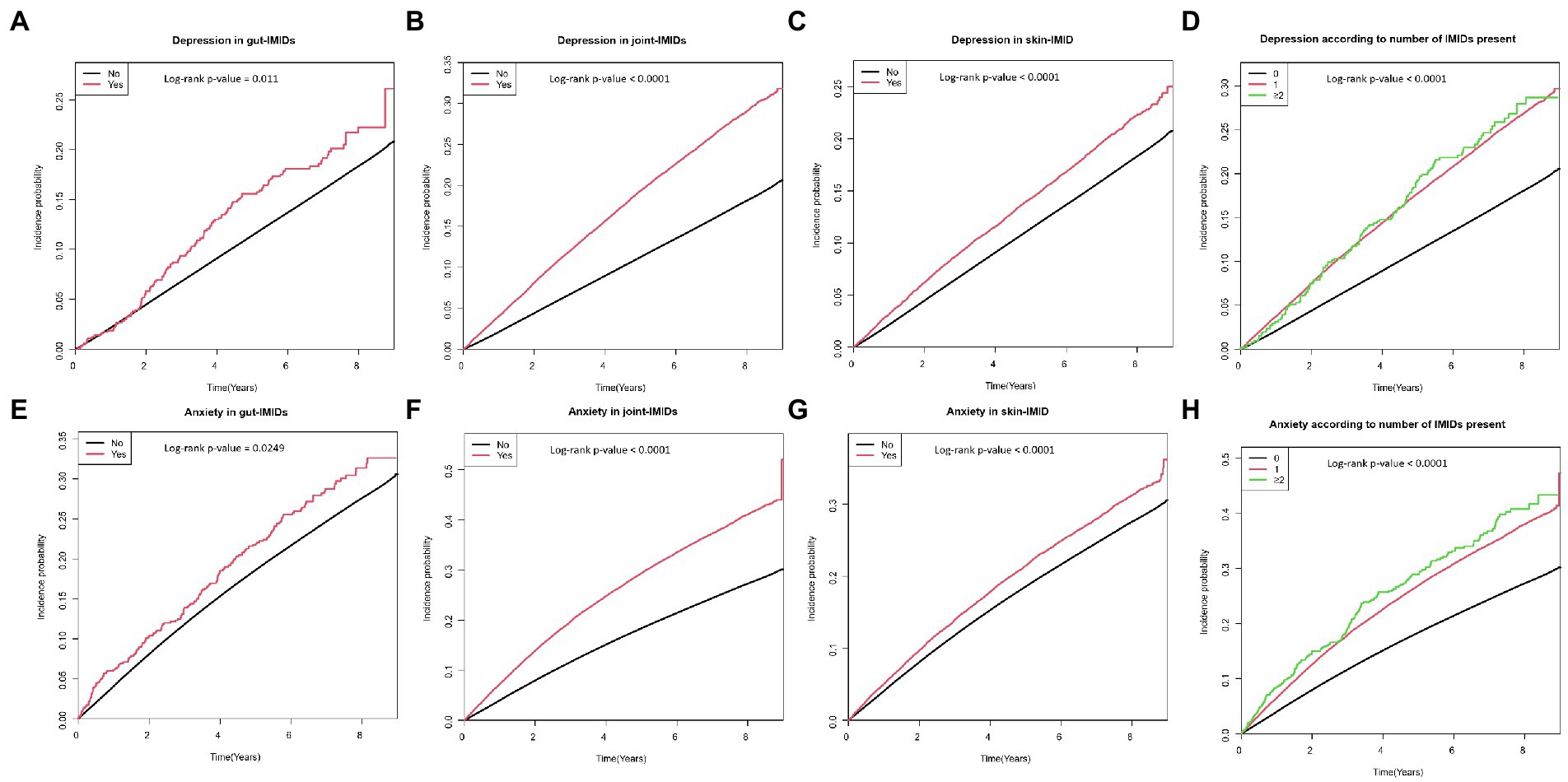
Figure 2. Cumulative incidence of depression in patients with (A) gut IMIDs, (B) joint IMIDs, (C) skin IMID, and (D) different numbers of IMIDs (0, 1, and ≥ 2); and cumulative incidence of anxiety in patients with (E) gut IMIDs, (F) joint IMIDs, (G) skin IMID, and (H) different numbers of IMIDs (0, 1, and ≥ 2). IMIDs, immune-mediated inflammatory disease.
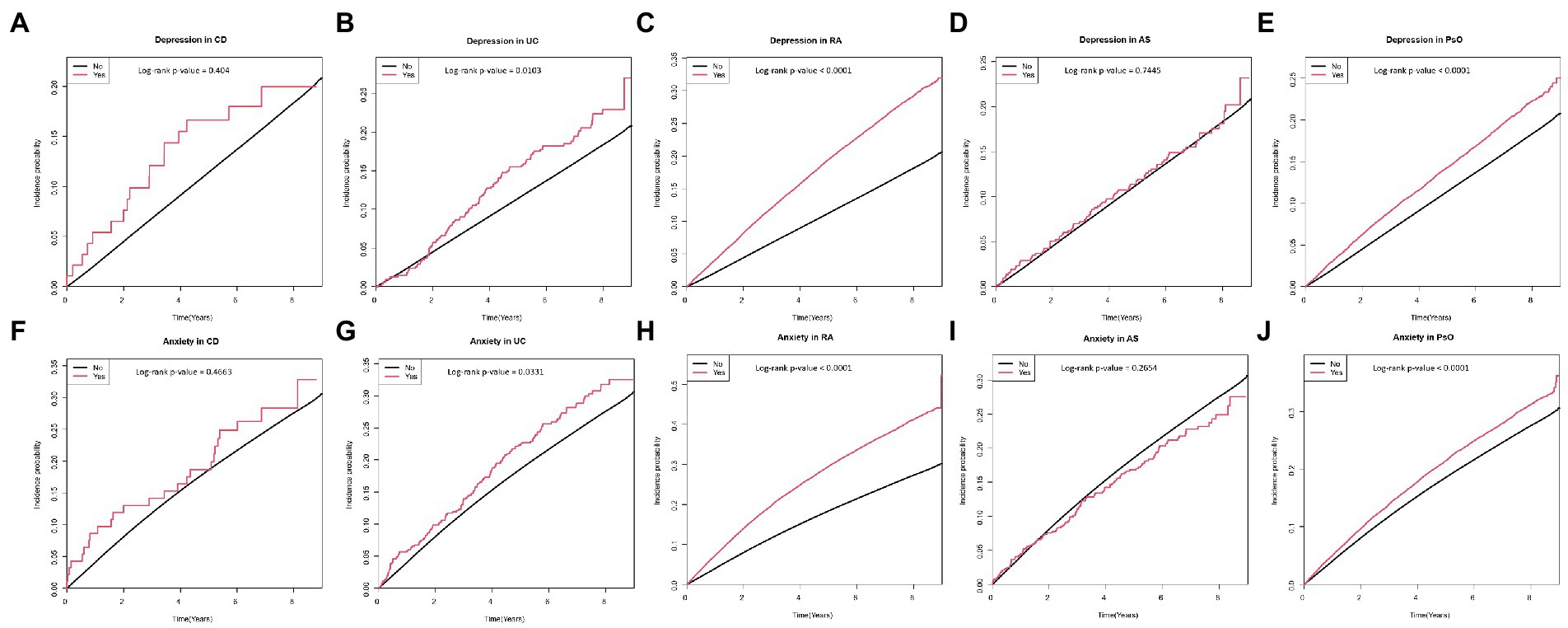
Figure 3. Cumulative incidence of depression in patients with (A) CD, (B) UC, (C) RA, (D) AS, and (E) PsO; and cumulative incidence of anxiety in patients with (F) CD, (G) UC, (H) RA, (I) AS, and (J) PsO. CD, crohn’s disease; UC, ulcerative colitis; RA, rheumatoid arthritis; AS, ankylosing spondylitis; PsO, psoriasis.
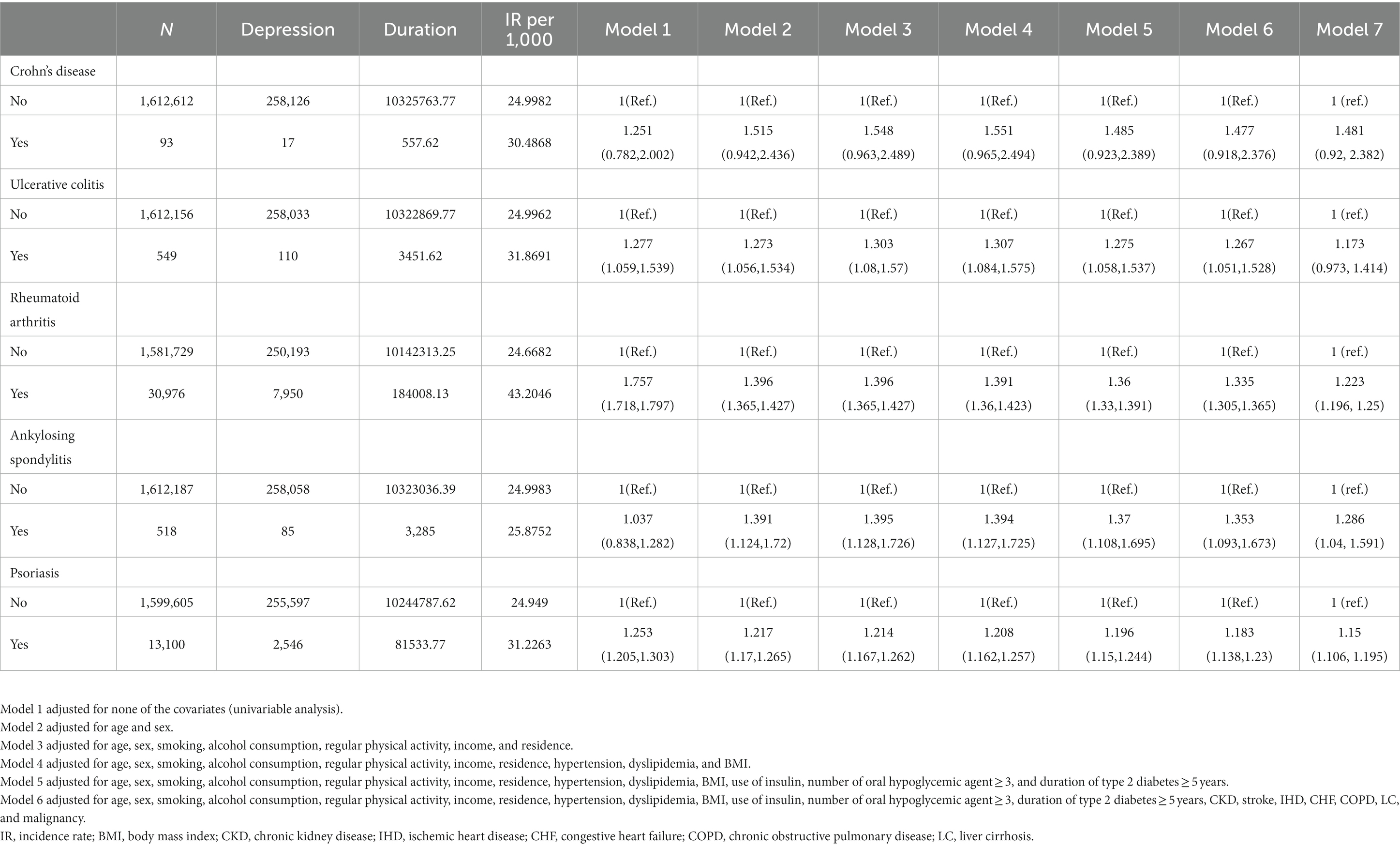
Table 3. Risk of depression according to the presence of individual immune-mediated inflammatory diseases.
Incidence and risk of anxiety according to the presence of IMIDs
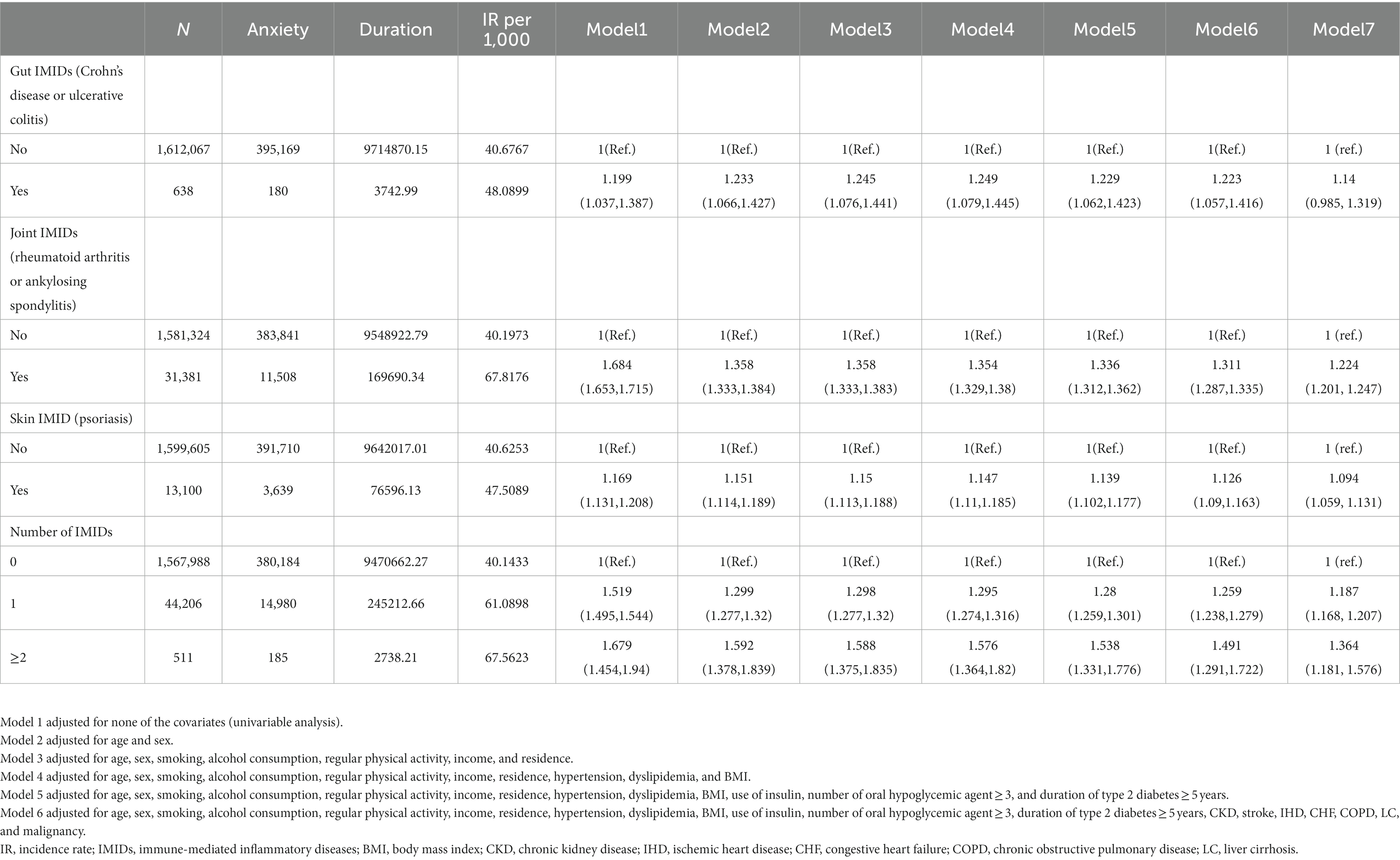
The incidence rates and HRs of anxiety, according to the existence of IMIDs are summarized in Table 4. The incidence rates of anxiety in patients with T2DM with gut IMIDs, joint IMIDs, and skin IMID were 48.09, 67.82, and 47.51 per 1,000 person-years, respectively. The incidence rates of anxiety stratified by specific diagnoses are reported in Supplementary Table 2. The cumulative incidence of anxiety according to the existence of organ-based IMIDs is depicted in Figures 2E–G. The univariable Cox proportional hazard model (model 1) revealed that existence of gut IMIDs, joint IMIDs, and skin IMID, respectively, was significantly associated with a higher risk of anxiety. In the final model (model 6) adjusted for potential confounders, existence of gut IMIDs (adjusted HR: 1.223 [95% CI: 1.057–1.416]), joint IMIDs (adjusted HR: 1.311 [95% CI: 1.287–1.335]), and skin IMID (adjusted HR: 1.126 [95% CI: 1.090–1.163]) was significantly associated with a higher risk of anxiety, respectively. According to the number of IMIDs present, patients with one IMID (adjusted HR: 1.259 [95% CI: 1.238–1.279]) had a significantly higher risk of anxiety than those without any IMIDs, and patients with ≥ 2 IMIDs (adjusted HR: 1.491 [95% CI: 1.291–1.722]) also had a significantly higher risk of anxiety than those without any IMIDs, with a larger effect size. When incident depression was additionally adjusted (model 7), similar associations were observed as in model 6. Existence of gut IMIDs (adjusted HR: 1.14 [95% CI: 0.985–1.319]) showed a trend of being associated with a higher risk of anxiety, and existence of joint IMIDs (adjusted HR: 1.224 [95% CI: 1.201–1.247]), and skin IMID (adjusted HR: 1.094 [95% CI: 1.059–1.131]) was significantly associated with a higher risk of anxiety. The cumulative incidence and HR of anxiety, according to the existence of individual IMIDs, are shown in Figures 3F–J and Table 5.
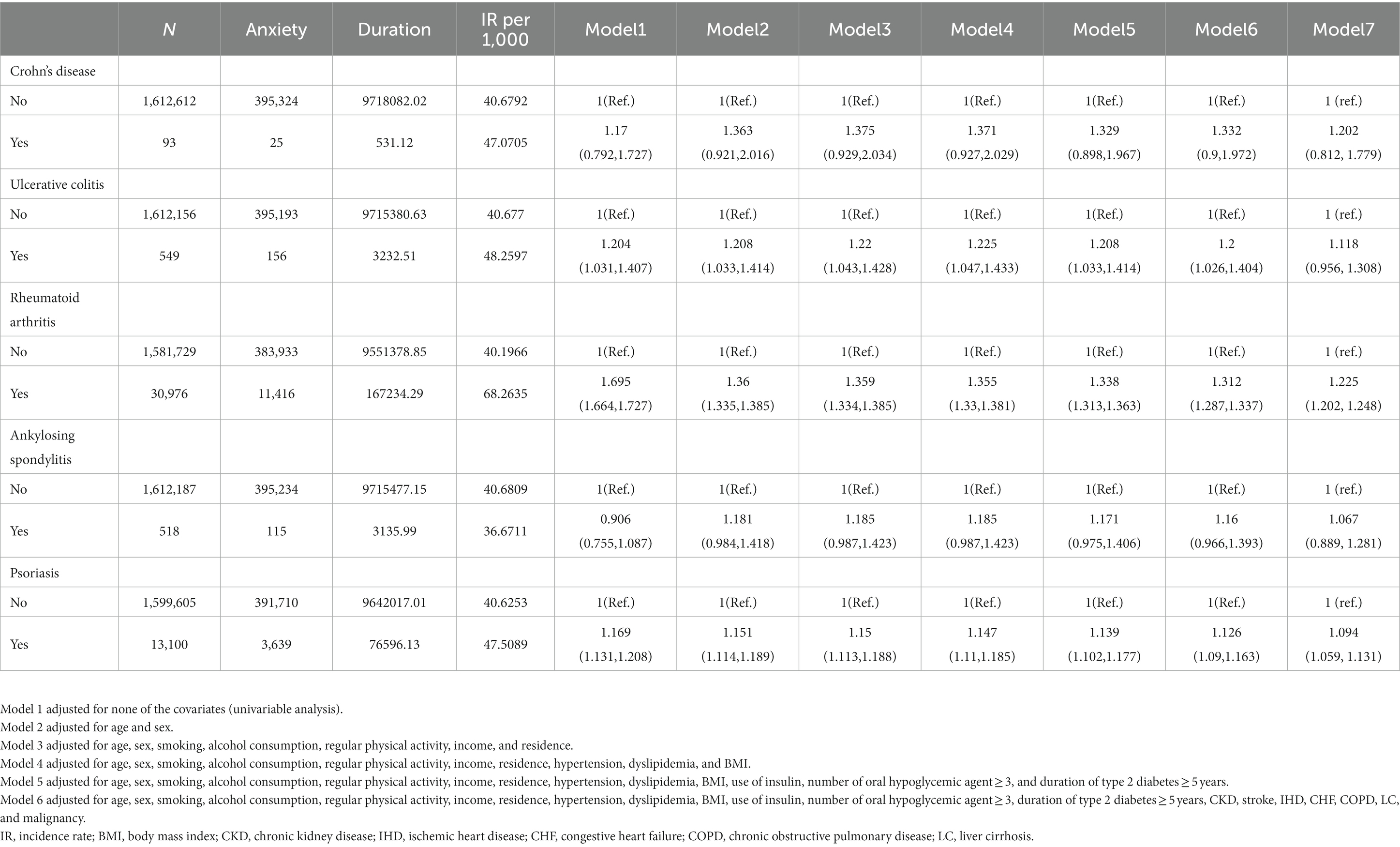
Table 5. Risk of anxiety according to the presence of individual immune-mediated inflammatory diseases.
Incidence and risk of developing both depression and anxiety according to the presence of IMIDs
As depression and anxiety often co-occur, we additionally analyzed the association between presence of IMIDs and occurrence of both depression and anxiety (Table 6). Similar associations were observed as in the analyses where depression and anxiety were analyzed separately. In model 6, existence of joint IMIDs (adjusted HR: 1.266 [95% CI: 1.151–1.394]), and skin IMID (adjusted HR: 1.252 [95% CI: 1.075–1.459]) was significantly associated with a higher risk of occurrence of both depression and anxiety. Existence of gut IMIDs (adjusted HR: 1.5 [95% CI: 0.78–2.883]) showed a trend of being associated with a higher risk of occurrence of both depression and anxiety. According to the number of IMIDs present, patients with one IMID (adjusted HR: 1.247 [95% CI: 1.148–1.354]) had a significantly higher risk of occurrence of both depression and anxiety than those without any IMIDs, and patients with ≥ 2 IMIDs (adjusted HR: 2.234 [95% CI: 1.24–4.025]) also had a significantly higher risk of occurrence of both depression and anxiety than those without any IMIDs, with a larger effect size.

Table 6. Risk of developing both depression and anxiety according to the presence of immune-mediated inflammatory diseases.
Subgroup analyses
The results of the subgroup analyses are shown in Figure 4. The impact of gut IMIDs on the incidence of depression did not differ significantly between subgroups of patients stratified by multiple risk factors (Figure 4A). The effect of joint IMIDs on the incidence of depression was more pronounced in men (p-interaction < 0.0001), patients without hypertension (p-interaction = 0.032), those not using insulin (p-interaction = 0.0013), and those who had a disease duration of T2DM of < 5 years (p-interaction = 0.0016; Figure 4B). The influence of skin IMID on the incidence of depression was more prominent in current smokers (p-interaction = 0.0181; Figure 4C).
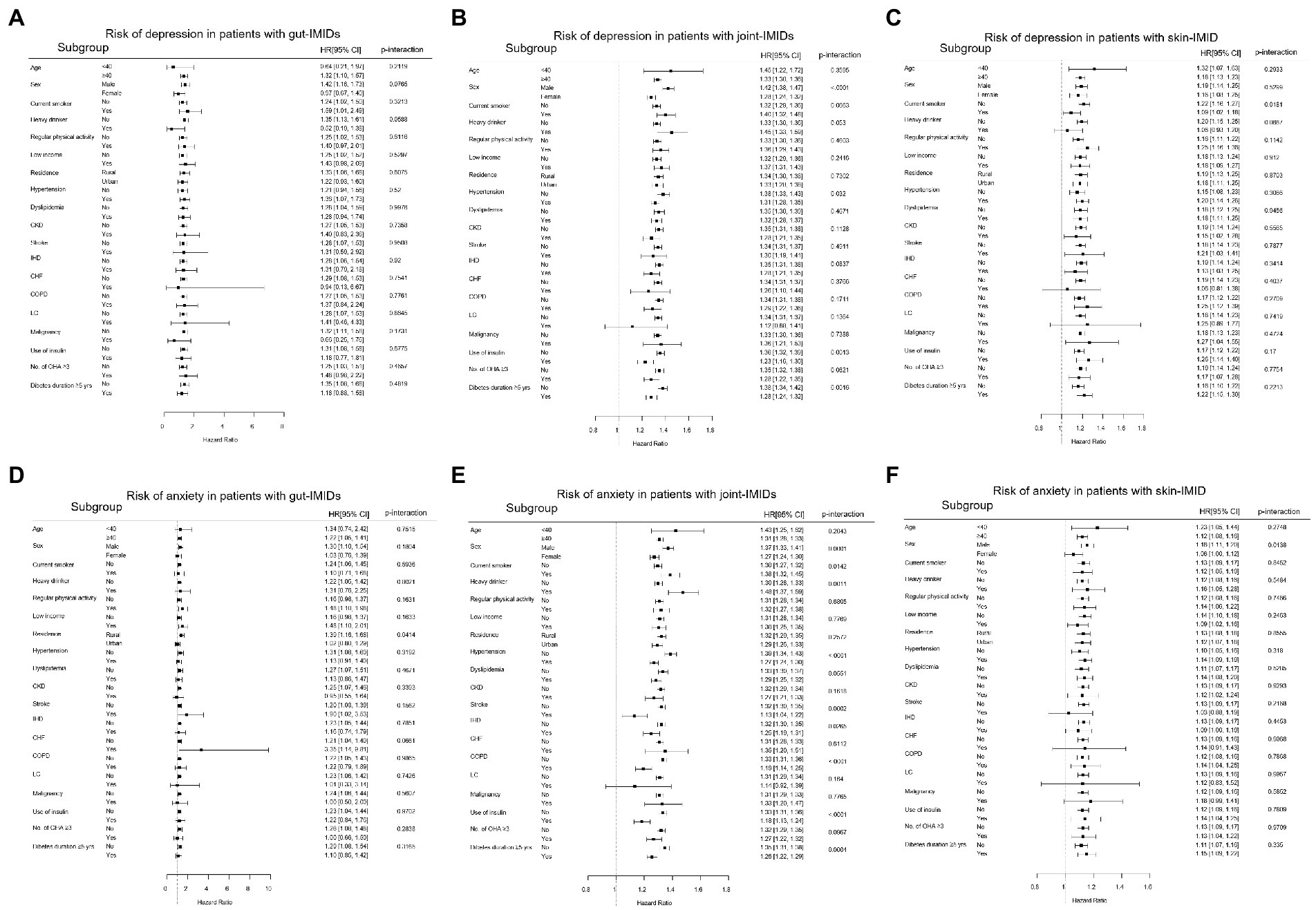
Figure 4. Subgroup analyses for the risk of depression in patients with (A) gut IMIDs, (B) joint IMIDs, and (C) skin IMID; and the risk of anxiety in patients with (D) gut IMIDs, (E) joint IMIDs, and (F) skin IMID. IMIDs, immune-mediated inflammatory disease; CKD, chronic kidney disease; IHD, ischemic heart disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; LC, liver cirrhosis; OHA, oral hypoglycemic agents.
The impact of gut IMIDs on the development of anxiety was more prominent in patients of rural residence (p-interaction = 0.0414; Figure 4D). The impact of joint IMIDs on the incidence of anxiety was more prominent in male patients (p-interaction = 0.0001), those who were current smokers (p-interaction = 0.0142) and heavy alcohol drinkers (p-interaction = 0.0011), those without hypertension (p-interaction < 0.0001), stroke (p-interaction = 0.0002), ischemic heart disease (p-interaction = 0.0265) and COPD (p-interaction < 0.0001), patients not using insulin (p-interaction < 0.0001), and those who had a disease duration of T2DM of <5 years (p-interaction = 0.0004; Figure 4E). The influence of skin IMID on the incidence of anxiety was more prominent in men (p-interaction = 0.0138; Figure 4F).
The subgroup analysis for the influence of individual IMIDs on the development of depression and anxiety is shown in Figure 5.
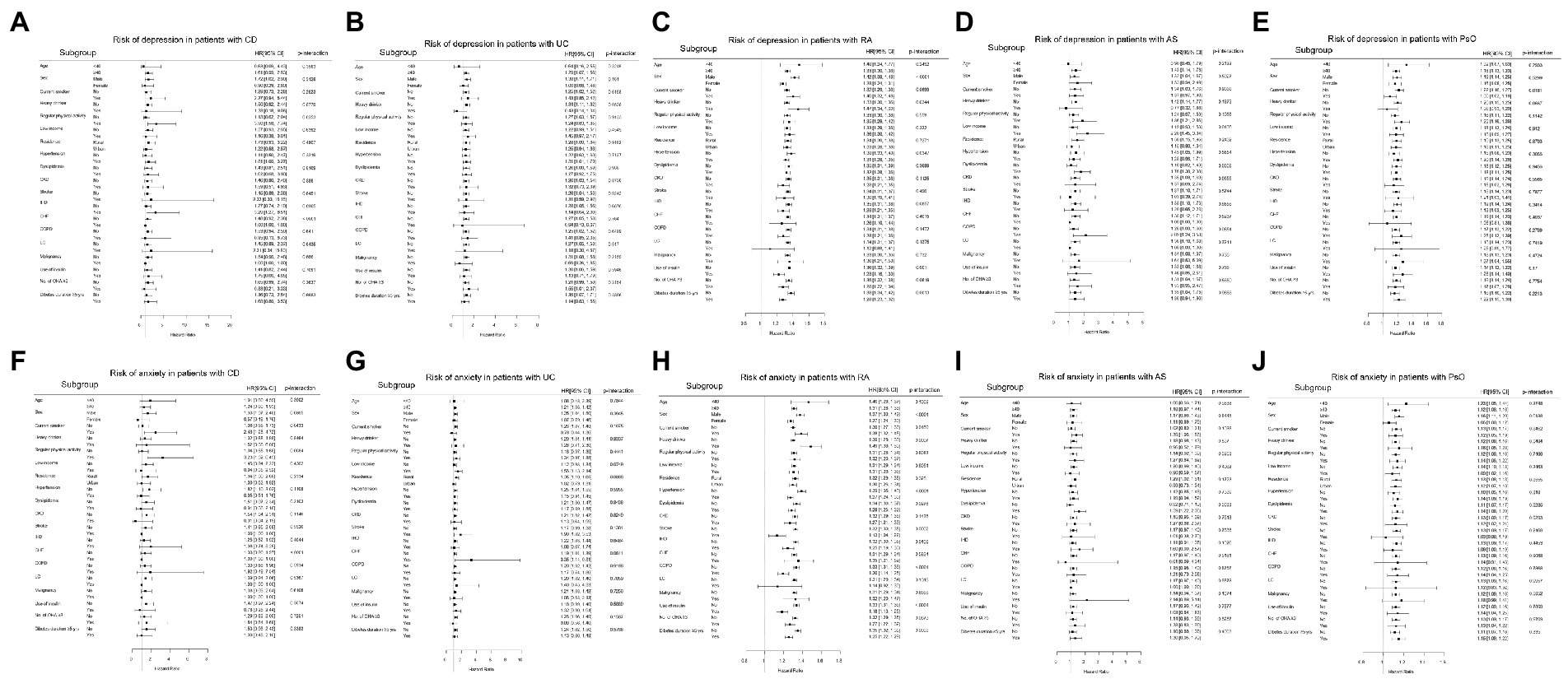
Figure 5. Subgroup analyses for the risk of depression in patients with (A) CD, (B) UC, (C) RA, (D) AS, and (E) PsO; and the risk of anxiety in patients with (F) CD, (G) UC, (H) RA, (I) AS, and (J) PsO. CD, crohn’s disease; UC, ulcerative colitis; RA, rheumatoid arthritis; AS, ankylosing spondylitis; PsO, psoriasis; CKD, chronic kidney disease; IHD, ischemic heart disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; LC, liver cirrhosis; OHA, oral hypoglycemic agents.
Discussion
In this large-scale population-based study, we found that existence of gut IMIDs, joint IMIDs, and skin IMID is associated with a significantly higher risk of developing depression and anxiety in patients with T2DM as compared to their respective counterparts that did not have those IMIDs. Moreover, according to the number of IMIDs present, patients with T2DM who had ≥ 2 IMIDs had a higher risk of depression and anxiety than those who had one IMID, both of whom had higher risk than those without any IMIDs. We also identified subgroups of patients in whom the impacts of IMIDs were more prominent. Our findings are noteworthy because it is the first to comprehensively assess the effect of organ-based IMIDs on depression and anxiety in patients with T2DM, which are associated with poor outcomes in patients with T2DM (42–44). These data provide better insights in stratifying the risk of depression and anxiety in patients with T2DM, which can lead to closer monitoring for depression and anxiety in high risk patients.
According to the existence of IMIDs in an organ-based manner, joint IMIDs had the largest effect sizes on depression and anxiety (adjusted HRs 1.335 and 1.311, respectively), followed by the gut IMIDs (adjusted HRs of 1.284 and 1.223, respectively). Skin IMID had the smallest effect sizes on depression and anxiety (adjusted HRs of 1.183 and 1.126, respectively). Considering that patients with T2DM are already at a higher risk of depression (1.67-fold higher risk) and anxiety (1.73-fold higher risk) compared with the general population (1, 2), the HRs of each organ-based IMID are substantial. These associations reflect that comorbid chronic inflammatory disorders are crucial factors associated with psychological distress in patients with T2DM, while the additional risks may be different depending on the affected organs.
Similar with the analyses where depression and anxiety were analyzed separately, when association between IMIDs and co-occurrence of depression and anxiety was assessed, joint IMIDs and skin IMID were significantly associated with a higher risk co-occurrence of depression and anxiety, and gut IMIDs had a trend of being associated with a higher risk co-occurrence of depression and anxiety. Given that IMIDs are chronic diseases (4), these results are accordant with the previous knowledge that depression and anxiety commonly co-occur in patients with chronic disease, such as COPD (45). Then, a question rises on whether depression and anxiety affect one another. When incident anxiety was additionally adjusted in the analyses of incident depression (model 7 in Table 2 and Table 3), and vice versa (model 7 in Table 4 and Table 5), although the effect sizes were attenuated, similar associations were observed as in model 6, suggesting that the associations of IMIDs with incident depression or anxiety are independent of incident anxiety or depression, respectively.
Mechanistically, a link between inflammatory process and neuropsychiatric conditions have been consistently reported (46, 47). In inflammatory conditions, pro-inflammatory cytokines access the central nervous system and interact with a cytokine network in the brain, influencing the brain function relevant to the development of psychological distress including depression and anxiety (48). Moreover, from an organ-based perspective, joint-brain axis, gut-brain axis, and gut-brain-skin axis have been proposed to explain the crosstalk of joint, gut, and skin inflammation with psychological distress, respectively (49–51). Together with this background knowledge, our data suggest that it is important to perceive IMIDs, a disease group typified by chronic inflammation (4), as risk factors of depression and anxiety in patients with T2DM.
The HRs for depression and anxiety were higher in patients with T2DM who had ≥ 2 IMIDs (42% higher risk and 49% higher risk, respectively) than in those with one IMID (30% higher risk and 26% higher risk, respectively). This suggests that higher inflammatory burden could be associated with a higher risk of developing depression and anxiety. Indeed, previous studies have shown that concentrations of inflammatory markers such as C-reactive protein and interleukin-6 correlate with the scores of depression and anxiety, supporting our findings (52, 53). Based on our data, the risk of depression and anxiety in patients with T2DM can be stratified in the following order: patients with ≥ 2 IMIDs, those with joint IMIDs, those with gut IMIDs, and those with skin IMID.
When individual IMIDs were analyzed separately, Crohn’s disease had a trend of being associated with a higher risk of depression and anxiety, but did not reach statistical significance. The wide 95% CIs of the HRs of Crohn’s disease suggest that this is probably due to the small number of patients with CD. The HR itself (48% higher risk and 33% higher risk for depression and anxiety, respectively), was numerically the highest compared to that of every other IMID. Therefore, although the association between Crohn’s disease and risk of depression and anxiety did not reach statistical significance, patients with Crohn’s disease could still be considered as having higher risk of depression and anxiety clinically.
Interestingly, ankylosing spondylitis showed a trend of being associated with a lower risk of anxiety in the univariable model, which was not observed in other individual IMIDs. We assume that this finding is attributable to the high proportion of males in patients with ankylosing spondylitis. The proportion of males (91.7%) was numerically the highest in patients with ankylosing spondylitis, compared with patients with other IMIDs. Given that female sex is a risk factor of anxiety (54, 55), the high proportion of males (i.e., low proportion of females) in patients with ankylosing spondylitis could explain why these patients showed a trend for a lower risk of anxiety. Indeed, this association was reversed in the subsequent models (model 2–6) where sex was adjusted and showed a trend of being associated with a higher risk of anxiety.
Some limitations should be noted in our study. First, this was a retrospective observational study. Although we found interesting associations between IMIDs and depression and anxiety, causality cannot be inferred. In addition, we lack mechanistic explanations for why some IMIDs pose higher risk than the others and why the impacts of IMIDs are more pronounced in particular subgroups of patients. Second, as this was a Korean nationwide population-based study, it is unclear whether our results are generalizable to different ethnic populations. Third, we lack data on the severity of IMIDs and were unable to analyze the association between the severity of IMIDs and depression and anxiety. Fourth, markers of inflammation such as erythrocyte sedimentation rate and C-reactive protein were unavailable in the NHIS database. Fifth, we could not determine whether comorbid IMIDs lower the threshold for psychological distress screening in this study, because the primary outcome was defined using ICD-10 codes from the claims database. Sixth, the diagnoses of depression and anxiety were not systematically screened and detected. Under-diagnosis of mental disorders is common in primary care and therefore, there is a possibility of bias resulting from under-diagnosis of depression and anxiety. Despite these limitations, the use of a nationwide database that covers the entire Korean population allowed for a large sample of patients with T2DM and an adequate number of outcome events, which are usually difficult to achieve in clinical trials. This enabled us to sufficiently evaluate the impact of IMIDs on the risk of depression and anxiety in patients with T2DM.
In conclusion, in patients with T2DM, the presence of IMIDs was associated with higher risks of depression and anxiety (Figure 6). The risk was higher in patients with ≥ 2 IMIDs than in those with one IMID. According to organ-based classification, joint IMIDs posed the highest risk, followed by gut IMIDs, and then the skin IMID. Our data suggest that patients with T2DM with concomitant IMIDs should be recognized as high risk patients for developing depression and anxiety, thus needing more stringent screening for depression and anxiety. This is of importance given the clinical implication that psychological distress has on patient-reported outcomes and prognosis of T2DM. Further prospective studies evaluating optimal screening schedule for depression and anxiety in these high risk patients are warranted.
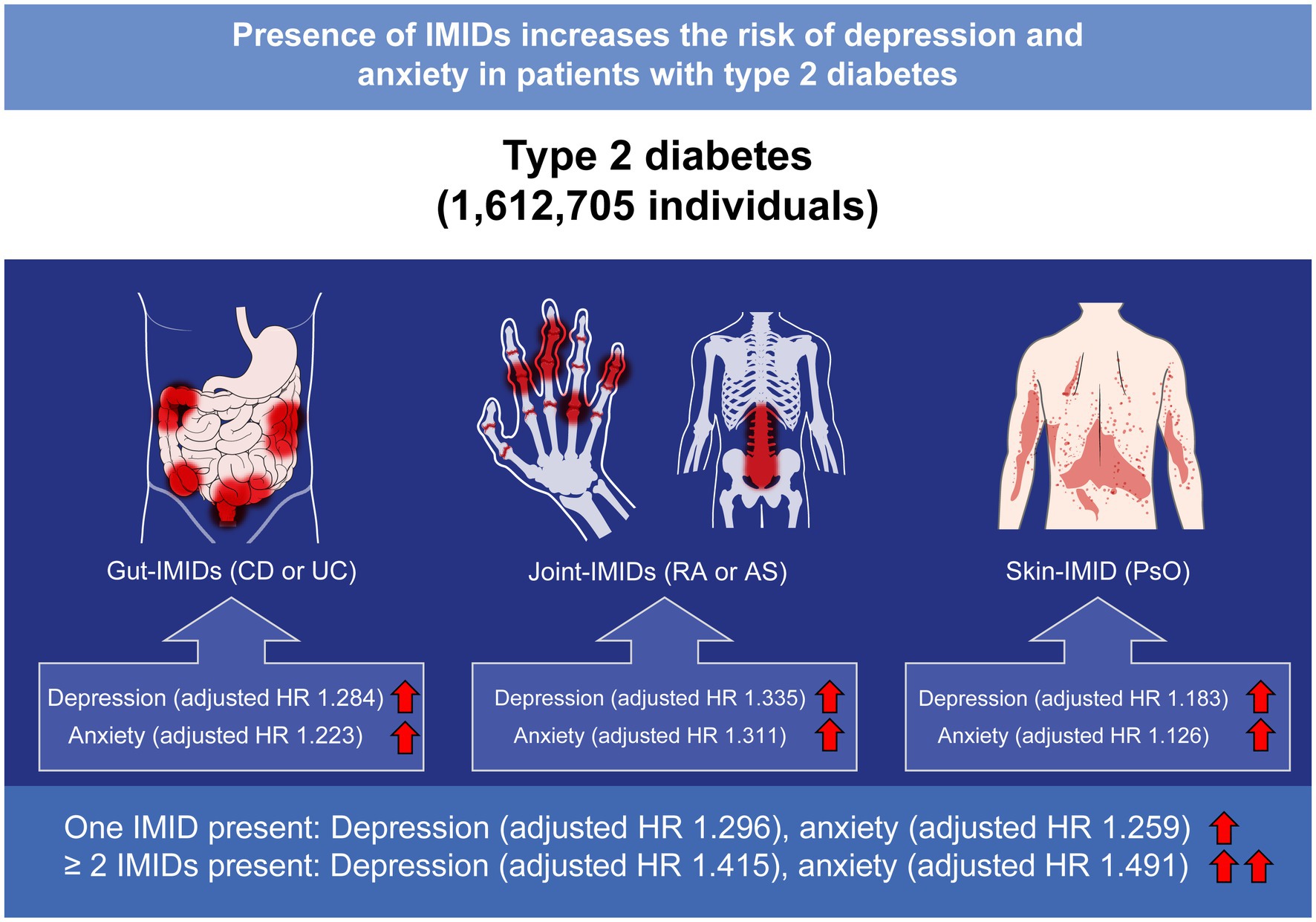
Figure 6. Summary of the present study. IMIDs, immune-mediated inflammatory disease; CD, Crohn’s disease; UC, ulcerative colitis; RA, rheumatoid arthritis; AS, ankylosing spondylitis; PsO, psoriasis; HR, hazard ratio.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of Gangnam Severance Hospital (IRB No: 3-2020-0269). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
OK, YK, JC, and KH: concept, design, administrative, and technical or material support. OK, YK, JC, KH, M-CP, and RK: acquisition, analysis, or interpretation of data. OK, YK, JC, and KH: drafting of the manuscript. OK, YK, JC, KH, M-CP, RK, J-HK, YY, and HP: critical revision of the manuscript for important intellectual content. KH: statistical analysis. OK: obtained funding. JC and KH: supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the research fund of Rheumatology Research Foundation (RRF-2022-02).
Acknowledgments
We would like to thank Seung Woo Lee and Ju-Hwan Yoo for the statistical support, and Medical Illustration and Design (MID), a part of the Medical Research Support Services of Yonsei University College of Medicine, for providing excellent support with medical illustration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1103911/full#supplementary-material
References
1. Wang, F, Wang, S, Zong, QQ, Zhang, Q, Ng, CH, Ungvari, GS, et al. Prevalence of comorbid major depressive disorder in type 2 diabetes: a meta-analysis of comparative and epidemiological studies. Diabet Med. (2019) 36:961–9. doi: 10.1111/dme.14042
2. Amiri, S, and Behnezhad, S. Diabetes and anxiety symptoms: a systematic review and meta-analysis. Int J Psychiatry Med. (2019):91217419837407. doi: 10.1177/0091217419837407
3. Snoek, FJ, Bremmer, MA, and Hermanns, N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol. (2015) 3:450–60. doi: 10.1016/s2213-8587(15)00135-7
4. Schett, G, McInnes, IB, and Neurath, MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med. (2021) 385:628–39. doi: 10.1056/NEJMra1909094
5. Baumgart, DC, and Sandborn, WJ. Crohn's disease. Lancet. (2012) 380:1590–605. doi: 10.1016/s0140-6736(12)60026-9
6. Danese, S, and Fiocchi, C. Ulcerative colitis. N Engl J Med. (2011) 365:1713–25. doi: 10.1056/NEJMra1102942
7. McInnes, IB, and Schett, G. The pathogenesis of rheumatoid arthritis. N Engl J Med. (2011) 365:2205–19. doi: 10.1056/NEJMra1004965
8. Taurog, JD, Chhabra, A, and Colbert, RA. Ankylosing spondylitis and axial Spondyloarthritis. N Engl J Med. (2016) 374:2563–74. doi: 10.1056/NEJMra1406182
9. Nestle, FO, Kaplan, DH, and Barker, J. Psoriasis. N Engl J Med. (2009) 361:496–509. doi: 10.1056/NEJMra0804595
10. Park, SH. Update on the epidemiology of inflammatory bowel disease in Asia: where are we now? Intest Res. (2022) 20:159–64. doi: 10.5217/ir.2021.00115
11. Cho, CW, You, MW, Oh, CH, Lee, CK, and Moon, SK. Long-term disease course of Crohn's disease: changes in disease location, phenotype, activities, and predictive factors. Gut Liver. (2022) 16:157–70. doi: 10.5009/gnl210118
12. Park, SB, Yoon, JY, and Cha, JM. What are the different phenotypes of inflammatory bowel disease in Asia? Gut Liver. (2022) 16:676–85. doi: 10.5009/gnl210385
13. Park, J, and Cheon, JH. Incidence and prevalence of inflammatory bowel disease across Asia. Yonsei Med J. (2021) 62:99–108. doi: 10.3349/ymj.2021.62.2.99
14. Low, D, Swarup, N, Okada, T, and Mizoguchi, E. Landscape of inflammatory bowel disease in Singapore. Intest Res. (2022) 20:291–6. doi: 10.5217/ir.2021.00089
15. Shafiee, NH, Manaf, ZA, Mokhtar, NM, and Raja Ali, RA. Anti-inflammatory diet and inflammatory bowel disease: what clinicians and patients should know? Intest Res. (2021) 19:171–85. doi: 10.5217/ir.2020.00035
16. Choi, K, Chun, J, Han, K, Park, S, Soh, H, Kim, J, et al. Risk of anxiety and depression in patients with inflammatory bowel disease: a Nationwide, population-based study. J Clin Med. (2019) 8. doi: 10.3390/jcm8050654
17. Marrie, RA, Hitchon, CA, Walld, R, Patten, SB, Bolton, JM, Sareen, J, et al. Increased burden of psychiatric disorders in rheumatoid arthritis. Arthritis Care Res (Hoboken). (2018) 70:970–8. doi: 10.1002/acr.23539
18. Shen, CC, Hu, LY, Yang, AC, Kuo, BI, Chiang, YY, and Tsai, SJ. Risk of psychiatric disorders following ankylosing spondylitis: a Nationwide population-based retrospective cohort study. J Rheumatol. (2016) 43:625–31. doi: 10.3899/jrheum.150388
19. Kurd, SK, Troxel, AB, Crits-Christoph, P, and Gelfand, JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. (2010) 146:891–5. doi: 10.1001/archdermatol.2010.186
20. Esser, N, Legrand-Poels, S, Piette, J, Scheen, AJ, and Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. (2014) 105:141–50. doi: 10.1016/j.diabres.2014.04.006
21. Kim, JA, Yoon, S, Kim, LY, and Kim, DS. Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for Health Research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. (2017) 32:718–28. doi: 10.3346/jkms.2017.32.5.718
22. Lim, WH, Choi, EK, Han, KD, Rhee, TM, Lee, HJ, Lee, SR, et al. Proteinuria detected by urine dipstick test as a risk factor for atrial fibrillation: a Nationwide population-based study. Sci Rep. (2017) 7:6324. doi: 10.1038/s41598-017-06579-0
23. Nam, GE, Kim, W, Han, K, Lee, CW, Kwon, Y, Han, B, et al. Body weight variability and the risk of cardiovascular outcomes and mortality in patients with type 2 diabetes: a Nationwide cohort study. Diabetes Care. (2020) 43:2234–41. doi: 10.2337/dc19-2552
24. Kwon, OC, Han, K, Chun, J, Kim, R, Hong, SW, Kim, JH, et al. Effects of immune-mediated inflammatory diseases on cardiovascular diseases in patients with type 2 diabetes: a nationwide population-based study. Sci Rep. (2022) 12:11548. doi: 10.1038/s41598-022-15436-8
25. Kang, EA, Chun, J, Kim, JH, Han, K, Soh, H, Park, S, et al. Periodontitis combined with smoking increases risk of the ulcerative colitis: a national cohort study. World J Gastroenterol. (2020) 26:5661–72. doi: 10.3748/wjg.v26.i37.5661
26. Kim, J, Chun, J, Lee, C, Han, K, Choi, S, Lee, J, et al. Increased risk of idiopathic pulmonary fibrosis in inflammatory bowel disease: a nationwide study. J Gastroenterol Hepatol. (2020) 35:249–55. doi: 10.1111/jgh.14838
27. Je, Y, Han, K, Chun, J, Kim, Y, Kim, JH, Youn, YH, et al. Association of Waist Circumference with the risk of inflammatory bowel disease: a Nationwide cohort study of 10 million individuals in Korea. J Crohns Colitis. (2022). doi: 10.1093/ecco-jcc/jjac193
28. Kim, SY, Servi, A, Polinski, JM, Mogun, H, Weinblatt, ME, Katz, JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. (2011) 13:R32. doi: 10.1186/ar3260
29. Lee, EE, Shin, A, Lee, J, Lee, JH, Ha, YJ, Lee, YJ, et al. All-cause and cause-specific mortality of patients with rheumatoid arthritis in Korea: a nation-wide population-based study. Joint Bone Spine. (2022) 89:105269. doi: 10.1016/j.jbspin.2021.105269
30. Lee, JS, Oh, JS, Kim, YJ, Hong, S, Lee, CK, Yoo, B, et al. Reasons for the high cesarean delivery rate among women with ankylosing spondylitis: using the Korean National Health Insurance Database. J Rheumatol. (2020) 47:668–73. doi: 10.3899/jrheum.190754
31. Han, JH, Lee, JH, Han, KD, Seo, HM, Bang, CH, Park, YM, et al. Epidemiology and medication trends in patients with psoriasis: a Nationwide population-based cohort study from Korea. Acta Derm Venereol. (2018) 98:396–400. doi: 10.2340/00015555-2877
32. Lee, CK, Ha, HJ, Oh, SJ, Kim, JW, Lee, JK, Kim, HS, et al. Nationwide validation study of diagnostic algorithms for inflammatory bowel disease in Korean National Health Insurance Service database. J Gastroenterol Hepatol. (2020) 35:760–8. doi: 10.1111/jgh.14855
33. Cho, SK, Sung, YK, Choi, CB, Kwon, JM, Lee, EK, and Bae, SC. Development of an algorithm for identifying rheumatoid arthritis in the Korean National Health Insurance claims database. Rheumatol Int. (2013) 33:2985–92. doi: 10.1007/s00296-013-2833-x
34. Ham, SP, Oh, JH, Park, HJ, Kim, JU, Kim, HY, Jung, SY, et al. Validity of diagnostic codes for identification of psoriasis patients in Korea. Ann Dermatol. (2020) 32:115–21. doi: 10.5021/ad.2020.32.2.115
35. Hong, S, Park, JH, Han, K, Lee, CB, Kim, DS, and Yu, SH. Association between obesity and cardiovascular disease in elderly patients with diabetes: a retrospective cohort study. J Clin Endocrinol Metab. (2022) 107:e515–27. doi: 10.1210/clinem/dgab714
36. Kang, YM, Cho, YK, Lee, SE, Park, JY, Lee, WJ, Kim, YJ, et al. Cardiovascular diseases and life expectancy in adults with type 2 diabetes: a Korean National Sample Cohort Study. J Clin Endocrinol Metab. (2017) 102:3443–51. doi: 10.1210/jc.2017-00643
37. Thuluvath, PJ, Alukal, JJ, and Zhang, T. Impact of hyponatremia on morbidity, mortality, and resource utilization in portal hypertensive ascites: a Nationwide analysis. J Clin Exp Hepatol. (2022) 12:871–5. doi: 10.1016/j.jceh.2021.10.145
38. Park, S, Chun, J, Han, KD, Soh, H, Choi, K, Kim, JH, et al. Increased end-stage renal disease risk in patients with inflammatory bowel disease: a nationwide population-based study. World J Gastroenterol. (2018) 24:4798–808. doi: 10.3748/wjg.v24.i42.4798
39. Lee, SR, Choi, EK, Rhee, TM, Lee, HJ, Lim, WH, Kang, SH, et al. Evaluation of the association between diabetic retinopathy and the incidence of atrial fibrillation: a nationwide population-based study. Int J Cardiol. (2016) 223:953–7. doi: 10.1016/j.ijcard.2016.08.296
40. Soh, H, Chun, J, Han, K, Park, S, Choi, G, Kim, J, et al. Increased risk of herpes zoster in young and metabolically healthy patients with inflammatory bowel disease: a Nationwide population-based study. Gut Liver. (2019) 13:333–41. doi: 10.5009/gnl18304
41. Kang, JI, Sung, NY, Park, SJ, Lee, CG, and Lee, BO. The epidemiology of psychiatric disorders among women with breast cancer in South Korea: analysis of national registry data. Psychooncology. (2014) 23:35–9. doi: 10.1002/pon.3369
42. Lustman, PJ, Anderson, RJ, Freedland, KE, de Groot, M, Carney, RM, and Clouse, RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. (2000) 23:934–42. doi: 10.2337/diacare.23.7.934
43. Anderson, RJ, Grigsby, AB, Freedland, KE, de Groot, M, McGill, JB, Clouse, RE, et al. Anxiety and poor glycemic control: a meta-analytic review of the literature. Int J Psychiatry Med. (2002) 32:235–47. doi: 10.2190/klgd-4h8d-4ryl-twq8
44. Egede, LE, Nietert, PJ, and Zheng, D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care. (2005) 28:1339–45. doi: 10.2337/diacare.28.6.1339
45. Phan, T, Carter, O, Waterer, G, Chung, LP, Hawkins, M, Rudd, C, et al. Determinants for concomitant anxiety and depression in people living with chronic obstructive pulmonary disease. J Psychosom Res. (2019) 120:60–5. doi: 10.1016/j.jpsychores.2019.03.004
46. Dantzer, R, O'Connor, JC, Freund, GG, Johnson, RW, and Kelley, KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
47. Gabbay, V, Klein, RG, Alonso, CM, Babb, JS, Nishawala, M, De Jesus, G, et al. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord. (2009) 115:177–82. doi: 10.1016/j.jad.2008.07.022
48. Capuron, L, and Miller, AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. (2011) 130:226–38. doi: 10.1016/j.pharmthera.2011.01.014
49. Süß, P, Rothe, T, Hoffmann, A, Schlachetzki, JCM, and Winkler, J. The joint-brain Axis: insights from rheumatoid arthritis on the crosstalk between chronic peripheral inflammation and the brain. Front Immunol. (2020) 11:612104. doi: 10.3389/fimmu.2020.612104
50. Rutsch, A, Kantsjö, JB, and Ronchi, F. The gut-brain Axis: how microbiota and host Inflammasome influence brain physiology and pathology. Front Immunol. (2020) 11:604179. doi: 10.3389/fimmu.2020.604179
51. Wang, X, Li, Y, Wu, L, Xiao, S, Ji, Y, Tan, Y, et al. Dysregulation of the gut-brain-skin axis and key overlapping inflammatory and immune mechanisms of psoriasis and depression. Biomed Pharmacother. (2021) 137:111065. doi: 10.1016/j.biopha.2020.111065
52. Osimo, EF, Pillinger, T, Rodriguez, IM, Khandaker, GM, Pariante, CM, and Howes, OD. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. (2020) 87:901–9. doi: 10.1016/j.bbi.2020.02.010
53. Howren, MB, Lamkin, DM, and Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. (2009) 71:171–86. doi: 10.1097/PSY.0b013e3181907c1b
54. Blanco, C, Rubio, J, Wall, M, Wang, S, Jiu, CJ, and Kendler, KS. Risk factors for anxiety disorders: common and specific effects in a national sample. Depress Anxiety. (2014) 31:756–64. doi: 10.1002/da.22247
Keywords: immune-mediated inflammatory diseases, type 2 diabetes, depression, anxiety, risk
Citation: Kwon OC, Kim Y, Chun J, Han K, Park M-C, Kim R, Kim J-H, Youn YH and Park H (2023) Association of immune-mediated inflammatory diseases with depression and anxiety in patients with type 2 diabetes: A nationwide population-based study. Front. Med. 10:1103911. doi: 10.3389/fmed.2023.1103911
Edited by:
Rossella Messina, University of Bologna, ItalyReviewed by:
Sridhar R Gumpeny, Endocrine and Diabetes Centre, IndiaAndreas Schmitt, Diabetes Zentrum Mergentheim, Germany
Copyright © 2023 Kwon, Kim, Chun, Han, Park, Kim, Kim, Youn and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaeyoung Chun, Y2h1bmptZEB5dWhzLmFj; Kyungdo Han, aGtkOTE3QG5hdmVyLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Oh Chan Kwon
Oh Chan Kwon Yuna Kim
Yuna Kim Jaeyoung Chun
Jaeyoung Chun Kyungdo Han
Kyungdo Han Min-Chan Park
Min-Chan Park Ryul Kim
Ryul Kim Jie-Hyun Kim
Jie-Hyun Kim Young Hoon Youn
Young Hoon Youn Hyojin Park
Hyojin Park on behalf of Gastroenterology
on behalf of Gastroenterology Neurology and Rheumatology National Data Science Research (GUARANTEE) Group
Neurology and Rheumatology National Data Science Research (GUARANTEE) Group