
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 09 February 2023
Sec. Ophthalmology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1103593
Purpose: To compare the changes in anatomical structure and visual function after idiopathic macular hole (iMH) treatment with internal limiting membrane (ILM) peeling and inverted ILM flap and determine the value of the inverted ILM flap for the treatment of iMH.
Methods: Forty-nine patients with iMH (49 eyes) were included in this study and followed up for 1 year (12 months) after treatment with inverted ILM flap and ILM peeling respectively. The main foveal parameters assessed included the preoperative minimum diameter (MD), intraoperative residual fragments, and postoperative ELM reconstruction. Visual function was assessed using best-corrected visual acuity.
Results: The hole closure rate was 100% for 49 patients; 15 patients were treated with the inverted ILM flap, and 34 patients underwent ILM peeling. There were no differences between the postoperative best-corrected visual acuities and the rates of ELM reconstruction for the flap and peeling groups with different MDs. In the flap group, ELM reconstruction was associated with the preoperative MD, presence of an ILM flap, and hyperreflective changes in the inner retina 1 month after surgery. In the peeling group, ELM reconstruction was associated with the preoperative MD, intraoperative residual fragments at the hole edge, and hyperreflective changes in the inner retina.
Conclusion: The inverted ILM flap and the ILM Peeling were both able to obtain high closure rate. However, the inverted ILM flap showed no obvious advantages related to anatomical morphology and visual function over ILM peeling.
Idiopathic macular holes (iMHs) are full-thickness anatomical defects at the fovea caused by traction of the vitreous and internal limiting membrane (ILM). The conventional treatment for iMH is pars plana vitrectomy (PPV) combined with ILM peeling to release traction (1), and postoperative macular hole closure rates of up to 100% have been reported (2–4). However, for the patients with the refractory iMHs (the large iMH and myopic MHs [with or without retinal detachment]) the postoperative closure rate after ILM peeling is only 61–100%, which is relatively low (5, 6). Michalewska (7) proposed the inverted ILM flap in 2014 to significantly improve the postoperative closure rate of refractory macular holes. A study of large iMHs suggested that the macula hole closure rate increased to more than 90% with ILM flap surgery (8–10), which markedly reduced the risk of postoperative recurrence of macular holes.
Subsequently, several studies have investigated the visual function and anatomical changes in patients with large iMHs after the inverted ILM flap. Rizzo et al. (11) found that the inverted ILM flap for large iMHs results in better visual outcomes. A systematic review meta-analysis recommended the inverted ILM flap for large iMHs (12, 13). However, the meta-analysis had limited reliability, and the postoperative ELM reconstruction status was not evaluated. Some previous studies have also reported that the inverted ILM flap improves the closure rate of large iMHs but not visual function compared with ILM peeling (14, 15). Yan et al. found no difference between the closure rates after the inverted ILM flap and ILM peeling for large iMH (16). However, these studies had fewer cases and shorter follow-ups. Therefore, the value of the inverted ILM flap for the treatment of macula holes needs to be established.
Therefore, the purpose of this study was to compare the changes in anatomical structure and visual function after ILM peeling and the inverted ILM flap for the treatment of iMH with different minimum diameters (MDs) to establish the value of the inverted ILM flap for iMH.
This was a retrospective study that included 49 eyes of 49 people who visited the Department of Retina Center, Affiliated Eye Hospital of Wenzhou Medical University, Hangzhou, Zhejiang Province, China, from January 2018 to January 2020. The participants had idiopathic full-thickness macular holes. The exclusion criteria included secondary MHs, such as those caused by trauma, high myopia (axial length [AL] ≥ 26.00 mm or refractive error of ≥ –6.00 D); MH with retinal detachment; other retinal vascular diseases, such as diabetic retinopathy, retinal vascular occlusion, and retinal inflammatory diseases; last follow-up visit within 12 months; and history of intraocular surgery.
This study was approved by the Institutional Review Board of Wenzhou Medical University, and the procedure complied with the principles of the Declaration of Helsinki. All patients signed an informed consent form. At the least, the informed consent form requested patient permission to allow researchers to collect information during clinical visits and use it for scientific research and article publication.
All patients underwent tests for BCVA (measured with the Snellen visual acuity chart and converted to logMAR for recording) and refractive error (spherical equivalent), intraocular lens assessments with IOL-Master 700, spectral domain optical coherence tomography (OCT) (SD-OCT), and intraoperative OCT (iOCT). All eyes were diagnosed with full-thickness macular holes by SD-OCT, and all patients were assessed at baseline and followed up 1, 3, 6, and 12 months after surgery.
The foveal microstructures in the eyes with iMHs were evaluated using SD-OCT (Heidelberg, Spectralis OCT). High-density central horizontal scanning in EDI mode was adopted and used for the central 5.8 × 5.8 mm with a 120-μm line spacing. The preoperative MD and the size of the external limiting membrane (ELM) defect were measured by software embedded in the OCT scanner (Figure 1). The postoperative foveal microstructures and characteristics included the macular hole reconstruction layer, hyperreflective changes in the inner retina, ILM flap, and subretinal cavity (Figure 1). All measurements were conducted by one professional technician; three measurements were taken and an average was obtained.
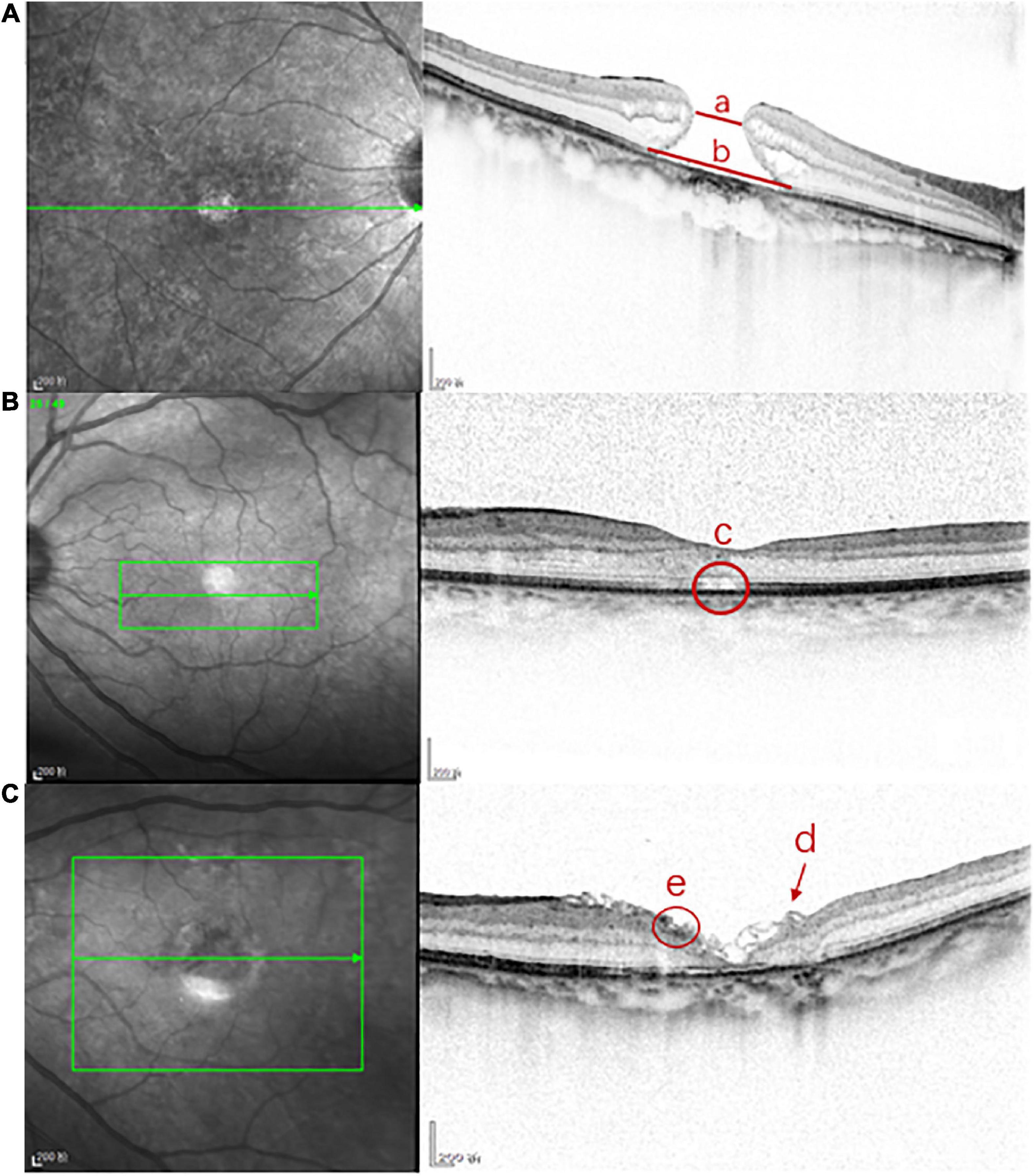
Figure 1. The foveal microstructures in iMH were evaluated by SD-OCT. (A) Line a is the preoperative minimum diameter of iMH, line b is the preoperative length of ELM defect of iMH; (B) c is the postoperative subretinal cavity; (C) the arrow d is the postoperative ILM flap in FLAP group, the circle e is the postoperative hyperreflective change of the inner retina.
The intraoperative foveal microstructures were evaluated using the Optovue iVue OCT System (Optovue, Inc., Fremont, CA, USA). The scanning speed was 26,000 times/min, and the wavelength was 830 nm. Images acquired before and after ILM peeling were analyzed for qualitative changes. iOCT was mainly used to observe the residual fragments (RFs) of the macular hole after ILM peeling, which were categorized into RFs at the hole edge (RFHEs) and RFs outside the hole edge (Figure 2).
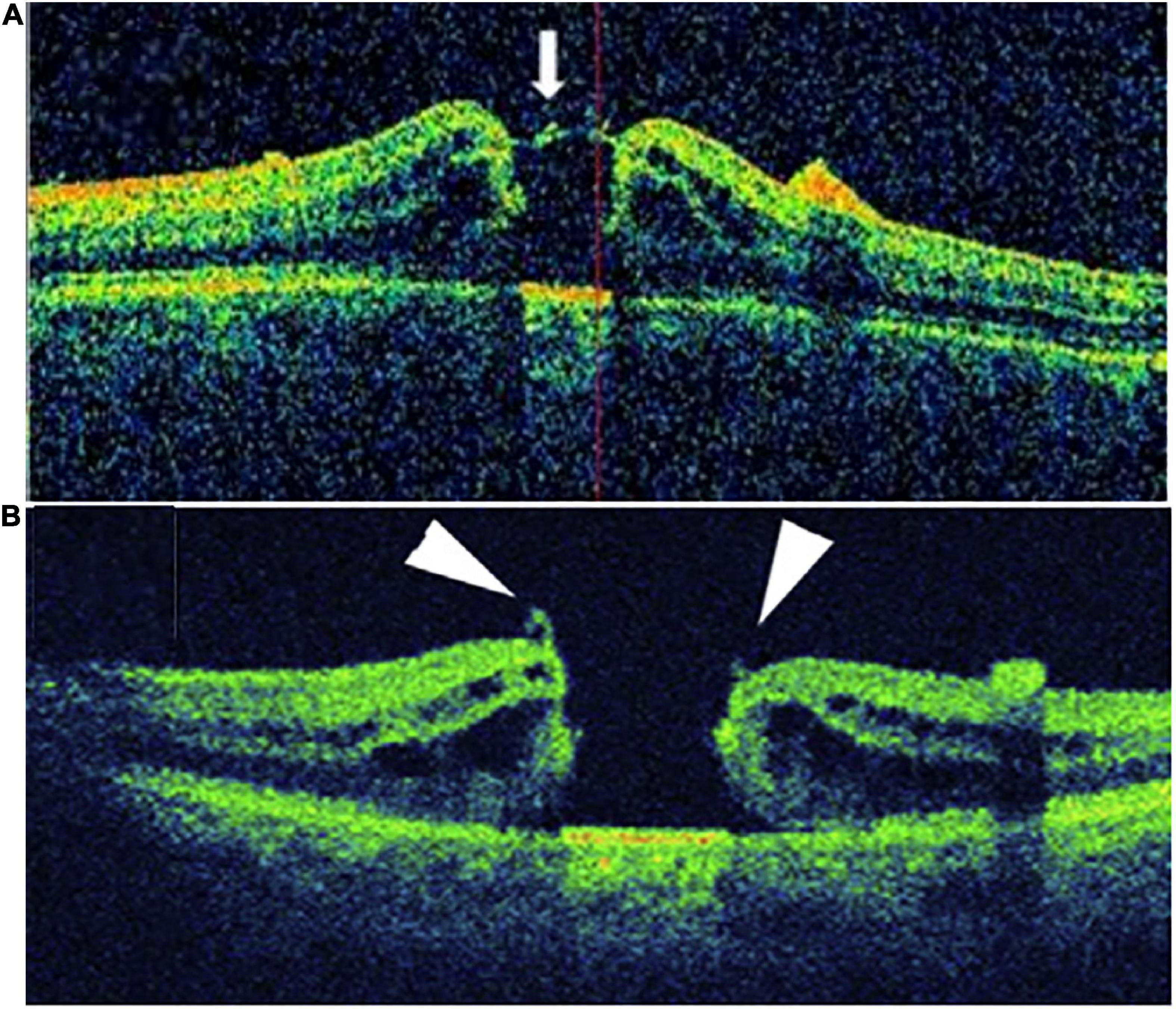
Figure 2. Patients with RF were divided into two groups according to the location of the RF: (A) RF at the hole edge group (RFHE), (B) RF not at the hole edge group (non- RFHE).
All patients were treated through 23-gauge PPV with ILM peeling by one surgeon (Shen). All patients also underwent cataract surgery. Indocyanine green staining (0.02 ml [0.025 mg/ml]) for the ILM was performed after routine vitrectomy.
After indocyanine green staining, the ILM in the macular area was removed (range: 2 to 4 PD). The peeling was initiated from the temporal area of the macula. The ILM was peeled off 360 degrees centripetally and over the MH edge.
During the removal of the ILM, the ILM at the temporal hole margin was retained. The hole margin tension was released, and the flap was inverted to cover the hole from the temporal to the nasal side.
Air-fluid exchange was performed by disinfecting the air at the end of the surgery, and all patients were required to maintain a face-down position for 7 days postoperatively.
SPSS software was used for all statistical analyses (version 21; SPSS, Inc., Chicago, IL). P-values less than 0.05 were considered significant. Fisher’s exact and Chi-squared tests were used to compare the categorical data. The continuous variables are expressed as the mean ± standard deviation (range). The continuous data were evaluated for normal distribution using the Shapiro - Wilk test. The t-test was used to analyze the data conforming to the normal distribution, and the Mann-Whitney U test was used to analyze non-normally distributed data. Pearson’s or Spearman’s analysis was used for correlation analysis. The ROC curve was used to evaluate the MDs for the postoperative reconstruction of ELM, and the critical value of the MD was calculated. Based on the critical value, the patients were divided into two subgroups for comparison.
The ages of the 49 patients (49 eyes) with iMH ranged from 51 to 75 years (63.09 ± 5.96) (Table 1). Twelve of the participants were males. There were 15 patients in the flap group and 34 patients in the peeling group. The hole closure rate was 100% at 12 months postoperatively, and the ELM closure rate was 67%; the ELM closure rate was 60% for the flap group and 71% for the peeling group. There were significant differences between the preoperative and postoperative 12-month BCVAs of the peeling and flap groups (0.99 ± 0.44 vs. 0.46 ± 0.43 [P = 0.023] and 0.95 ± 0.39 vs. 0.54 ± 0.54, [P = 0.000], paired t-test).
The preoperative and postoperative anatomical morphology and visual function of the flap and peeling groups were compared (Table 2). There were no significant differences between the ALs, MDs, preoperative BCVAs, and postoperative ELM reconstruction rates and BCVAs of the groups (P > 0.05, independent sample t-test).
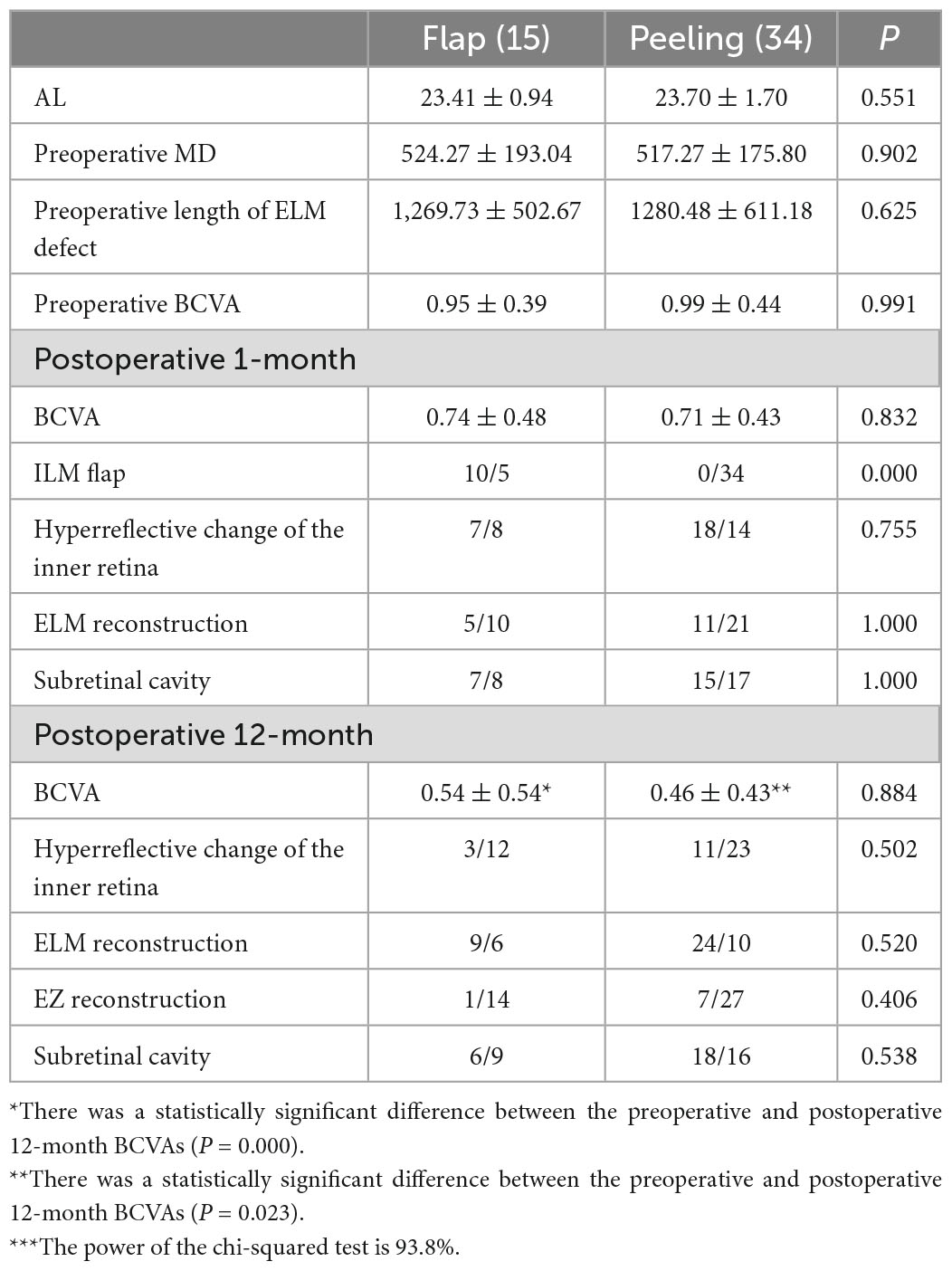
Table 2. The preoperative and postoperative anatomical morphology and visual function of the flap and peeling groups***.
The ROC curve suggested that the MD was predictive of postoperative ELM reconstruction (P = 0.000, AUC = 0.947), and the cut-off point of MD was 579 μm (Figure 3). The two groups were subdivided into four according to the MD. The preoperative and postoperative anatomical changes and visual function of the flap and peeling groups were compared for MD of > 579 μm (Table 3) and ≤ 579 μm (Table 4), respectively, but there were no statistical differences (P > 0.05, independent sample t-test and Shapiro - Wilk tests).
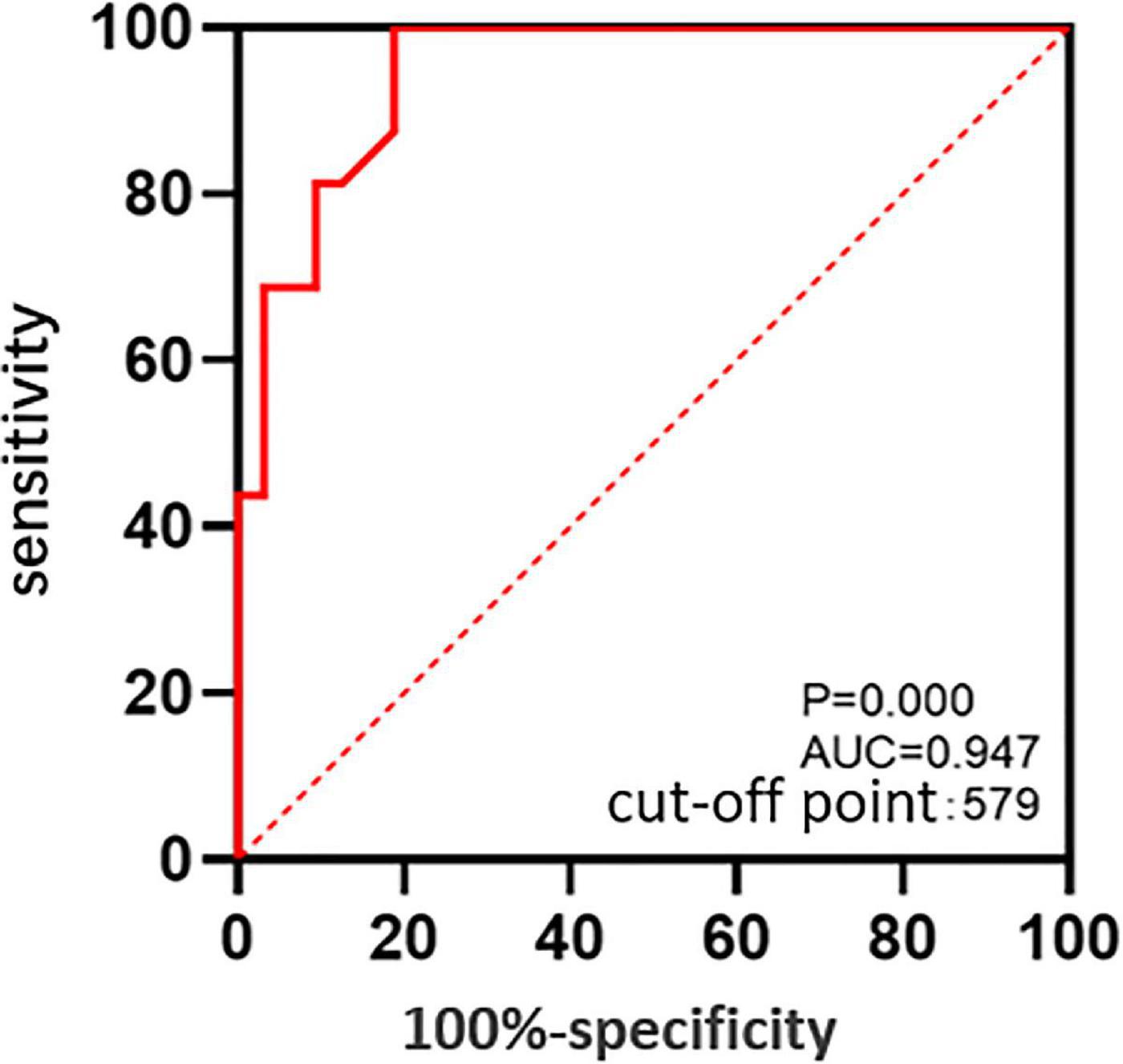
Figure 3. The ROC curve: The predictive value of the MD for postoperative reconstruction states of ELM.
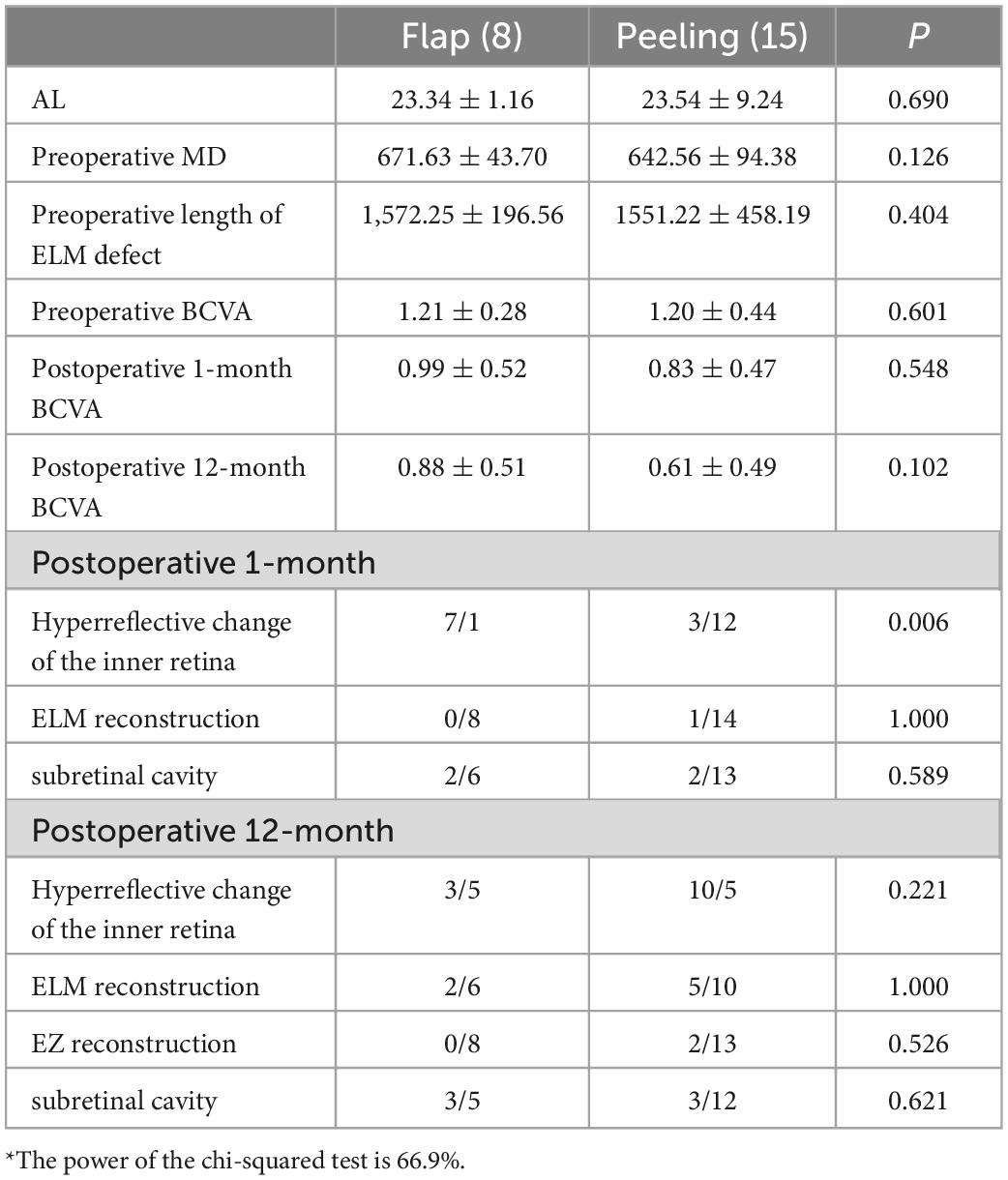
Table 3. Comparison of the preoperative and postoperative anatomical structures and visual function of the flap and peeling groups for MDs of > 579 μm*.
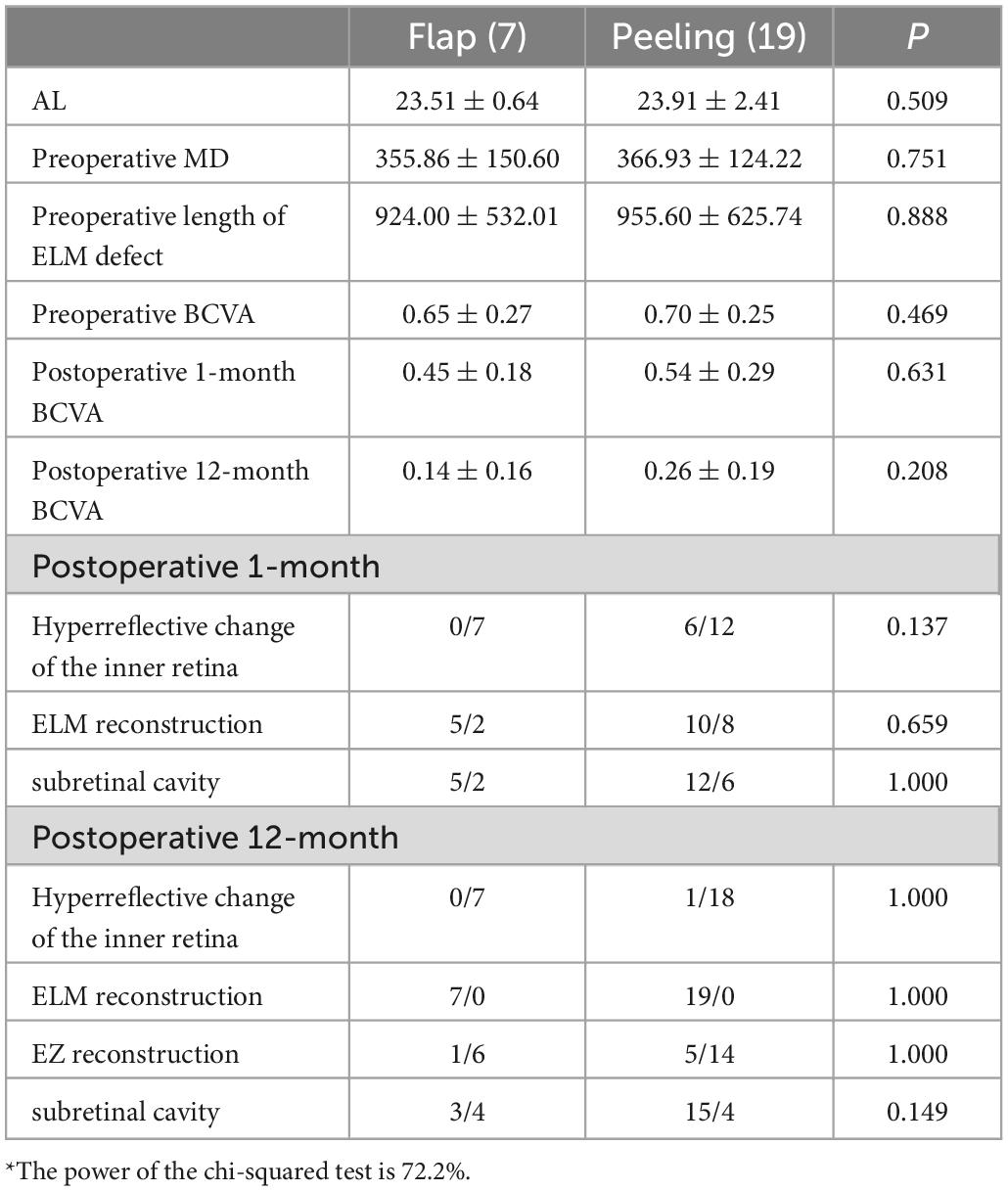
Table 4. Comparison of the preoperative and postoperative anatomical structures and visual function of the flap and peeling groups for MDs of ≤ 579 μm*.
The Flap group was divided into two based on the postoperative reconstruction of the ELM (Table 5, independent sample t-test and Mann–Whitney U tests); 9 patients were included in the ELM group. There were significant differences in the MD and preoperative BCVA between the two groups (P = 0.010, P = 0.010). The hyperreflective changes of the inner retina and ILM flap of the non-ELM group were significantly more than those of the ELM group 1 month after surgery (P = 0.001 and 0.044, respectively); the subretinal cavities were also significantly less in the non-ELM than in the ELM group (P = 0.007). The BCVAs were significantly better for the ELM group than for the non-ELM group (P = 0.013), and the hyperreflective changes of the inner retina in the non-ELM group were significantly more than those in the ELM group (P = 0.044).
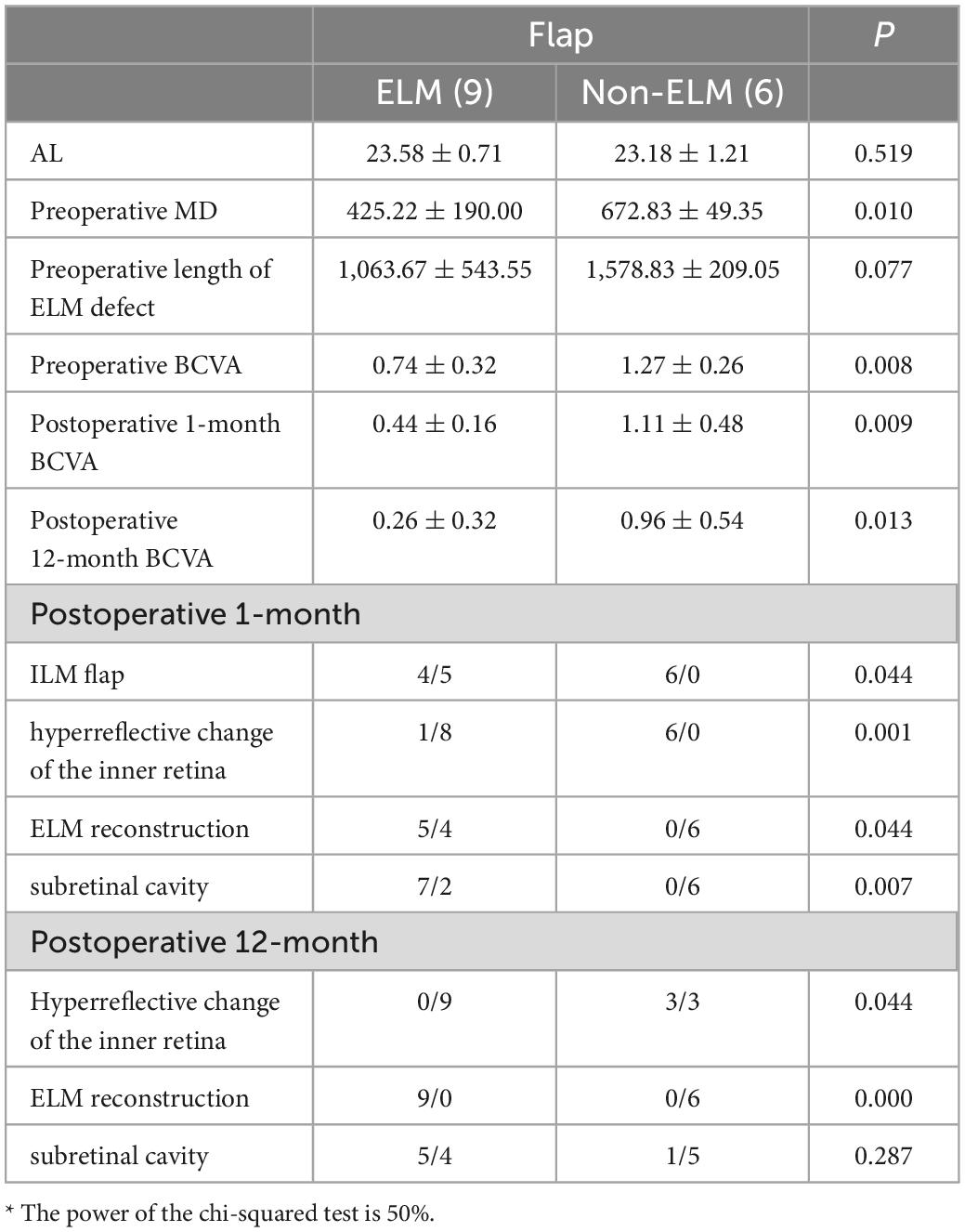
Table 5. Comparison of the preoperative and postoperative anatomical structures and visual function of the ELM and non-ELM subgroups of the flap group*.
ELM reconstruction 12 months after surgery was closely related to the preoperative MD (P = 0.009; R = −0.650), preoperative BCVA (P = 0.005, R = −0.684), presence of the ILM flap 1 month after surgery (P = 0.024, R = −0.577), and hyperreflective changes in the inner retina (P = 0.000, R = −0.873), respectively (Figure 4).
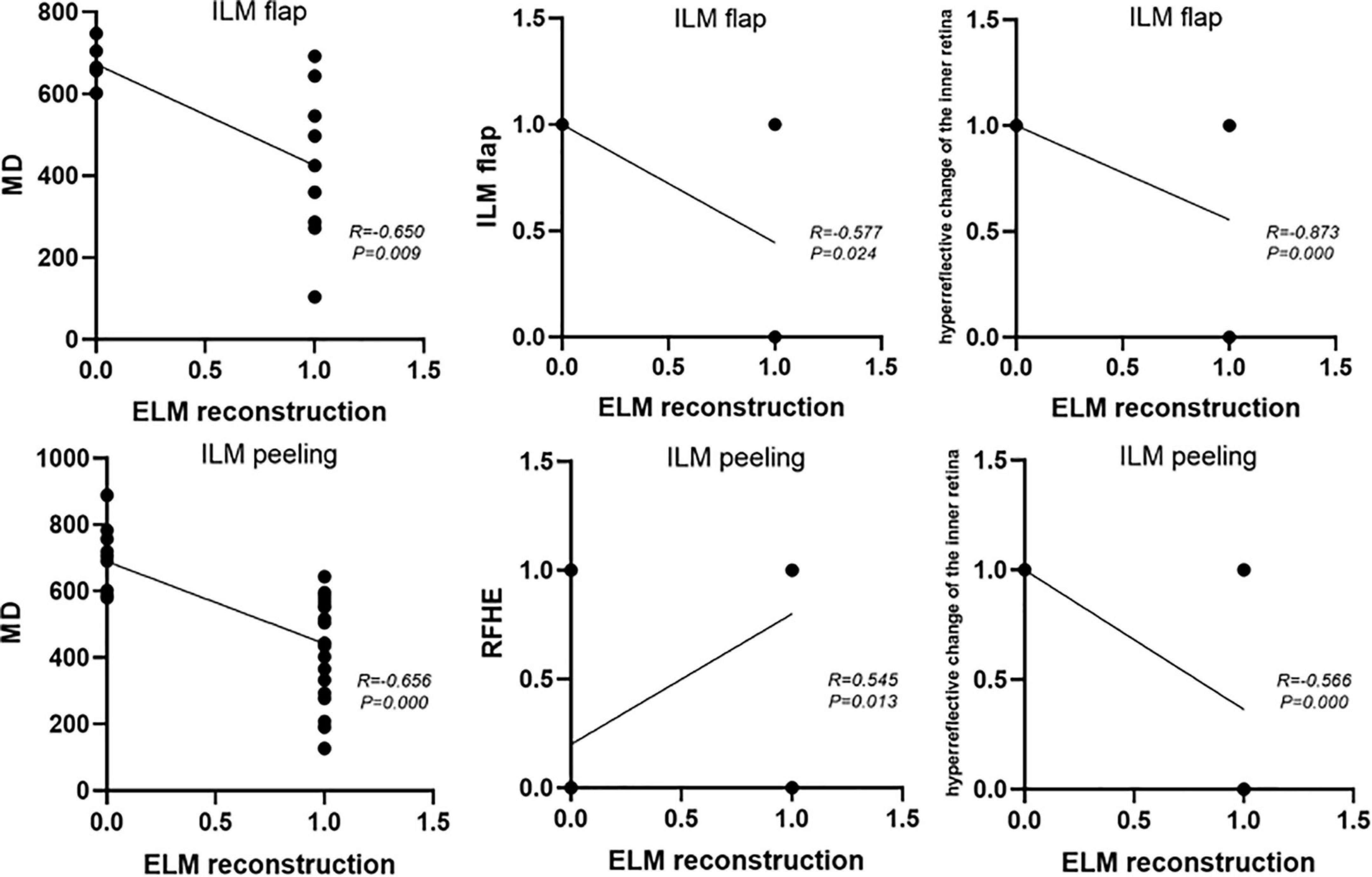
Figure 4. The factors affecting postoperative ELM reconstruction in the flap group and the peeling group.
The peeling group was divided into two according to the postoperative reconstruction of the ELM (Table 6, independent sample t-test and Shapiro - Wilk); 24 patients were included in the ELM group. There were significant differences in the preoperative MD, size of the ELM defect, and BCVA of the two groups (P = 0.000, P = 0.000, P = 0.006). iOCT showed significantly more RFHEs in the ELM group than in the non-ELM group (P = 0.031). After 1 month postoperatively, the hyperreflective changes in the inner retina in the non-ELM group were significantly more than those in the ELM group (P = 0.001), and the subretinal cavities were significantly less in the non-ELM group than in the ELM group (P = 0.001). BCVA was significantly better for the ELM group than for the non-ELM group 12 months postoperatively (P = 0.000), and the hyperreflective changes in the inner retina of the non-ELM group were significantly more than those of the ELM group (P = 0.000).
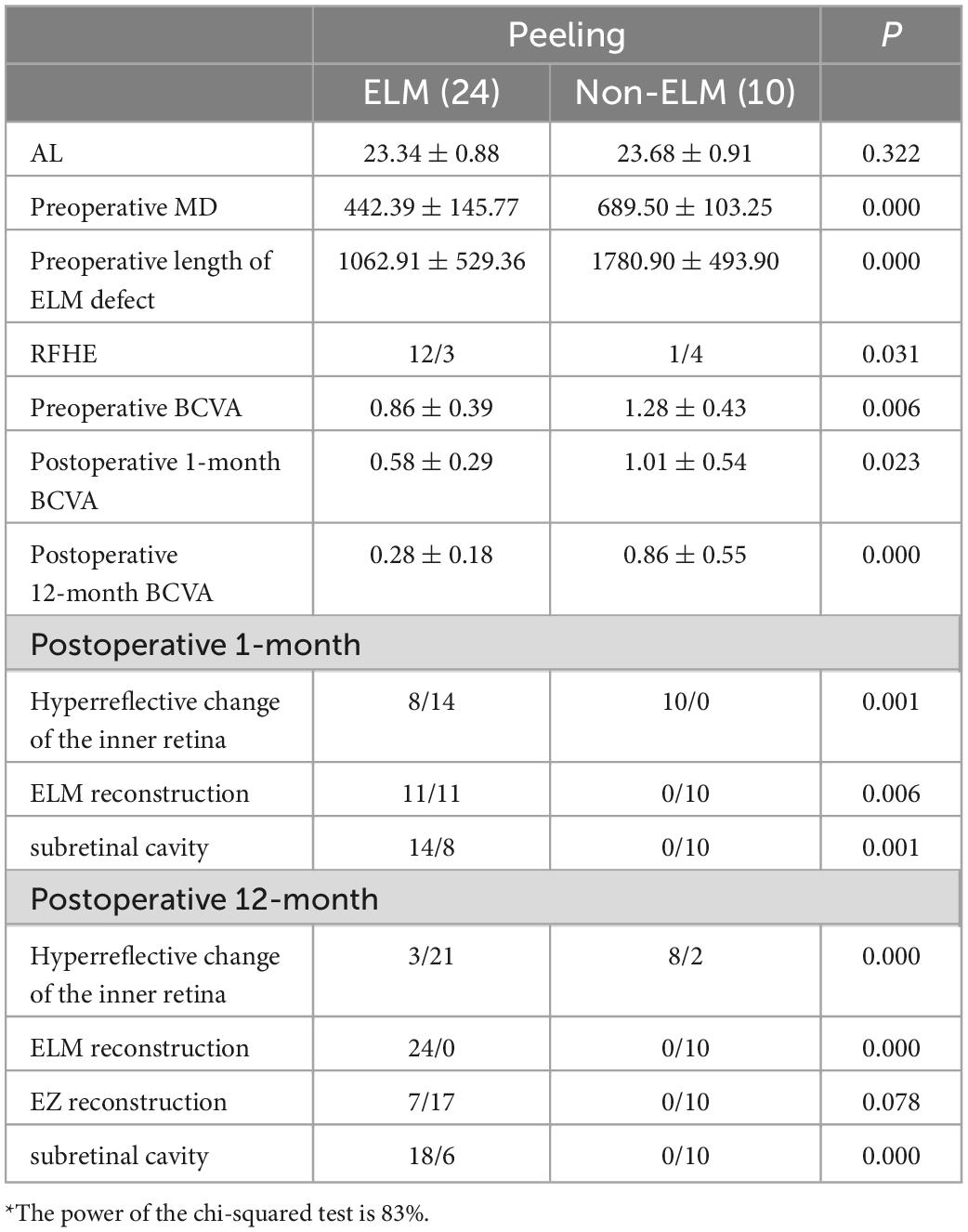
Table 6. Comparison of the preoperative and postoperative anatomical structures and visual function of the ELM and non-ELM groups in the peeling group*.
The postoperative 12-month ELM reconstruction was closely related to the preoperative MD (P = 0.000, R = –0.656), preoperative ELM defect size (P = 0.001, R = −0.548), preoperative BCVA (P = 0.010, R = −0.440), intraoperative RFHEs (P = 0.013, R = 0.545), and hyperreflective changes in the inner retina (P = 0.000, R = −0.566) (Figure 4).
In this longitudinal study, the inverted ILM flap improved the postoperative visual acuities of patients with macula holes. However, the postoperative hole closure rates, visual acuities, and ELM reconstruction rates after inverted ILM flap and ILM peeling were not different. There was no difference between the groups based on different MDs. We observed the anatomical reconstruction of the iMH in the flap and peeling groups, respectively. The patients with ILM flaps and hyperreflective changes of the inner retina 1 month postoperatively had more difficult ELM reconstruction 12 months postoperatively. In the peeling group, patients with intraoperative RFHEs and hyperreflective changes of the inner retina also had more difficult ELM reconstruction 12 months after surgery. We speculated that postoperative ELM reconstruction was related to ILM residues in iMHs.
This study showed that the flap and peeling groups could achieve 100% postoperative hole closure rates and improved postoperative visual function, but there was no difference in anatomical morphology and visual function between the flap and peeling groups with different MDs. This was contrary to previous findings (8–10). Since Michalewska reported the inverted ILM flap in 2014, researchers have generally believed that it can improve hole closure rates and visual function in patients with refractory MH (7, 17), but most previous studies involved few cases and did not assess postoperative ELM reconstruction. Recent studies have reported results similar to those of our study (10, 16, 18). Yan (16) suggested that there were no differences between the closure rates and visual acuities after the inverted ILM flap and ILM peeling for iMH. Hasegawa (19) believed that the inverted ILM flap improved the closure rate of large iMHs but not visual function compared with ILM peeling. The inverted ILM flap provides a scaffold for the proliferation and migration of Muller cells and this may account for these findings. However, the ILM flap inevitably fills the hole, and this hinders the rearrangement of the retinal structure and improvement of postoperative visual acuity (16).
The integrities of the EZ and ELM after MH surgery are important indicators. The ELM closure rates at 12 months postoperatively for the flap and peeling groups were not different. Therefore, we further divided the two groups into the ELM and non-ELM groups based on the presence or absence of postoperative ELM reconstruction to observe the differences in the postoperative anatomical reconstruction procedures and the cause of ELM reconstruction.
The Flap group was further divided into two subgroups based on the postoperative 12-month ELM reconstruction. The study showed a higher preoperative MD, more postoperative 1-month hyperreflective changes in the inner retina, and more ILM flaps in the non-ELM group than in the ELM group. The ELM reconstruction was closely related to the preoperative MD, presence of an ILM flap, and hyperreflective changes of the inner retina at 1 month postoperatively. The mechanism of macular hole closure is incompletely understood. Bringmann (20) reported that the closure of iMH was caused by the movement of Muller cells. Muller cells moved toward the fovea to form an accumulation of intermediate reflective tissue and enveloped the photoreceptor cell somata, promoting the centripetal bridging of the ELM. However, the ILM flap was still visible in the non-ELM group at 1 month after surgery in this study, suggesting the co-existence of the ILM and Muller cells in the fovea during the postoperative iMH closure. If these tissues are connected to the RPE, the collagen fibers of the ILM do not degenerate easily, and they form the postoperative hyperreflective changes of the inner retina, which can hinder the centripetal rearrangement of photoreceptors and ELM reconstruction more difficult. These findings showed that the ILM flap did not contribute to the ELM reconstruction of iMH.
The postoperative reconstruction in the peeling group was also observed. The non-ELM groups showed higher preoperative MDs, more intraoperative non-RFHEs, and more postoperative 1-month hyperreflective changes in the inner retina than the ELM group, and the ELM reconstruction was closely related to the preoperative MD, intraoperative RFHEs, and hyperreflective changes in the inner retina. This was consistent with the results of Kumar (21). Previous research reported that RF was similar to the inverted ILM flap mechanism (17); the RFs provided a bridge for the centripetal movement of Muller cells. However, Makoto (22) observed that postoperative BCVA was worse in patients with iMH and RFs than those without them. This may be related to the composition of RF. Son (23) collected some RFs after ILM peeling and found that they only contained RPE cells; however, Compera (24) and Kenawy (25) reported that RFs originated from vitreous cells and Muller cells after histological studies. In the patients in the ELM group, intraoperative RFs often appeared at the edge of the hole, and we speculated they were residual tissues after ILM peeling comprising ILM and retinal tissue. If the ILM was peeled off 360 degrees centripetally and over the MH edge during ILM peeling, the ILM at the edge of the hole would be completely peeled off; the RF residues at the edge of the hole would be the only remaining retinal tissue, which is conducive to postoperative recovery. However, this needs to be further confirmed by more accurate histological studies.
In this study, we speculated that postoperative ELM reconstruction is associated with ILM residues. The postoperative ELM closure rate may be significantly improved if ILM residue is reduced regardless of treatment with an ILM flap or ILM peeling. However, this still requires further histological studies.
This study has some limitations. First, it involved a few cases. Tables 3, 5 have smaller power because their samples are small, but the follow-up period for this study was long, and the data were relatively rare, which can provide clinical guiding significance to readers, and we look forward to the results of large samples in the future. Second, this was a retrospective study. Third, in this study, all patients in the peeling group were treated with centripetal ILM peeling, but iOCT detected RFs in 58.8% of the patients, which was similar to the 54.0–67.0% reported in a previous study. Among the patients with RFs, only 65.0% of RFs were located at the edge of the hole. Therefore, the approach to obtaining satisfactory RFs requires further discussion.
In conclusion, we found no difference between the postoperative BCVAs and rates of ELM reconstruction of the flap and peeling groups subcategorized by MDs. The inverted ILM flap treatment is not recommended for patients with iMHs, as the flap may hinder the normal postoperative reconstruction of iMHs. However, the presence of RFHEs after traditional ILM peeling is closely related to postoperative ELM reconstruction. This may suggest that complete ILM peeling is effective for treating iMHs with different MDs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
LS, JM, and YC contributed to the conception of the study. YC, YX, and XY performed the experiment. YX, JY, and CW contributed significantly to analysis and manuscript preparation. YX and CW performed the data analyses and wrote the manuscript. ZZ helped perform the analysis with constructive discussions. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes: results of a pilot study. Arch Ophthalmol. (1991) 109:654–9.
2. Park DW, Sipperley JO, Sneed SR, Dugel PU, Jacobsen J. Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology. (1999) 106:1392–7;discussion1397–8.
3. Mahalingam P, Sambhav K. Surgical outcomes of inverted internal limiting membrane flap technique for large macular hole. Indian J Ophthalmol. (2013) 61:601–3.
4. Wu TT, Kung YH. Comparison of anatomical and visual outcomes of macular hole surgery in patients with high myopia vs. non-high myopia: a case-control study using optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. (2012) 250:327–31. doi: 10.1007/s00417-011-1821-7
5. Marques RE, Sousa DC, Leal I, Faria MY, Marques-Neves C. Complete ILM peeling versus inverted flap technique for macular hole surgery: a meta-analysis. Ophthal Surg Lasers Imaging Retina. (2020) 51:187–A2. doi: 10.3928/23258160-20200228-08
6. Chatziralli I, Machairoudia G, Kazantzis D, Theodossiadis G, Theodossiadis P. Inverted internal limiting membrane flap technique for myopic macular hole: a meta-analysis. Surv Ophthalmol. (2021) 66:771–80.
7. Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. (2010) 117:2018–25.
8. Shanmugam MP, Ramanjulu R, Kumar M, Rodrigues G, Reddy S, Mishra D. Inverted ILM peeling for idiopathic and other etiology macular holes. Indian J Ophthalmol. (2014) 62:898–9. doi: 10.4103/0301-4738.141077
9. Yamashita T, Sakamoto T, Terasaki H, Iwasaki M, Ogushi Y, Okamoto F, et al. Best surgical technique and outcomes for large macular holes: retrospective multicentre study in Japan. Acta Ophthalmol. (2018) 96:e904–10.
10. Narayanan R, Singh SR, Taylor S, Berrocal MH, Chhablani J, Tyagi M, et al. Surgical outcomes after inverted internal limiting membrane flap versus conventional peeling for very large macular holes. Retina. (2019) 39:1465–9.
11. Rizzo S, Tartaro R, Barca F, Caporossi T, Bacherini D, Giansanti F. Internal limiting membrane peeling versus inverted flap technique for treatment of full-thickness macular holes: a comparative study in a large series of patients. Retina. (2018) 38(Suppl. 1):S73–8. doi: 10.1097/IAE.0000000000001985
12. Gu C, Qiu Q. Inverted internal limiting membrane flap technique for large macular holes: a systematic review and single-arm meta-analysis. Graefes Arch Clin Exp Ophthalmol. (2018) 256:1041–9. doi: 10.1007/s00417-018-3956-2
13. Shen Y, Lin X, Zhang L, Wu M. Comparative efficacy evaluation of inverted internal limiting membrane flap technique and internal limiting membrane peeling in large macular holes: a systematic review and meta-analysis. BMC Ophthalmol. (2020) 20:14. doi: 10.1186/s12886-019-1271-2
14. Ra H, Lee WK. Efficacy of the inverted internal limiting membrane flap technique with perfluorocarbon liquid-mediated selective staining for large macular hole repair. Curr Eye Res. (2019) 44:53–9. doi: 10.1080/02713683.2018.1524014
15. Yu Y, Liang X, Wang Z, Wang J, Liu X, Chen J, et al. Internal limiting membrane peeling and air tamponade for stage III and stage IV idiopathic macular hole. Retina. (2020) 40:66–74.
16. Yan Y, Zhao T, Sun C, Zhao H, Jia X, Wang Z. Anatomical and functional outcomes in eyes with idiopathic macular holes that underwent surgery using the inverted internal limiting membrane (ILM) flap technique versus the conventional ILM peeling technique. Adv Ther. (2021) 38:1931–45.
17. Tao J, Chen H, Zhu L, Pan D, Fang J, Chen Y, et al. Macular hole edge morphology predicts restoration of postoperative retinal microstructure and functional outcome. BMC Ophthalmol. (2020) 20:280. doi: 10.1186/s12886-020-01541-7
18. Iwasaki M, Kinoshita T, Miyamoto H, Imaizumi H. Influence of inverted internal limiting membrane flap technique on the outer retinal layer structures after a large macular hole surgery. Retina. (2019) 39:1470–7.
19. Hasegawa Y, Hata Y, Mochizuki Y, Arita R, Kawahara S, Kita T, et al. Equivalent tamponade by room air as compared with SF(6) after macular hole surgery. Graefes Arch Clin Exp Ophthalmol. (2009) 247:1455–9. doi: 10.1007/s00417-009-1120-8
20. Bringmann A, Jochmann C, Unterlauft JD, Wiedemann R, Rehak M, Wiedemann P. Different modes of foveal regeneration after closure of full-thic kness macular holes by (re)vitrectomy and autologous platelet concentrate. Int J Ophthalmol. (2020) 13:36–48. doi: 10.18240/ijo.2020.01.06
21. Kumar V, Yadav B. HOLE-DOOR SIGN: a novel intraoperative optical coherence tomography feature predicting macular hole closure. Retina. (2018) 38:2045–50. doi: 10.1097/IAE.0000000000001791
22. Inoue M, Itoh Y, Koto T, Kurimori HY, Hirakata A. Intraoperative OCT findings may predict postoperative visual outcome in eyes with idiopathic macular hole. Ophthalmol Retina. (2019) 3:962–70.
23. Son G, Lee JS, Lee S, Sohn J. Epiretinal proliferation associated with macular hole and intraoperative perifoveal crown phenomenon. Korean J Ophthalmol. (2016) 30:399–409. doi: 10.3341/kjo.2016.30.6.399
24. Compera D, Entchev E, Haritoglou C, Scheler R, Mayer WJ, Wolf A, et al. Lamellar hole-associated epiretinal proliferation in comparison to epiretinal membranes of macular pseudoholes. Am J Ophthalmol. (2015) 160:373–84.e1. doi: 10.1016/j.ajo.2015.05.010
Keywords: idiopathic macular hole, internal limiting membrane, the inverted ILM flap treatment, visual function, optical coherence tomography
Citation: Chen Y, Xu Y, Ye X, Yu J, Wang C, Zhang Z, Mao J and Shen L (2023) The effect comparison of ILM flap and traditional ILM peeling in iMH. Front. Med. 10:1103593. doi: 10.3389/fmed.2023.1103593
Received: 20 November 2022; Accepted: 16 January 2023;
Published: 09 February 2023.
Edited by:
Georgios Panos, Nottingham University Hospitals NHS Trust, United KingdomReviewed by:
Youxin Chen, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2023 Chen, Xu, Ye, Yu, Wang, Zhang, Mao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianbo Mao,  cm9ja2V0MjIyQHNpbmEuY29t; Lijun Shen,
cm9ja2V0MjIyQHNpbmEuY29t; Lijun Shen,  bGpzaGVueXNnQDE2My5jb20=
bGpzaGVueXNnQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.