
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 04 April 2023
Sec. Nephrology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1098871
This article is part of the Research Topic Renal Iron Handling In Health and Disease, Volume II View all 4 articles
 Yu-Wei Fang1,2
Yu-Wei Fang1,2 Jing-Tong Wang1
Jing-Tong Wang1 Tzu Yun Lin3
Tzu Yun Lin3 Chung-Jen Lee4
Chung-Jen Lee4 Tsrang-Neng Jang2,5
Tsrang-Neng Jang2,5 Ming-Hsien Tsai1,2*
Ming-Hsien Tsai1,2* Hung-Hsiang Liou3*
Hung-Hsiang Liou3*Introduction: A negative association between C-terminal fibroblast growth factor 23 (cFGF23) and hemoglobin (Hb) levels has been reported in patients with predialysis chronic kidney disease. In dialysis patients, the dominant form of serum FGF23 is intact FGF23 (iFGF23); however, its association with the Hb level remains unclear. Therefore, simultaneously monitoring iFGF23 and cFGF23 levels is crucial. In this study, we investigated the associations between both forms of FGF23 (iFGF23 and cFGF23) and renal anemia in chronic hemodialysis (CHD) patients.
Methods: We included 166 CHD patients from two hospitals in this cross-sectional, observational study. The primary predictors were serum iFGF23, cFGF23, and iFGF23/cFGF23 levels. The main outcome was the Hb level.
Results: Among the CHD patients included, 60.8% were men with a mean age of 59.4 ± 12.7 years. In the crude analysis, iFGF23 and iFGF23/cFGF23 levels showed a significant negative association (−0.27, p = 0.004 and −0.22, p = 0.034, respectively) with the Hb level. Even after adjusting for multiple variables (a parsimonious model), every increment of natural log transformation by 1 for (ln)iFGF23 and ln(iFGF23/cFGF23) levels showed a negative correlation with the Hb level (estimate: −0.27 [95%CI: −0.44, −0.10, p = 0.001]; −0.19 [95%CI: −0.37, −0.01, p = 0.042], respectively), whereas both were positively associated with erythropoietin-stimulating agent (ESA) hyporesponsiveness (odds ratio [OR]: [95%CI: 2.30, 1.26–4.17], p = 0.006; 1.95 [95%CI: 1.08–3.50], p = 0.025). Moreover, these abovementioned associations were more dominant in patients with diabetes who used angiotensin receptor blockers.
Discussion: In conclusion, a negative association between serum iFGF23 or iFGF23/cFGF23 level and the Hb level was observed in our CHD patients. Meanwhile, a higher iFGF23 or iFGF23/cFGF23 level may predispose patients to ESA hyporesponsiveness.
According to the data from the National Health and Nutrition Examination Survey, the prevalence of anemia can vary from 8.4 to 53.4% at different stages of chronic kidney disease (CKD) (1, 2). The causes of renal anemia are multifactorial and include decreased erythropoietin production, iron deficiency, malnutrition–inflammation status, 1,25-dihydroxyvitamin D (1,25(OH)2D) deficiency, and uremic toxin accumulation (3, 4). In clinical practice, renal anemia is usually accompanied by an impaired quality of life (5), a higher prevalence of cardiovascular disease (CVD) (6), congestive heart failure (7), and increased hospitalization and mortality rates (8).
The burgeoning link between CKD mineral bone disease and renal anemia has attracted attention in recent years. Fibroblast growth factor 23 (FGF23), an endocrine FGF subfamily protein, can increase urinary phosphate excretion and decrease intestinal phosphate absorption to counteract high phosphorus load in CKD patients (9, 10). Serum FGF23 can be found either in its biologically active form [intact FGF23 (iFGF23)] or its inactive fragment form [C-terminal FGF23 (cFGF23)].
Beyond its role in mineral homeostasis, FGF23 was demonstrated to impede prenatal and postnatal erythropoiesis in an animal experiment (11). In our previous study that focused on 53 incident stage 3–4 CKD patients, we first demonstrated that values higher than the median cFGF23 value were negatively correlated with hemoglobin (Hb) levels (12). Thereafter, several studies confirmed our findings either in mice or in non-dialysis CKD patients (13–18). In all of these studies, only cFGF23 was adopted. However, Shimada et al. demonstrated that the serum FGF23 in dialysis patients was mostly iFGF23 (19). This finding implies that increased production and impaired cleavage of FGF23 in CKD patients can lead to distorted proportions of active and inactive FGF23 (20). Impairments such as FGF23 cleavage and distorted FGF23 ratio have been reported to exaggerate along with the progression of CKD (19, 21). In fact, the iFGF23/cFGF23 ratio was higher in CKD patients than in the healthy population (20). Usui et al. reported that erythropoietin-stimulating agent (ESA) hyporesponsiveness was also associated with either the highest or lowest iFGF23 quintile in Japanese CHD patients (22).
Because the cleavage of FGF23 in chronic hemodialysis (CHD) alters the serum levels of iFGF23 and cFGF23 and the ratio of iFGF23/cFGF23, their relationship with serum Hb levels remains unclear. In this study, we examined the relationship between both forms of FGF23 (iFGF23 and cFGF23) and Hb levels in CHD patients.
A total of 466 patients who had undergone HD for more than 3 months at Hsin-Jen Hospital and Shin-Kong Wu Ho-Su Memorial Hospital and were aged between ≥20 years and <85 years were screened for this cross-sectional, observational study. Patients with a poor nutritional status (body mass index [BMI] <17 kg/m2), kidney transplant history, acute hepatitis, autoimmune diseases, malignancy, myocardial infarction, or stroke within 6 months before enrollment, or hospitalization due to infection within 3 months before enrollment were excluded from the study. This study was performed as per the ethical principles of the Declaration of Helsinki and was approved by the ethics committee of Shin-Kong Wu Ho-Su Memorial Hospital (No. 20160802R). All patients gave their informed consent to participate in the study.
At the time of enrollment, the following data were recorded: age; gender; dialysis vintage; body mass index; history of diabetes (DM), hypertension, and coronary artery disease; and usage of angiotensin receptor blockers (ARBs), beta-blockers, calcium channel blockers, lipid-lowering agents, and ESA.
Fasting blood samples were collected and centrifuged at 3,000× g for 10 min, after which the supernatant serum was stored in a refrigerator at −20°C prior to analyses. An autoanalyzer (Beckman AU) was used for the analysis of parameters such as the white blood cell count (103/uL), glucose (mg/dL), Hb (g/dL), blood urea nitrogen (mg/dL), creatinine (mg/dL), sodium (mEq/L), potassium (mEq/L), calcium (mg/dL), phosphate (mg/dL), alkaline phosphate (Alk-P, IU/L), albumin (g/dL), uric acid (mg/dL), total cholesterol (mg/dL), triglyceride (mg/dL), iron (μg/dL), ferritin (ng/mL), and iron saturation (iron/iron-binding capacity, %). High-sensitivity C-reactive protein levels (hs-CRP, mg/dL) were measured using the latex-enhanced immunoturbidimetric method (Simens ADVIA Chemistry Systems). Intact parathyroid hormone (iPTH, pg/mL) levels were measured using the Roche Elecsys assay (Roche Diagnostics, www.roche.com). FGF23 was measured using a two-site enzyme-linked immunosorbent assay that detects two epitopes in the carboxyl-terminal portion of FGF23 (cFGF23) and also detects epitopes within the amino-terminal and carboxyl-terminal portions of FGF-23 (iFGF23) (Quidel, www.quidel.com). 1,25(OH)2D [IDS, www.idspic.com], sclerostin (Biomedica, www.bmgrp.com), Dickkopf-1 (Biomedica, www.bmgrp.com), and α-Klotho (IBL America, www.ibl-america.com) levels were measured using a chemiluminescent immunoassay. The dialysis quality (Kt/V) was measured using the Daugirdas formula (23), and the cardiothoracic ratio was calculated by dividing the transverse diameter of the heart by the maximum inner width of the thoracic cavity using chest radiography.
The ESA prescribed for our CHD patients was either epoetin beta or darbepoetin alfa. However, the dose we administered was based on a protocol provided by the Bureau of National Health Insurance, according to which the ESA dose was escalated and had an upper limit. Thereafter, we defined the ESA dose in our study using the mean dosage prescribed 6 months before the recruitment. To standardize the ESA dose, we converted darbepoetin alfa to epoetin beta by adopting a ratio of 1 ug:200 IU. In our CHD patients, mean Hb levels of <10 g/dL and mean-standardized ESA doses of >6,000 u/week were regarded as ESA hyporesponsiveness, a definition we also adopted in other studies (22, 24).
Clinical parameters are expressed as the mean ± standard deviation or median (25th, 75th centile; IQR) for continuous variables and frequencies with proportions for categorical variables. Pearson's correlation coefficient was used to examine correlations between variables with normally distributed data, whereas Spearman's rank correlation coefficient was used for variables whose data distributions deviated from the normal distribution. Wilcoxon signed-rank test or t-test was used to compare the means of continuous variables according to whether the data distributions were normal or not, and test was used to compare categorical variables between the groups. Some factors were naturally log-transformed (ln) to approximate a normal distribution.
A simple linear regression model was used to determine the risks associated with Hb levels. In the parsimonious model, the variables were chosen via the stepwise model selection method when p < 0.15 of the F-statistic for entry and p > 0.05 for removal. A separate regression analysis was used to model the changes in Hb levels as a function of ln(iFGF23) and ln(iFGF23/cFGF23) using a modified stepwise procedure with four modeling steps. In addition, logistic regression was used to model ESA hyporesponsiveness as a function of ln(iFGF23) and ln(iFGF23/cFGF23). A two-sided p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS for Windows version 9.4 (SAS Institute Inc., Cary, NC, USA).
Among the CHD patients screened, only 166 patients with a mean age of 59.4 ± 12.7 years were recruited. The median dialysis vintage of these patients was 6.1 years [interquartile range (IQR): 2.8–11.7; 61% (n = 101) were men, 49% (n = 81) were diabetic, and 55% (n = 91) had a history of coronary artery disease]. With a mean Hb level of 10.3 ± 1.2 g/dL, 40.3% (n = 67) of the patients had Hb levels of <10 g/dL (Table 1). A high missing rate (15.6%) of the hs-CRP data was noted. Serum iFGF23, cFGF23, and iFGF23/cFGF23 levels were measured, and the median levels were 700 pg/mL (IQR: 459–1,180) for iFGF23, 1124 RU/mL (IQR: 835–1,966) for cFGF23, and 0.52 (IQR: 0.32–0.93) for iFGF23/cFGF23. In addition, the distributions of iFGF23, cFGF23, and iFGF23/cFGF23 were all right-skewed (Figures 1A–C), and there was no significant correlation between iFGF23 and cFGF23 (Figure 1D). Moreover, Hb levels were negatively associated with ln(iFGF23), ln(iFGF23/cFGF23), or ln(iFGF23/α-klotho). These findings are illustrated in Figure 2.
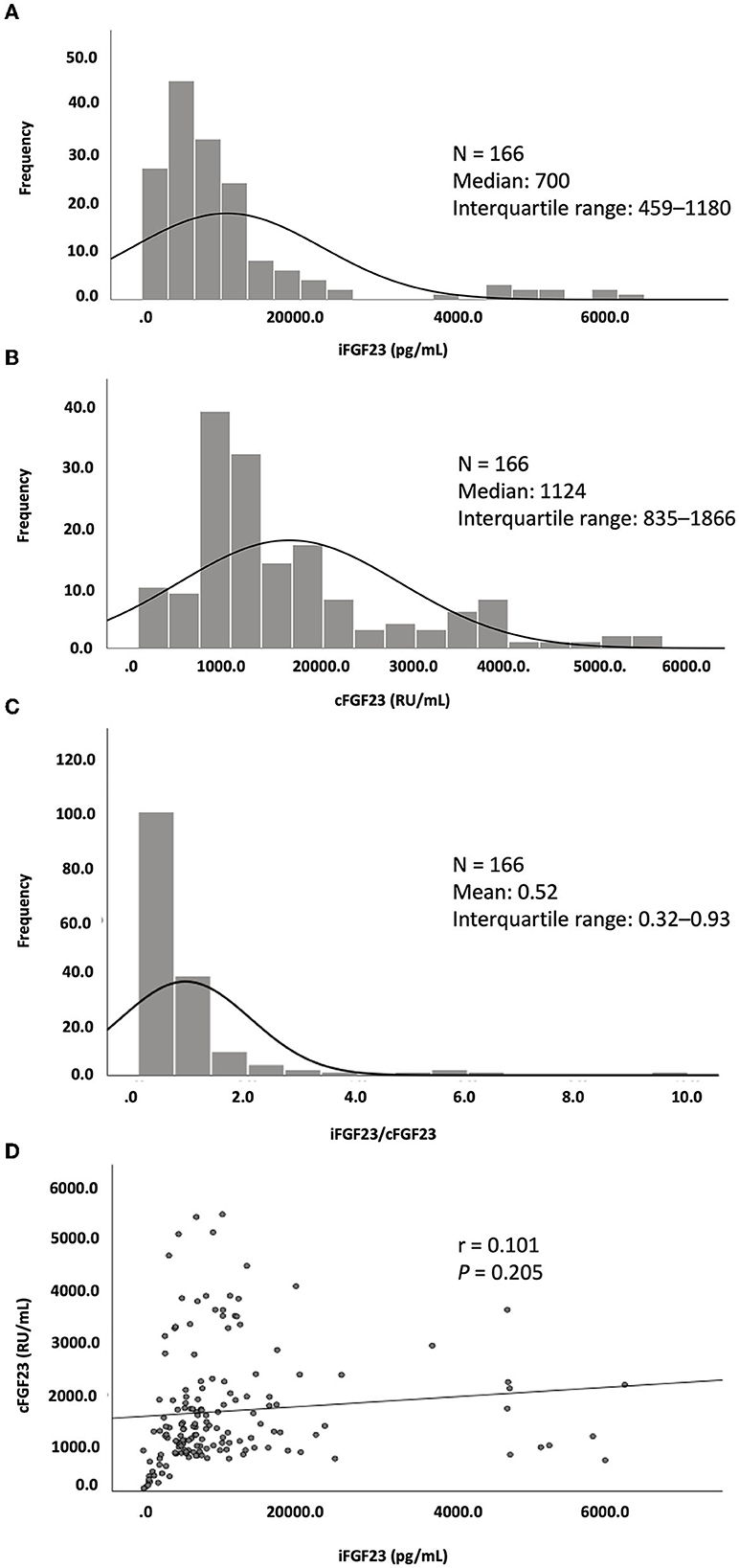
Figure 1. Distribution of (A) iFGF23, (B) cFGF23, and (C) iFGF23/cFGF23 and (D) the correlation between iFGF23 and cFGF23.
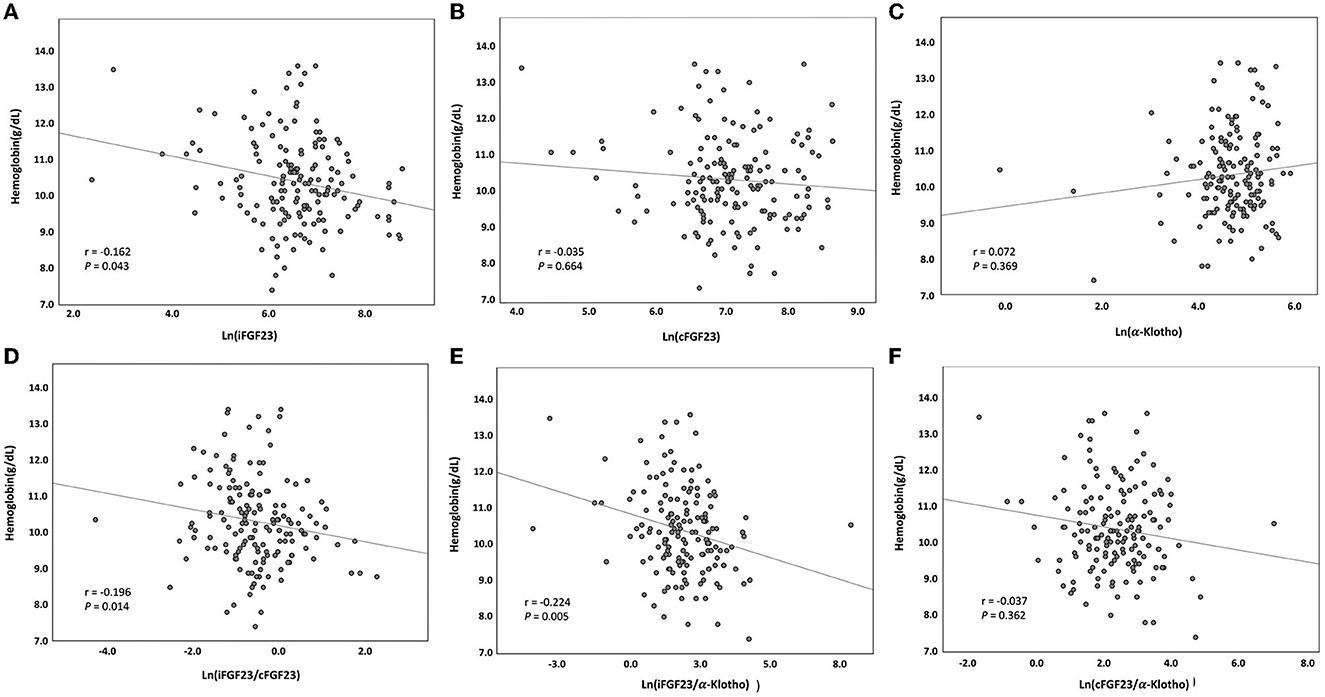
Figure 2. Serum hemoglobin level vs. serum (A) ln(iFGF23), (B) ln(cFGF23), (C) ln(α-klotho), (D) ln(iFGF23/cFGF23), (E) ln(iFGF23/α-klotho), and (F) ln(cFGF23/α-klotho) levels. The lines indicate best-fit regression lines derived using the least mean square method.
When stratified by Hb level, in the population of patients with Hb <10 g/dL, there were significantly more diabetic people (58.2% [n = 39] vs. 42.4% [n = 42], p = 0.045), higher iFGF23/α-klotho ratios [7.7 (IQR: 4.2–21.4) vs. 6.0 (IQR: 3.6–11.7), p = 0.020], higher hs-CRP levels [0.44 mg/dL (IQR: 0.1–1.07) vs. 0.17 (IQR: 0.05–0.41), p = 0.005], higher mean-standardized ESA doses [5,667 u/wk (IQR: 5,000–6,667) vs. 4,000 u/wk (IQR: 2,833–5,000), p < 0.001], lower serum Cr levels (9.2 ± 1.8 mg/dL vs. 10.1 ± 2.1 mg/dL, p = 0.011), and lower serum K levels (4.6 ± 0.6 mg/dL vs. 4.8 ± 0.6 mg/dL, p = 0.022). No significant differences in serum albumin, iFGF23, cFGF23, Alk-P, iPTH, 1,25(OH)2D, iron, ferritin, or transferrin saturation were observed (Table 1).
When we applied linear regression to analyze the risk factors for Hb level, the crude analysis results revealed that diabetes [−0.38; 95% confidence interval (CI): −0.74, −0.01, p = 0.043], ln(iFGF23) (−0.27; 95%CI: −0.45, −0.84, p = 0.004), ln(iFGF23/cFGF23) (−0.22; 95% CI: −0.43, −0.02, p = 0.034), ln(iFGF23/α-klotho) (−0.24; 95% CI: −0.39, −0.09, p = 0.001), creatinine (0.11; 95% CI: 0.02, 0.20, p = 0.019), sodium (0.06; 95% CI: <0.001, 0.13 p = 0.048), albumin (0.87; 95%CI: 0.28, 1.46, p = 0.004), ln(alk-P) (0.46; 95% CI: 0.09, 0.83 p = 0.014), ln(ferritin) (−0.32; 95% CI: −0.49, −0.15, p < 0.001), and the use of ARBs (−0.40; 95% CI: −0.77, −0.02, p = 0.037) were significant determinants in our CHD patient cohort.
After multivariable adjustment using the forward stepwise model selection method, among the 39 parameters listed in Table 2, only eight parameters were identified as the main determinants (a parsimonious model). These parameters were age (0.15; 95% CI: 0.01, 0.29 every 10 years), ln(iFGF23) (−0.27; 95% CI: −0.44, −0.10), sodium (0.07; 95% CI: 0.02, 0.13), phosphate (0.15; 95% CI: 0.01, 0.31), albumin (0.88; 95% CI: 0.30, 1.46), Alk-P (0.63; 95% CI: 0.30, 0.95), ln(ferritin) (−0.42; 95% CI: −0.58, −0.26), and use of ARBs (−0.49; 95% CI: −0.82, −0.16).
After adjusting for demographic data, comorbidities, dialysis-related parameters, and bone markers using stepwise multivariable adjusting models, increments of not only ln(iFGF23) (−0.27, 95% CI, −0.44, −0.10) but also ln(iFGF23/cFGF23) (−0.19, 95% CI, −0.37, −0.01) were negatively associated with Hb levels, and these associations remained significant in crude analyses and all adjustment models (Table 3).
As shown in Figure 3, after multivariable adjustments, discrete associations between high ln(iFGF23) and low Hb levels were noted in women aged ≤65 years who had a history of hypertension, CVD, or ARB usage and whose serum Cr level was ≤9 mg/dL, sodium >135 mEq/L, potassium ≤4.5 mEq/L, phosphate ≤5 mEq/L, ferritin ≤400 ng/mL, or alk-P >70 U/L (all p-values <0.05).
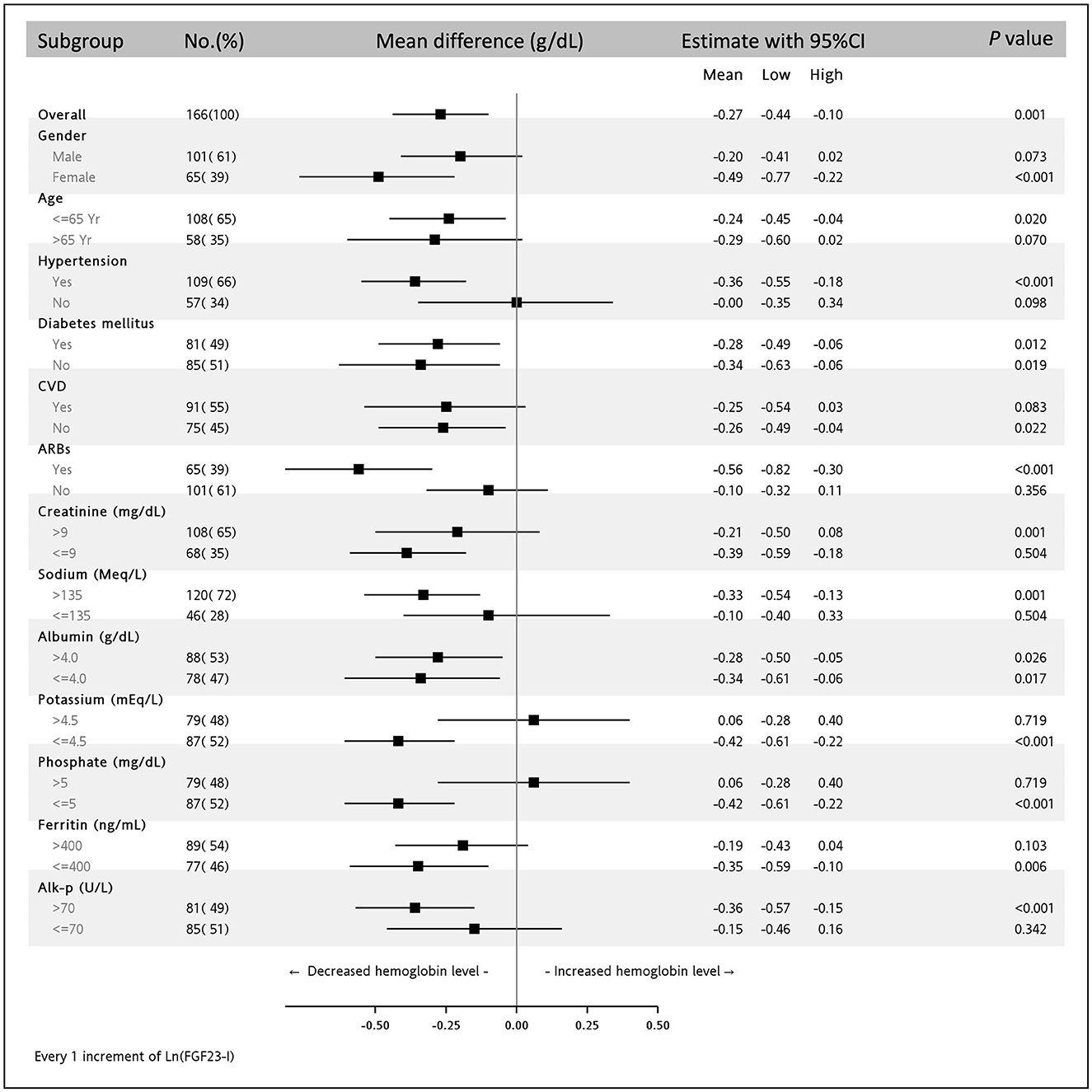
Figure 3. Subgroup analysis of the association between ln(iFGF23) and serum hemoglobin level in the multivariable adjusting model. The parsimonious adjusting model is Model 3 in Table 3.
A further subgroup analysis of DM and ARBs use is shown in Table 4. It shows that only the subgroup of CHD patients with DM and ARB use retained a significant negative association between ln(iFGF23) or ln(iFGF23/cFGF23) and H, in which the estimates of ln(iFGF23) and ln(iFGF23/cFGF23) were −0.45 (−0.78, −0.12) and −0.29 (−0.58, −0.01), respectively.
Table 5 shows that 13.9% of enrolled patients (23/166) had ESA hyporesponsiveness. Moreover, in the stepwise regression models, both ln(iFGF23) and ln(iFGF23/cFGF23) were positively associated with ESA hyporesponsiveness in the parsimonious model (odds ratio [OR]: 2.30, 95%CI, 1.26–4.17; 1.95, 95%CI, 1.08–3.50, respectively).
In this study, negative associations of serum iFGF23 level and iFGF23/cFGF23 ratio with Hb levels were found in CHD patients. Furthermore, serum iFGF23 level and iFGF23/cFGF23 ratios were positively associated with ESA hyporesponsiveness. These findings were observed in patients with predialysis CKD as well as those who had undergone HD. According to our subgroup analysis, women aged ≤65 years who used ARBs and had a higher serum iFGF23 level or a lower serum ferritin level were independently correlated with lower Hb levels. This negative association between serum iFGF23 or iFGF23/cFGF23 and Hb levels was more dominant in our CHD patients who were diabetics and used ARBs.
Iron deficiency has been recognized as one of the causes of anemia (25). In fact, iron deficiency is associated with higher FGF23 levels in women with a history of heavy uterine bleeding and in patients undergoing HD (26, 27). In HD patients, high FGF23 levels can be lowered by supplementing intravenous iron or iron-containing phosphate binders (27–29). However, after adjusting for the iron status of our CHD patients, iFGF23 levels and iFGF23/cFGF23 ratios were still negatively correlated with Hb levels. In addition to iron, FGF23 itself has been demonstrated to induce anemia by intervening in erythropoiesis (11, 14, 30, 31). Coe et al. reported that both erythroid progenitor cell counts in the bone marrow and serum erythropoietin levels were higher in mice in which FGF23 was genetically inactivated compared with wide-type mice (11). Conversely, high FGF23 and low Hb levels, which were noted in five out of six nephrectomized mice, could be rescued by applying an FGF23-blocking peptide (14). Several studies have demonstrated an inverse correlation between FGF23 and erythropoiesis in clinical settings (29, 32–35). Recently, Usui et al. reported that Japanese CHD patients who had either the highest or the lowest iFGF23 quintile showed hyporesponsiveness to ESA (22). In their study, CHD patients in the lowest quintile had a poorer nutritional status than other participants. This could be the reason why we observed ESA hyporesponsiveness only in patients with higher FGF23 levels in our cohort since malnourished patients were excluded from our study.
Inflammation status in CHD patients has been shown to be another risk factor for anemia (25). Moreover, chronic inflammation in CKD patients has been reported to enhance the overproduction of iFGF23 (36). However, after adjusting for inflammatory markers, iFGF23 levels and iFGF23/cFGF23 ratios were all negatively correlated with Hb levels in our CHD patients.
Alon et al. demonstrated that 1,25(OH)2D could enhance the proliferative response of erythroid-origin stem cells to erythropoietin as well as increase erythropoietin receptor expression (37). In clinical practice, 1,25(OH)2D deficiency is regarded as an independent risk factor for renal anemia in CKD (38). However, after adjusting for serum 1,25(OH)2D in our CHD patients whose serum 1,25(OH)2D levels were relatively lower, iFGF23 or iFGF23/cFGF23 and Hb levels remained negatively correlated.
Inhibition of the RAA system, which could increase the renal plasma flow, led to reduced erythropoietin production in spontaneously hypertensive rats (39). Angiotensin II, through activation of the angiotensin II type 1 receptor, can also induce erythroid progenitor cell proliferation, whereas ARBs can antagonize such effects (40). In a retrospective study, type 2 diabetic CKD patients who were treated with ARBs had lower Hb levels (41). Our data also confirmed this finding (but in CHD patients). Moreover, hyporesponsiveness to erythropoietin-stimulating agents was demonstrated in chronic dialysis patients who used ARBs (42). According to our subgroup analysis, the negative correlation between iFGF23 and iFGf23/cFGF23 and renal anemia is presented only in diabetic CHD patients who used ARBs. Nevertheless, the mechanism underlying this finding is yet to be explored (43).
Blood-circulating FGF23 can be found either in its biologically active intact form or as inactive C-terminal fragments (19, 44). Owing to the heterogeneity of study designs caused due to the adoption of different FGF23 assays, the interpretation of FGF23 levels in clinical settings is controversial (19). In predialysis CKD patients, a negative correlation was delineated only between serum cFGF23 and Hb levels (12, 13, 15). However, the impaired cleavage of FGF23 in CKD patients makes serum iFGF23 and cFGF23 levels and the iFGF23/cFGF23 ratio distinct from those in normal subjects (19, 21, 22). In fact, the major form of serum FGF23 in peritoneal dialysis patients was found to be iFGF23 by Shimada et al. (19). Moreover, the iFGF23/cFGF23 ratio in CKD patients was found to be higher than that in the normal population (20). During the dialysis sessions, the smaller molecular weight of cFGF23 rendered it more dialyzable than iFGF23. In addition, iFGF23 remained biologically active even after dialysis (19). In the J-DOPPS cohort, Usui et al. demonstrated that iFGF23 had a negative impact on ESA resistance in CHD patients, although they did not assess cFGF23 in their study (30). In the present study, we analyzed iFGF23 and cFGF23 levels and the iFGF23/cFGF23 ratio and found that only the iFGF23 level and the iFGF23/cFGF23 ratio were correlated with the severity of anemia in CHD patients. Meanwhile, we also detected that this significant association of the iFGF23/cFGF23 ratio with the Hb level was mostly affected by the iFGF23 level.
In our CHD patients, the correlations between ln(iFGF23) and ln(iFGF23/cFGF23) and Hb were statistically significant but not so powerful (r = −0.162, p = 0.043; r = −0.196, p = 0.014). In addition, according to the subgroup analysis, this association was more predominant in diabetic CHD patients who used ARBs. These findings indicate that beyond FGF23, there could be some coexisting factors that are involved in renal anemia, and these factors are to be explored in greater detail.
The present study has several limitations. First, it was a cross-sectional study; thus, a causal relationship between iFGF23 and anemia in CHD patients could not be inferred. Second, the number of CHD patients included in this study was relatively small. Nevertheless, a significant correlation between the iFGF23 level and iFGF23/cFGF23 ratio and Hb level was observed. To avoid over-adjusting, we applied a stepwise model selection method to reduce the dimensionality in the multivariable model. Third, even if we avoided administering excessive and limited doses of ESAs by administering the mean-standardized ESA dose, it is still difficult to exclude the bias that may have been introduced. However, we still found that iFGF23 exerted a negative impact on ESA hyporesponsiveness in our CHD patients. Finally, although the patients were enrolled from two different hospitals, our data are not representative of CHD patients nationwide. Therefore, to verify our findings, multiple, large-scale multicenter studies need to be conducted. Finally, no healthy subjects or predialysis CKD patients were included as a control group in this study.
In the present study, we extend the role of FGF23 in anemia from predialysis CKD patients to CHD patients. Instead of cFGF23 in predialysis CKD patients, the iFGF23 level and the iFGF23/cFGF23 ratio were negatively associated with the Hb levels of CHD patients. Further studies are required to confirm our findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Shin-Kong Wu Ho-Su Memorial Hospital Ethics Committee (No. 20160802R). The patients/participants provided their written informed consent to participate in this study.
Y-WF, M-HT, and H-HL: conceptualization. C-JL and M-HT: formal analysis. Y-WF: funding acquisition. Y-WF, J-TW, and TL: investigation. M-HT and H-HL: methodology and writing—reviewing and editing. J-TW, TL, and T-NJ: project administration. T-NJ, Y-WF, M-HT, and H-HL: resources. C-JL: validation. Y-WF and M-HT: writing—original draft. All authors contributed equally to this study and approved the manuscript.
This study was sponsored by the Shin-Kong Wu Ho-Su Memorial Hospital (2019SKHADR006) and the National Science and Technology Council (105-2628-B-341-001-MY3).
The authors would like to thank the hemodialysis patients at Hsin-Jen Hospital and Shin-Kong Wu Ho-Su Memorial Hospital who provided their samples for our study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS ONE. (2014) 9:e84943. doi: 10.1371/journal.pone.0084943
2. Inker LA, Grams ME, Levey AS, Coresh J, Cirillo M, Collins JF, et al. Relationship of estimated gfr and albuminuria to concurrent laboratory abnormalities: an individual participant data meta-analysis in a global consortium. Am J Kidney Dis. (2019) 73:206–17. doi: 10.1053/j.ajkd.2018.08.013
3. Portoles J, Martin L, Broseta JJ, Cases A. Anemia in chronic kidney disease: from pathophysiology and current treatments, to future agents. Front Med (Lausanne). (2021) 8:642296. doi: 10.3389/fmed.2021.642296
4. Fishbane S, Spinowitz B. Update on anemia in ESRD and earlier stages of CKD: core curriculum 2018. Am J Kidney Dis. (2018) 71:423–35. doi: 10.1053/j.ajkd.2017.09.026
5. van Haalen H, Jackson J, Spinowitz B, Milligan G, Moon R. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: analysis of multinational real-world data. BMC Nephrol. (2020) 21:88. doi: 10.1186/s12882-020-01746-4
6. Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the anemia in chronic heart failure: outcomes and resource utilization (anchor) study. Circulation. (2006) 113:2713–23. doi: 10.1161/CIRCULATIONAHA.105.577577
7. Dhingra R, Gaziano JM, Djoussé L. Chronic kidney disease and the risk of heart failure in men. Circul Heart failure. (2011) 4:138–44. doi: 10.1161/CIRCHEARTFAILURE.109.899070
8. Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton). (2009) 14:240–6. doi: 10.1111/j.1440-1797.2008.01065.x
9. Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. (2011) 79:1370–8. doi: 10.1038/ki.2011.47
10. Musgrove J, Wolf M. Regulation and effects of Fgf23 in chronic kidney disease. Annu Rev Physiol. (2020) 82:365–90. doi: 10.1146/annurev-physiol-021119-034650
11. Coe LM, Madathil SV, Casu C, Lanske B, Rivella S, Sitara D. Fgf-23 is a negative regulator of prenatal and postnatal erythropoiesis. J Biol Chem. (2014) 289:9795–810. doi: 10.1074/jbc.M113.527150
12. Tsai MH, Leu JG, Fang YW, Liou HH. High fibroblast growth factor 23 levels associated with low hemoglobin levels in patients with chronic kidney disease stages 3 and 4. Medicine. (2016) 95:e3049. doi: 10.1097/MD.0000000000003049
13. Nam KH, Kim H, An SY, Lee M, Cha MU, Park JT, et al. Circulating fibroblast growth factor-23 levels are associated with an increased risk of anemia development in patients with nondialysis chronic kidney disease. Sci Rep. (2018) 8:7294. doi: 10.1038/s41598-018-25439-z
14. Agoro R, Montagna A, Goetz R, Aligbe O, Singh G, Coe LM, et al. Inhibition of fibroblast growth factor 23 (fgf23) signaling rescues renal anemia. FASEB J. (2018) 32:3752–64. doi: 10.1096/fj.201700667R
15. Czaya B, Faul C. The role of fibroblast growth factor 23 in inflammation and anemia. Int J Mol Sci. (2019) 20:4195. doi: 10.3390/ijms20174195
16. Bielesz B, Reiter T, Hammerle FP, Winnicki W, Bojic M, Gleiss A, et al. The role of iron and erythropoietin in the association of fibroblast growth factor 23 with anemia in chronic kidney disease in humans. J Clin Med. (2020) 9:2640. doi: 10.3390/jcm9082640
17. Francis C, Courbon G, Gerber C, Neuburg S, Wang X, Dussold C, et al. Ferric citrate reduces fibroblast growth factor 23 levels and improves renal and cardiac function in a mouse model of chronic kidney disease. Kidney Int. (2019) 96:1346–58. doi: 10.1016/j.kint.2019.07.026
18. Mehta R, Cai X, Hodakowski A, Lee J, Leonard M, Ricardo A, et al. Fibroblast growth factor 23 and anemia in the chronic renal insufficiency cohort study. Clin J Am Soc Nephrol. (2017) 12:1795–803. doi: 10.2215/CJN.03950417
19. Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. (2010) 95:578–85. doi: 10.1210/jc.2009-1603
20. Wolf M, White KE. Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Curr Opin Nephrol Hypertens. (2014) 23:411–9. doi: 10.1097/01.mnh.0000447020.74593.6f
21. Stubbs JR, He N, Idiculla A, Gillihan R, Liu S, David V, et al. Longitudinal evaluation of fgf23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J Bone Miner Res. (2012) 27:38–46. doi: 10.1002/jbmr.516
22. Usui T, Zhao J, Fuller DS, Hanafusa N, Hasegawa T, Fujino H, et al. Association of erythropoietin resistance and fibroblast growth factor 23 in dialysis patients: results from the Japanese dialysis outcomes and practice patterns study. Nephrology. (2021) 26:46–53. doi: 10.1111/nep.13765
23. Hasegawa T, Zhao J, Fuller DS, Bieber B, Zee J, Morgenstern H, et al. Erythropoietin hyporesponsiveness in dialysis patients: possible role of statins. Am J Nephrol. (2017) 46:11–7. doi: 10.1159/000477217
24. DeLoughery TG. Iron deficiency anemia. Med Clin North Am. (2017) 101:319–32. doi: 10.1016/j.mcna.2016.09.004
25. Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Mineral Res. (2013) 28:1793–803. doi: 10.1002/jbmr.1923
26. Honda H, Michihata T, Shishido K, Takahashi K, Takahashi G, Hosaka N, et al. High fibroblast growth factor 23 levels are associated with decreased ferritin levels and increased intravenous iron doses in hemodialysis patients. PLoS ONE. (2017) 12:e0176984. doi: 10.1371/journal.pone.0176984
27. Shima H, Miya K, Okada K, Minakuchi J, Kawashima S. Sucroferric oxyhydroxide decreases serum phosphorus level and fibroblast growth factor 23 and improves renal anemia in hemodialysis patients. BMC Res Notes. (2018) 11:363. doi: 10.1186/s13104-018-3483-6
28. Maruyama N, Otsuki T, Yoshida Y, Nagura C, Kitai M, Shibahara N, et al. Ferric citrate decreases fibroblast growth factor 23 and improves erythropoietin responsiveness in hemodialysis patients. Am J Nephrol. (2018) 47:406–14. doi: 10.1159/000489964
29. Eser B, Yayar O, Buyukbakkal M, Erdogan B, Ercan Z, Merhametsiz O, et al. Fibroblast growth factor is associated to left ventricular mass index, anemia and low values of transferrin saturation. Nefrologia. (2015) 35:465–72. doi: 10.1016/j.nefro.2015.06.025
30. Lewerin C, Ljunggren Ö, Nilsson-Ehle H, Karlsson MK, Herlitz H, Lorentzon M, et al. Low serum iron is associated with high serum intact Fgf23 in elderly men: the Swedish MROS study. Bone. (2017) 98:1–8. doi: 10.1016/j.bone.2017.02.005
31. Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (adhr) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. (2011) 108:E1146–55. doi: 10.1073/pnas.1110905108
32. Fishbane S, Block GA, Loram L, Neylan J, Pergola PE, Uhlig K, et al. Effects of ferric citrate in patients with nondialysis-dependent ckd and iron deficiency anemia. J Am Soc Nephrol. (2017) 28:1851–8. doi: 10.1681/ASN.2016101053
33. Fukao W, Hasuike Y, Yamakawa T, Toyoda K, Aichi M, Masachika S, et al. Oral versus intravenous iron supplementation for the treatment of iron deficiency anemia in patients on maintenance hemodialysis-effect on fibroblast growth factor-23 metabolism. J Ren Nutr. (2018) 28:270–7. doi: 10.1053/j.jrn.2017.12.009
34. Iguchi A, Yamamoto S, Yamazaki M, Tasaki K, Suzuki Y, Kazama JJ, et al. Effect of ferric citrate hydrate on fgf23 and Pth levels in patients with non-dialysis-dependent chronic kidney disease with normophosphatemia and iron deficiency. Clin Exp Nephrol. (2018) 22:789–96. doi: 10.1007/s10157-017-1510-x
35. David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. (2016) 89:135–46. doi: 10.1038/ki.2015.290
36. Alon DB, Chaimovitz C, Dvilansky A, Lugassy G, Douvdevani A, Shany S, et al. Novel role of 1,25(Oh)(2)D(3) in induction of erythroid progenitor cell proliferation. Exp Hematol. (2002) 30:403–9. doi: 10.1016/S0301-472X(02)00789-0
37. Patel NM, Gutiérrez OM, Andress DL, Coyne DW, Levin A, Wolf M. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int. (2010) 77:715–20. doi: 10.1038/ki.2009.551
38. Koike H, Ito K, Miyamoto M, Nishino H. Effects of long-term blockade of angiotensin converting enzyme with captopril (sq14,225) on hemodynamics and circulating blood volume in SHR. Hypertension. (1980) 2:299–303. doi: 10.1161/01.HYP.2.3.299
39. Mrug M, Stopka T, Julian BA, Prchal JF, Prchal JT. Angiotensin II stimulates proliferation of normal early erythroid progenitors. J Clin Invest. (1997) 100:2310–4. doi: 10.1172/JCI119769
40. Inoue A, Babazono T, Iwamoto Y. Effects of the renin-angiotensin system blockade on hemoglobin levels in type 2 diabetic patients with chronic kidney disease. Am J Hypertens. (2008) 21:317–22. doi: 10.1038/ajh.2007.53
41. Schwarzbeck A, Wittenmeier KW, Hällfritzsch U. Anaemia in dialysis patients as a side-effect of sartanes. Lancet. (1998) 352:286. doi: 10.1016/S0140-6736(05)60259-0
42. Watanabe T, Temma Y, Okada J, Yamada E, Ishida E, Horiguchi K, et al. Angiotensin receptor blockers significantly reduce hemoglobin level in patients with type 2 diabetes mellitus not suffered chronic cardiac failure and chronic kidney disease. Endocr J. (2021) 68:503–7. doi: 10.1507/endocrj.EJ20-0773
43. Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, et al. Mutant Fgf-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. (2002) 143:3179–82. doi: 10.1210/endo.143.8.8795
Keywords: hemodialysis, intact fibroblast growth factor 23, C-terminal fibroblast growth factor 23, anemia, erythropoiesis
Citation: Fang Y-W, Wang J-T, Lin TY, Lee C-J, Jang T-N, Tsai M-H and Liou H-H (2023) High intact fibroblast growth factor 23 levels associated with low hemoglobin levels in patients on chronic hemodialysis. Front. Med. 10:1098871. doi: 10.3389/fmed.2023.1098871
Received: 15 November 2022; Accepted: 13 March 2023;
Published: 04 April 2023.
Edited by:
Chia-Ter Chao, National Taiwan University Hospital, TaiwanReviewed by:
Jamshid Roozbeh, Shiraz University of Medical Sciences, IranCopyright © 2023 Fang, Wang, Lin, Lee, Jang, Tsai and Liou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Hsien Tsai, Y2hhb3NteXRoLnR3QGdtYWlsLmNvbQ==; Hung-Hsiang Liou, aGgyNTg1MjdAbXMyMy5oaW5ldC5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.