
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Med. , 23 February 2023
Sec. Regulatory Science
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1096992
This article is part of the Research Topic Pharmacovigilance and Drug Repositioning Research using Pharmacoepidemiology View all 7 articles
 Haruka Shida1
Haruka Shida1 Kazuhiro Kajiyama1
Kazuhiro Kajiyama1 Sono Sawada1†
Sono Sawada1† Chieko Ishiguro1†
Chieko Ishiguro1† Mikiko Kubo2
Mikiko Kubo2 Ryota Kimura2
Ryota Kimura2 Mai Hirano3
Mai Hirano3 Noriyuki Komiyama3
Noriyuki Komiyama3 Toyotaka Iguchi3
Toyotaka Iguchi3 Yukio Oniyama2
Yukio Oniyama2 Yoshiaki Uyama1*
Yoshiaki Uyama1*The Pharmaceuticals and Medical Devices Agency (PMDA) has conducted many pharmacoepidemiological studies for postmarketing drug safety assessments based on real-world data from medical information databases. One of these databases is the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB), containing health insurance claims of almost all Japanese individuals (over 100 million) since April 2009. This article describes the PMDA’s regulatory experiences in utilizing the NDB for postmarketing drug safety assessment, especially focusing on the recent cases of use of the NDB to examine the practical utilization and safety signal of a drug. The studies helped support regulatory decision-making for postmarketing drug safety, such as considering a revision of prescribing information of a drug, confirming the appropriateness of safety measures, and checking safety signals in real-world situations. Different characteristics between the NDB and the MID-NET® (another database in Japan) were also discussed for appropriate selection of data source for drug safety assessment. Accumulated experiences of pharmacoepidemiological studies based on real-world data for postmarketing drug safety assessment will contribute to evolving regulatory decision-making based on real-world data in Japan.
Spontaneous reports of adverse drug reactions are a valuable source for postmarketing drug safety assessments in Japan (1). However, it is also known to have some limitations in drug safety assessment based on these reports such as underreporting, more serious cases frequently reported and a lack of denominator (2, 3). Meanwhile, in recent years, secondary utilization of data from the database has received significant attention for the regulatory purpose of postmarketing drug safety assessment in accordance with advancements in the medical information database infrastructure and further advancement of regulatory assessment of drug safety in Japan (4). The Pharmaceuticals and Medical Devices Agency (PMDA) has conducted pharmacoepidemiological studies for postmarketing drug safety assessment based on real-world data (RWD) from medical information databases (5). One of those databases is the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB), which was established by the Japanese Ministry of Health, Labour, and Welfare (MHLW) based on “Act on Assurance of Medical Care for Elderly People” (6). The NDB is one of the world’s largest health-related databases and contains nationwide electronic health insurance claims data and specific health check-up data gathered from all medical institutions, including hospitals, clinics, pharmacies, and dental clinics, and covers the medical information of almost all Japanese individuals (over 100 million) since April 2009 (6).
This article describes PMDA’s regulatory experiences in utilizing the NDB for postmarketing drug safety assessment, especially focusing on the recent cases of use of the NDB for examining the practical utilization and safety signal of a drug. The permission to use the NDB for drug safety assessment has been granted to the PMDA by the expert committee of MHLW since March 2016.
Drug-overuse headache was a concern for triptan-based drugs, a class of migraine medication, based on Japanese and foreign spontaneous reports. In recent years, although the prevalence of triptan overuse has been elucidated gradually based on healthcare databases in foreign countries (7), no data were available on the real-world situation of drug use in clinical practice in Japan. This study aimed to identify the practical use and prescription patterns of triptan-based drugs, including their overuse.
We utilized the NDB to identify patients who were prescribed triptan-based drugs, including eletriptan hydrobromide, sumatriptan succinate, sumatriptan, zolmitriptan, naratriptan hydrochloride, and rizatriptan benzoate, between August 1, 2010, and March 31, 2016. For each patient, the total monthly dose equivalent of sumatriptan succinate was calculated based on the single or maximum daily dose of each triptan-based drug. The definitions to identify a patients with triptan-overuse were created based on the diagnostic criteria of “Triptan-overuse headache” in the International Classification of Headache Disorders 3rd edition (ICHD-3) (i.e., regular intake of one or more triptans, in any formulation, on ≥10 days/month for >3 months) (8). Actually, in order to consider the amount of intake in the prescription data, the definition was slightly modified with two variations. The first definition intended for “suspected overuse” and was defined as a patient who met at least one of the following criteria; (1) the total monthly dose exceeded 10 times the single dose of sumatriptan succinate for four or more consecutive months, or (2) the total monthly dose in a certain month exceeded four or higher integral multiple (X ≥ 4) of 10 times the single dose of sumatriptan succinate and additionally prescribed triptan-based drugs within X months after. The second definition intended for “overuse” and was defined by changing the term “10 times the single dose of sumatriptan succinate” in “suspected overuse” to “10 times the maximum daily dose of sumatriptan succinate.” It should be noted that these definitions were used for this study without validation because of the exploratory nature of the study purpose. All analyses were performed by using SAS 9.4 statistical software (SAS Institute, Cary, NC, USA).
Overall, 2,078,556 patients with triptan-based drug prescriptions were included in the analysis. Of those, 102,871 (4.95%) were “suspected overuse” and 16,426 (0.79%) were “overuse” cases. These results indicate that there were a certain number of patients with suspected overuse of triptan-based drugs in Japan. MHLW/PMDA evaluated the results of this study as well as the situations in foreign countries in consultation with external experts and revised the important precautions section and the clinically significant adverse reactions section in the package inserts (PIs) of triptan-based drugs to include information about a medication-overuse headache (9).
In July 2018, valsartan-containing drugs approved for hypertension were voluntarily recalled by the marketing authorization holder (MAH) due to contamination with an impurity, N-nitrosodimethylamine (NDMA), a known carcinogen (10). More recently, no potential relationship between cancer risk and carcinogenic contaminant during valsartan prescription have been reported (11), but there was little safety information available at the time of this study in Japan. This study aimed to elucidate the prescribing patterns and cumulative dose per patient of valsartan-containing drugs to provide information related to the assessment of patient health effects caused by NDMA.
We utilized the NDB to identify patients with prescriptions for recalled products between June 1, 2014 and March 31, 2016. We estimated the cumulative dose per patient based on each prescription’s daily dose (mg) and period (days). We then calculated summary statistics, including mean, standard deviation, median, interquartile range (IQR), and minimum and maximum values for the cumulative dose, and illustrated the frequency distribution chart. All analyses were performed by using SAS 9.4 statistical software (SAS Institute, Cary, NC, USA).
A total of 18,823 patients with prescriptions for recalled products were identified. The median cumulative dose was 33,600 mg (IQR: 10,080–51,920 mg), with minimum and maximum values of 40 mg and 360,000 mg, respectively. As shown in the frequency distribution chart of Figure 1, if the maximum daily dose (160 mg in Japan) of valsartan-containing drugs was prescribed for the entire study period (670 days), the maximum cumulative dose was assumed to reach at the range of 100.000-110,000 mg. The 764 patients fell into this maximum range, and 98% out of the total patients were under this range. The study elucidated the prescribing patterns and cumulative dose per patient of the recalled products and provided useful information related to assessing patient health effects caused by NDMA.
In December 2019, the Health Sciences Authority (HSA) in Singapore announced that certain metformin-containing drugs approved for diabetes were voluntarily recalled by MAH owing to contamination with NDMA impurities (12). Similar contamination with NDMA was found in metformin-containing drugs marketed in Japan (13, 14). Based on the National Institute of Health Sciences (NIHS) risk assessment in Japan, the MHLW concluded that no additional safety-related regulatory actions were required (15). This study aimed to examine the daily dose and cumulative prescription days of metformin-containing drugs in a real-world setting to support the assumptions used in the risk assessment.
We utilized the NDB to identify patients who were prescribed metformin-containing drugs between August 1, 2010 and March 31, 2018, and examined the earliest date of prescription of metformin-containing drugs per patient as the first prescription. We then excluded patients with first prescriptions between August 1, 2010, and March 31, 2011. As the date of the first prescription affects both the follow-up period and cumulative prescription days, we divided the target population into seven sub-cohorts based on the fiscal year of the first prescription date. We calculated the cumulative number of prescription days per patient and its summary statistics for each sub-cohort. In the case of overlapping prescription periods for consecutive prescriptions, days of overlap were ignored. Moreover, we calculated the daily dose per patient by dividing the cumulative dose by cumulative prescription days and its summary statistics for each sub-cohort. All analyses were performed by using SAS 9.4 statistical software (SAS Institute, Cary, NC, USA).
A total of 4,239,798 patients with prescriptions of metformin-containing drugs were identified in the main cohort. The median daily dose was 708.4 mg (IQR: 509.1–916.0 mg), and the daily doses for approximately 97% of patients were <1,500 mg. Table 1 shows the number of patients and the summary statistics of the cumulative prescription days for each sub-cohort. The third quartile of cumulative prescription days was approximately 6 years in patients in the sub-cohort for 2011 which was the longest follow-up period of up to 7 years. These results indicate that in most patients, metformin-containing drugs have been continually prescribed for nearly a decade in Japan. The results of this study supported the appropriateness of the risk assessment by NIHS, showing that the daily dose of metformin-containing drugs in clinical practice was consistent with the assumption of the risk assessment (1,500 mg/day) (15).
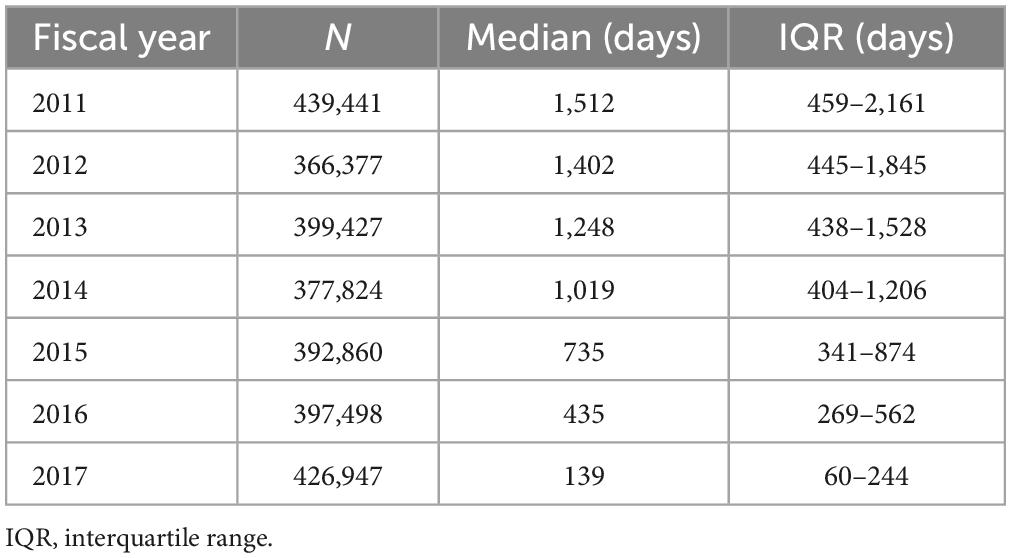
Table 1. Number of patients and summary statistics of cumulative prescription days of metformin-containing drugs for each sub-cohort.
Inconsistent findings regarding the increased risk of retinal detachment (RD) associated with fluoroquinolones (antibiotics), have been reported (16–20). Under these circumstances, the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency recommended in 2014 to update the product information of fluoroquinolones to warn prescribers regarding impaired vision in the section of special warnings and precautions for use in consideration of the severity of RD (21). In Japan, there have been no spontaneous reports regarding the relationship between RD and fluoroquinolones as of July 2019, and recent report based on the healthcare database shows no association between fluoroquinolones and RD (22). This study aimed to check the safety signal indicating the association between RD and fluoroquinolones with reference to those of other antibiotics in Japan.
In utilizing the NDB, a sequence symmetry analysis (SSA) (23) was conducted in patients with both prescriptions of antibiotics (fluoroquinolones, cephems, macrolides, penicillins, penems, aminoglycosides, or tetracyclines) and the surgery for RD between April 1, 2011, and March 31, 2016. Surgery for RD was defined as the combination of diagnosis and surgical procedure in the same month. Patients whose dates of the first exposure and first outcome were within 90-days were included. Patients who met at least one of the following criteria were excluded: (1) exposure or outcome that occurred from August 1, 2010 to March 31, 2011; and (2) the first exposure and the first outcome occurred on the same date. The crude sequence ratios (CSRs), the number of patients prescribed with an antibiotic prior to RD divided by the number of patients diagnosed as RD before prescribing antibiotics and the adjusted sequence ratios [ASRs, CSR divided by null-effect sequence ratio (23)] were calculated with their 95% confidence interval (CI). These parameters were calculated based on patient numbers accounted for each administration route of fluoroquinolones prescription (i.e., “systemic fluoroquinolones,” “topical fluoroquinolones except for ophthalmic use,” and “ophthalmic fluoroquinolones”). Note that “ophthalmic fluoroquinolones” was independently categorized because some dosage forms of fluoroquinolones were approved and used for the perioperative sterilization of the ocular, which may be a confounding factor for RD. All analyses were performed by using SAS 9.4 statistical software (SAS Institute, Cary, NC, USA).
A total of 81,686 patients with prescriptions of fluoroquinolones were identified, and the ASR for fluoroquinolones and RD was 2.76 (95% CI: 2.72–2.80); none of the other antibiotics increased the ASR (Supplementary Table 1). As shown in Table 2, the increased ASR was observed only for ophthalmic fluoroquinolone but not for other route administrations, such as systemic and other topical (i.e., percutaneous) administrations. The identified signal in the ophthalmic fluoroquinolone was also not likely associated with fluoroquinolone because ophthalmic fluoroquinolones are usually prescribed for perioperative sterilization before surgery for RD. PMDA evaluated these results as well as other relevant information, such as spontaneous adverse event reports in Japan, and concluded that no additional safety measures were required at that time (in January 2020).
The results of four pharmacoepidemiological studies using the NDB helped support regulatory decision-making for postmarketing drug safety, such as considering a revision of prescribing information, confirming the appropriateness of safety measures, and checking safety signals in real-world situations. These studies have further enriched our knowledge and experiences in utilizing NDB for regulatory purposes and have expanded the possibility of NDB utilization in drug safety assessment, in addition to studies for a particular safety risk assessment of a drug compared with other drugs (24–26).
In addition to studies using the NDB described in this manuscript, PMDA has also conducted various studies using another database called the Medical Information Database Network (MID-NET®), which is known as a reliable and valuable database in Japan (27, 28), for postmarketing drug safety assessment. As shown in Figure 2, NDB and MID-NET® have different characteristics in terms of data categories and patient follow-up. For example, the NDB is the largest database with nation-based medical claims and allows the follow-up of a patient regardless of their visiting hospitals and types of health insurance (6). In contrast, the MID-NET® includes not only claims data but also electronic medical records with laboratory test results in collaborative hospitals, although a patient can only be followed within a hospital (27, 28). For all four studies presented in this article, it was necessary to identify patients to the extent possible for understanding the real-world situation of drug use. For the study for detection of safety signal, the outcome of RD was defined only with claims data but not with laboratory test results. Therefore, we selected the NDB as a data source for these studies. MID-NET® is usually selected for a study in which laboratory test results are necessary for analysis (29–32). As described above, an understanding of the characteristics of each database is important to select a data source fit to a study purpose. Accumulating practical experiences in utilizing these databases in PMDA will enable more accurate database studies and increase the confidence of obtained real-world evidence (RWE) to be used as a basis for regulatory decision-making.
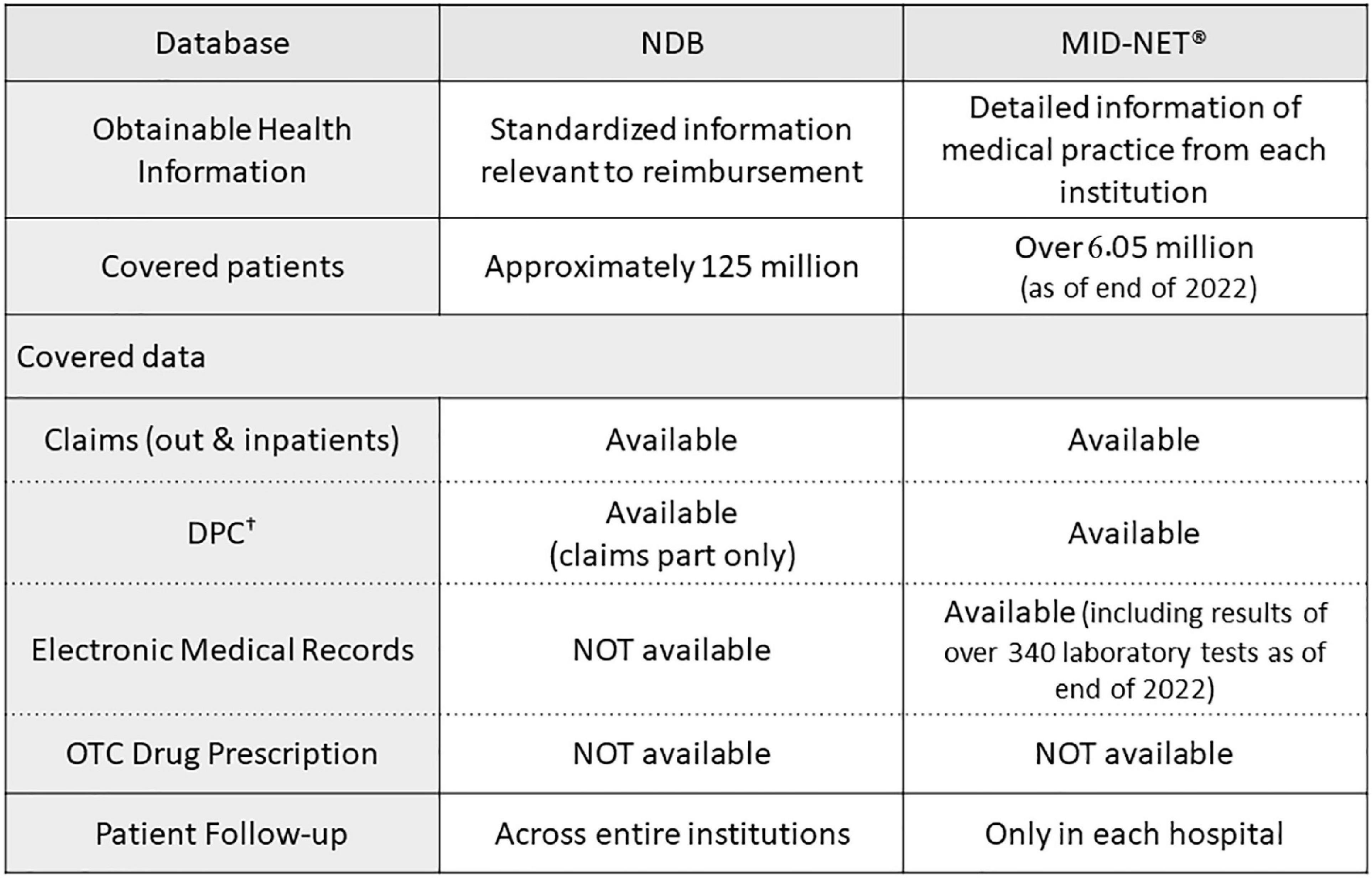
Figure 2. Comparisons of characteristics between NDB and MID-NET®. †Known as the diagnostic procedure combination (DPC) data, similar to diagnosis related groups (DRGs) in USA. (Acute Inpatient Medical Care System data).
In conclusion, the PMDA will continue to utilize the NDB for conducting pharmacoepidemiological studies based on RWD for postmarketing drug safety assessment. Accumulated experiences through those studies will contribute to evolving regulatory decision-making based on RWE.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Since these studies were conducted as an official activity of the PMDA under the Pharmaceuticals and Medical Devices Agency Law [Article 15–5–(c) and (f)] (33), they were not subject to review by the institutional review boards (25). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
HS, SS, CI, MK, RK, MH, NK, and YU designed the studies. HS, SS, and KK performed the analysis and analyzed the data. HS, SS, KK, CI, MK, RK, MH, NK, TI, YO, and YU wrote the manuscript. All authors contributed to the final manuscript, read and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1096992/full#supplementary-material
1. Ishiguro C, Misu T, Iwasa E, Izawa T. Analysis of safety-related regulatory actions by Japan’s pharmaceutical regulatory agency. Pharmacoepidemiol Drug Saf. (2017) 26:1314–20. doi: 10.1002/pds.4252
2. Hazell L, Shakir S. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. (2006) 29:385–96. doi: 10.2165/00002018-200629050-00003
3. Noguchi Y, Tachi T, Teramachi H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief Bioinform. (2021) 22:bbab347. doi: 10.1093/bib/bbab347
4. Nishioka K, Makimura T, Ishiguro A, Nonaka T, Yamaguchi M, Uyama Y. Evolving acceptance and use of RWE for regulatory decision making on the benefit/risk assessment of a drug in Japan. Clin Pharmacol Ther. (2022) 111:35–43. doi: 10.1002/cpt.2410
5. Ishiguro C, Takeuchi Y, Uyama Y, Tawaragi T. The MIHARI project: establishing a new framework for pharmacoepidemiological drug safety assessments by the Pharmaceuticals and Medical Devices Agency of Japan. Pharmacoepidemiol Drug Saf. (2016) 25:854–9. doi: 10.1002/pds.4032
7. Zebenholzer K, Gall W, Wöber C. Use and overuse of triptans in Austria - a survey based on nationwide healthcare claims data. J Headache Pain. (2018) 19:34. doi: 10.1186/s10194-018-0864-0
8. Headache Classification Committee of the International Headache Society [IHS]. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. (2013) 33:629–808. doi: 10.1177/0333102413485658
9. Pharmaceuticals and Medical Devices Agency. Summary of Investigation Results Triptans (in Japanese). (2019). Available online at: https://www.pmda.go.jp/files/000229838.pdf (accessed November 9, 2022).
10. Pharmaceuticals and Medical Devices Agency. Administrative Notice (in Japanese). (2018). Available online at: https://www.pmda.go.jp/files/000226196.pdf (accessed November 11, 2022).
11. Salim H, Jones A. Angiotensin II receptor blockers (ARBs) and manufacturing contamination: a retrospective National Register Study into suspected associated adverse drug reactions. Br J Clin Pharmacol. (2022) 88:4812–27. doi: 10.1111/bcp.15411
12. Health Sciences Authority. HSA Update. (2019). Available online at: https://www.hsa.gov.sg/docs/default-source/default-document-library/hsaupdate_hsa-recalls-3-out-of-46-metformin-medicines_final3aa0739771ba4f5789784858c7b5f5b7.pdf (accessed June 2, 2022).
13. Ministry of Health, Labour and Welfare of Japan. Press Release: Notice of Voluntary Collection of Pharmaceutical Products (Class I) (Metgluco Tablets 250mg/500 mg, Sumitomo Pharma Co., Ltd.) (in Japanese). (2020). Available online at: https://www.mhlw.go.jp/stf/newpage_11052.html (accessed October 12, 2022).
14. Ministry of Health, Labour and Welfare of Japan. Press Release: Notice of Voluntary Collection of Pharmaceutical Products (Class I) (Metformin Hydrochloride Tablets 250mgMT“JG”, NIHON GENERIC Co., Ltd) (in Japanese). (2020). Available online at: https://www.mhlw.go.jp/stf/newpage_11036.html (accessed October 12, 2022).
15. Ministry of Health, Labour and Welfare of Japan. Administrative Notice. Results of the Health Effect Assessment of the Use of Metformin-Containing Products in Which N-dimethylnitrosamine Was Detected (in Japanese). (2020). Available online at: https://www.pmda.go.jp/files/000237205.pdf (accessed October 14, 2022).
16. Chui C, Man K, Cheng C, Chan E, Lau W, Cheng V, et al. An investigation of the potential association between retinal detachment and oral fluoroquinolones: a self-controlled case series study. J Antimicrob Chemother. (2014) 69:2563–7. doi: 10.1093/jac/dku145
17. Eftekhari K, Ghodasra D, Haynes K, Chen J, Kempen J, VanderBeek B. Risk of retinal tear or detachment with oral fluoroquinolone use: a cohort study. Pharmacoepidemiol Drug Saf. (2014) 23:745–52. doi: 10.1002/pds.3623
18. Fife D, Zhu V, Voss E, Levy-Clarke G, Ryan P. Exposure to oral fluoroquinolones and the risk of retinal detachment: retrospective analyses of two large healthcare databases. Drug Saf. (2014) 37:171–82. doi: 10.1007/s40264-014-0138-y
19. Kapoor K, Hodge D, St Sauver J, Barkmeier A. Oral fluoroquinolones and the incidence of rhegmatogenous retinal detachment and symptomatic retinal breaks: a population-based study. Ophthalmology. (2014) 121:1269–73. doi: 10.1016/j.ophtha.2013.12.006
20. Pasternak B, Svanström H, Melbye M, Hviid A. Association between oral fluoroquinolone use and retinal detachment. JAMA. (2013) 310:2184–90. doi: 10.1001/jama.2013.280500
21. European Medicins Agency. PRAC Recommendations on Signals. (2014). Available online at: https://www.ema.europa.eu/en/documents/prac-recommendation/prac-recommendations-signals-adopted-prac-meeting-10-13-june-2014_en.pdf (accessed October 5, 2022).
22. Londhe A, Holy C, Weaver J, Fonseca S, Villasis-Keever A, Fife D. Risk of retinal detachment and exposure to fluoroquinolones, common antibiotics, and febrile illness using a self-controlled case series study design: Retrospective analyses of three large healthcare databases in the US. PLoS One. (2022) 17:e0275796. doi: 10.1371/journal.pone.0275796
23. Lai E, Pratt N, Hsieh C, Lin S, Pottegård A, Roughead E, et al. Sequence symmetry analysis in pharmacovigilance and pharmacoepidemiologic studies. Eur J Epidemiol. (2017) 32:567–82. doi: 10.1007/s10654-017-0281-8
24. Komamine M, Kajiyama K, Ishiguro C, Uyama Y. Cardiovascular risks associated with dipeptidyl peptidase-4 inhibitors monotherapy compared with other antidiabetes drugs in the Japanese population: A nationwide cohort study. Pharmacoepidemiol Drug Saf. (2019) 28:1166–74. doi: 10.1002/pds.4847
25. Pharmaceuticals and Medical Devices Agency. MIHARI Project (in Japanese). (2023). Available online at: https://www.pmda.go.jp/safety/surveillance-analysis/0045.html (accessed November 9, 2022).
26. Sawada S, Kajiyama K, Shida H, Kimura R, Nakazato Y, Iguchi T, et al. Cardiovascular risk of urate-lowering drugs: A study using the National Database of Health Insurance Claims and Specific Health Checkups of Japan. Clin Transl Sci. (2022) [Epub ahead of print]. doi: 10.1111/cts.13439
27. Yamaguchi M, Inomata S, Harada S, Matsuzaki Y, Kawaguchi M, Ujibe M, et al. Establishment of the MID-NET® medical information database network as a reliable and valuable database for drug safety assessments in Japan. Pharmacoepidemiol Drug Saf. (2019) 28:1395–404. doi: 10.1002/pds.4879
28. Yamada K, Itoh M, Fujimura Y, Kimura M, Murata K, Nakashima N, et al. The utilization and challenges of Japan’s MID-NET® medical information database network in postmarketing drug safety assessments: A summary of pilot pharmacoepidemiological studies. Pharmacoepidemiol Drug Saf. (2019) 28:601–8. doi: 10.1002/pds.4777
29. Kajiyama K, Ishiguro C, Ando T, Kubota Y, Kinoshita N, Oniyama Y, et al. Nested Case-Control Study Utilizing MID-NET® on Thrombocytopenia Associated With Pegfilgrastim in Patients Treated With Antineoplastic Agents. Clin Pharmacol Ther. (2021) 110:473–9. doi: 10.1002/cpt.2263
30. Hasegawa T, Sawada S, Ishiguro C, Ando T, Kobayashi K, Komiyama N, et al. Assessing the Risk of Decrease in Kidney Function in Patients Prescribed Direct-Acting Antivirals for Hepatitis C Utilizing the MID-NET® Medical Information Database Network in Japan. Ther Innov Regul Sci. (2022) 56:625–31. doi: 10.1007/s43441-022-00400-5
31. Sawada S, Ando T, Hirano M, Komiyama N, Iguchi T, Oniyama Y, et al. Effect of Hepatitis C Drugs on Blood Coagulability in Patients on Warfarin Using the Medical Information Database Network (MID-NET®) in Japan. Ther Innov Regul Sci. (2021) 55:539–44. doi: 10.1007/s43441-020-00247-8
32. Kinoshita Y, Kajiyama K, Ishiguro C, Nonaka T, Kimura R, Kikuchi Y, et al. Characterizing Granulocytopenia Associated with Thiamazole in Patients with Hyperthyroidism Based on Real-World Data from the MID-NET® in Japan. Clin Pharmacol Ther. (2023) [Epub ahead of print]. doi: 10.1002/cpt.2850
33. Pharmaceuticals and Medical Devices Agency. Act on the Pharmaceuticals and Medical Devices Agency (Act No. 192 of 2002) (in Japanese). (2022). Available online at: https://elaws.e-gov.go.jp/document?lawid=414AC0000000192 (accessed June 2, 2022).
Keywords: real-world data, pharmacoepidemiology, national claims database, drug safety, regulatory decision-making
Citation: Shida H, Kajiyama K, Sawada S, Ishiguro C, Kubo M, Kimura R, Hirano M, Komiyama N, Iguchi T, Oniyama Y and Uyama Y (2023) Use of National Database of Health Insurance Claims and Specific Health Checkups for examining practical utilization and safety signal of a drug to support regulatory assessment on postmarketing drug safety in Japan. Front. Med. 10:1096992. doi: 10.3389/fmed.2023.1096992
Received: 13 November 2022; Accepted: 07 February 2023;
Published: 23 February 2023.
Edited by:
Yoshihiro Noguchi, Gifu Pharmaceutical University, JapanReviewed by:
Jason Robert Guertin, Laval University, CanadaCopyright © 2023 Shida, Kajiyama, Sawada, Ishiguro, Kubo, Kimura, Hirano, Komiyama, Iguchi, Oniyama and Uyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshiaki Uyama,  dXlhbWEteW9zaGlha2lAcG1kYS5nby5qcA==
dXlhbWEteW9zaGlha2lAcG1kYS5nby5qcA==
†Present address: Sono Sawada, IQVIA Solutions Japan K.K., Tokyo, Japan; Chieko Ishiguro, Section of Clinical Epidemiology, Department of Data Science, Center for Clinical Sciences, National Center for Global Health and Medicine, Tokyo, Japan
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.