- 1Department of Pediatrics, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
Background: The long-term overall survival of children with T-cell acute lymphoblastic leukemia (T-ALL) is limited to approximately 80–85% because of a high incidence of relapse after achieving remission with intensive chemotherapy and hematopoietic stem cell transplantation (HSCT). Novel treatment strategies inducing long-term remission are needed to improve the outcome. Histone deacetylase inhibitors (HDACis) have been reported to be effective in a series of T-ALL cases. Preclinical studies suggested that T-ALL cells are sensitive to Chidamide, which is a selective HDACi.
Methods: This preliminary clinical study evaluated the efficacy and safety of Chidamide in combination with chemotherapy or post-HSCT for children with T-ALL at a dose of 0.5 mg/kg weight of patient twice per week for at least 6 months.
Results: In total, 27 children with a mean age of 7.88 years were included. The high-risk proportion was 66.7%. After a median follow-up period of 37.8 months (9.5–67.9 months), the overall survival and event-free survival in the patients treated with Chidamide were 94.1 and 95.2%, respectively. All patients except two maintained persistent remission with <0.01% blast cells in minimal residual disease.
Conclusion: The combination therapy with Chidamide in a case series of T-ALL shows the promising clinical efficacy and good safety in children.
Clinical trial registration: https://www.chictr.org.cn/, identifier ChiCTR2000030357.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) accounts for approximately 12–15% of all ALL cases in childhood. The long-term overall survival (OS) of children with T-ALL is limited to approximately 80–85% because of a high incidence of relapse after achieving remission with intensive chemotherapy (1–3). Currently, the first-line induction therapy is a combination of intensive chemotherapy of Dexamethasone/prednisone, vincristine, pegaspargase, daunorubicin, 6-mercaptopurine, and cytarabine, while consolidation therapy includes cyclophosphamide, cytarabine, 6-mercaptopurine, pegaspargase, vincristine, nelarabine, and high-dose methotrexate. Progress has been achieved in the nelarabine treatment of T-ALL (4). Lately, venetoclax and bortezomib in combination with chemotherapy has been reported to be effective in relapsed or refractory (R/R) T- ALL (5). However, the complete remission (CR) rate for nelarabine has been only 31–36%. The duration of response was short except in a small proportion of patients who underwent allogeneic hematopoietic stem cell transplantation (HSCT) (6). Consequently, novel treatment strategies for T-ALL that can maintain long-term remission are needed to improve the outcome.
Studies have suggested that high expression of Histone deacetylase (HDAC) in childhood T-ALL is associated with poor prognosis, which indicates that HDAC inhibitors (HDACis) may be used to treat T-ALL (7). HDACis have been recommended for relapsed or refractory T-cell lymphoma and have been reported to be effective in cases (8). HDACi reverses the inhibition of the transcription of tumor-suppressor and related genes, which is caused by HDAC acetylating the Lysine residues on histone proteins (9, 10). It has been approved by the National Medical Products Administration for the treatment of relapsed and refractory peripheral T-cell lymphoma, and it has been approved by the FDA for clinical study (8, 11). Chidamide in combination with chemotherapy has been proved to be effective in R/R T lymphoblastic lymphoma/leukemia in adults and young adolescents (8). Furthermore, Chidamide does not significantly increase the adverse effects of chemotherapy (12).
Based on these rationales, for the treatment of children with T-ALL at our institution, Chidamide has been added to the chemotherapy and for post-HSCT relapse prophylaxis. Here, we report the clinical efficacy and safety of the Chidamide in a case series of 27 patients with first-time diagnosed T-ALL.
Materials and methods
Patient population
From July 1, 2016, to May 31, 2021, 235 children were diagnosed with T-ALL in the SCCLG-2016 cooperation group. The SCCLG-2016 ALL protocol (Supplementary Information 1) was adopted (ChiCTR2000030357). 27 patients, who treated with Chidamide (Epidaza®), were included in the present study. Patients provided informed consent to receive Chidamide. We excluded patients who received Chidamide for <24 weeks and who abandoned treatment at any point of therapy without achieving any endpoint. The research protocol was approved by the ethics committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University. All data were prospectively collected and analyzed.
Diagnosis, response assessment and risk stratification
The diagnosis of T-ALL was based on morphological French-American-British criteria: negative staining for myeloperoxidase or Sudan black and immunophenotypical expression of T- cell surface antigens CD7 or CD2, often with CD5, CD3, CD4, or CD8 in more than 20% of blast cells and nuclear deoxynucleotide transferase or intracytoplasmic CD3 in more than 10% of blast cells. Unequivocal cases were reviewed centrally. Immunophenotyping was performed according to the European Group for the Immunological Characterization of Leukemias or World Health Organization 2008 criteria.
CR was defined as bone marrow blast cells ≤5%, absence of extramedullary disease and recovery of peripheral blood counts (absolute neutrophil count ≥ 1.0 × 109/L and platelets ≥ 100 × 109/L). Minimal residual disease (MRD) in bone marrow aspiration specimens was assessed by multiparameter flow cytometry with a sensitivity of 0.01%. flow cytometry MRD was analyzed according to a previous French multicenter study for pediatric and adult ALL. MRD was analyzed by Kaluza software (Beckman Coulter, United States) or CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA). Reagents were provided by BD Biosciences (Becton, Dickinson, China) and Beckman Coulter Commercial Enterprise (China) Co., Ltd.
After the seven-day prednisone test and dexamethasone-based four-drug induction (Supplementary Information 1), patients with T-ALL were classified into the intermediate-risk (IR) and high-risk (HR) groups based on the response to early treatment. Central nervous system leukemia (CNSL) was diagnosed when white blood cell count (WBC) was higher than 5/mL with blasts were found in cerebrospinal fluid or when clinical symptoms of cranial nerve palsy, brain or eye involvement, or hypothalamic syndrome were found. HR criteria were poor prednisone response with the presence of ≥1 × 109 blasts/L in PB on Day 8 after seven-day prednisone monotherapy and one dose of triple intrathecal therapy (TIT), no partial bone marrow remission (M3 [≥25% blasts] or MRD ≥ 10%) on Day 15, no bone marrow remission (M2 [5–25% blasts]/M3, MRD ≥ 1%) after induction phase 1 (Day 33), and/or positive MLL gene rearrangement, low diploidy, iAMP21 or IKZF1 deletion, MEF2D rearrangement and TCF3-HLF gene fusion, and/or mediastinal tumor lesion evaluated on Day 33 without reduction to 1/3 of the original tumor size or tumor still exists before consolidation therapy. The remaining patients were considered IR.
Treatment
The treatment protocols for T-cell ALL were based on the ALL-IC-BFM 2002 (13), and one dose of 1000 mg/m2 of Cyclophosphamide (CTX) was added to the traditional BFM induction therapy (Supplementary Information 1). Some modifications in systemic therapy and, mostly, in the CNSL prophylactic therapy were made in both the IR group and the HR group. Prophylactic cranial radiotherapy was not recommended. Central nervous system (CNS) involvement was treated with additional TIT. Cranial radiotherapy of 18 Gy was indicated in TIT refractory CNSL only.
Transplantation conditioning regimens for T-ALL were myeloablative, which included: Busulfan 12.8 mg/kg, Fludarabine 120–150 mg/m2, Cyclophosphamide 120 mg/kg, and Semustine 250 mg/m2 (or cranial radiotherapy of 9 Gy). Total-body irradiation (TBI) was recommended in peripheral blood stem cell (PBSC) match sibling donor transplantation.
Chidamide was used at a dose of 0.5 mg/kg patient weight every 4 days in combination with chemotherapy for patients with T-ALL undergoing maintenance therapy or started at 4–8 weeks post-HSCT for 2 years.
Statistical analyses
Overall survival was measured as the time from the diagnosis of T-ALL to death or last follow-up. OS was calculated from diagnosis to death (from any cause). Event-free survival (EFS) was measured as the time from achieving CR to relapse, treatment related death or last follow-up. The “event-free” duration was defined as the time from diagnosis to the date of treatment-related death or relapse or until the date of the last contact in CR. For all analyses, patients lost to follow-up were censored at the time of their withdrawal. The survival curves were analyzed using the Kaplan–Meier method.
Follow-up data were updated as of March 2022. Toxicity was graded based on the modified Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Statistical analysis was performed by using SPSS 23.0 (SPSS Institute, Cary, NC, USA). P < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
In total, 27 patients were treated with Chidamide, of whom 12 started post-HSCT and 15 received Chidamide during post-remission maintenance therapy. The median follow-up period was 37.8 months (9.5–67.9 months). 18 patients were not older than 10 years old. Male to Female ratio was 8: 1. Three of them were diagnosed with CNS involvement and stratified as HR at the first diagnosis. According to the risk stratification criteria, 18 patients were treated according to the protocol of HR T-ALL, among which 12 patients received HSCT. The characteristics of individual patients treated with Chidamide are shown in Table 1.
Risk-stratified outcomes
Up to the observation time points, 25 of the children receiving Chidamide were in continuous remission. The survival curves are shown in Figure 1. At the end of the 36-month follow-up period, the OS rate was 94.1%, while the EFS rate was 95.2%. In the IR group, both the OS and EFS rates of patients with Chidamide were 100%. One IR patient relapsed in central nervous system and bone marrow subsequently in 30 months after the end of chemotherapy. In the HR group, the OS rates of patients with Chidamide was 90.9%, while the EFS rate was 92.9%. One ETP-ALL patients got relapse in 14 months post-HSCT and deceased in 4 months later.
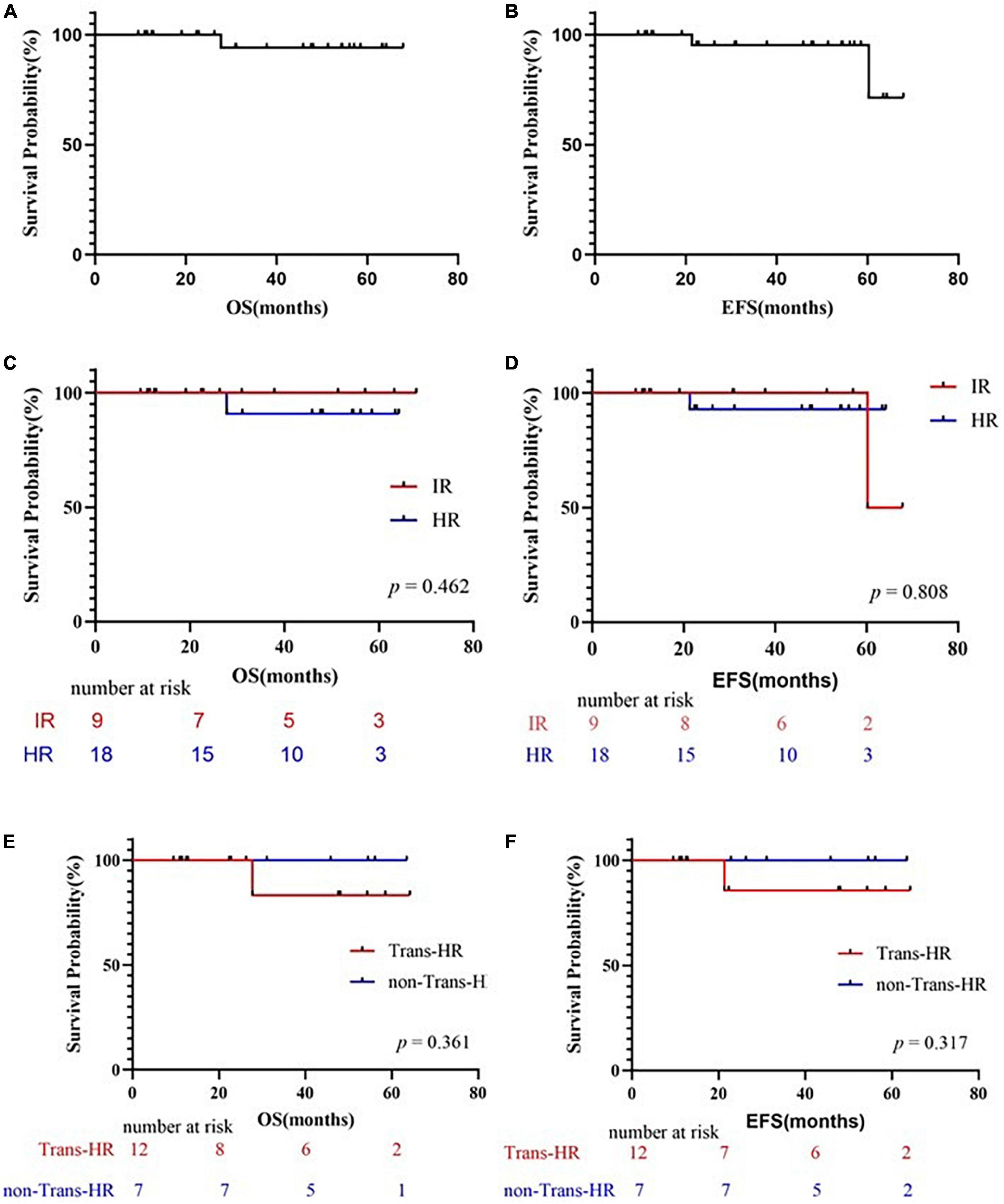
Figure 1. Survival curves. (A) Probability of overall survival (OS) for patients treated with Chidamide. (B) Probability of EFS for patients treated with Chidamide. (C) Comparing the probability of OS for patients treated with Chidamide in IR group and HR group. (D) Comparing the probability of EFS for patients treated with Chidamide in IR group and HR group. (E) Comparing the probability of OS for patients treated with or without HSCT. (F) Comparing the probability of EFS for patients treated with or without HSCT. OS, overall survival; EFS, event-free survival; IR, intermediate-risk; HR, high-risk; HSCT, hematopoietic stem cell transplantation; Trans, treated with HSCT; non-Trans-HR, HR but not treated with HSCT.
HSCT management and outcomes
Most of the HSC donors were unrelated donors (UDs; 9/12; 75%). One was HLA-matched unrelated donors (MUDs), and 8 were mismatched unrelated donors. Two donors were matched sibling donors (MSDs). One donor was haploidentical related donors. The sources of HSCs used were cord blood (n = 7), peripheral blood stem cell (n = 4), and mixed graft (n = 1, P27). The conditioning regimens (Table 2) were classified as myeloablative regimens. TBI was used in three children, which accepted MSD transplantation (n = 2) or MUD transplantation (n = 1) (Table 2). Five cases of severe conditioning toxicity, not related to Chidamide, were reported: veno-occlusive disease (P24), profuse diarrhea (P19), gastrointestinal bleeding (P24), septicemia and subsequent acute cardiac insufficiency (P24), hepatic toxicity (P24), and grade III to IV hemorrhagic cystitis (P8, 24, 27), severe pneumonia (P12), peripheral neuritis after cyclophosphamide (P19, 27). Severe conditioning regimen adverse effects were well controlled and non-fatal. Graft failure was not reported. Donor lymphocyte infusion was not used among the included patients. TIT was not given post-HSCT
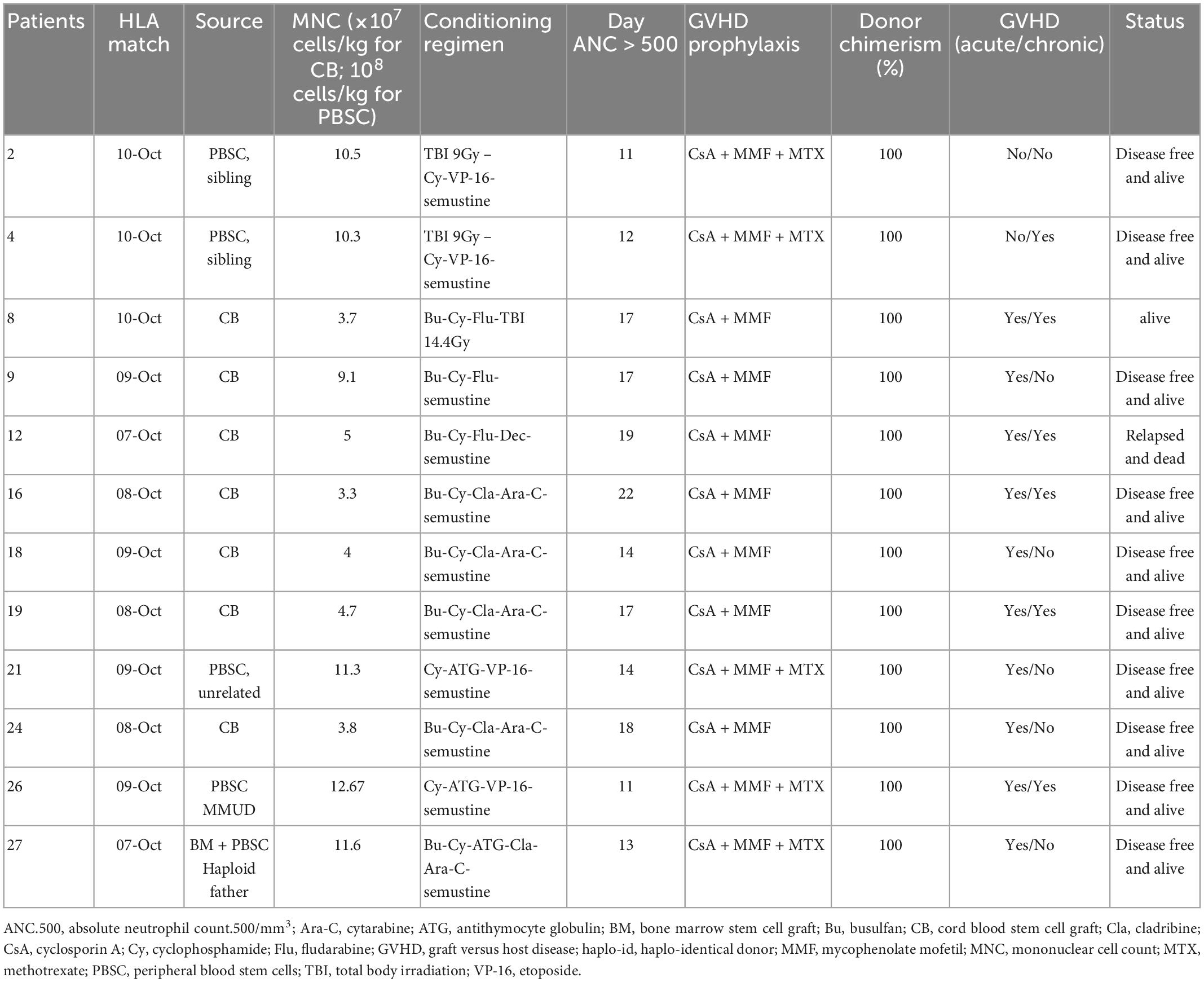
Table 2. Hematopoietic stem cell transplantation (HSCT) management and outcomes of the 12 high-risk patients.
In total, 12 patients accepted HSCT at their first complete remission, and accepted Chidamide post-HSCT regularly. The overall survival and event free survival rate were 83.33 and 85.7% at 64.2 months after transplantation (Figures 1E, F), respectively, with a median follow-up of 27.8 months in surviving children (range, 9.5–64.2 months). One deceased patient (P12) suffered from CNSL relapse at the sixth month post-HSCT, which proceeded to bone marrow relapse at the end. This relapsed patient died before second remission and did not receive second HSCT. Infection was a major complication in the population accepted HSCT. Bacterial, fungal, or virus infections occurred after HSCT in 42% (5/12) of the patients with successful engraftment, but only 2 of these patients had infections during Chidamide therapy after HSCT (P8: lung invasive fungal disease; P12: sinusitis and non-tuberculosis mycobacteria pneumonia). 10 out of the 12 transplanted patients suffered from acute graft versus host disease (GVHD) (83.3%), but only 2 of them had severe GVHD. The incidence of chronic GVHD was 50% (6/12).
Treatment-related adverse events
No death was reported over the first year of Chidamide combination therapy and subsequent deaths were all related to disease progression. The adverse events observed during treatment were nausea or vomiting (n = 6), neutropenia (n = 9), thrombocytopenia (n = 9), renal impairment (n = 1) and infection (Herpes Zoster, n = 1). The adverse events were all reversible when administration of Chidamide was stopped, and Chidamide therapy was continued with previous doses until relapse, or the end of maintenance therapy, or 2 years post-HSCT.
Discussion
In this study, we prospectively investigated the safety and efficacy of the combination of Chidamide with chemotherapy post-remission or post-HSCT in pediatric T-ALL patients. We show that the addition of Chidamide to the traditional regimen has promising effects in relapse prophylaxis. The most important findings in this study are (i) that the relapse rate in the high-risk group with or without HSCT was reduced and (ii) that a few severe adverse effects were found in the Chidamide group for post-HSCT and post-remission maintenance in pediatric T-ALL.
The long-term survival of patients with T-ALL relapse has been lower than 40%, even with intensive chemotherapy or post-HSCT (14). The significance of relapse prophylaxis has been recognized. However, the effectivity of relapse prophylaxis is limited, and the optimal strategy is controversial. The results in this study indicate the efficacy of Chidamide in relapse prophylaxis.
Preclinical study results indicated the promising efficacy of Chidamide. Chidamide may regulate immune function (15) and increase the sensitivity of T-ALL cells to chemotherapy drugs independent of NOTCH mutation (16). The mechanistic investigations indicated the higher efficacy of HDACi in HR and R/R T-ALL. In clinical studies, Chidamide in combination with chemotherapy has been proved effective in R/R T-cell lymphoblastic lymphoma/leukemia in adults and young adolescents (8, 11, 12). The efficacy of Chidamide in peripheral T-cell lymphoma suggests that Chidamide may play a role in more immature T-LBL/ALL (17, 18). In our study, all T-ALL patients except one showed persistent remission after maintenance chemotherapy resulting in a reduced relapse rate, similar to its effects in AML or R/R T-cell lymphoblastic lymphoma/leukemia studies (19), indicating a promising relapse prophylaxis option for T-ALL (20). The relapse rates in the IR and HR groups were similar. The results support that Chidamide can be included in remission prevention regimens and reduce the intensity of chemotherapy for T-ALL in the future (8, 21, 22). High-risk and refractory T-ALL are more likely to benefit from Chidamide combination chemotherapy.
Our results showed a lower incidence of relapse than previous studies in both adults and children. In the last decade, EFS and OS rates for T-ALL children in several large cohorts have been about 73–80% (23–25). For those who survived the early intensive chemotherapy, relapse rate and HSCT related mortality were still high (23–25). In this study, the EFS rate for T-ALL patients was up to 95.2 ± 4.6%, which was much higher than in previous reports. The exclusion of early fatal cases irrelevant to Chidamide was one important factor for this result. The most important risk factor for early mortality in our center was fatal complications during intensive chemotherapy in the first 6 months, especially in the HR group. The Chidamide group had a higher proportion of HR and HSCT acceptance than general T-ALL population, but Chidamide increased neither relapse related mortality nor HSCT related mortality. The good OS and EFS results in this study indicate the promising efficacy and safety of Chidamide.
There is concern that Chidamide cannot penetrate the brain blood barrier to the cerebrospinal fluid, which makes it difficult to prevent CNSL relapse through Chidamide. According to previous studies, multiple triple drugs intrathecal injections during intensive chemotherapy and maintenance chemotherapy, as adopted in our protocol, are the primary and reliable effective CNSL prophylaxis.
In previous study, for T-ALL patients who underwent alternative donor HSCT using a mismatched, umbilical cord blood, or haploidentical donor, OS rate at 5 years was only 34%, while non-relapse mortality and relapse was 26 and 41%, respectively (26), even the participation of TBI in conditioning regimens. Lately, it was reported that the 5-year leukemia free survival of T-ALL patients receiving HSCT at first complete remission was as high as 73.5%. However, once relapse occurred, the 5-year leukemia-free survival (LFS) was only 44.4% even if second remission was attained (27). If they accepted umbilical cord blood transplantation (UCBT) at first complete remission, TBI-based myeloablative conditioning presented LFS rate of 85.4%, as it reduced the risk of relapse, while other conditioning regimens prognosed worse (62.2%) (27). So, the LFS after UCBT for T-ALL were 65.8–85.4% (27, 28). The key points of long-term survival would be proper conditioning regimens, transplantation at the first complete remission and relapse prophylaxis. Donor cell transfusion would be an effective option, except for those with GVHD or those accepted UCBT, just like patients in this study. There is a new trend that post-HSCT antitumor medication participates in the relapse prophylaxis of leukemia therapy. Molecular targeted drugs, like tyrosine kinase inhibitors, demethylation therapy, Bcl-2 inhibitors, have been recommended (29–31). However, for most of T-ALL, there is no recommended medicine for post-HSCT relapse prophylaxis. Chidamide has been a new attempt, which has showed great potential according to the low post-HSCT relapse rate and the low HSCT related complications incidence.
With respects to observation end-points, since the follow-up period is now only 3 to 4 years, there is a possibility that some patients have not achieved the end-point of relapse. However, according to the Berlin–Frankfurt–Munster (BFM) protocol-based CCLG-ALL2008 protocol in China, relapse cases were reported mostly ultra-early (65%, in the first 18 months after first diagnosis), and early (30%, in the second 18 months after first diagnosis). Only less than 5% of relapse cases occurred after more than 36 months (32). These previous data indicated that the follow-up period of the present study is effective for observation for relapse rates. Further studies should include prolonged observation periods.
The dose of Chidamide was 0.5 mg/kg in this study. We adopted this dosage from previous studies, which included children younger than 18 years old (8, 12). The plasma concentration of Chidamide was not monitored. The adverse events observed during treatment were nausea or vomiting, neutropenia, thrombocytopenia, renal impairment and infections, which were similar to previous reports. It did not significantly increase the adverse effects of maintenance chemotherapy. Since the patients’ compliance was good, by monitoring WBC, hemoglobin levels, and platelet count, we adjusted the dosages of Chidamide. When grade 3 or worse bone marrow suppression occurred, we reduced the dosage or discontinued treatment. WBC between 2 × 109/L and 4 × 109/L is safe and efficient for maintenance therapy.
There are some limitations in this study. Considering the limited number of cases, a larger number of patients should be enrolled to further analyze the efficacy of Chidamide in T- ALL relapse prophylaxis. Furthermore, it was difficult to analyze the efficacy differences between subgroups based on, for example, gene mutation, risk stratification, and early T-cell precursor ALL (33). To further analyze the efficacy of Chidamide in different T-ALL subtypes, we need more large-scale prospective data.
In conclusion, we reported a series of cases of firstly diagnosed T-ALL who received Chidamide during maintenance regimens or post-HSCT with a remission rate of 95% in more than 3 years, showing promising efficacy in relapse prophylaxis. Maintenance regimens containing Chidamide are well tolerated in T-ALL children. The benefit of Chidamide in prolonged EFS deserves further study and confirmation by large-scale prospective clinical trials.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
J-PF designed the study. X-YL and X-WH analyzed the data and wrote the manuscript. X-YL, X-WH, and KH contributed to the retrieval and analysis of essential data. All authors contributed to the charts, critical revision, and final approval of the manuscript.
Funding
This work was supported in part by grants from the Bethune Medicine Scientific Research Fund Project (No. SCE111DS), the Science and Technology Project of Guangzhou (201803010032), and the 5010 Project of Clinical Trial of Sun Yat-sen University (2007016), and support from funding (SYSKY-2022-103-01).
Acknowledgments
We acknowledge the efforts of the staffs in each of the nine collaborative centers. We thank the hardworking nurses and loving parents who participated in this study. We admire the care and support from New Sunshine Charity Foundation and the staffs from the Follow-up center of Sun Yat-sen Memorial Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1096529/full#supplementary-material
Abbreviations
T-ALL, T-cell acute lymphoblastic leukemia; HSCT, hematopoietic stem cell transplantation; HDACi, histone deacetylase inhibitor; OS, overall survival; EFS, event-free survival; R/R, relapsed or refractory; CR, complete remission; IR, intermediate-risk; HR, high-risk; MRD, minimal residual disease; CNSL, central nervous system leukemia; WBC, white blood cell count; TIT, triple intrathecal therapy; CTX, cyclophosphamide; CNS, central nervous system; TBI, total-body irradiation; PBSC, peripheral blood stem cell; MUD, matched unrelated donor; MSD, matched sibling donor; GVHD, graft versus host disease; LFS, leukemia-free survival; UCBT, umbilical cord blood transplantation.
References
1. Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, et al. Childhood T-cell acute lymphoblastic leukemia: the Dana-farber cancer institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. (2003) 21:3616–22. doi: 10.1200/JCO.2003.10.116
2. Asselin BL, Devidas M, Wang C, Pullen J, Borowitz MJ, Hutchison R, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the children’s oncology group (POG 9404). Blood. (2011) 118:874–83. doi: 10.1182/blood-2010-06-292615
3. Schrauder A, Reiter A, Gadner H, Niethammer D, Klingebiel T, Kremens B, et al. Superiority of allogeneic hematopoietic stem-cell transplantation compared with chemotherapy alone in high-risk childhood T-cell acute lymphoblastic leukemia: results from ALL-BFM 90 and 95. J Clin Oncol. (2006) 24:5742–9. doi: 10.1200/JCO.2006.06.2679
4. Abaza Y, Kantarjian HM, Faderl S, Jabbour E, Jain N, Thomas D, et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma. Am J Hematol. (2018) 93:91–9. doi: 10.1002/ajh.24947
5. Richard-Carpentier G, Jabbour E, Short NJ, Rausch CR, Savoy JM, Bose P, et al. Clinical Experience with venetoclax combined with chemotherapy for relapsed or refractory T-cell acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. (2020) 20:212–8. doi: 10.1016/j.clml.2019.09.608
6. Gökbuget N, Basara N, Baurmann H, Beck J, Brüggemann M, Diedrich H, et al. High single-drug activity of nelarabine in relapsed T-lymphoblastic leukemia/lymphoma offers curative option with subsequent stem cell transplantation. Blood. (2011) 118:3504–11. doi: 10.1182/blood-2011-01-329441
7. West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. (2014) 124:30–9. doi: 10.1172/JCI69738
8. Guan W, Jing Y, Dou L, Wang M, Xiao Y, Yu L. Chidamide in combination with chemotherapy in refractory and relapsed T lymphoblastic lymphoma/leukemia. Leuk Lymphoma. (2020) 61:855–61. doi: 10.1080/10428194.2019.1691195
9. Mao J, Li S, Zhao H, Zhu Y, Hong M, Zhu H, et al. Effects of chidamide and its combination with decitabine on proliferation and apoptosis of leukemia cell lines. Am J Transl Res. (2018) 10:2567–78.
10. Kitadate A, Ikeda S, Abe F, Takahashi N, Shimizu N, Matsue K, et al. Histone deacetylase inhibitors downregulate CCR4 expression and decrease mogamulizumab efficacy in CCR4-positive mature T-cell lymphomas. Haematologica. (2018) 103:126–35. doi: 10.3324/haematol.2017.177279
11. Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. (2015) 26:1766–71. doi: 10.1093/annonc/mdv237
12. Shi Y, Jia B, Xu W, Li W, Liu T, Liu P, et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. (2017) 10:69.
13. Stary J, Zimmermann M, Campbell M, Castillo L, Dibar E, Donska S, et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol. (2014) 32:174–84. doi: 10.1200/JCO.2013.48.6522
14. Gökbuget N, Stanze D, Beck J, Diedrich H, Horst H, Hüttmann A, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. (2012) 120:2032–41. doi: 10.1182/blood-2011-12-399287
15. Conte M, De Palma R, Altucci L. HDAC inhibitors as epigenetic regulators for cancer immunotherapy. Int J Biochem Cell Biol. (2018) 98:65–74. doi: 10.1016/j.biocel.2018.03.004
16. Gryder BE, Sodji QH, Oyelere AK. Targeted cancer therapy: giving histone deacetylase inhibitors all they need to succeed. Future Med Chem. (2012) 4:505–24. doi: 10.4155/fmc.12.3
17. Jain N, Lamb AV, O’Brien S, Ravandi F, Konopleva M, Jabbour E, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood. (2016) 127:1863–9. doi: 10.1182/blood-2015-08-661702
18. Ma M, Wang X, Tang J, Xue H, Chen J, Pan C, et al. Early T-cell precursor leukemia: a subtype of high risk childhood acute lymphoblastic leukemia. Front Med. (2012) 6:416–20. doi: 10.1007/s11684-012-0224-4
19. Cao LH, Yu WJ, Fan CH, Lin YQ, Zhou MY, Lou JY, et al. Acute myeloid leukemia treated with chidamide combined regimens: two cases report. Zhonghua Xue Ye Xue Za Zhi. (2017) 38:967.
20. Sanda T, Li X, Gutierrez A, Ahn Y, Neuberg DS, O’Neil J, et al. Interconnecting molecular pathways in the pathogenesis and drug sensitivity of T-cell acute lymphoblastic leukemia. Blood. (2010) 115:1735–45. doi: 10.1182/blood-2009-07-235143
21. Gui L, Cao J, Ji D, Zhang H, Fan Q, Zhu J, et al. Chidamide combined with cyclophosphamide, doxorubicin, vincristine and prednisone in previously untreated patients with peripheral T-cell lymphoma. Chin J Cancer Res. (2021) 33:616–26.
22. Zhang W, Su L, Liu L, Gao Y, Wang Q, Su H, et al. The combination of chidamide with the CHOEP regimen in previously untreated patients with peripheral T-cell lymphoma: a prospective, multicenter, single arm, phase 1b/2 study. Cancer Biol Med. (2021) 18:841–8. doi: 10.20892/j.issn.2095-3941.2020.0413
23. Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the children’s oncology group. Blood. (2008) 111:2548–55. doi: 10.1182/blood-2007-02-070342
24. Stark B, Avrahami G, Nirel R, Abramov A, Attias D, Ballin A, et al. Extended triple intrathecal therapy in children with T-cell acute lymphoblastic leukaemia: a report from the Israeli national ALL-studies. Br J Haematol. (2009) 147:113–24. doi: 10.1111/j.1365-2141.2009.07853.x
25. Kobos R, Shukla N, Renaud T, Prockop SE, Boulad F, Steinherz PG. High-dose cyclophosphamide for the treatment of refractory T-cell acute lymphoblastic leukemia in children. J Pediatr Hematol Oncol. (2014) 36:e265–70. doi: 10.1097/MPH.0000000000000080
26. Hamilton BK, Rybicki L, Abounader D, Adekola K, Advani A, Aldoss I, et al. Allogeneic hematopoietic cell transplantation for adult T cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant. (2017) 23:1117–21. doi: 10.1016/j.bbmt.2017.04.003
27. Ishida H, Kato M, Kawahara Y, Ishimaru S, Najima Y, Kako S, et al. Prognostic factors of children and adolescents with T-cell acute lymphoblastic leukemia after allogeneic transplantation. Hematol Oncol. (2022) 40:457–68. doi: 10.1002/hon.2980
28. Kawahara Y, Ishimaru S, Tanaka J, Kako S, Hirayama M, Kanaya M, et al. Impact of KIR-ligand mismatch on pediatric T-cell acute lymphoblastic leukemia in unrelated cord blood transplantation. Transplant Cell Ther. (2022) 28:598.e1–8. doi: 10.1016/j.jtct.2022.05.037
29. Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. (2020) 38:2993–3002. doi: 10.1200/JCO.19.03345
30. Xuan L, Liu Q. Maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. (2021) 14:4. doi: 10.1186/s13045-020-01017-7
31. Kim K, Jabbour E, Short NJ, Kebriaei P, Kantarjian H, Ravandi F. Current approaches to philadelphia chromosome-positive B-cell lineage acute lymphoblastic leukemia: role of tyrosine kinase inhibitor and stem cell transplant. Curr Oncol Rep. (2021) 23:95. doi: 10.1007/s11912-021-01086-y
32. Liu XM, Chen XJ, Zou Y, Wang SC, Wang M, Zhang L, et al. Outcome of children with T cell acute lymphoblastic leukemia treated with Chinese children leukemia group acute lymphoblastic leukemia (CCLG-ALL) 2008 protocol. Zhonghua Er Ke Za Zhi. (2019) 57:761–6.
Keywords: Chidamide, T-cell acute lymphoblastic leukemia, children, chemotherapy, hematopoietic stem cell transplantation
Citation: Li X-Y, Han X-W, Huang K, Zhang Y-T, Xu H-G, Zhou D-H, Xu L-H and Fang J-P (2023) Chidamide as maintenance after chemotherapy or hematopoietic stem cell transplantation in 27 children with T-cell lymphoblastic leukemia: A real-world prospective study. Front. Med. 10:1096529. doi: 10.3389/fmed.2023.1096529
Received: 12 November 2022; Accepted: 16 January 2023;
Published: 02 February 2023.
Edited by:
Marcos De Lima, The Ohio State University, United StatesReviewed by:
Juana Serrano Lopez, Health Research Institute Foundation Jimenez Diaz (IIS-FJD), SpainAiming Pang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Li, Han, Huang, Zhang, Xu, Zhou, Xu and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Pei Fang,  ZmFuZ2pwZWlAbWFpbC5zeXN1LmVkdS5jbg==
ZmFuZ2pwZWlAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work
 Xin-Yu Li
Xin-Yu Li Xia-Wei Han
Xia-Wei Han Ke Huang
Ke Huang Ya-Ting Zhang
Ya-Ting Zhang Hong-Gui Xu2
Hong-Gui Xu2 Lu-Hong Xu
Lu-Hong Xu Jian-Pei Fang
Jian-Pei Fang