
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 30 May 2023
Sec. Dermatology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1093827
This article is part of the Research Topic Case Reports in Dermatology View all 23 articles
 Satoko Minakawa1,2*
Satoko Minakawa1,2* Yasushi Matsuzaki1
Yasushi Matsuzaki1 Shogo Yao1
Shogo Yao1 Chihiro Sagara1
Chihiro Sagara1 Eijiro Akasaka1
Eijiro Akasaka1 Hiroshi Koga3
Hiroshi Koga3 Norito Ishii3
Norito Ishii3 Takashi Hashimoto4
Takashi Hashimoto4 Daisuke Sawamura1
Daisuke Sawamura1We report a case of autoimmune bullous disease (AIBD) with IgG and IgM autoantibodies against epidermal basement membrane zone (BMZ), which showed recurrence of mucocutaneous lesions after coronavirus disease 2019 (COVID-19) mRNA vaccination. A 20-year-old Japanese woman with a 4-year history of epidermolysis bullosa acquisita (EBA) presented to our clinic. She noticed fever and rash on the same day and visited at our hospital 2 days later. Physical examination revealed blisters, erosions and erythema on the face, shoulder, back, upper arms, and lower lip. A skin biopsy from the forehead showed subepidermal blister. Direct immunofluorescence showed linear depositions of IgG, IgM, and C3c in the epidermal BMZ. By indirect immunofluorescence of 1M NaCl-split normal human skin, circulating IgG autoantibodies were bound to the dermal side of the split at 1:40 serum dilution, and circulating IgM antibodies were bound to the epidermal side of the spilt. After the increase of prednisolone dose to 15 mg/day, the mucocutaneous lesions resolved in a week. The present case is the first case of possible EBA with IgG and IgM anti-BMZ antibodies, in which the mucocutaneous lesions were recurred after COVID-19 mRNA vaccination. Clinicians should be aware that bullous pemphigoid-like AIBDs, including EBA and IgM pemphigoid, might be developed after COVID-19 mRNA vaccination.
Autoimmune bullous diseases (AIBDs), a group of tissue specific autoimmune disease of the skin, are classified into various subtypes with the different immunoglobulin types and autoantigens (1). Epidermolysis bullosa acquisita (EBA) is characterized clinically by blisters and scars of the skin and erosive mucosal lesions, and immunologically by IgG autoantibodies against type VII collaegen (2, 3). In many EBA cases, oral administration of corticosteroids are effective but do not lead to complete remission.
AIBDs have been reported to be induced or exacerbated after immunization with various vaccines, including vaccines for measles, varicella zoster, influenza, hepatitis B and human papillomavirus (4).
The rapid flare of a bullous diseases after vaccination is not novel. Bullous pemphigoid (BP) has been reported to occur within 24 hours after vaccination (5). In addition, among the 12 patients who newly developed subepidermal blistering lesions after the first or second coronavirus disease 2019 (COVID-19) mRNA vaccination, the diagnosis of BP was confirmed in eight patients by the results of direct immunofluorescence (DIF), indirect immunofluorescence (IIF) of 1 M NaCl-split skin and/or enzyme-linked immunosorbent assays (ELISA) of BP180 (6).
In this report, we present with a case of EBA with IgG and IgM autoantibodies against epidermal basement membrane zone (BMZ), which showed recurrence of mucocutaneous lesions 2 days after COVID-19 mRNA vaccination.
A 16-year-old Japanese female presented with a 1-month history of erosions on the lips. She had no medical history. Physical examination revealed blisters, erosions and erythema on the face (Figure 1a), lips, back, shoulders (Figure 1b) and arms (Figure 1c). Histopathology for a skin biopsy from the face showed subepidermal blisters. DIF showed linear depositions of IgG, IgM, and C3c in the epidermal BMZ (data not shown).
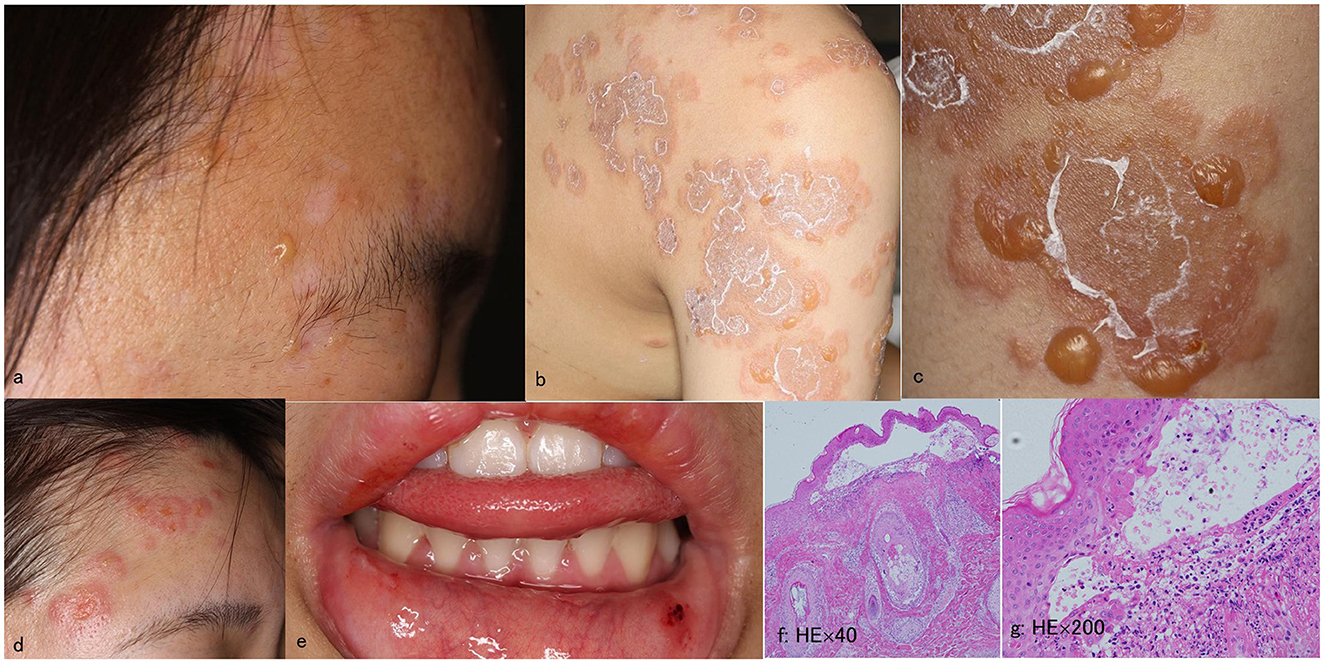
Figure 1. Clinical and histopathological features at the first visit and at the time of relapse. (a–e) Clinical features of blisters, erosions, and erythema on the forehead (a), shoulder (b), and upper arms (c) at the first visit, and those on the face (d), and lower lip (e) at the time of relapse. (f, g) Histopathology of the skin biopsy from the forehead at the time of relapse showing subepidermal blisters with infiltration of neutrophils in the blister and dermis [original magnifications; (f) ×40 and (g) ×200].
Circulating IgG anti-BMZ autoantibodies were detected by IIF of normal human skin, which bound to the dermal side of 1 M NaCl-split normal human skin at 1:40 serum dilution (Figure 2a). Circulating IgM autoantibodies were bound to the epidermal side of 1M NaCl-split normal human skin at 1:40 serum dilution (Figure 2b). IgG ELISAs (MBL, Japan) showed positive results for type VII collagen (index 39.76; cut-off < 6.14), but negative for desmoglein 1, desmoglein 3, BP180 and BP230. Immunoblotting of normal human dermal extract detected IgG antibodies to type VII collagen (Figure 2c), while IgG immunoblotting analyses of other substrates were negative. IgM immunoblotting analyses of normal human epidermal extracts, recombinant proteins of NC16a and C-terminal domains of BP180 and concentrated culture supernatant of HaCaT cells were negative.
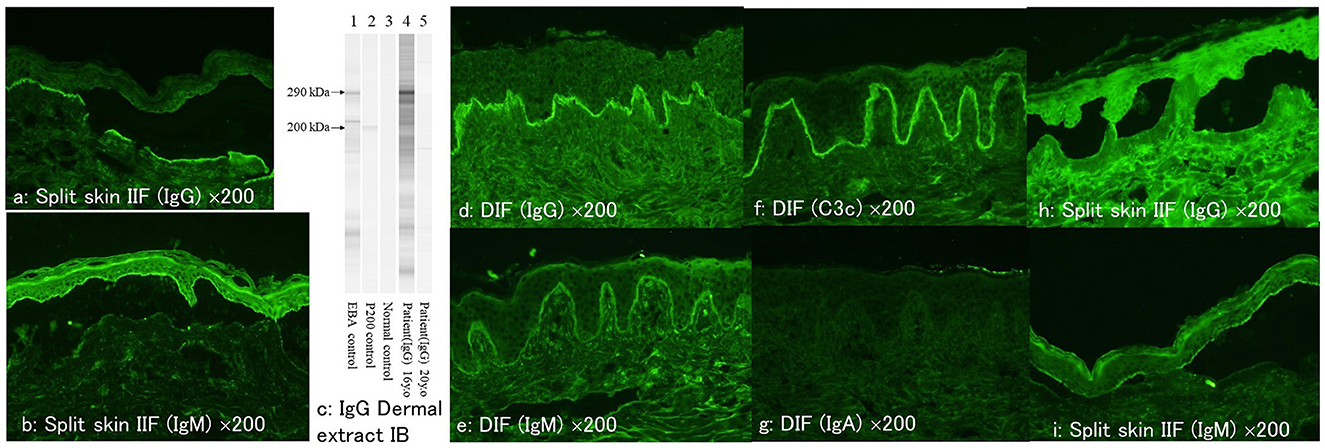
Figure 2. The result of various immunological tests at the first visit and at the time of relapse. (a, b) At the first visit, indirect immunofluorescence of 1M NaCl-split skin showing positive reactivity with dermal side for IgG antibodies (a) and with epidermal side for IgM antibodies (b). (c) The results of IgG immunoblotting of normal human dermal extract. The serum at the first visit (lane 4), but not the serum at the relapse (lane 5), reacted with the 290 kDa type VII collagen. (d–g) Direct immunofluorescence at the time of relapse showing positive results for IgG (d), IgM (e), and C3c (f) but negative for IgA (g). (h, i) At the time of relapse, indirect immunofluorescence of 1M NaCl-split skin showing positive reactivity with dermal side for IgG antibodies (h) and with epidermal side for IgM antibodies (i) (all of the immunofluorescence figures: original magnification, ×200).
The diagnosis of EBA with IgM antibodies to unknown antigen was made, and the initiation of oral prednisolone 30 mg/day gradually improved the skin and oral mucosal symptoms. However, the skin lesions were intaractable, and the patient stayed on prednisolone for the 4 years until the recurrence, when the patient was treated with prednisolone 2 mg/day.
Four years later, after the patient received first dose of COVID-19 mRNA vaccination, she first noticed fever on day 1 on the same day and then bullous skin lesions 2 days later. Then the patient visited at our hospital. Physical examination revealed blisters, erosions and erythema on the face (Figure 1d), shoulder, back, upper arms, and lower lip (Figure 1e). A skin biopsy from the forehead histopathologically showed subepidermal blisters (Figures 1f, g). DIF showed linear depositions of IgG, IgM, and C3c, but not IgA, in the epidermal BMZ (Figures 2d–g).
By IIF of 1M NaCl-split normal human skin, circulating IgG autoantibodies were bound to the dermal side of the split at 1:40 serum dilution (Figure 2h), and circulating IgM antibodies were bound to the epidermal side of the spilt (Figure 2i). IgG type VII collagen ELISA was negative (index 5.78; cut-off < 6.14). Immunoblotting of normal human dermal extract did not detect IgG antibodies to type VII collagen (Figure 2c). IgM immunoblotting analyses using the 4 substrates were negative. The results of various immunological tests both at the first visit 4 years before and after the COVID-19 mRNA vaccination were summarized in Table 1.
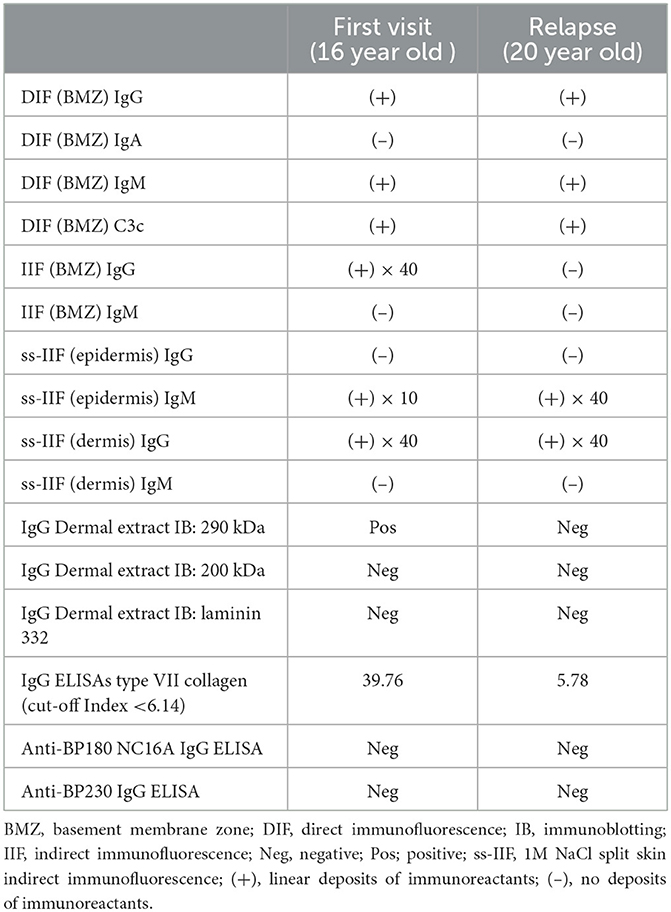
Table 1. The summary of the results of various immunological tests both at the first visit 4 years before and at the time of relapse after the COVID-19 vaccination.
After the increase of prednisolone dose form 2 to 15 mg/day, the mucocutaneous lesions resolved in a week.
COVID-19 mRNA vaccination has been reported to induce not only AIBDs but also many other skin conditions (7). From the positive results in DIF and IIF analyses, as well as the clinical features of prominent blisters, we considered that the present case might have had a relapse of EBA by COVID-19 mRNA vaccination 4 years after the first visit, although the 2 days may not be too short for the recurrence of autoimmune disease, and we could not detect specific autoantigens at this recurrent time.
Regarding the precise diagnosis for the present case, at the first visit, the results of various immunological tests, including positive IgG reactivity with type VII collagen, made the diagnosis of EBA, although IgM anti-BMZ antibodies co-existed.
The mucocutaneous lesions recurred after COVID-19 mRNA vaccination were most likely the recurrence of EBA from the positive IgG reactivity with dermal side of 1 M NaCl-split skin, although we could not detect IgG reactivity with type VII collagen. The reason for the failure to detect anti-type VII collagen might be either that the antibody titer was too low or that the epitope on type VII collagen had changed. The possibility of the change from EBA to the other AIBDs with IgG reactivity with dermal side, i.e., anti-laminin 332-type mucous membrane pemphigoid (MMP) or ant-p200 pemphigoid, is less conceivable.
Recently, Hirano et al. (8) proposed IgM pemphigoid, which showed anti-epidermal BMZ antibodies exclusive of IgM type, as a novel AIBDs entity. However, the disease entity of IgM pemphigoid has not been widely accepted. The clinical and histopathological characteristics, as well as the therapies and disease course, in IgM pemphigoid remain unknown. IgM autoantibodies in IgM pemphigoid tend to react with non-NC16A domain of BP180, probably C-terminal domain (8). Another possibility is that this case had IgM pemphigoid, and IgM anti-BMZ antibodies caused the mucocutaneous lesions both at the onset and at the time of relapse.
An older study suggested that IgM deposits (granular and linear) are present in the sun-exposed skin of healthy adults (n = 10/41) (9). Other studies also identified linear IgM deposits in various bullous and non-bullous skin disorders (9). Although the true pathogenic role of the IgM antibodies is unknown, the fact that IgM antibodies were present at both the initial and recurrence stages, while IgG anti-type VII collagen antibodies were not detected at the recurrence stage might suggest the pathogenic relevance of the IgM antibodies.
Previous studies reported that various autoimmune skin diseases, including AIBDs, developed skin lesions about 1 week after COVID-19 vaccination with some variation (10, 11). This case showed the recurrence only 2 days after vaccination, and therefore it may be difficult to conclude that the recurrence of the skin lesions is attributed to vaccination. Indeed, 2 days are too short for pathogenic plasma cells to produce autoantibodies.
However, there may be different mechanisms for the quick recurrence of the skin lesions. For example, the autoantibodies were persisted in the serum in this patient, and some triggers caused by vaccination might reactivate the pathogenic activity of the autoantibodies. One possibility might be similar to the mechanism in vancomycin-induced linear IgA disease, in which recurrence of the skin lesions occurred after few days of vancomycin intake, through the activation of preexisting IgA anti-collagen VII antibodies by binding of vancomycin to IgA antibodies (12).
In addition, a previous report described that the relapses of some skin diseases were seen very early (even on day 2) after COVID-19 vaccination. Therefore, we considered that this case might recur the previous autoimmune skin disease after vaccination (13).
Characteristic clinical features in our patient were prominent vesicular erythematous skin lesions on the face and severe oral mucosal lesions. Skin lesions on the face are rarely seen in both common BP and EBA (2, 3, 14–17). In addition, oral mucosal lesions are frequently seen in EBA, but are uncommon in BP (16, 17). Therefore, the prominent facial skin lesions and severe oral mucosal lesions in our patient might be cause by COVID-19 vaccination or IgM anti-BMZ autoantibodies.
EBA is a heterogeneous disease (18). In our case, the lesions are present on the mucosa and the face which is not common in normal BP. The cutaneous manifestations in EBA can be classified into two major clinical subtypes: non-inflammantory (classical or mechanobullous) and inflammatory EBA, which is characterized by cutaneous inflammation, resembling BP, linear IgA disease, MMP, or Brunsting-Perry pemphigoid (15).
The facial involvement is not uncommon, especially in the Brunsting-Perry pemphigoid-like variant of EBA, which is confined to the head and neck, was originally described in 1957 in seven patients with localized cicatricial pemphigoid (19). Similar findings have also been reported in patients with BP and EBA (20, 21). All these disorders have a common subepidermal clefting or blistering and linear IgG/complement deposition by DIF with autoantibodies against variable BMZ components such as BP180, BP230, laminin-332, and collagen VII (22).
The cutaneous manifestations of an individual EBA patients may change during the course of disease, or the same patients may present with two different forms simultaneously (14). Many EBA patients have not to lead to complete remission by the treatment (23). If disease activity cannot be controlled. Oral lesions are most commonly observed in patients with non-inflammatory EBA and those with MMP-like or LAD-like inflammatory EBA. Hence, we diagnosed her as the recurrence of non-inflammatory EBA.
In viral infections, IgM develops early stage. IgM levels increased during the first week after SARS-CoV-2 infection, peaked 2 weeks and then reduced to near-background levels in most patients (24). IgG was detectable after 1 week and was maintained at a high level for a long period (24).
In conclusion, the present case is the first case of possible EBA with IgG and IgM anti-BMZ antibodies, in which the mucocutaneous lesions were recurred 2 days after COVID-19 mRNA vaccination. Clinicians should be aware that BP-like AIBDs, including EBA and IgM pemphigoid, might be developed after COVID-19 mRNA vaccination. However, because corticosteroid treatment might have a beneficial effect on COVID-19, abrupt termination or quick dose reduction of systemic corticosteroids should be avoided in severe AIBDs (25).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The Committee of Medical Ethics of Hirosaki University Graduate School of Medicine approved the research study. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the patient for the publication of this case report.
TH and SM contributed to conception and organized the data. SY and CS contributed to collect samples. NI and HK performed the statistical analysis. SM wrote the first draft of the manuscript. TH, YM, and EA editing the manuscript. DS decided to submit report for publication. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported in part by A Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (project 20k08684 to SM) and by the 2022 Hirosaki University Research Support System.
We are grateful to the patient for providing written informed consent to publication. We express our gratitude to Mrs. Mayumi Tamada and Mrs. Kyoko Hiromatsu, Department of Dermatology, Kurume University School of Medicine, Fukuoka, Japan for immunoblotting analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AIBDs, autoimmune bullous diseases; BMZ, basement membrane zone; BP, bullous pemphigoid; COVID-19, coronavirus disease 2019; DIF, direct immunofluorescence; EBA, epidermolysis bullosa acquisita; ELISA, enzyme-linked immunosorbent assays; IIF, indirect immunofluorescence; MMP, mucous membrane pemphigoid; ss-IIF, salt split skin indirect immunofluorescence.
1. Hashimoto T, Tsuruta D, Koga H, Fukuda S, Ohyama B, Komai A, et al. Summary of results of serological tests and diagnoses for 4774 cases of various autoimmune bullous diseases consulted to Kurume University. Br J Dermatol. (2016) 175:953–65. doi: 10.1111/bjd.14692
2. Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. (2017) 13:157–69. doi: 10.1080/1744666X.2016.1221343
3. Koga H, Prost-Squarcioni C, Iwata H, Jonkman MF, Ludwig RJ, Bieber K. Epidermolysis bullosa acquisita: the 2019 update. Front Med. (2018) 5:362. doi: 10.3389/fmed.2018.00362
4. Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. (2022) 86:1160–4. doi: 10.1016/j.jaad.2021.04.061
5. Agharbi F-Z, Eljazouly M, Basri G, Faik M, Benkirane A, Albouzidi A, et al. Bullous pemphigoid induced by the AstraZeneca COVID-19 vaccine. Ann Dermatol Venereol. (2022) 149:56–7. doi: 10.1016/j.annder.2021.07.008
6. Tomayko MM, Damsky W, Fathy R, McMahon DE, Turner N, Valentin MN, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. (2021) 148:750–1. doi: 10.1016/j.jaci.2021.06.026
7. McMahon DE, Kovarik CL, Damsky W, Rosenbach M, Lipoff JB, Tyagi A, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. (2022) 86:113–21. doi: 10.1016/j.jaad.2021.09.002
8. Hirano Y, Iwata H, Tsujuwaki M, Mai S, Mai Y, Imafuku K, et al. Super-resolution imaging detects BP180 autoantigen in immunoglobulin M pemphigoid. Dermatol. (2022) 49:374–8. doi: 10.1111/1346-8138.16260
9. Leibold AM, Bennion S, David-Bajar K, Schleve MJ. Occurrence of positive immunofluore scence in the dermo-epidermal junction of sun-exposed skin of normal adults. J Cutan Pathol. (1994) 21:200–6. doi: 10.1111/j.1600-0560.1994.tb00261.x
10. Sprow G, Afarideh M, Dan J, Feng R, Keyes E, Grinnell M, et al. Autoimmune skin disease exacerbations following COVID-19 vaccination. Front Immunol. (2022) 13:899526. doi: 10.3389/fimmu.2022.899526
11. Gambichler T, Hamdani N, Budde H, Sieme M, Skrygan M, Scholl L, et al. Bullous pemphigoid after SARS-CoV-2 vaccination: spike-protein-directed immunofluorescence confocal microscopy and T-cell-receptor studies. Br J Dermatol. (2022) 186:728–31. doi: 10.1111/bjd.20890
12. Yamagami J, Nakamura Y, Nagao K, Funakoshi T, Takahashi H, Tanikawa A, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. (2018) 138:1473–80. doi: 10.1016/j.jid.2017.12.035
13. Ishikawa O, Nishio M, Ishibuchi T. Clinical analyses of 22 cases with cutaneous reactions after COVID-19 vaccination. Jpn J Dermatol. (2021) 131:2595–604. (in Japanese). doi: 10.14924/dermatol.131.2595
14. Palestine RF, Kossard S, Dicken CH. Epidermolysis bullosa acquisita: a heterogeneous disease. J Am Acad Dermatol. (1981) 5:43–53. doi: 10.1016/S0190-9622(81)70076-8
15. Ludwig RJ. Clinical presentation, pathogenesis, diagnosis, and treatment of epidermolysis bullosa acquisita. ISRN Dermatol. (2013) 2013:812029. doi: 10.1155/2013/812029
16. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. (2013) 381:320–32. doi: 10.1016/S0140-6736(12)61140-4
17. Daniel BS, Murrell DF. Review of autoimmune blistering diseases: the pemphigoid diseases. J Eur Acad Dermatol Venereol. (2019) 33:1685–94. doi: 10.1111/jdv.15679
18. Ishii N, Hamada T, Dainichi T, Karashima T, Nakama T, Yasumoto S, et al. Epidermolysis bullosa acquisita: what's new? J Dermatol. (2010) 37:220–30. doi: 10.1111/j.1346-8138.2009.00799.x
19. Brunsting LA, Perry HO. Benign pemphigold; a report of seven cases with chronic, scarring, herpetiform plaques about the head and neck. AMA Arch Dermatol. (1957) 75:489–501. doi: 10.1001/archderm.1957.01550160015002
20. Asfour L, Chong H, Mee J, Groves R, Singh M. Epidermolysis bullosa acquisita (Brunsting-Perry pemphigoid variant) localized to the face and diagnosed with antigen identification using skin deficient in type VII collagen. Am J Dermatopathol. (2017) 39:e90–6 doi: 10.1097/DAD.0000000000000829
21. Reis Gavazzoni Dias MF, Aparecida Guedes Vilar E, de Oliveira Bento C, et al. Brunsting-Perry type pemphigoid causing secondary cicatricial alopecia in 2 patients. Skin Appendage Disord. (2018) 4:308–11. doi: 10.1159/000485570
22. Rahbar Z, Cohen JN, McCalmont TH, LeBoit PE, Connolly MK, Berger T, et al. Cicatricial pemphigoid Brunsting-Perry variant masquerading as neutrophil-mediated cicatricial alopecia. J Cutan Pathol. (2022) 49:408–11. doi: 10.1111/cup.14177
23. Kim JH, Kim YH, Kim S, Noh EB, Kim S-E, Vorobyev A, et al. Serum levels of anti-type VII collagen antibodies detected by enzyme-linked immunosorbent assay in patients with epidermolysis bullosa acquisita are correlated with the severity of skin lesions. J Eur Acad Dermatol Venereol. (2013) 27:e224–30. doi: 10.1111/j.1468-3083.2012.04617.x
24. Hou H, Wang T, Zhang B, Luo Y, Mao L, Wang F, et al. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl Immunol. (2020) 9:e01136. doi: 10.1002/cti2.1136
Keywords: autoimmune blistering diseases, EBA, immunofluorescence, enzyme-linked immunosorbent assay (ELISA), type VII collagen
Citation: Minakawa S, Matsuzaki Y, Yao S, Sagara C, Akasaka E, Koga H, Ishii N, Hashimoto T and Sawamura D (2023) Case report: A case of epidermolysis bullosa acquisita with IgG and IgM anti-basement membrane zone antibodies relapsed after COVID-19 mRNA vaccination. Front. Med. 10:1093827. doi: 10.3389/fmed.2023.1093827
Received: 09 November 2022; Accepted: 02 May 2023;
Published: 30 May 2023.
Edited by:
Robert Gniadecki, University of Alberta, CanadaCopyright © 2023 Minakawa, Matsuzaki, Yao, Sagara, Akasaka, Koga, Ishii, Hashimoto and Sawamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoko Minakawa, minakawas@yahoo.co.jp
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.