
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 23 February 2023
Sec. Geriatric Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1090829
Background: Stroke-associated infection (SAI) is a common complication after a stroke. The incidence of infection was higher in people with sarcopenia than in the general population. However, the relationship between pre-stroke sarcopenia risk and SAI in older patients has not been confirmed. This study aimed to investigate the association between pre-stroke sarcopenia risk and SAI in older patients with acute ischemic stroke (AIS).
Methods: This retrospective study was conducted by the Peking University People’s Hospital. We evaluated the pre-stroke sarcopenia risk by applying the SARC-F questionnaire. Multivariate logistic regression was applied to explore the association between pre-stroke sarcopenia risk and SAI.
Results: A total of 1,002 elder patients with AIS (592 men; 72.9 ± 8.6 years) were enrolled in our study. Pre-stroke sarcopenia risk was found in 29.1% of the cohort. The proportion of patients with pre-stroke sarcopenia risk was larger in the SAI group than in the non-SAI group (43.2 vs. 25.3%, p < 0.001). In multivariate logistic analysis, pre-stroke sarcopenia risk was shown to be independently associated with SAI (OR = 1.454, 95% CI: 1.008–2.097, p = 0.045) after adjusting for potential factors. This association remained consistent across the subgroups based on age, sex, body mass index, smoking status, drinking status, diabetes, hypertension, and dyslipidemia.
Conclusion: Pre-stroke sarcopenia risk was independently associated with SAI in older patients with AIS. Our findings highlight the significance of pre-stroke sarcopenia identification in the prevention and management of SAI in this population.
Stroke-associated infection (SAI) is a common complication after stroke (1). The rate of SAI was reported to be 30% (95% CI: 24–36%), with pneumonia and urinary tract infections (UTI) being the most common (2). SAI is associated with short-term mortality and adverse neurological functions (3, 4). It also has long-lasting negative effects on patients’ survival (5).
Sarcopenia was estimated in 15.8% of patients with acute stroke (6). Notably, pre-stroke sarcopenia is independently associated with severe disability and high mortality rates (7, 8). In addition, skeletal muscle has been considered an essential regulator of immune system function (9). Developing sarcopenia increased 3.88 times the risk of community-acquired pneumonia (10). Moreover, the risk of infection was increased by 49.6% [odds ratio (OR) = 1.496, 95% confidence interval (CI): 1.102–2.031] in patients with diabetes and sarcopenia than in those without (11). Therefore, it is necessary to pay attention to the risk of SAI in patients with sarcopenia and acute ischemic stroke (AIS).
Muscle mass and quality evaluation occupy an important position in diagnosing sarcopenia (12). However, acute stroke often led to a sudden disorder of physical function and consciousness. The diagnosis of sarcopenia is difficult to be performed in such a clinical setting. SARC-F is a brief questionnaire for sarcopenia screening and frees researchers from measuring muscle function (13–15). Therefore, we aimed to determine whether the sarcopenia risk defined by SARC-F is a risk factor for SAI in older patients after AIS.
We retrospectively enrolled the older patients suffering from AIS in the Peking University People’s Hospital from September 2019 to February 2022. The inclusion criteria were as follows: age ≥ 60 years, admission within 24 h of symptom onset, and a neurological deficit symptom with infarction lesion on imaging. The exclusion criteria included the following: (1) intracranial hemorrhage or subarachnoid hemorrhage; (2) cannot complete the SARC-F questionnaire due to dementia, aphasia, and loss of consciousness; (3) active infection within the last 2 weeks; (4) premorbid stroke-related disability; (5) active malignancy; and (6) recent history of trauma or surgery.
Data were abstained at the time of presentation, including age, sex, body mass index (BMI), smoking status, drinking status, stroke etiology, National Institutes of Health Stroke Scale (NIHSS) score, diabetes, hypertension, dyslipidemia, hyperuricemia, coronary artery disease, chronic heart failure, and chronic obstructive pulmonary disease. According to the Trial of Org 10,172 in Acute Stroke Treatment classification, AIS was categorized into five etiologies: large-artery atherosclerosis, cardioembolism, small vessel occlusion, other determined etiologies, and undetermined etiology (16). Blood samples were taken within 24 h of admission. Laboratory data were collected, including hemoglobin (HB), fast blood glucose, serum creatinine, estimated glomerular filtration rate (eGFR), uric acid, total bilirubin, direct bilirubin, serum album (ALB), alanine aminotransferase, total cholesterol, total glyceride, and D-dimmer.
To evaluate pre-stroke sarcopenia risk, patients were asked to complete the SARC-F questionnaire by recalling within 2 days of admission. SARC-F is a simple questionnaire for rapidly identifying people at risk of sarcopenia (13). It has five items: strength, assistance in walking, rising from a chair, climbing stairs, and falls. Each item will be assigned a score of 0–2 (15), depending on the individual physical performance before stroke onset. The SARC-F score range is 0 to 10. Patients were categorized as having pre-stroke sarcopenia risk if the SARC-F score ≥ 4 (15).
Stroke-associated infection was defined as any new infection within the first week of AIS onset (17, 18). Patients diagnosed with the infection must meet the modified Centers for Disease Control and Prevention criteria (19). SAI was classified into three types, including pneumonia, UTI, and other infection. Pneumonia was suspected when there were relevant clinical symptoms and leukocytosis (>11 × 109/L) and confirmed with an infiltrate on the chest radiograph. UTI was diagnosed according to the urinary tract symptoms and positive microbiological cultures (using midstream urine). Other infection diagnoses were made based on their corresponding diagnostic criteria.
Statistical analyses were conducted using IBM SPSS Statistics version 22.0 (IBM Corp), R project for Statistical Computer version 4.1.2,1 and MedCalc Statistical Software version 20.1.0 (MedCalc Software Ltd). A p-value of <0.05 was defined as statistically significant. The differences between the two groups in continuous variables were evaluated by the t-test or Mann–Whitney U test. The comparisons of categorical variables were performed using Fisher’s exact test and Pearson’s chi-square test. Univariate logistic regression analysis was used to screen for the potential factors associated with SAI. Then, the factors with a value of p of <0.05 were added to stepwise multivariate logistic regression to explore the independent factors of SAI.
A total of 1,002 patients were enrolled in our research cohort after applying the selection criteria (Figure 1). There were 215 (21.5%) patients suffering from SAI during hospitalization, 104 of which were diagnosed with pneumonia, 66 with UTI, 42 with other infections, and 3 with both pneumonia and UTI. Patient’s characteristics classified by pre-stroke sarcopenia risk are shown in Table 1. The sarcopenia risk group was older (p < 0.001) and had a higher NIHSS score (p < 0.001) than its counterpart. Patients with sarcopenia risk had a higher level of serum creatinine and lower levels of HB, eGFR, and ALB than those without (all p ≤ 0.05). In addition, the proportion of coronary artery disease was bigger in the sarcopenia risk group (p = 0.005).
Figure 2 shows the distribution of the SARC-F score before stroke onset in patients. Pre-stroke sarcopenia risk identified by the SARC-F questionnaire (≥4 points) was found in 29.2% of older patients with AIS. The proportion of patients with pre-stroke sarcopenia risk was larger in the SAI group than in the non-SAI group (43.2 vs. 25.3%, p < 0.001). Similarly, the ratios of pre-stroke sarcopenia risk were higher in both patients with pneumonia (41.1 vs. 25.3%) and UTI (47.8 vs. 25.3%) than those without SAI (all p ≤ 0.05). Patients with other infections had a higher rate of sarcopenia risk compared with those without infection (38.1 vs. 25.3%), though the difference was not significant (p = 0.065).
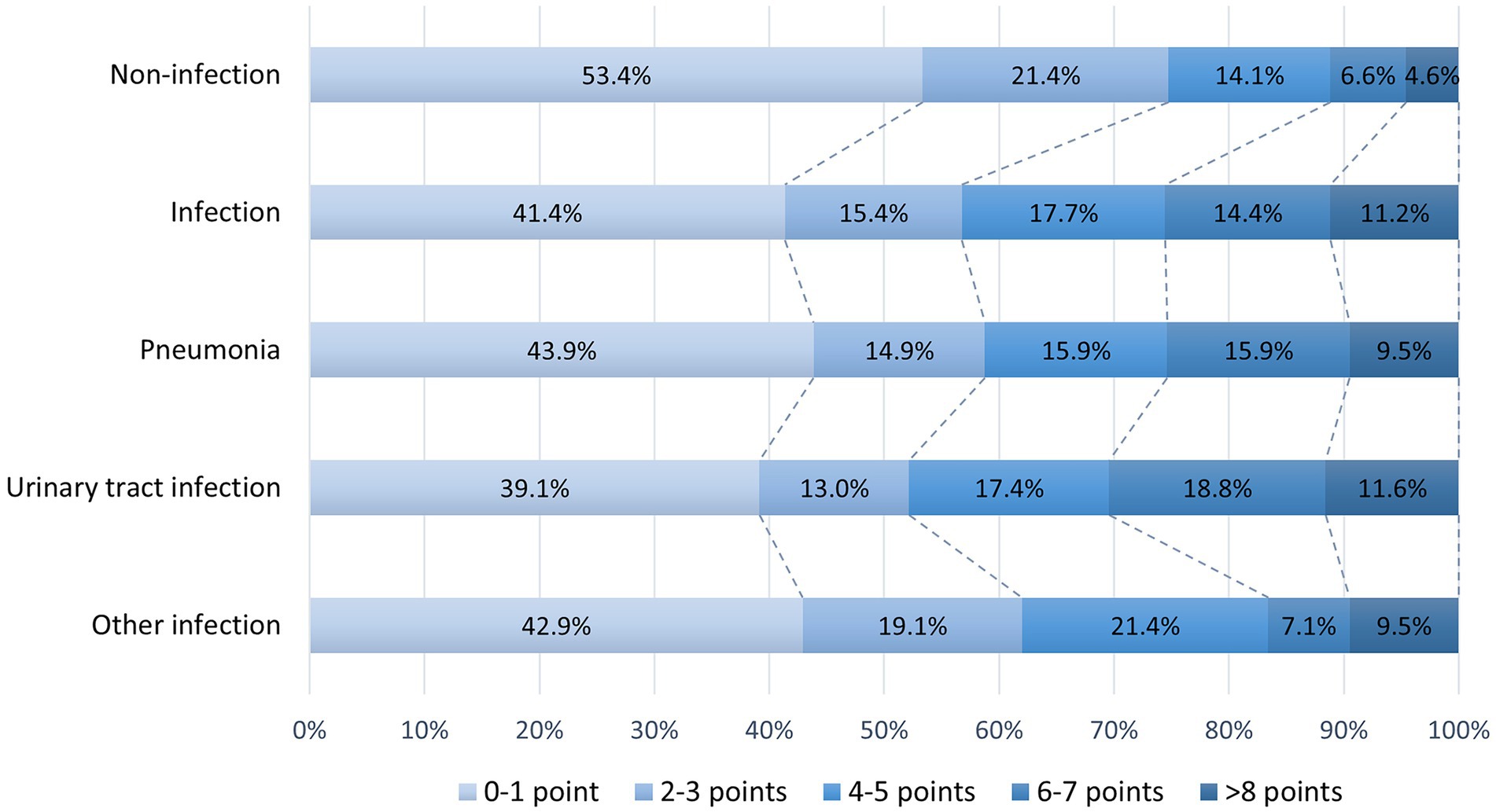
Figure 2. Distribution of SARC-F scores in patients with and without SAI. SAI, stroke-associated infection.
In univariate logistic regression analysis, the OR of SAI was 2.252 (95% CI, 1.645–3.084, p < 0.001) for patients with pre-stroke sarcopenia risk. The association between pre-stroke sarcopenia risk and SAI persisted (OR = 1.454, 95% CI: 1.008–2.097, p ≤0.05) after adjustment for age, BMI, NIHSS score at admission, diabetes, and ALB (Table 2). Subgroup analyses were performed to investigate whether the effect of sarcopenia risk on SAI occurrence was consistent among different patients (Figure 3). Pre-stroke sarcopenia risk did not increase the risk of SAI in patients with NIHSS score > 16 (p = 0.605). However, pre-stroke sarcopenia risk was significantly associated with SAI among diverse groups categorized by age, sex, BMI, smoking status, drinking status, diabetes, hypertension, and dyslipidemia (all p < 0.05) (Figure 3).
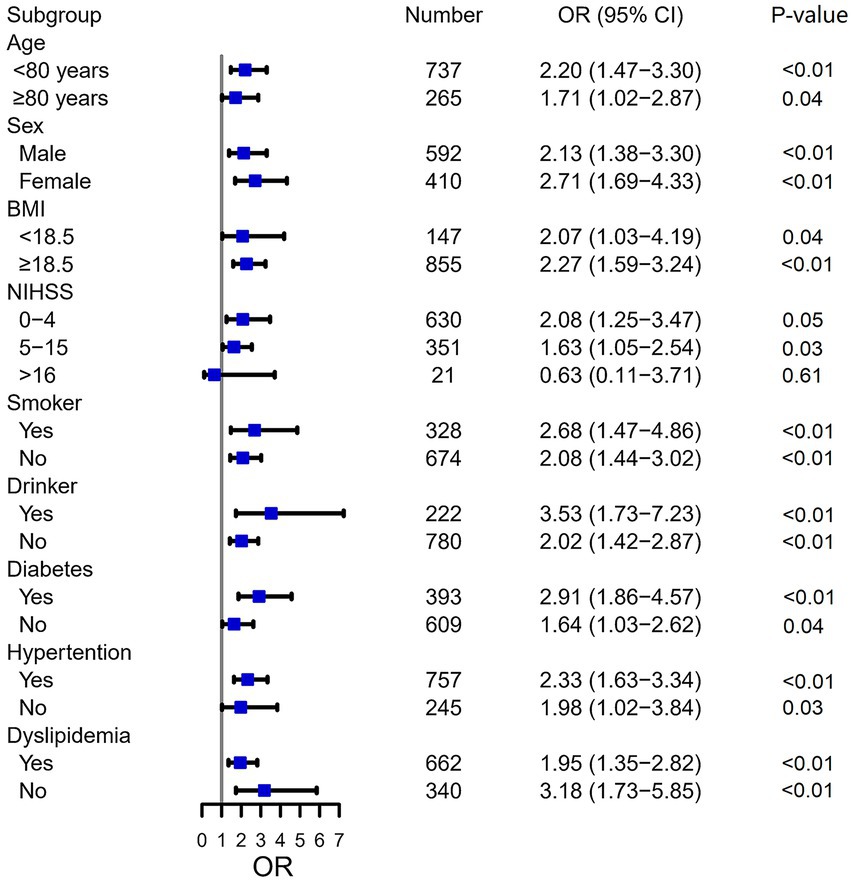
Figure 3. Subgroup analysis of sarcopenia risk for SAI. SAI, stroke-associated pneumonia; OR, odds ratio; CI, confidence interval; BMI, body mass index; NIHSS, National Institutes of Health Stroke Scale.
Our research revealed that pre-stroke sarcopenia risk was independently associated with SAI in older patients with AIS. Moreover, the association remained significant in diverse subgroups categorized by clinical characteristics. These results indicate the significance of pre-stroke sarcopenia identification in the prevention and management of SAI in elders.
In Chinese hospitalized elder adults, the prevalence of sarcopenia for men and women was 29.7% (95% CI 18.4–41.1%) and 23% (95% CI 17.1–28.8%), respectively (20). Consistently, pre-stroke sarcopenia risk was found in 29.2% of older patients with AIS in the present study. Sarcopenia is an age-related process occurring in older adults (21). Hence, it is not surprising that patients with pre-stroke sarcopenia risk were older than those without sarcopenia risk. Additionally, patients with sarcopenia risk had lower levels of ALB and HB compared with their counterparts in our study. The low level of ALB and HB tends to suggest malnutrition, which is an independent risk factor for sarcopenia in the elderly (22).
Age, NIHSS score, diabetes, and ALB were confirmed to be associated with SAI in the previous research (17, 23). After adjusting for these potential factors, pre-stroke sarcopenia risk was still an independent predictor (OR = 1.454, 95% CI: 1.008–2.097) for SAI in the present study. In line with our results, sarcopenia was shown to be an essential predictor for postoperative infections (24–26). In addition, sarcopenia doubles the risk of nosocomial infection in elder people admitted to acute care after 3 weeks of hospitalization (27). Skeletal muscle is increasingly accepted as a regulator of the immune system (9). It can modulate immune functions by secreting myokines, such as IL-7 and IL-15 (9). IL-7 is considered an essential signal for the survival and expansion of mature T cells (28). IL-15 plays a key role in the proliferation, activation, and distribution of natural killer cells and CD8 T cells (29). Moreover, stroke can result in an impairment of the defense mechanisms (1). Thus, we presumed that sarcopenia might aggravate the impaired immune response to pathogens in older patients with AIS.
Apart from impaired immune function, the decline in muscle quantity and quality might be another non-negligible cause of SAI. The tongue muscle is considered an essential swallowing-related muscle. Sarcopenia is associated with swallowing disorder in older adults (30, 31), partly due to a decrease in the mass of tongue muscles (32). Moreover, swallowing dysfunction increased the incidence of aspiration in the elderly, leading to airway colonization of Gram-negative bacteria (33). Thus, sarcopenia may contribute to pneumonia in patients with AIS through the same pathogenesis. Besides pneumonia, UTI (6.9%) was the second most common infection in our cohort. Urinary incontinence rate was elevated in older women with sarcopenia, with the declining function of pelvic muscles being the possible reason (34). Notably, urinary incontinence may increase the risk of UTI in the older population (35, 36). Hence, patients with sarcopenia risk were more likely to have UTI in our study.
There are some limitations to our study. First, we eliminated the patients with aphasia, dementia, and consciousness disorder since they could not complete a questionnaire. The selected bias may limit the generalizability of our findings. Second, the pre-sarcopenia risk was diagnosed based on the SARC-F questionnaire. It was considered a screening tool with low sensitivity but high specificity (15). For this reason, the number of sarcopenia in our cohort may be underestimated. Third, this is a single-center retrospective study with a limited sample size. Multicenter prospective studies are expected to validate the association between pre-stroke sarcopenia risk and SAI in older patients with AIS.
Pre-stroke sarcopenia risk was independently associated with SAI in older patients with AIS. Our findings highlight the significance of pre-stroke sarcopenia identification in the prevention and management of SAI in older people.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking University People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JB was involved in the conception and design of the study. JZ contributed to the data analysis and interpretation. XS and XC collected the clinical data and wrote the manuscript. All authors discussed and approved the final manuscript.
We would like to thank all the participants of this study for their contribution.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Faura, J, Bustamante, A, Miró-Mur, F, and Montaner, J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation. (2021) 18:127. doi: 10.1186/s12974-021-02177-0
2. Westendorp, WF, Nederkoorn, PJ, Vermeij, JD, Dijkgraaf, MG, and de Beek, D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. (2011) 11:110. doi: 10.1186/1471-2377-11-110
3. Hong, KS, Kang, DW, Koo, JS, Yu, KH, Han, MK, Cho, YJ, et al. Impact of neurological and medical complications on 3-month outcomes in acute ischaemic stroke. Eur J Neurol. (2008) 15:1324–31. doi: 10.1111/j.1468-1331.2008.02310.x
4. Suda, S, Aoki, J, Shimoyama, T, Suzuki, K, Sakamoto, Y, Katano, T, et al. Stroke-associated infection independently predicts 3-month poor functional outcome and mortality. J Neurol. (2018) 265:370–5. doi: 10.1007/s00415-017-8714-6
5. Kwan, J, Pickering, RM, Kunkel, D, Fitton, C, Jenkinson, D, Perry, VH, et al. Impact of stroke-associated infection on long-term survival: a cohort study. J Neurol Neurosurg Psychiatry. (2013) 84:297–304. doi: 10.1136/jnnp-2012-302552
6. Inoue, T, Ueshima, J, Kawase, F, Kobayashi, H, Nagano, A, Murotani, K, et al. Trajectories of the prevalence of sarcopenia in the pre- and post-stroke periods: a systematic review. Nutrients. (2022) 15:113. doi: 10.3390/nu15010113
7. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
8. Yang, F, Li, N, Yang, L, Chang, J, Yan, A, and Wei, W. Association of pre-stroke Frailty with Prognosis of elderly patients with acute cerebral infarction: a cohort study. Front Neurol. (2022) 13:855532. doi: 10.3389/fneur.2022.855532
9. Nelke, C, Dziewas, R, Minnerup, J, Meuth, SG, and Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. (2019) 49:381–8. doi: 10.1016/j.ebiom.2019.10.034
10. Altuna-Venegas, S, Aliaga-Vega, R, Maguiña, JL, Parodi, JF, and Runzer-Colmenares, FM. Risk of community-acquired pneumonia in older adults with sarcopenia of a hospital from Callao, Peru 2010-2015. Arch Gerontol Geriatr. (2019) 82:100–5. doi: 10.1016/j.archger.2019.01.008
11. Zhang, Y, Weng, S, Huang, L, Shen, X, Zhao, F, and Yan, S. Association of sarcopenia with a higher risk of infection in patients with type 2 diabetes. Diabetes Metab Res Rev. (2022) 38:e3478. doi: 10.1002/dmrr.3478
12. Chen, LK, Liu, LK, Woo, J, Assantachai, P, Auyeung, TW, Bahyah, KS, et al. Sarcopenia in Asia: consensus report of the Asian working Group for Sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
13. Malmstrom, TK, and Morley, JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. (2013) 14:531–2. doi: 10.1016/j.jamda.2013.05.018
14. Woo, J, Leung, J, and Morley, JE. Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc. (2014) 15:630–4. doi: 10.1016/j.jamda.2014.04.021
15. Ida, S, Kaneko, R, and Murata, K. SARC-F for screening of sarcopenia among older adults: a meta-analysis of screening test accuracy. J Am Med Dir Assoc. (2018) 19:685–9. doi: 10.1016/j.jamda.2018.04.001
16. Adams, HP Jr, Bendixen, BH, Kappelle, LJ, Biller, J, Love, BB, Gordon, DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
17. Zhang, H, Wu, T, Tian, X, Lyu, P, Wang, J, and Cao, Y. High neutrophil percentage-to-albumin ratio can predict occurrence of stroke-associated infection. Front Neurol. (2021) 12:705790. doi: 10.3389/fneur.2021.705790
18. Deng, QW, Gong, PY, Chen, XL, Liu, YK, Jiang, T, Zhou, F, et al. Admission blood cell counts are predictive of stroke-associated infection in acute ischemic stroke patients treated with endovascular therapy. Neurol Sci. (2021) 42:2397–409. doi: 10.1007/s10072-020-04827-2
19. Garner, JS, Jarvis, WR, Emori, TG, Horan, TC, and Hughes, JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. (1988) 16:128–40. doi: 10.1016/0196-6553(88)90053-3
20. Chen, Z, Li, WY, Ho, M, and Chau, PH. The prevalence of sarcopenia in Chinese older adults: meta-analysis and meta-regression. Nutrients. (2021) 13:1441. doi: 10.3390/nu13051441
21. Cruz-Jentoft, AJ, and Sayer, AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
22. Gao, Q, Hu, K, Yan, C, Zhao, B, Mei, F, Chen, F, et al. Associated factors of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. Nutrients. (2021) 13:4291. doi: 10.3390/nu13124291
23. Hoffmann, S, Malzahn, U, Harms, H, Koennecke, HC, Berger, K, Kalic, M, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. (2012) 43:2617–23. doi: 10.1161/STROKEAHA.112.653055
24. Fujikawa, H, Araki, T, Okita, Y, Kondo, S, Kawamura, M, Hiro, J, et al. Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surg Today. (2017) 47:92–8. doi: 10.1007/s00595-016-1357-x
25. Lieffers, JR, Bathe, OF, Fassbender, K, Winget, M, and Baracos, VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. (2012) 107:931–6. doi: 10.1038/bjc.2012.350
26. Babu, JM, Kalagara, S, Durand, W, Antoci, V, Deren, ME, and Cohen, E. Sarcopenia as a risk factor for prosthetic infection after total hip or knee arthroplasty. J Arthroplast. (2019) 34:116–22. doi: 10.1016/j.arth.2018.09.037
27. Cosquéric, G, Sebag, A, Ducolombier, C, Thomas, C, Piette, F, and Weill-Engerer, S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. (2006) 96:895–901. doi: 10.1017/BJN20061943
28. Carrette, F, and Surh, CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. (2012) 24:209–17. doi: 10.1016/j.smim.2012.04.010
29. Conlon, KC, Lugli, E, Welles, HC, Rosenberg, SA, Fojo, AT, Morris, JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. (2015) 33:74–82. doi: 10.1200/JCO.2014.57.3329
30. Cha, S, Kim, WS, Kim, KW, Han, JW, Jang, HC, Lim, S, et al. Sarcopenia is an independent risk factor for dysphagia in community-dwelling older adults. Dysphagia. (2019) 34:692–7. doi: 10.1007/s00455-018-09973-6
31. Maeda, K, and Akagi, J. Sarcopenia is an independent risk factor of dysphagia in hospitalized older people. Geriatr Gerontol Int. (2016) 16:515–21. doi: 10.1111/ggi.12486
32. Ogawa, N, Mori, T, Fujishima, I, Wakabayashi, H, Itoda, M, Kunieda, K, et al. Ultrasonography to measure swallowing muscle mass and quality in older patients with sarcopenic dysphagia. J Am Med Dir Assoc. (2018) 19:516–22. doi: 10.1016/j.jamda.2017.11.007
33. Marik, PE, and Kaplan, D. Aspiration pneumonia and dysphagia in the elderly. Chest. (2003) 124:328–36. doi: 10.1378/chest.124.1.328
34. Erdogan, T, Bahat, G, Kilic, C, Kucukdagli, P, Oren, MM, Erdogan, O, et al. The relationship between sarcopenia and urinary incontinence. Eur Geriatr Med. (2019) 10:923–9. doi: 10.1007/s41999-019-00232-x
35. Melo, LSD, Ercole, FF, Oliveira, DU, Pinto, TS, Victoriano, MA, and Alcoforado, CLGC. Urinary tract infection: a cohort of older people with urinary incontinence. Rev Bras Enferm. (2017) 70:838–44. doi: 10.1590/0034-7167-2017-0141
Keywords: stroke-associated infection, pre-stroke sarcopenia risk, older people, acute ischemic stroke, SARC-F questionnaire
Citation: Song X, Chen X, Bai J and Zhang J (2023) Association between pre-stroke sarcopenia risk and stroke-associated infection in older people with acute ischemic stroke. Front. Med. 10:1090829. doi: 10.3389/fmed.2023.1090829
Received: 05 December 2022; Accepted: 27 January 2023;
Published: 23 February 2023.
Edited by:
Marios Kyriazis, National Gerontology Centre, CyprusReviewed by:
I. G. P. Suka Aryana, Udayana University, IndonesiaCopyright © 2023 Song, Chen, Bai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Bai, ZG9jYmFpQHllYWgubmV0; Jun Zhang, emhhbmdqdW5AcGt1cGguZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.