
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 20 January 2023
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1085988
This article is part of the Research Topic Translational Research in Severe COVID-19 and Long-Term Symptoms Post-COVID-19 View all 14 articles
Purpose: Long COVID, also known as post-acute sequelae of COVID-19, refers to the constellation of long-term symptoms experienced by people suffering persistent symptoms for one or more months after SARS-CoV-2 infection. Blood biomarkers can be altered in long COVID patients; however, biomarkers associated with long COVID symptoms and their roles in disease progression remain undetermined. This study aims to systematically evaluate blood biomarkers that may act as indicators or therapeutic targets for long COVID.
Methods: A systematic literature review in PubMed, Embase, and CINAHL was performed on 18 August 2022. The search keywords long COVID-19 symptoms and biomarkers were used to filter out the eligible studies, which were then carefully evaluated.
Results: Identified from 28 studies and representing six biological classifications, 113 biomarkers were significantly associated with long COVID: (1) Cytokine/Chemokine (38, 33.6%); (2) Biochemical markers (24, 21.2%); (3) Vascular markers (20, 17.7%); (4) Neurological markers (6, 5.3%); (5) Acute phase protein (5, 4.4%); and (6) Others (20, 17.7%). Compared with healthy control or recovered patients without long COVID symptoms, 79 biomarkers were increased, 29 were decreased, and 5 required further determination in the long COVID patients. Of these, up-regulated Interleukin 6, C-reactive protein, and tumor necrosis factor alpha might serve as the potential diagnostic biomarkers for long COVID. Moreover, long COVID patients with neurological symptoms exhibited higher levels of neurofilament light chain and glial fibrillary acidic protein whereas those with pulmonary symptoms exhibited a higher level of transforming growth factor beta.
Conclusion: Long COVID patients present elevated inflammatory biomarkers after initial infection. Our study found significant associations between specific biomarkers and long COVID symptoms. Further investigations are warranted to identify a core set of blood biomarkers that can be used to diagnose and manage long COVID patients in clinical practice.
The coronavirus disease (COVID-19) was defined as an infectious disease caused by the SARS-CoV-2 virus (1). While the majority of people recovered fully from COVID-19, 45% of COVID survivors might suffer from a variety of unresolved symptoms, which persisted for nearly 4 months after SARS-CoV-2 infection and are referred to as long COVID (2). Older adults might be less likely to experience long COVID than younger adults (3). Additionally, the incidence of experiencing long COVID symptoms post-infection is significantly greater among women versus men (4).
Long COVID manifests as a complex set of symptoms, including neurological, neuropsychiatric, cardiopulmonary, and gastrointestinal (3). Across the studies that have reported the prevalence of long COVID symptoms, among the neurological and neuropsychiatric symptoms more frequently associated with long COVID are fatigue (29–58%), headache (10–44%), and anxiety or depression (22–28%) (5–8). Shortness of breath or difficulty breathing (21–24%) and loss of taste or smell (12–15%) are also frequently reported by long COVID patients with pulmonary symptoms (7, 8). Interestingly, in patients who experienced long COVID syndrome, neurological, neuropsychiatric, cardiopulmonary, and gastrointestinal, and other complications (primarily rheumatological complications) were significantly more likely observed in female than in male patients (9). Furthermore, persistent pulmonary or neurological manifestations seen in long COVID may affect an individual’s ability to perform their work, as well as routine daily living activities, such as household chores (6).
One urgent public health question is how to monitor and relieve these long COVID symptoms (3). In this regard, it is desirable to have access to non- or minimally invasive biomarkers, such as those that are often measured in readily available patient blood samples. Clinically-relevant circulating biomarkers may serve as valuable indicators of patients’ normal physiological conditions or disease severity. For example, the up-regulated levels of neurofilament light chain (NFL) and glial fibrillary acidic protein (GFAP) in serum may indicate neuronal damage in the progression of neurodegenerative diseases, such as Alzheimer’s disease (10) or Parkinson’s disease (11). In addition, Interleukin (IL) 6 was not only identified as a prognostic biomarker for disease monitoring in cancer patients with severe COVID-19 (12) but also served as a target for treating COVID-19-related systemic inflammation, such as acute respiratory distress syndrome and cytokine release syndrome (13, 14). While the literature on this topic is evolving fast, to-date diagnostic biomarkers for long COVID remain unclear. This study aims to systematically evaluate the published peer-reviewed literature with the goal of identifying blood biomarkers that may serve as indicators or therapeutic targets for long COVID.
A systematic literature review in PubMed, Embase, and CINAHL was performed on 18 August 2022. The publication time limit for this search was not specified in order to capture all relevant literature. The search keywords included two broad categories of (1) long COVID-19 symptoms and (2) biomarkers, each with a defined yet broad subset of keywords, as documented in Supplementary Table 1. Duplicate records retrieved from these three databases were removed. Then, all relevant articles from reference lists were identified. Articles must be available in full text. After screening the titles and abstracts, final eligibility is determined based on the full content.
The inclusion criteria for this systematic review were as follows: (1) Types of study: the primary source of quantitative studies in a peer-reviewed journal published in English. All original studies, including randomized or non-randomized controlled clinical trials, case reports/case series, and correspondences, were included. For mixed-method studies, if quantitative data could be extracted separately, the studies were included. (2) Types of participants: adult long COVID patients were allowed. No restrictions were imposed on the participants’ sex, ethnicity, and clinical symptoms. (3) Types of outcome measures: biomarker data were reported. Articles that did not provide biomarkers or did not have statistically significant data were excluded. Unpublished theses, dissertations, review articles, conference proceedings, and studies using animal models were also excluded.
According to the inclusion and exclusion criteria, data extraction was completed by two authors (SM and S-HL) and verified by another author (Y-JL). The following headlines were extracted from the articles: authors, study location, number of total patients, patients age (median/mean), long COVID timeframe, comparison groups, types of symptoms, biomarker measurements, and conclusions (Supplementary Table 2).
The quality of the studies was assessed using the modified REporting recommendations for tumor MARKers prognostic studies (REMARK), which provides a valuable reference when reporting or analyzing medical studies related to diseases markers or prognostic markers (12, 15, 16). Two independent reviewers (S-HL and T-AL) verified the total scores. The percentages of studies reviewed that met the criteria for methodological quality are shown in Supplementary Table 3, and outcomes were summarized in the respective section of results.
Through the database search, 574 studies from PubMed, CINAHL, and Embase were identified. The search process adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (17) flow diagram, as shown in Figure 1. After screening each article’s title and abstract, 133 full-text articles were assessed for eligibility. Twenty-eight studies met the eligibility criteria and were included in this systematic review. The 28 articles listed in ascending alphabetical order (Supplementary Table 2) were published between 2021 and 2022. The eligible studies were conducted in the United States (9, 32.1%), Spain (4, 14.3%), Italy (3, 10.7%), Germany (2, 7.1%), and 1 (3.6%) each in Australia, Brazil, Colombia, Costa Rica, Egypt, India, Ireland, Mexico, Singapore, and Turkey. Among 3,374 participants, 1,569 (46.5%) were long COVID patients, 1,419 (42.1%) were participants who completely recovered from COVID-19, 255 (7.6%) were healthy participants (vaccinated and unvaccinated), and 104 (3.1%) were patients with COVID-19. Of 193 biomarkers tested in the 28 studies, 113 (58.5%) were significantly associated with long COVID symptoms. Long COVID timeframe was defined according to definitions used in the reviewed articles: less than 3 months after SARS-CoV-2 infection in 8 (28.6%) studies, 3–6 months in 9 (32.1%) studies, and 6 or more months in 3 (10.7%) studies. There is one (3.6%) study with a various range (22 to 322 days), and the rest 7 (25%) studies did not provide the definition.
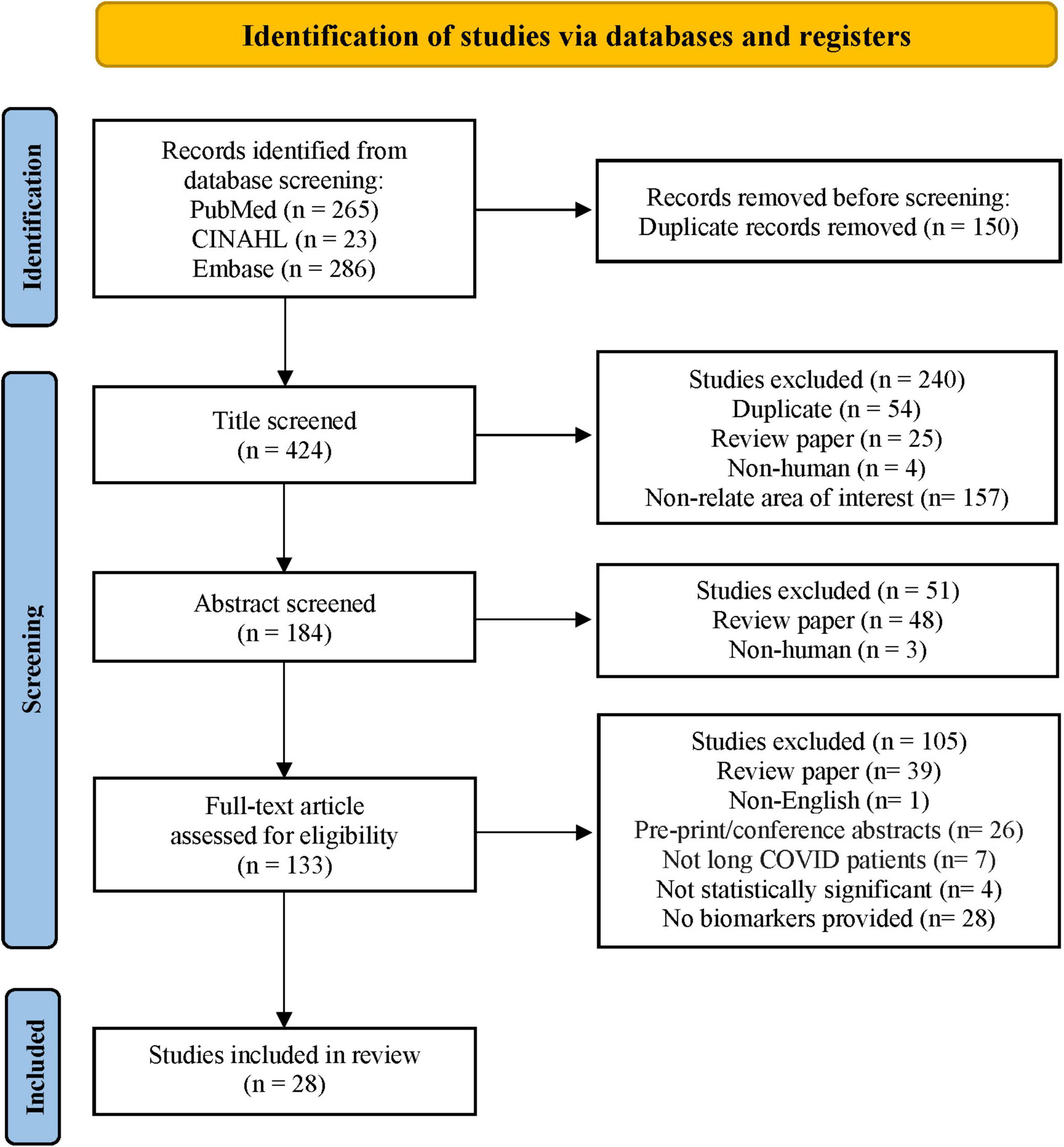
Figure 1. Flow diagram of the literature search. Adapted from Page et al. (17).
All the eligible studies were further evaluated by the modified REMARK questionnaire (18). As shown in Supplementary Table 3 and Figure 2, the majority of studies used a prospective design (96.4%), provided a rationale for the sample sizes (96.4%), described the characteristics of the study population (96.4%), and provided information on the measurement of biomarkers (82.1%). 67.9% defined clinical outcomes, 60.7% provided a list of candidate variables, and 57.1% defined patients’ enrollment period. Few articles blinded the measurements of biomarkers to patient outcomes (10.7%).
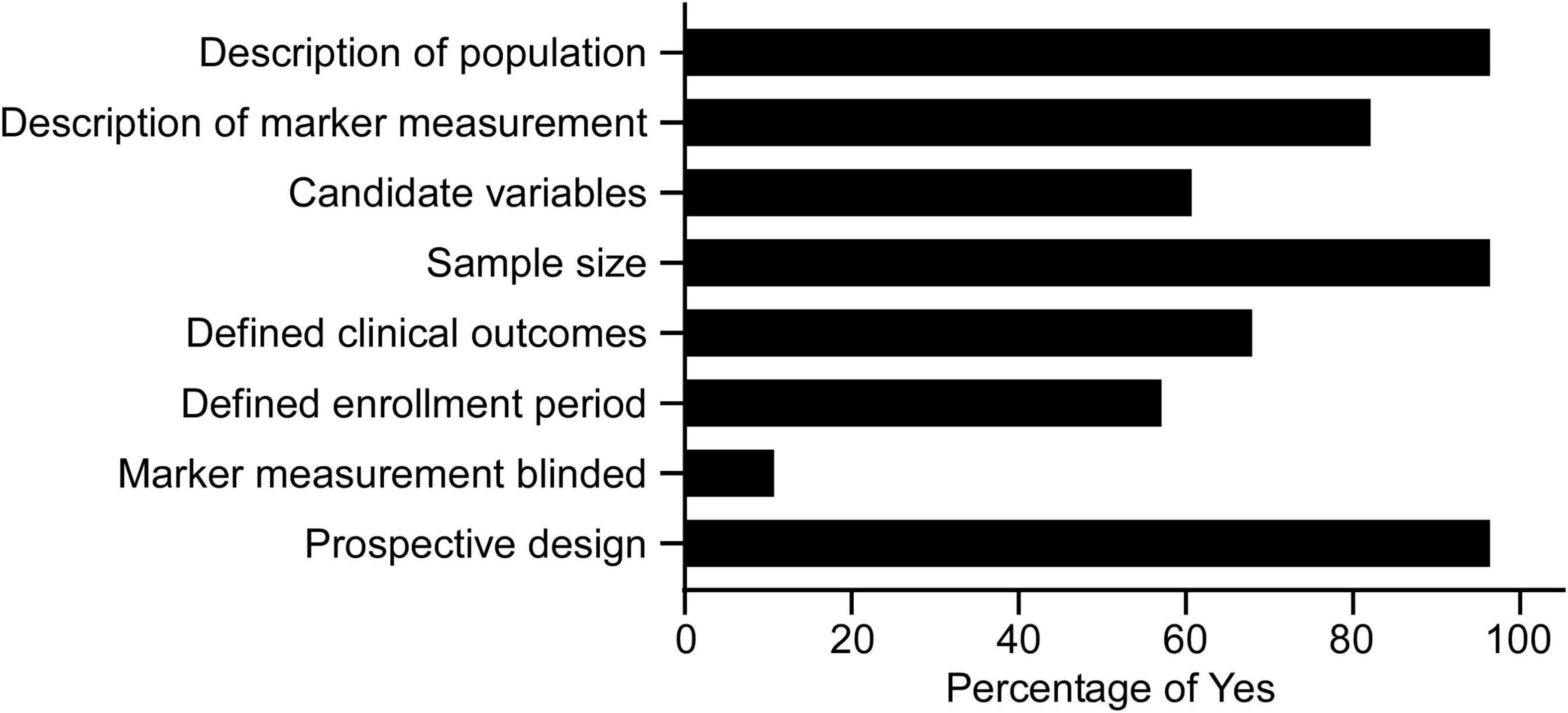
Figure 2. Study quality of the 28 articles in the systematic review assessed by the modified REMARK questionnaire (Supplementary Table 3).
Among 113 biomarkers, 69.9% (79 of 113) biomarkers were significantly increased, 25.7% (29 of 113) biomarkers were decreased, and 4.4% (5 of 113) biomarkers required further determination in long COVID patients. To facilitate the understanding of biological mechanisms in long COVID related biomarkers, the biomarkers were divided into six categories based on their biological function: (1) Cytokines/Chemokines (38, 33.6%); (2) Biochemical markers (24, 21.2%); (3) Vascular markers (20, 17.7%); (4) Neurological markers (6, 5.3%); (5) Acute phase protein (5, 4.4%); and (6) Others (20, 17.7%) (Table 1). With respect to immune response, long COVID patients exhibited higher levels of pro-inflammatory cytokines/chemokines [IL-6, tumor necrosis factor alpha (TNF-α), IL-17, IL-4, and C-C motif chemokine ligand (CCL) 2] and acute phase proteins [C-reactive protein (CRP) and ferritin]. For biochemical markers associated with metabolism, COVID-19 patients with elevated levels of lactate dehydrogenase (LDH) tended to experience long COVID symptoms. Furthermore, in terms of neurological and vascular markers, patients with increased NFL and vascular endothelial growth factor (VEGF) plus decreased hemoglobin showed worse long COVID symptoms.
Among 28 studies, 20 (71.4%) studies reported biomarkers between long COVID and completely recovered patients, and 12 (42.9%) studies demonstrated biomarkers between long COVID patients and healthy participants (Table 2). Compared with recovered COVID patients, long COVID patients showed higher levels of IL-6 (6 of 20, 30%), CRP (3 of 20, 15%), and TNF-α (3 of 20, 15%); lower levels of hemoglobin (2 of 20, 10%). Notably, cytokine/chemokine and biochemical markers accounted for 23.9% (11 of 46) and 39.1% (18 of 46), respectively. Moreover, matched with healthy participants, increased levels of IL-6 (4 of 12, 33.3%), TNF-α (2 of 12, 16.7%), IL-17 (2 of 12, 16.7%), and CCL3 (2 of 12, 16.7%) were associated with long COVID patients. In particular, 44.2% (38 of 86) are cytokine/chemokine, and 20.9% (18 of 86) are vascular markers. The Venn diagram comparison analysis of the differently regulated biomarkers among various groups revealed that IL-6, CRP, and TNF-α remain up-regulated in long COVID patients and may be important indicators of long COVID syndrome (Figure 3).
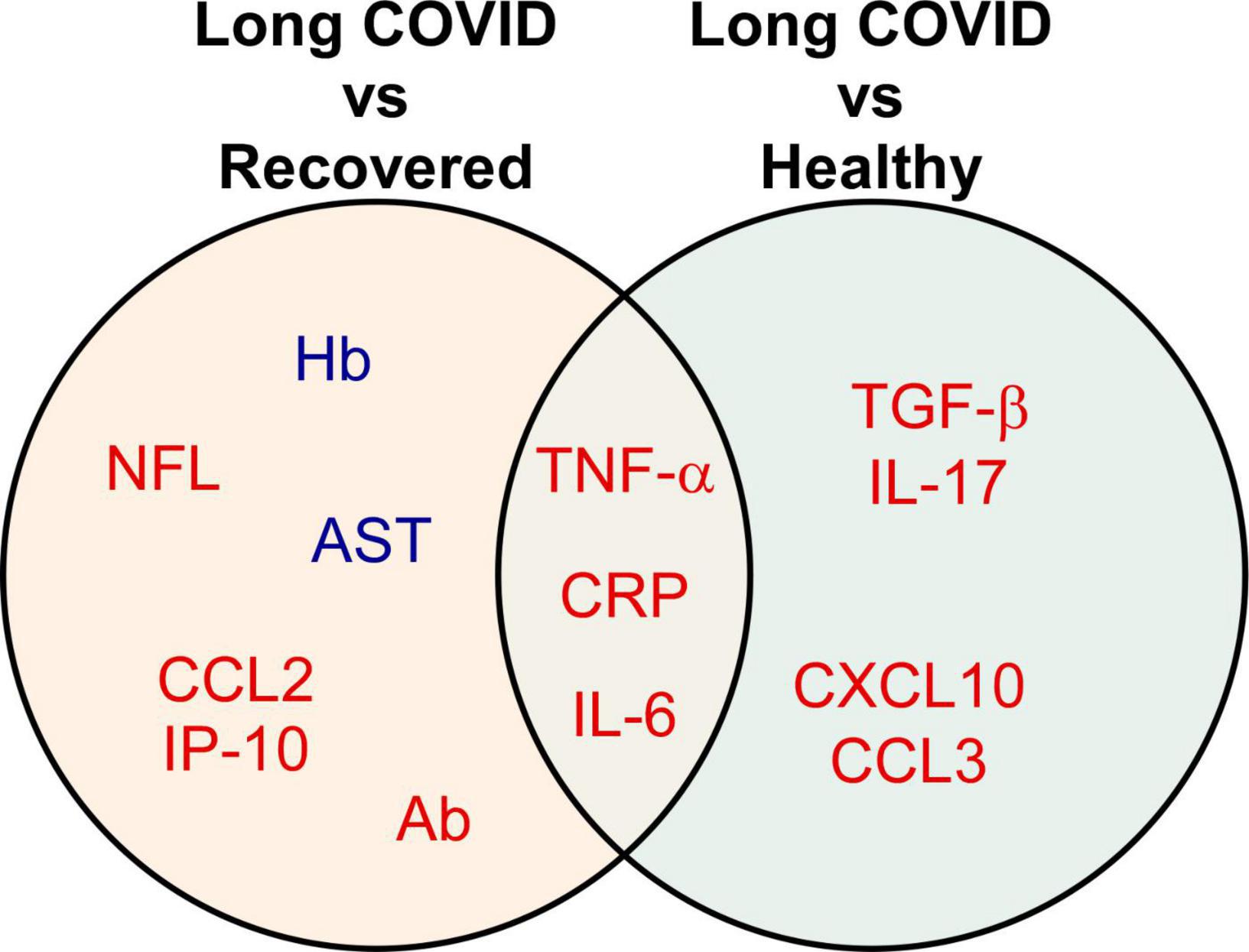
Figure 3. Biomarkers significantly associated with different comparison groups. The Venn diagram presents the 13 biomarkers that were reported by two or more eligible studies. Red indicates up-regulated, while blue refers to down-regulated biomarkers. A detailed list of abbreviations in Figure 3 can be found in Supplementary Table 2.
Among 28 studies, 57.1% (16 of 28) of the studies reported biomarkers in patients with multiple symptoms, followed by 39.3% (11 of 28) with neurological symptoms and 17.9% (5 of 28) with pulmonary symptoms (Table 3). A total of 113 blood biomarkers that were related to long COVID symptoms after SARS-CoV-2 infection, 92 (81.4%), 22 (19.5%), and 15 (13.3%) biomarkers, respectively, showed a significant association with multiple, neurological, and pulmonary symptoms. The major classification of biomarkers was cytokines/chemokines: 35.9% (33 of 92) in multiple symptoms, 22.7% (5 of 22) in neurological symptoms, and 33.3% (5 of 15) in pulmonary symptoms. As shown in Figure 4, through the Venn diagram comparative analysis of these biomarkers, increased CRP was found to be a significant indicator of multiple, neurological, and pulmonary long COVID symptoms. Additionally, several up-regulated vascular biomarkers associated with angiogenesis [VEGF or Platelet derived growth factor BB (PDGF-BB)] and coagulation [D-dimer, von Willebrand factor antigen (VWF:Ag), von Willebrand factor propeptide (VWF:pp), soluble thrombomodulin (sTM), or Factor VIII:C] were reported in patients with multiple symptoms. Elevated neurological biomarkers related to nerve injuries, such as NFL and GFAP, may serve as diagnostic biomarkers for long COVID neurological symptoms, especially for long COVID headaches (19, 20). Moreover, in long COVID pulmonary symptoms, compared with healthy control, long COVID patients with pulmonary fibrosis exhibited higher Transforming growth factor beta (TGF-β) (21, 22). As a result, these biomarkers may serve as indicators of distinct long COVID symptoms.
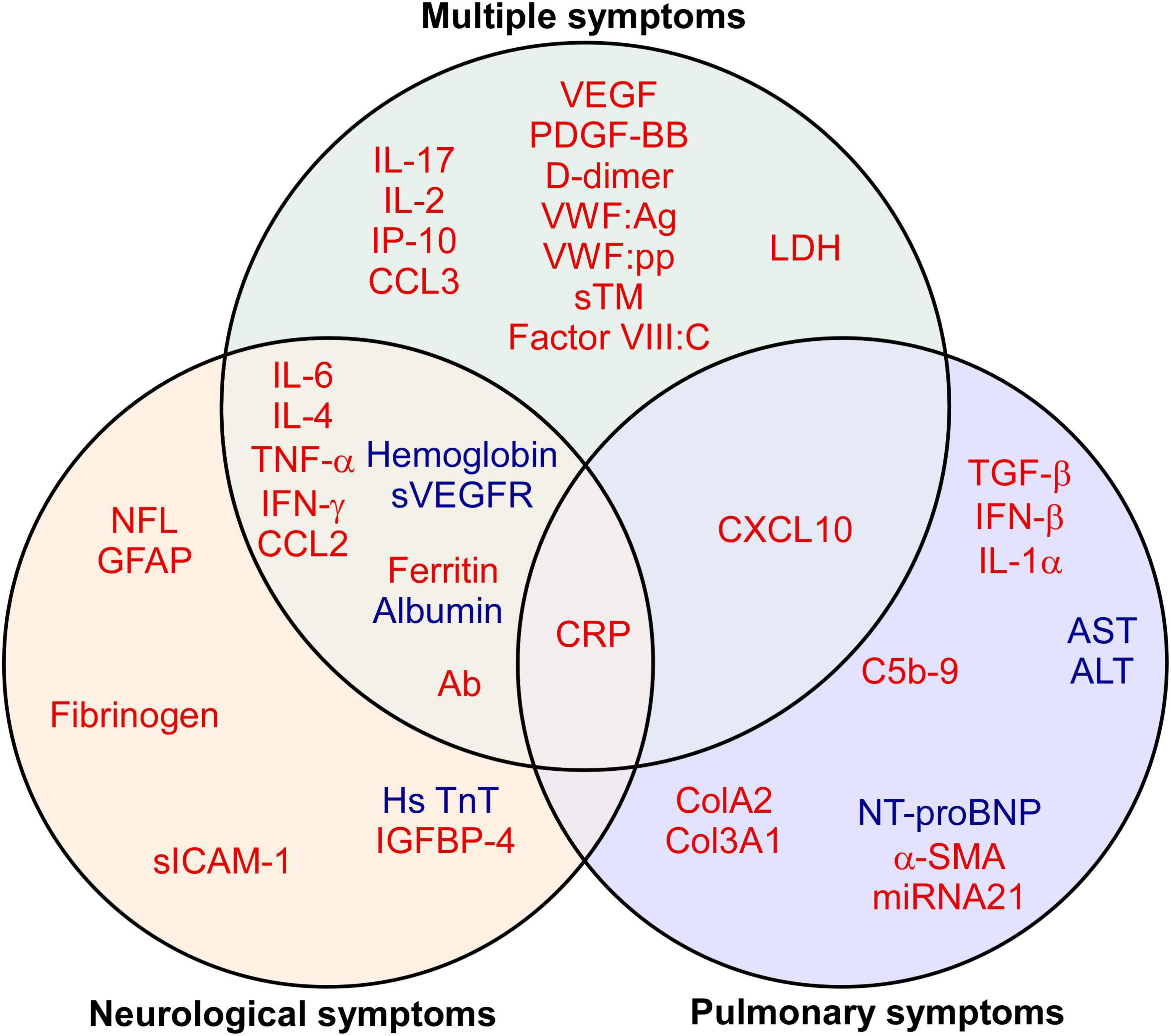
Figure 4. Biomarkers of long COVID symptoms. The Venn diagram presents the 41 biomarkers that were reported by two or more eligible studies. Red indicates up-regulated, while blue refers to down-regulated biomarkers. A detailed list of abbreviations in Figure 4 can be found in Supplementary Table 2.
Of 193 putative biomarkers tested, 113 were found in this review to be statistically significantly associated with long COVID. To provide a functional view of the biomarkers, we divided the 113 biomarkers into six categories based on their biological function: cytokine/chemokine, biochemical markers, vascular markers, neurological markers, acute phase protein, and others. Through a comprehensive evidence synthesis of biomarkers in long COVID, the up-regulated IL-6, CRP, and TNF-α were found to be a potential core set of biomarkers for long COVID.
Severe systemic inflammation and substantial tissue damage in acute COVID are accelerated by pro-inflammatory cytokines/chemokines (23), which involve many pathophysiological mechanisms, like leukocyte trafficking (24), cytokine storm (25), and normal tissue necroptosis (26). Systemic inflammatory markers, such as IL-6 and CRP, were associated with disease severity and mortality among COVID-19 patients (27). Moreover, consistent with our findings (Figures 3, 4), the prolonged IL-6, TNF-α, and CRP were also implicated in systemic and neurological long COVID sequelae (28).
Neurological symptoms are the most common long COVID clinical manifestations (7). NFL and GFAP are skeleton proteins that maintain the stability of neuron axons and astrocytes. The expression of these neural peptides in circulation may serve as biomarkers associated with neuronal degeneration and damage (29, 30). Long COVID patients with elevated serum NFL and GFAP showed worse headaches and persistent neuropathic pain (19, 20). Furthermore, Peluso et al. reported that the serum levels of NFL and GFAP in post-acute COVID patients are positively correlated with IL-6, TNF-α, and CCL2 (19) that may induce immune cells and activate detrimental neuroinflammation (31). This indirect mechanism demonstrates that pro-inflammatory cytokines/chemokines may exacerbate substantial neuronal damage.
Pulmonary fibrosis is one of the complications of severe COVID cases (32). Similar to the long COVID patients with pulmonary symptoms, elevated levels of IL-6, CRP, and TGF-β were identified in patients at increased risk of developing pulmonary fibrosis after SARS-CoV-2 infection (Figure 4) (33, 34). TGF-β is a multifunctional cytokine that plays a crucial role in tissue repair after injury. Upon a pulmonary viral infection, epithelial cell injury may induce the activation of M2 macrophages to secrete TGF-β, stimulating fibroblast proliferation and collagen synthesis and leading to fibrosis (22, 35). Recently, Zhou et al. demonstrated that Pirfenidone, an Food and Drug Administration (FDA)-approved TGF-β/collagen-targeted drug, attenuated the post-COVID-19 pulmonary fibrosis manifestation (36). Hence, a combination therapy targeting the anti-inflammatory (such as IL-6 blockades) (13) and anti-fibrotic pathways (such as Pirfenidone) (36) may be a potential therapeutical strategy for long COVID with pulmonary fibrosis.
In this review, we have evaluated and summarized the long COVID-related biomarkers. However, because of the heterogeneity of long COVID, no laboratory test could definitively distinguish long COVID from other diseases. A panel of markers may effectively differentiate long COVID cases from others and serve as potential biomarkers for early detection of long COVID. As shown in Figures 3, 4, in addition to the use of a core set of biomarkers (IL-6, CRP, and TNF-α), IL-4, Interferon (IFN) gamma, CCL2, Ferritin, Hemoglobin, NFL, and GFAP may be added in the penal of long COVID patients with neurological symptoms. Likewise, C-X-C motif chemokine ligand 10 (CXCL10), TGF-β, IFN-β, and IL-1α may be included in the panel in patients with pulmonary symptoms. Holistic patient-centered care and the improved management of long COVID may require the integration of symptom management approaches, the current panel of long COVID biomarkers, as well as additional more specific biomarkers that are yet to be identified. Furthermore, some biomarkers may also be affected by participants’ existing clinical conditions. For example, NFL may serve as not only a biomarker for long COVID neurological symptoms in this study but also a biomarker for neurodegenerative diseases, such as Alzheimer’s disease (10) and Parkinson’s disease (11). Therefore, future application of this panel of long COVID biomarkers may need to consider the patient’s clinical history to avoid concomitant pathologies of other diseases.
Our study highlights the first systematic review to synthesize unique expression patterns of inflammatory biomarkers in long COVID, and assess whether they can serve as diagnostic or prognostic markers. Categorization of the biomarkers into six categories based on biological function may further inform our understanding of the clinicopathology of long COVID. The findings may guide and help clinicians to identify a core set of blood biomarkers that can be used to monitor and manage long COVID in clinical practice. Nevertheless, there are several limitations to our approach. First, as shown in the quality assessment (Supplementary Table 3) of the manuscript, 96.4% (27 of 28) of the eligible articles provided different sampling criteria to exclude participants with some existing disease conditions based on the clinical history of patients. Moreover, most of the biomarkers in the eligible studies were measured after the onset of the long COVID symptoms. Therefore, the main biomarkers found to be overexpressed in long COVID, such as IL-6, CRP, and TNF-α, although important in COVID, likely lack specificity to serve as predictors for long COVID. The identified biomarkers are real and reflect the biology of viral infections which do activate the inflammasome, leading to the production of important cytokines/chemokines (IL-1β, TNF-α, IL-6, etc.) (37, 38). This may be the main reason why existing studies predominantly measured only these main inflammatory biomarkers. Unfortunately, many other stressors, such as viruses, bacteria, inhaled nano/particles, industrial toxins, etc., do share common mechanistic features that involve inflammation via inflammasome activation. For this reason, it is necessary that future studies on long COVID employ broader screening platforms that are likely to yield unique and likely specific biomarkers for SARS-CoV-2. For example, a recent study proposed that IL-26 may be a COVID-19-specific biomarker, but none of the studies to date have measured IL-26 (39). Furthermore, there is a lack of consistency on specific long COVID symptoms. 57.1% of the eligible studies examined biomarkers for patients with multiple symptoms of long COVID. Additionally, the duration of facing persistent long COVID symptoms varied within and across studies. Incongruent with long COVID symptoms and timeframes may contribute to distinct biomarkers. Finally, vaccination may affect patients’ physiological variables and the levels of biomarkers in serum. However, there is no sufficient evidence to know the effect of vaccination because only one of the 28 eligible studies separated the vaccinated participants from the unvaccinated ones. Such issues should be addressed in future longitudinal studies on biomarker expression among long COVID patients to understand the causal relationship between long COVID symptom development and acute COVID inflammation.
Long COVID patients present elevated inflammatory biomarkers after initial infection. Our study found that people with higher levels of IL-6, CRP, and TNF-α after SARS-CoV-2 infection for one or more months may experience long-term COVID symptoms. This systematic review could identify a panel of blood biomarkers that can be used to manage long COVID patients in clinical practice.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Y-JL supervised the entire project, designed, analyzed manuscripts and wrote manuscript. S-HL and SM designed, analyzed manuscripts, and wrote manuscript. T-AL analyzed manuscripts and wrote manuscript. C-TK analyzed manuscripts. DB provided scientific input and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded in part by the University of Massachusetts Lowell (Faculty start-up D50210000000022 to Y-JL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1085988/full#supplementary-material
1. Vodnar D, Mitrea L, Teleky B, Szabo K, Calinoiu L, Nemes S, et al. Coronavirus disease (COVID-19) caused by (SARS-CoV-2) infections: a real challenge for human gut microbiota. Front Cell Infect Microbiol. (2020) 10:575559. doi: 10.3389/fcimb.2020.575559
2. O’Mahoney L, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. (2023) 55:101762. doi: 10.1016/j.eclinm.2022.101762
3. Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale K, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. (2022) 28:1706–14. doi: 10.1038/s41591-022-01909-w
4. Chen C, Haupert S, Zimmermann L, Shi X, Fritsche L, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. (2022) 226:1593–607. doi: 10.1093/infdis/jiac136
5. Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. (2022) 11:269. doi: 10.3390/pathogens11020269
6. Huang L, Li X, Gu X, Zhang H, Ren L, Guo L, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. (2022) 10:863–76. doi: 10.1016/S2213-2600(22)00126-6
7. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo P, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. (2021) 11:16144. doi: 10.1038/s41598-021-95565-8
8. Natarajan A, Shetty A, Delanerolle G, Zeng Y, Zhang Y, Raymont V, et al. A systematic review and meta-analysis of Long COVID symptoms. medRxiv [Preprint]. (2022). doi: 10.1101/2022.03.08.22272091
9. Sylvester S, Rusu R, Chan B, Bellows M, O’Keefe C, Nicholson S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: a review. Curr Med Res Opin. (2022) 38:1391–9. doi: 10.1080/03007995.2022.2081454
10. Teunissen C, Verberk I, Thijssen E, Vermunt L, Hansson O, Zetterberg H, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. (2022) 21:66–77. doi: 10.1016/S1474-4422(21)00361-6
11. Bäckström D, Linder J, Jakobson MS, Riklund K, Zetterberg H, Blennow K, et al. NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology. (2020) 95:e827–38. doi: 10.1212/WNL.0000000000010084
12. Lee T, Wang S, Kuo C, Li C, McCullough L, Bello D, et al. Prognostic serum biomarkers in cancer patients with COVID-19: a systematic review. Transl Oncol. (2022) 21:101443. doi: 10.1016/j.tranon.2022.101443
13. Menzella F, Fontana M, Salvarani C, Massari M, Ruggiero P, Scelfo C, et al. Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation. Crit Care. (2020) 24:589. doi: 10.1186/s13054-020-03306-6
14. Zhang C, Wu Z, Li J, Zhao H, Wang G. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. (2020) 55:105954. doi: 10.1016/j.ijantimicag.2020.105954
15. Sauerbrei W, Taube S, McShane L, Cavenagh M, Altman D. Reporting recommendations for tumor marker prognostic studies (REMARK): an abridged explanation and elaboration. J Natl Cancer Inst. (2018) 110:803–11. doi: 10.1093/jnci/djy088
16. Altman D, McShane L, Sauerbrei W, Taube S. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. (2012) 9:e1001216. doi: 10.1371/journal.pmed.1001216
17. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Lai Y, Hanneman S, Casarez R, Wang J, McCullough L. Blood biomarkers for physical recovery in ischemic stroke: a systematic review. Am J Transl Res. (2019) 11:4603–13.
19. Peluso M, Sans H, Forman C, Nylander A, Ho H, Lu S, et al. Plasma markers of neurologic injury and inflammation in people with self-reported neurologic postacute sequelae of SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e200003. doi: 10.1212/NXI.0000000000200003
20. Magdy R, Eid R, Fathy W, Abdel-Aziz M, Ibrahim R, Yehia A, et al. Characteristics and risk factors of persistent neuropathic pain in recovered COVID-19 patients. Pain Med. (2022) 23:774–81. doi: 10.1093/pm/pnab341
21. Ali M, Abdullah F, Naveed A, Ahmed S, Khan A, Hasan A. Role of circulatory miRNA-21 and associated signaling pathways in the pathogenesis of pulmonary fibrosis among individuals recovered after COVID-19 infection. Human Gene. (2022) 34:201093. doi: 10.1016/j.humgen.2022.201093
22. Colarusso C, Maglio A, Terlizzi M, Vitale C, Molino A, Pinto A, et al. Post-COVID-19 patients who develop lung fibrotic-like changes have lower circulating levels of IFN-β but higher levels of IL-1α and TGF-β. Biomedicines. (2021) 9:1931. doi: 10.3390/biomedicines9121931
23. Lucas C, Wong P, Klein J, Castro T, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. (2020) 584:463–9.
24. Alon R, Sportiello M, Kozlovski S, Kumar A, Reilly E, Zarbock A, et al. Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19. Nat Rev Immunol. (2021) 21:49–64. doi: 10.1038/s41577-020-00470-2
25. Vanderbeke L, Van Mol P, Van Herck Y, De Smet F, Humblet-Baron S, Martinod K, et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun. (2021) 12:4117. doi: 10.1038/s41467-021-24360-w
26. Bohmwald K, Gálvez N, Canedo-Marroquín G, Pizarro-Ortega M, Andrade-Parra C, Gómez-Santander F, et al. Contribution of cytokines to tissue damage during human respiratory syncytial virus infection. Front Immunol. (2019) 10:452. doi: 10.3389/fimmu.2019.00452
27. Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. (2020) 127:104370. doi: 10.1016/j.jcv.2020.104370
28. Stefanou M, Palaiodimou L, Bakola E, Smyrnis N, Papadopoulou M, Paraskevas G, et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther Adv Chronic Dis. (2022) 13:20406223221076890. doi: 10.1177/20406223221076890
29. DeKosky S, Kochanek P, Valadka A, Clark R, Chou S, Au A, et al. Blood biomarkers for detection of brain injury in COVID-19 patients. J Neurotrauma. (2021) 38:1–43. doi: 10.1089/neu.2020.7332
30. Frontera J, Boutajangout A, Masurkar A, Betensky R, Ge Y, Vedvyas A, et al. Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID subjects with normal cognition, mild cognitive impairment, or Alzheimer’s dementia. Alzheimers Dement. (2022) 18:899–910. doi: 10.1002/alz.12556
31. Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. (2017) 17:49–59. doi: 10.1038/nri.2016.123
32. Polak S, Van Gool I, Cohen D, von der Thusen J, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. (2020) 33:2128–38. doi: 10.1038/s41379-020-0603-3
33. Mohammadi A, Balan I, Yadav S, Matos W, Kharawala A, Gaddam M, et al. Post-COVID-19 pulmonary fibrosis. Cureus. (2022) 14:e22770. doi: 10.7759/cureus.22770
34. Vaz de Paula C, Nagashima S, Liberalesso V, Collete M, da Silva F, Oricil A, et al. COVID-19: immunohistochemical Analysis of TGF-beta signaling pathways in pulmonary fibrosis. Int J Mol Sci. (2021) 23:168. doi: 10.3390/ijms23010168
35. Huang X, Xiu H, Zhang S, Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. (2018) 2018:1264913. doi: 10.1155/2018/1264913
36. Zhou X, Yang D, Kong X, Wei C, LvQiu S, Wang L, et al. Case report: pirfenidone in the treatment of post-COVID-19 pulmonary fibrosis. Front Med. (2022) 9:925703. doi: 10.3389/fmed.2022.925703
37. Conway E, Mackman N, Warren R, Wolberg A, Mosnier L, Campbell R, et al. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. (2022) 22:639–49. doi: 10.1038/s41577-022-00762-9
38. Subramaniam S, Kothari H, Bosmann M. Tissue factor in COVID-19-associated coagulopathy. Thromb Res. (2022) 220:35–47. doi: 10.1016/j.thromres.2022.09.025
39. Cardenas E, Ekstedt S, Piersiala K, Petro M, Karlsson A, Kagedal A, et al. Increased IL-26 associates with markers of hyperinflammation and tissue damage in patients with acute COVID-19. Front Immunol. (2022) 13:1016991. doi: 10.3389/fimmu.2022.1016991
40. Littlefield K, Watson R, Schneider J, Neff C, Yamada E, Zhang M, et al. SARS-CoV-2-specific T cells associate with inflammation and reduced lung function in pulmonary post-acute sequalae of SARS-CoV-2. PLoS Pathog. (2022) 18:e1010359. doi: 10.1371/journal.ppat.1010359
41. Maamar M, Artime A, Pariente E, Fierro P, Ruiz Y, Gutierrez S, et al. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: a cross-sectional study. Curr Med Res Opin. (2022) 38:901–9. doi: 10.1080/03007995.2022.2042991
42. Pasini E, Corsetti G, Romano C, Scarabelli T, Chen-Scarabelli C, Saravolatz L, et al. Serum metabolic profile in patients with long-covid (PASC) syndrome: clinical Implications. Front Med. (2021) 8:714426. doi: 10.3389/fmed.2021.714426
43. Ferrando S, Dornbush R, Lynch S, Shahar S, Klepacz L, Karmen C, et al. Neuropsychological, medical, and psychiatric findings after recovery from acute COVID-19: a cross-sectional study. J Acad Consult Liaison Psychiatry. (2022) 63:474–84. doi: 10.1016/j.jaclp.2022.01.003
44. Martone A, Tosato M, Ciciarello F, Galluzzo V, Zazzara M, Pais C, et al. Sarcopenia as potential biological substrate of long COVID-19 syndrome: prevalence, clinical features, and risk factors. J Cachexia Sarcopenia Muscle. (2022) 13:1974–82. doi: 10.1002/jcsm.12931
45. Aparisi A, Ybarra-Falcon C, Iglesias-Echeverria C, Garcia-Gomez M, Marcos-Mangas M, Valle-Penacoba G, et al. Cardio-pulmonary dysfunction evaluation in patients with persistent post-COVID-19 headache. Int J Environ Res Public Health. (2022) 19:3961. doi: 10.3390/ijerph19073961
46. Fernandez-de-Las-Penas C, Ryan-Murua P, Rodriguez-Jimenez J, Palacios-Cena M, Arendt-Nielsen L, Torres-Macho J. Serological biomarkers at hospital admission are not related to long-term post-covid fatigue and dyspnea in COVID-19 survivors. Respiration. (2022) 101:658–65. doi: 10.1159/000524042
47. Wechsler J, Butuci M, Wong A, Kamboj A, Youngblood B. Mast cell activation is associated with post-acute COVID-19 syndrome. Allergy. (2022) 77:1288–91. doi: 10.1111/all.15188
48. Zhao J, Schank M, Wang L, Dang X, Cao D, Khanal S, et al. Plasma biomarkers for systemic inflammation in COVID-19 survivors. Proteomics Clin Appl. (2022) 16:e2200031. doi: 10.1002/prca.202200031
49. Giron L, Peluso M, Ding J, Kenny G, Zilberstein N, Koshy J, et al. Markers of fungal translocation are elevated during post-acute sequelae of SARS-CoV-2 and induce NF-kappaB signaling. JCI Insight. (2022) 7:e160989. doi: 10.1172/jci.insight.164813
50. Holmes E, Wist J, Masuda R, Lodge S, Nitschke P, Kimhofer T, et al. Incomplete systemic recovery and metabolic phenoreversion in post-acute-phase nonhospitalized COVID-19 patients: implications for assessment of post-acute COVID-19 syndrome. J Proteome Res. (2021) 20:3315–29. doi: 10.1021/acs.jproteome.1c00224
51. Aparisi A, Ybarra-Falcon C, Garcia-Gomez M, Tobar J, Iglesias-Echeverria C, Jaurrieta-Largo S, et al. Exercise ventilatory inefficiency in post-COVID-19 syndrome: insights from a prospective evaluation. J Clin Med. (2021) 10:2591.
52. Patterson B, Guevara-Coto J, Yogendra R, Francisco E, Long E, Pise A, et al. Immune-based prediction of COVID-19 severity and chronicity decoded using machine learning. Front Immunol. (2021) 12:700782. doi: 10.3389/fimmu.2021.700782
53. Peluso M, Lu S, Tang A, Durstenfeld M, Ho H, Goldberg S, et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. (2021) 224:1839–48. doi: 10.1093/infdis/jiab490
54. Sun B, Tang N, Peluso M, Iyer N, Torres L, Donatelli J, et al. Characterization and biomarker analyses of post-COVID-19 complications and neurological manifestations. Cells. (2021) 10:386. doi: 10.3390/cells10020386
55. Acosta-Ampudia Y, Monsalve D, Rojas M, Rodriguez Y, Zapata E, Ramirez-Santana C, et al. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J Infect Dis. (2022) 225:2155–62. doi: 10.1093/infdis/jiac017
56. Ong S, Fong S, Young B, Chan Y, Lee B, Amrun S, et al. Persistent symptoms and association with inflammatory cytokine signatures in recovered coronavirus disease 2019 patients. Open Forum Infect Dis. (2021) 8:ofab156. doi: 10.1093/ofid/ofab156
57. Queiroz M, Neves P, Lima S, Lopes J, Torres M, Vallinoto I, et al. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front Cell Infect Microbiol. (2022) 12:922422. doi: 10.3389/fcimb.2022.922422
58. Torres-Ruiz J, Lomelin-Gascon J, Lira-Luna J, Perez-Fragoso A, Tapia-Conyer R, Nunez-Aguirre M, et al. FANSY POSTCOV: a composite clinical immunological predictive index for post-COVID-19 syndrome unveils distinctive features in a cohort study of mild to critical patients. Clin Transl Med. (2021) 11:e623. doi: 10.1002/ctm2.623
59. Flaskamp L, Roubal C, Uddin S, Sotzny F, Kedor C, Bauer S, et al. Serum of post-COVID-19 syndrome patients with or without ME/CFS differentially affects endothelial cell function in vitro. Cells. (2022) 11:2376. doi: 10.3390/cells11152376
60. Hanson B, Visvabharathy L, Ali S, Kang A, Patel T, Clark J, et al. Plasma biomarkers of neuropathogenesis in hospitalized patients with COVID-19 and those with postacute sequelae of SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e1151. doi: 10.1212/NXI.0000000000001151
61. Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. (2021) 19:2546–53. doi: 10.1111/jth.15490
62. Haffke M, Freitag H, Rudolf G, Seifert M, Doehner W, Scherbakov N, et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J Transl Med. (2022) 20:138. doi: 10.1186/s12967-022-03346-2
Keywords: biomarker, long COVID, IL-6, CRP, TNF-α
Citation: Lai Y-J, Liu S-H, Manachevakul S, Lee T-A, Kuo C-T and Bello D (2023) Biomarkers in long COVID-19: A systematic review. Front. Med. 10:1085988. doi: 10.3389/fmed.2023.1085988
Received: 31 October 2022; Accepted: 02 January 2023;
Published: 20 January 2023.
Edited by:
Gloria Pérez-Rubio, Instituto Nacional de Enfermedades Respiratorias (INER), MexicoReviewed by:
Patrizia Pignatti, Scientific Clinical Institute Maugeri (ICS Maugeri), ItalyCopyright © 2023 Lai, Liu, Manachevakul, Lee, Kuo and Bello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Ju Lai,  eXVuanVfbGFpQHVtbC5lZHU=
eXVuanVfbGFpQHVtbC5lZHU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.