- 1Department of Geriatrics, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Department of Cardiovascular Medicine, Xiangya Hospital, Central South University, Changsha, Hunan, China
Background: As a cellular process, senescence functions to prevent the proliferation of damaged, old and tumor-like cells, as well as participate in embryonic development, tissue repair, etc. This study aimed to analyze the themes and topics of the scientific publications related to cellular senescence in the past three decades by machine learning.
Methods: The MeSH term “cellular senescence” was used for searching publications from 1990 to 2021 on the PubMed database, while the R platform was adopted to obtain associated data. A topic network was constructed by latent Dirichlet allocation (LDA) and the Louvain algorithm.
Results: A total of 21,910 publications were finally recruited in this article. Basic studies (15,382, 70.21%) accounted for the most proportion of publications over the past three decades. Physiology, drug effects, and genetics were the most concerned MeSH terms, while cell proliferation was the leading term since 2010. Three senolytics were indexed by MeSH terms, including quercetin, curcumin, and dasatinib, with the accumulated occurrence of 35, 26, and 22, separately. Three clusters were recognized by LDA and network analyses. Telomere length was the top studied topic in the cluster of physiological function, while cancer cell had been a hot topic in the cluster of pathological function, and protein kinase pathway was the most popular topic in the cluster of molecular mechanism. Notably, the cluster of physiological function showed a poor connection with other clusters.
Conclusion: Cellular senescence has obtained increasing attention over the past three decades. While most of the studies focus on the pathological function and molecular mechanism, more researches should be conducted on the physiological function and the clinical translation of cellular senescence, especially the development and application of senotherapeutics.
1. Introduction
Cellular senescence refers to a form of stable cell cycle arrest in response to various stimuli (1). As a cellular process, senescence functions to prevent the proliferation of damaged, old and tumor-like cells, as well as participate in embryonic development, tissue repair, etc. (2, 3). However, cellular senescence is also associated with cancer and age-related diseases, including diabetes, hypertension, Alzheimer’s disease, etc. (4). Due to its significant role in pathophysiological processes, studies related to cellular senescence have dramatically increased in recent years. Nevertheless, the priorities and trends of these publications, which can reflect the research hot spots and suggest future research direction, have not been systematically analyzed and discussed.
Bibliometric analysis is often applied to quantitatively analyze academic literature and provide information about recent research topics and trends in a certain field, such as atrial fibrillation (5), diabetes (6), etc. However, bibliometric analysis on cellular senescence has not been conducted yet, because of the deficiency of effective tools to handle large amounts of literature, and previous bibliometric studies are usually conducted in high-cited articles (7). Natural language processing (NLP), a series of machine learning methods for analyzing human language, has been employed to process medical information in recent years (8). Latent Dirichlet allocation (LDA) is a classic method of NLP, and the application of LDA allows scientists to extract specific themes from a large number of publications (9, 10), which may contribute to the development of bibliometric analysis.
In this study, we analyze the themes and topics of the scientific publications related to cellular senescence in the past three decades by combining the machine learning method LDA with bibliometric analysis. These results may provide an overview of previous researches, reveal hot topics about cellular senescence and, more essentially, provide potential directions for future research.
2. Materials and methods
Scientific publications indexed under the medical subject headings (MeSH) term “cellular senescence” from 1990 to 2021 were searched on PubMed at 23 December 2022 (Supplementary material 1). Details of the search results were downloaded in the format of PubMed, and the R package “Bibliometrix” was used to extract metadata including the publication year, the publication type, journal, article abstract, MeSH terms, etc. (11). Publications with “Publication Year” of 2022 or 2023 were excluded for further analyses. The impact factor of journal was determined by journal citation reports 2021. The country/region publication number was determined by the listed countries/regions in each publication, and the thermal world map was created by Gunn Map 2.1 MeSH terms with occurrence more than 10 times were included in MeSH analyses. Since this was a bibliometric analysis, ethical approval was not necessary.
Latent Dirichlet allocation, a classic topic modeling method to describe the characteristics of a large number of unstructured texts, was applied to identify the research topics with greater specificity from the abstract of the downloaded articles according to previous studies (12, 13). Using Python, a glossary of feature terms was created by LDA based on how often the vocabulary words coexisted in the abstract of the downloaded articles. Research topics were classified based on these feature terms subsequently, and we set the number of research topics to 50 according to previous research. Then, the two most probable research topics of each article were identified according to how often the feature terms appeared in this article. In addition, the Louvain algorithm was adopted for cluster analysis to conduct a topic network and further reveal the relationship between these topics.
R codes and Python codes used in this article were available on GitHub (14).2 Excel and R platforms were applied for the visualization of the work, while the topic network was performed by Gephi3 (15).
3. Results
3.1. Characteristics of the publications
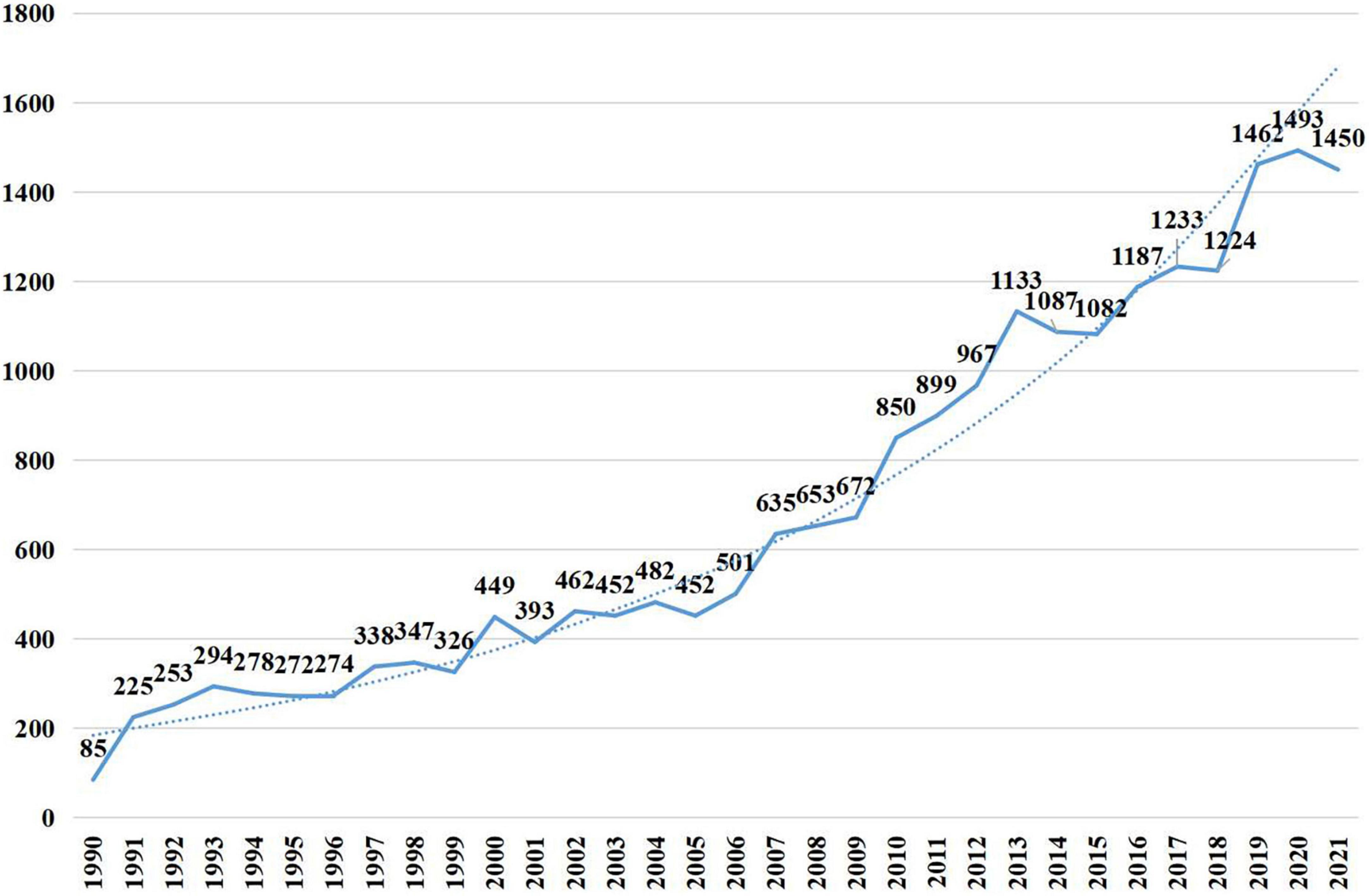
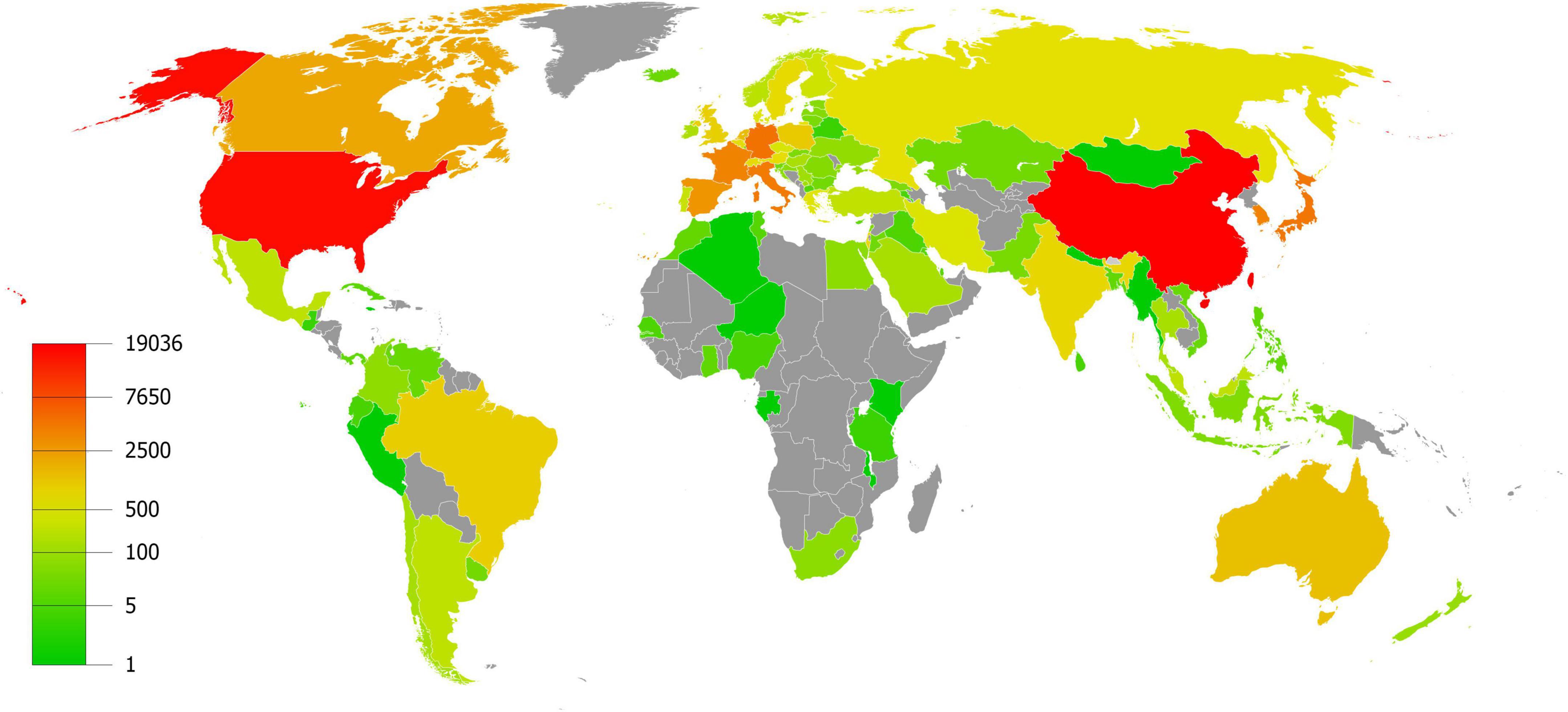
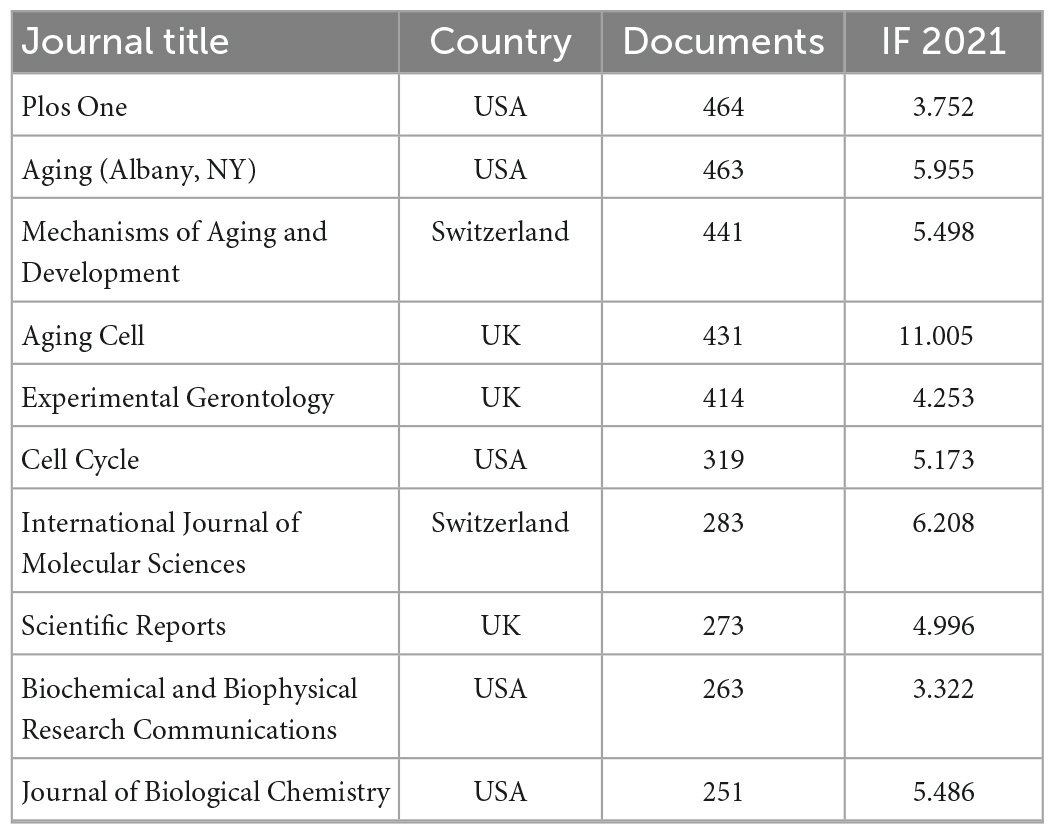
A total of 21,910 publications indexed under the MeSH term “cellular senescence” were included in our study. On average, 336 articles were published per year from 1990 to 2005. The number of publications continuously increased since 2005 and reached 1,493 per year in 2020 (Figure 1). Overall, the average growth rate from 1990 to 2021 was 9.58%. As shown in Figure 2, the publications were roughly classified into six types, among which basic studies (15,382, 70.21%) accounted for the most proportion of publications over the past three decades. The proportion of review and meta-analysis, comment, letter or editorial, and clinical study slightly increased, while the proportion of case report and other articles declined. In addition, the number of publications in different countries/regions was shown in Figure 3. China, USA, Germany, Japan, and Italy were the top five countries/regions with the largest number of publications. Moreover, the top 10 journals with the most publications were listed in Table 1.
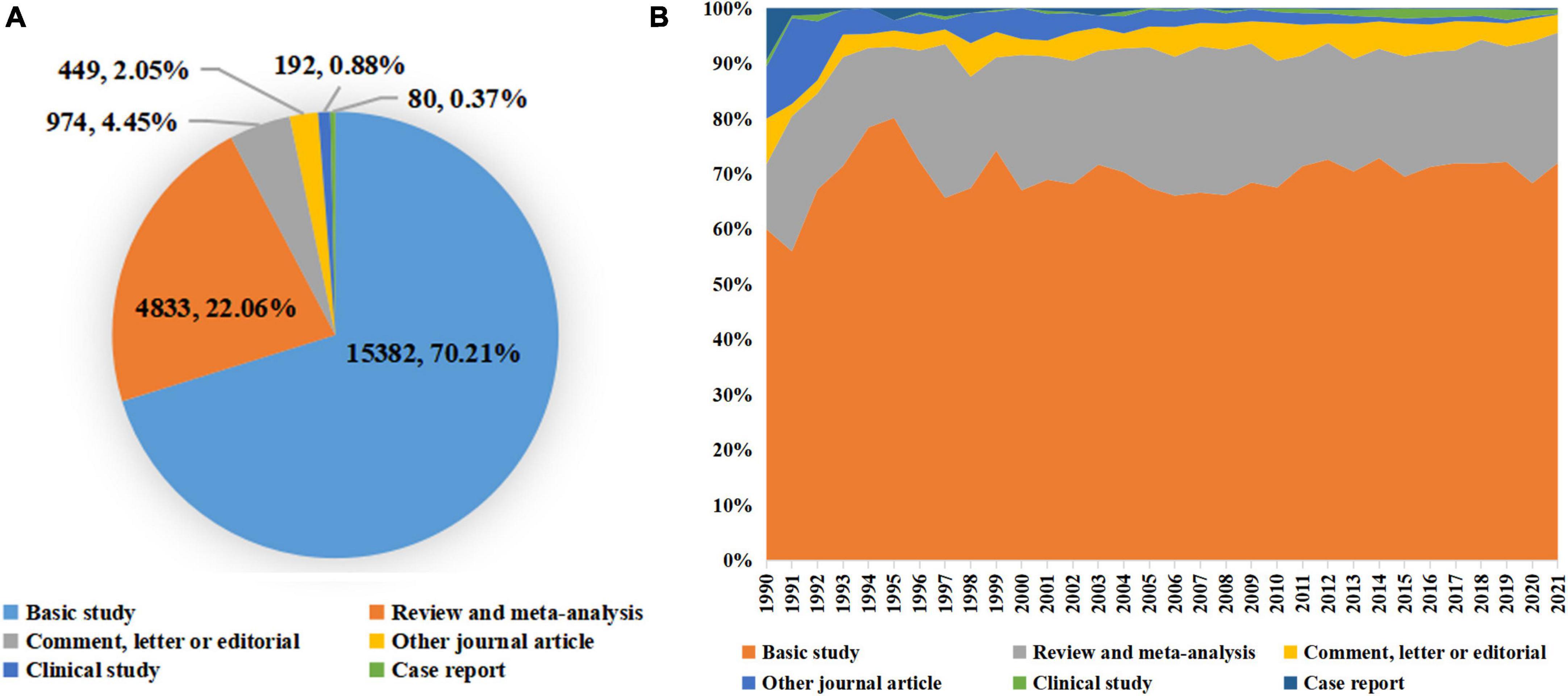
Figure 2. Distribution of publication types. (A) The total number and proportion of different publication types on cellular senescence in the past 30 years; (B) the distribution of different publication types per year.
3.2. MeSH analyses
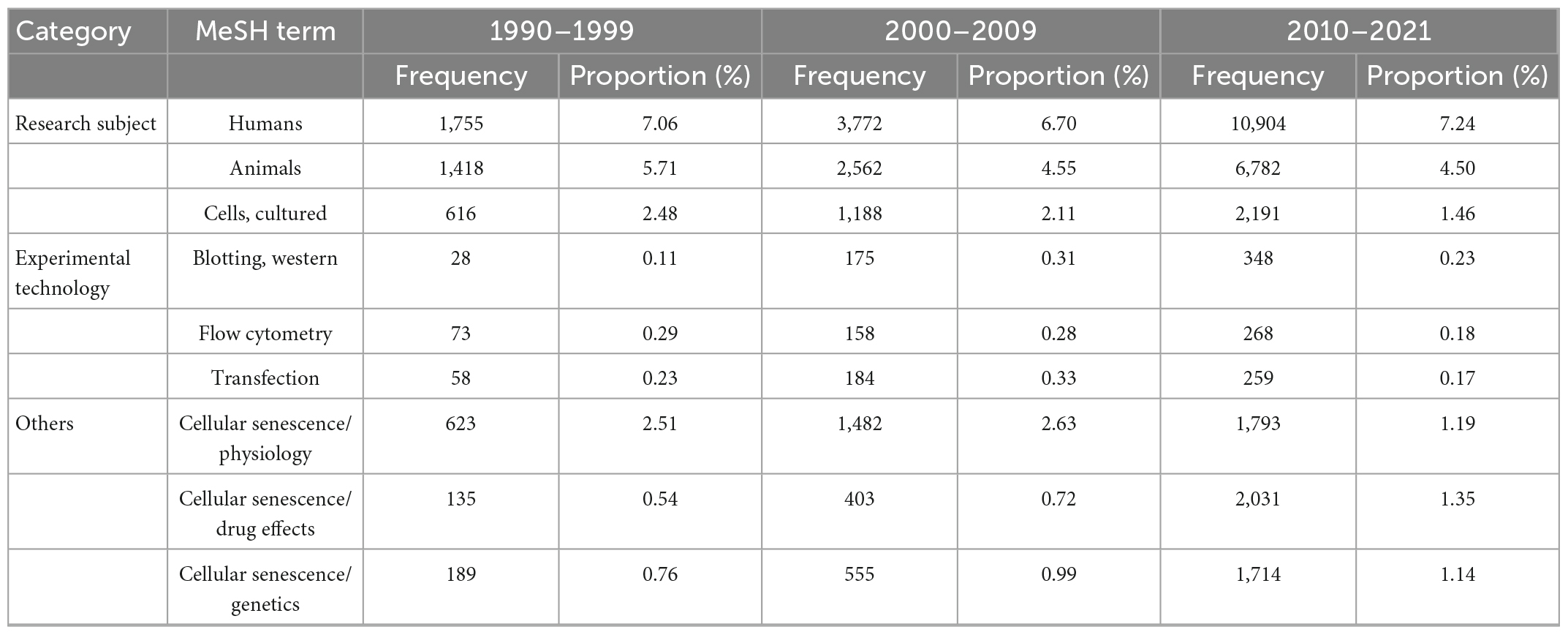
For MeSH analyses, 3,256 MeSH terms with a cumulative occurrence of 231, a total of 729 times were included. These terms were divided into three categories: (1) research subject; (2) experimental technology; and (3) others.
The top three MeSH terms of each category were listed in Table 2. Human was the main term of research subject, which was followed by animals and cells. The most common experimental technology was western blot, flow cytometry and transfection. For other MeSH terms, physiology, drug effects and genetics were the most concerned terms. Among which the proportion of drug effects increased most rapidely in the last decade.
Furthermore, Figure 4 illustrated the accumulated and annual occurrence of the top 15 MeSH terms related to the pathogenesis of cellular senescence. Cell proliferation and signal transduction were the top two terms over the past three decades and remained popular until 2021. Additionally, the MeSH term telomere shortening appeared in 2010, which increased dramatically over the past decades and had become one of the most focused topics in 2021.
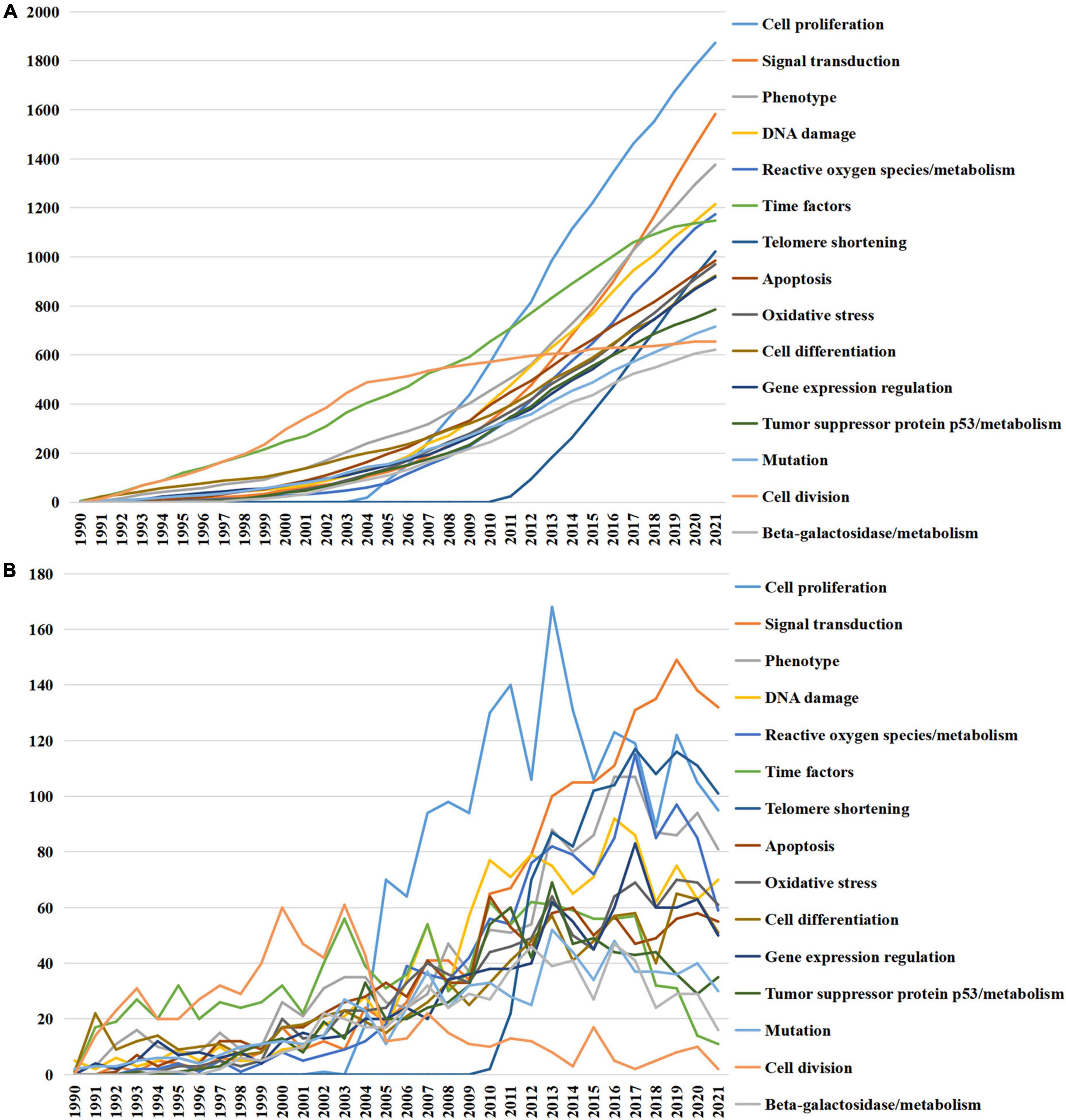
Figure 4. Top 15 medical subject headings (MeSH) terms related to the pathogenesis of cellular senescence. (A) Accumulated occurrences; (B) annual occurrences.
To further reveal the updated research hot spots, Top 10 MeSH terms emerging after 2015 were emphasized and presented in Figure 5. The conception of cell self renewal, immunosenescence, senescence-associated secretory phenotype (SASP) were proposed, CRISPR-Cas systems and RNA-seq technique were applied, and the roles of resveratrol, dasatinib, long non-coding RNA and extracellular vesicle were investigated.
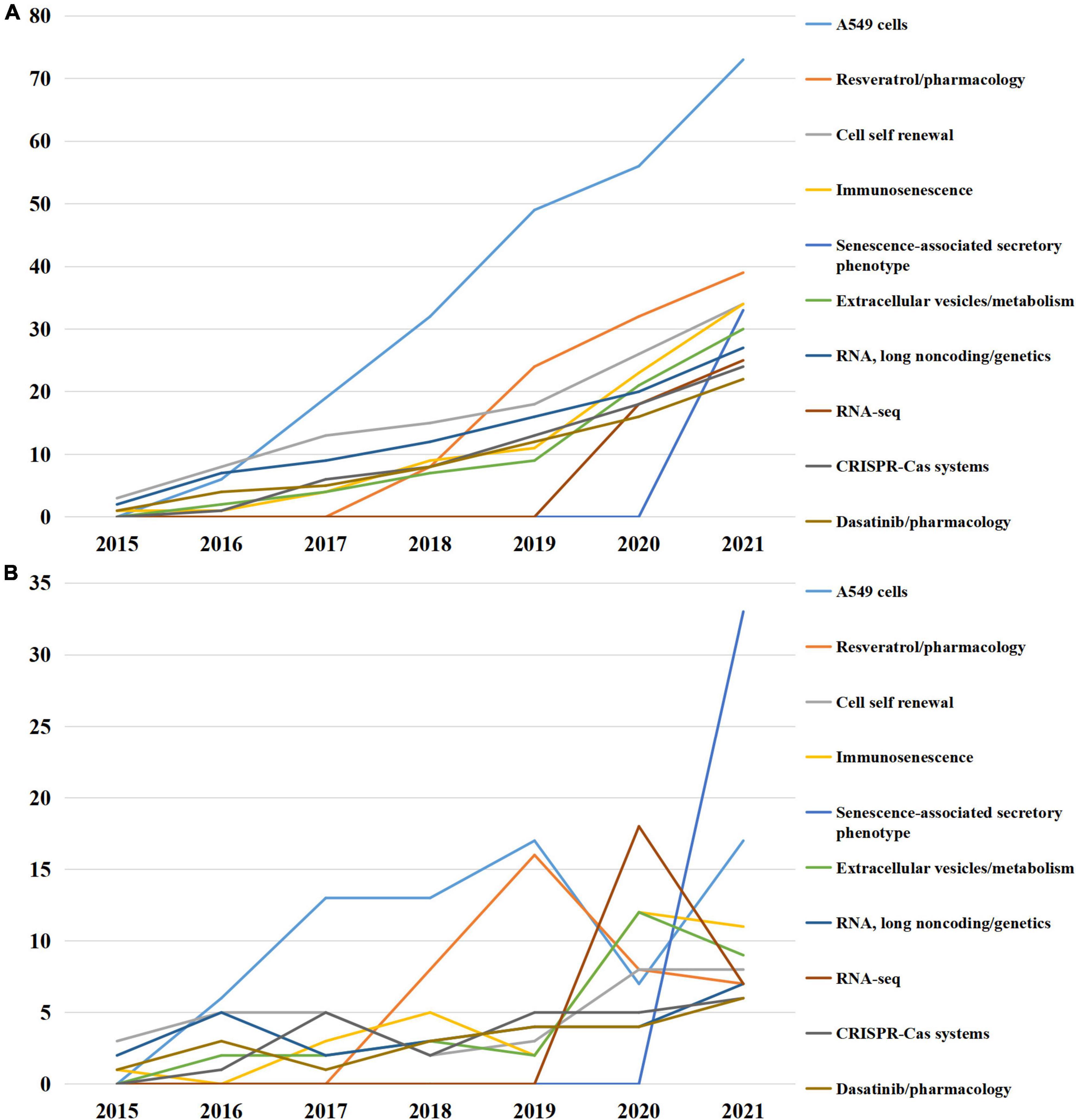
Figure 5. Top 10 medical subject headings (MeSH) terms emerging after 2015. (A) Accumulated occurrences; (B) annual occurrences.
Since dasatinib, as one of the classic senolytics, had attracted the attention of researchers recently, we searched for other senolytics in the MeSH term list. Currently, only three senolytics were indexed by MeSH terms, including quercetin, curcumin and dasatinib (Figure 6). These senolytics were still not well-studied in the field of cellular senescence, with the accumulated occurrence of 35, 26, and 22, separately. As for senomorphics, the other treatment strategy for senescent cells, the only drug indexed by MeSH terms was metformin, with accumulated occurrence of 58 (Figure 6).

Figure 6. Medical subject headings (MeSH) terms in relate to senolytics and senomorphics. (A) Accumulated occurrences; (B) annual occurrences.
In all, these findings revealed the trend of the hot research fields over the past three decades.
3.3. LDA analyses
Latent Dirichlet allocation analyses were performed to obtain more detailed research topics from the abstract of the publications and illustrate relationships between prominent topics by creating a network. A total of 20,486 publications were included for LDA analyses except those without an abstract. As shown in Figure 7, three clusters were identified by the Louvain algorithm and presented in different colors, including physiological function (marked in red), pathological function (marked in green), and molecular mechanism (marked in blue).
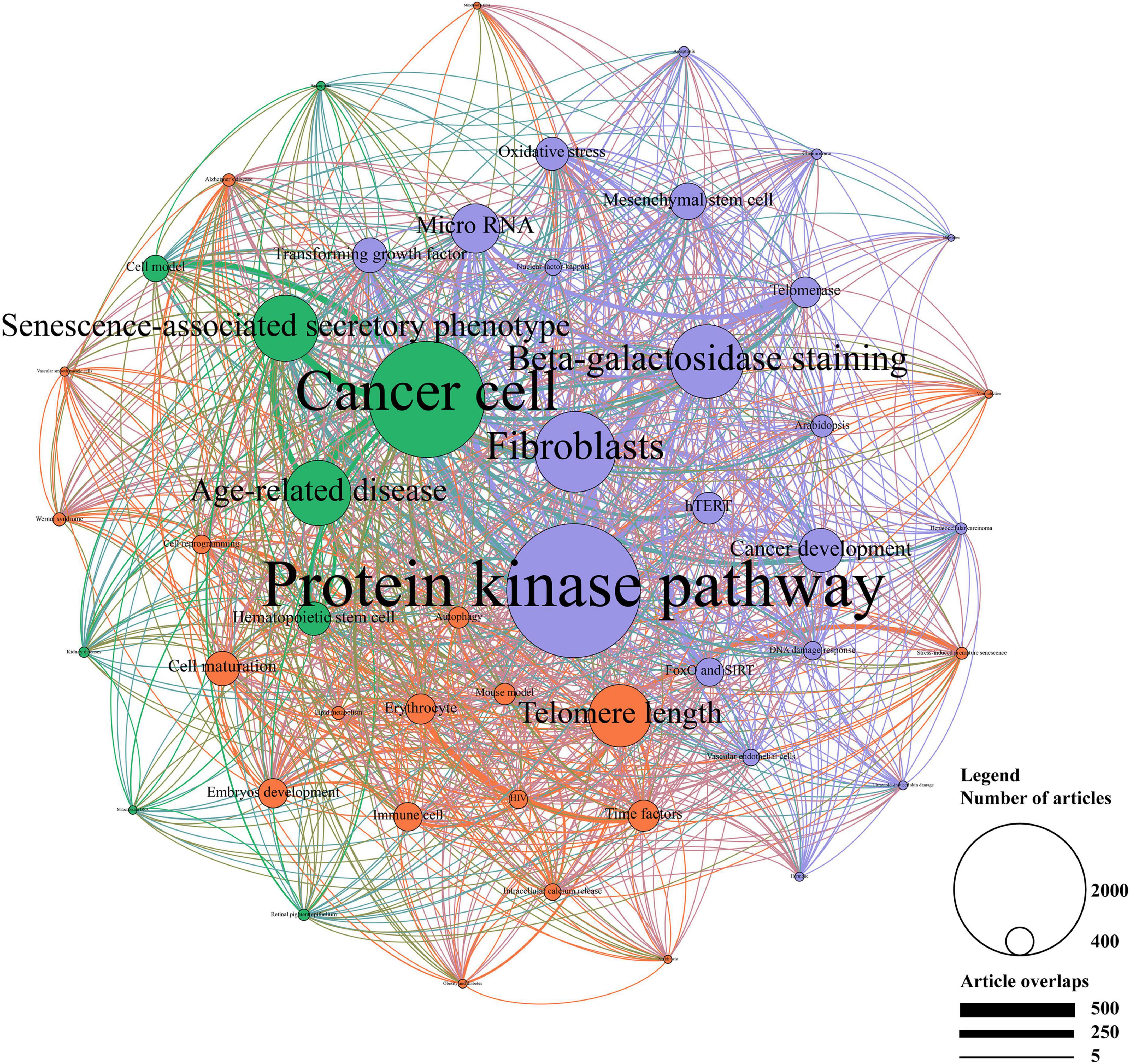
Figure 7. Topic cluster network by latent Dirichlet allocation (LDA). The red cluster represents “physiological function”, the green cluster represents “pathological function”, and the blue cluster represents “molecular mechanism”. The size of the circle represents the number of publications in each topic, while the degree of line thickness represents the weight of connection between topics.
In the cluster of physiological function, telomere length, cell maturation, time factors, erythrocyte, and embryos development were the top five studied topics. Of these, erythrocyte and time factors showed a strong connection. The cluster of pathological function mainly focused on cancer cell and SASP, followed by age-related disease, hematopoietic stem cell and cell model. Strong correlations were shown between these topics, especially between age-related disease, cancer cell, and SASP. In addition, protein kinase pathway, fibroblasts, beta-galactosidase staining, microRNA, and cancer development accounted for the most important part of the molecular mechanism. Moreover, protein kinase pathway showed a strong connection with fibroblasts and microRNA.
According to the whole network, protein kinase pathway, cancer cell, fibroblasts, beta-galactosidase, and SASP were the primarily focused research topics. Topics in the cluster of physiological function accounted for a relatively small proportion of the network, where more studies should be conducted. Compact connections were shown between the cluster of pathological function and the cluster of molecular mechanism, among which protein kinase pathway and cancer cell showed the strongest association. In the contrast, the cluster of physiological function showed a poor connection with other clusters. Together, LDA analyses revealed the detailed research topics as well as their relationships.
4. Discussion
Plenty of studies had been conducted to investigate the roles of cellular senescence since it was discovered in 1961 by Hayflick and Moorhead (16). With increasing attention to this field, plenty of articles reviewed the research progresses of cellular senescence in various diseases, such as cardiovascular diseases (17), endocrine diseases (18), Alzheimer’s disease (19), kidney injury (20), etc. More generally, Amaya-Montoya et al. summarized the basic mechanisms of cellular senescence and the current state of development of therapies against cellular senescence (21), demonstrating a very optimistic opinion in the development of senolytics and senomorphics. However, these comments were conducted based on manual literature acquisition and reading without quantitative analyses, which might influence the objectivity of their opinions on research hot spots. The study, for the first time, quantitatively analyzed 21,910 publications related to cellular senescence during the past three decades by machine learning. Although our research failed to elaborate the detailed mechanisms of cellular senescence and the role of cellular senescence in various diseases, it revealed research hot spots from the perspective of metrology, which may provide potential directions for future research. According to our analyses, the current research situation of senotherapeutics is far from satisfactory, but we are still confident about the future development of senotherapeutics.
The number of studies on cellular senescence increased rapidly over the past three decades. Nearly 2/3 of the investigations were basic studies, while clinical studies only accounted for a small proportion. Though experimental studies may be more appropriate to explore the mechanisms of cellular senescence, their real significance appears when they can be translated into clinical practices. Indeed, some clinical studies have been done on cellular senescence, which promotes the development of this field. The term “senolytics” was created in 2015, and the associated term “senotherapeutics” was added to the MeSH term in 2022, indicating the significance of translational medicine and clinical practice. Thus, more translational and clinical studies should be encouraged in the future.
Medical subject headings analyses were used to figure out the trend of research fields (22). Cell proliferation, as the top theme with rapid risen, is closely correlated to cellular senescence. The arrest of cell proliferation is the characteristic of senescent cells and SASP of senescent cells regulates the proliferation of nearby cells as well (1). The MeSH term telomere shortening was another theme increasing dramatically since it appeared. Telomere shortening caused by cell proliferation is a well-recognized form of replicative senescence (23). It is obvious that these MeSH terms will continue to be research hot spots in the future.
Several hallmarks of senescent cells, including p53, beta-galactosidase and SASP were identified in MeSH analyses. Senescent cells should be defined not by a single trait, but multiple hallmarks. A guide recommend to verify cellular senescence by three different traits, including cell cycle arrest, structural change and additional trait. p53, p16, and p21 are the main markers of cell cycle arrest, while the beta-galactosidase is one of the most widely used markers for structural change. As for additional trait, senescent cells may produce a complex secretome, such as IL-1β, IL-6, and ICAM-1, known as SASP, which is used as a secondary marker to access cellular senescence (24, 25). However, several problems need to be addressed. First, the multiple hallmarks do reflect the complex and heterogeneous mechanisms of cellular senescence, but an universal and robust hallmark is still needed to identify senescence. Furthermore, there are different types of cellular senescence, however, there are only common hallmarks of cellular senescence, indicating the different types of cellular senescence may be hard to identify because of lacking specific hallmarks.
Notably, the significance of senotherapeutics was induced by MeSH analyses. We found that the drug effect of cellular senescence has attracted increasing attention, as shown by the proportion of drug effect in MeSH terms increased from 0.54 to 1.35% during the past three decades. This phenomenon suggested the importance people attached to senotherapeutics, which can be classified into three strategies (senolytics, senomorphics, and senescence-targeting immunotherapeutics). Senolytics selectively killed senescent cells, and senomorphics prevented the progression of young cells to senescent cells by targeting SASPs, while senescence-targeting immunotherapeutics mediated the clearance of senescent cells (26). By searching for novel MeSH terms that did not appear until 2015, we found immunosenescence in the top 10 MeSH terms, indicating that the role of immunity in cellular senescence has been focused by researchers. As we known, the declination of immunological function is closely related to the increasing of senescent cells (27). Strategies targeting senescent immune cells using specific antibodies that recognize senescence surface markers have been applied in anticancer therapies (28). In addition, reversing the abnormal function of senescent immune cells to restore their immune functions is another strategy targeting immune cells, which has also been investigated recently (29). These results indicated that senescence-targeting immunotherapeutics played pivotal roles in delaying the progress of senescence. Meanwhile, the significance of senolytics and senomorphics were also embodied in novel MeSH terms that did not appear until 2015. Dasatinib, as one of the classic senolytics, was added to the MeSH term in 2016, while SASP, as the target of senomorphics, was added to the MeSH term in 2022. Senolytics mainly targeted signal pathways which are activated in senescent cells to kill them (30). Various senolytics, such as quercetin and dasatinib, have been identified and applied to diminish senescent cells in different disease models including myocardial infarction and osteoporosis (31, 32). In contrast to senolytics, senomorphics suppressed the function of SASPs instead of kill senescent cells. Researches showed that cellular senescence is one of the fundamental mechanisms in both age-related and diabetic phenotypes (33). Diabetes can induce cellular senescence and SASP to impair cardiac function independent of age (34). Metformin, as a well-known drug for diabetes, can inhibit SASP by decreasing the expression of IL-1β, IL-6, C-X-C motif chemokine 5 (CXCL5) and NF-kB (35). The function of metformin in aging process has been tested in clinical trials (36), which may become a precursor for future clinical application of senomorphics. It is worth to note that, as we mentioned above, the complexity, various types, and specificity of SASP has not been fully acknowledged. Thus the development of senomorphics may be limited until a comprehensive system is established in the field of SASP. Furthermore, we searched associated senolytics and senomorphics in MeSH terms. Although only three of the senolytics and one senomorphics were added to MeSH terms currently, with relatively small amounts of accumulated occurrence, it did not hinder the fact that researchers have noticed the significance of senotherapeutics in the clinical practice of cellular senescence.
Since the MeSH term was used to catalog publications rather than displaying the detailed content of the publication, plenty of information was ignored in MeSH analyses (37). Therefore, LDA and Louvain algorithm were applied to further investigate the relevant research topics from the abstract of the publications as well as illustrate their relationship. Studies on cellular senescence mainly focused on three fields: physiological function, pathological function and molecular mechanism.
Cellular senescence is usually recognized as a process of damage that leads to multisystem disease or aging. Recently, the physiological function of cellular senescence attracted more attention (2). Telomere length accounted for the predominant proportion in this cluster and was also closely associated with the cluster of pathological function, which was consistent with the result of MeSH analyses. The role of telomere in cellular senescence had been reviewed in a previous article (38). In addition, recent studies highlighted the significant role of cellular senescence in embryo development. Markers and features of senescence had been observed in developing embryo. As the embryo develops, senescent signal changes in a highly organized manner, indicating that senescence in embryo development is a tightly controlled programmed process (39, 40). The specific mechanism of senescence in embryo development remains to be further studied.
Cancer cell, SASP, and age-related disease were the main topics in the cluster of physiological function. The old population is more likely to suffer from chronic diseases, such as diabetes, hypertension, Alzheimer’s disease, etc. The chronic and continuous progress of senescence in cells contributes to the pathogenesis of these diseases mentioned above (41). It is suggested that anti-senescence therapies may be conducive to preventing age-related diseases, which have been discovered and soon become hot research topics (42). However, the safety and efficacy of anti-senescence therapies have not been clearly understood, which demands further clinical studies to validate. It is well-known that cancer is still one of the biggest killers of human, and senescence plays an important role in cancer development and escape (43). Inducing senescence in cancer cells limits the proliferation of cancer cells and may be an optional choice to suppress cancer development. But this therapy may also lead to the relapse of cancer cells from other therapies. The combined use of pro-senescence and senolytic therapies may solve this problem.
Protein kinase pathway was the predominant topic in the molecular mechanism cluster. p38-mitogen-activated protein kinase (MAPK) pathway has been widely investigated in cellular senescence (44). The activation of p38-MAPK activates SASP signal pathway, and compounds targeting p38-MAPK present senolytic function in cancer (23). In addition, microRNAs are reported to promote aging in mouse liver (45). They are associated with other pathways including oxidative stress, p53, telomere shortening, etc. (46). Therefore, microRNAs may be an important linkage between cellular senescence and aging, which need to be further studied.
In conclusion, publications related to cellular senescence increase rapidly during the past 30 years. More research should be focused on the physiological function of cellular senescence and clinical translation, especially the development and application of senotherapeutics.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RS conceived and design of the study. ZL contributed to the design of the study. CL collected and analyzed the data and drafted the manuscript. RS and ZL revised the manuscript. All authors made substantive intellectual contributions to this study to qualify as authors and read and approved the final manuscript.
Funding
This work was supported by grants from Postgraduate Autonomous Exploration and Innovation Project of Central South University (No. 2022ZZTS0038 to CL), Postgraduate Scientific Research Innovation Project of Hunan Province (No. CX20220130 to CL), National Natural Science Foundation of China (No. 82000301 to ZL), and Natural Science Foundation of Hunan Province, China (No. 2021JJ40938 to ZL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1072359/full#supplementary-material
Abbreviations
LDA, latent Dirichlet allocation; NLP, natural language processing; MAPK, mitogen-activated protein kinase; MeSH, medical subject headings; CRISPR, clustered regularly interspaced short palindromic repeats; Cas, CRISPR-associated; SASP, senescence-associated secretory phenotype.
Footnotes
- ^ http://lert.co.nz/map/
- ^ https://www.jianguoyun.com/p/DUIaWw8Q88SoCxi5xPEEIAA
- ^ https://gephi.org/
References
1. Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. (2018) 128:1238–46. doi: 10.1172/JCI95148
2. Rhinn M, Ritschka B, Keyes WM. Cellular senescence in development, regeneration and disease. Development (Cambridge). (2019) 146:dev151837. doi: 10.1242/dev.151837
3. Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol Rev. (2019) 99:1047–78. doi: 10.1152/physrev.00020.2018
4. Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. (2014) 28:99–114. doi: 10.1101/gad.235184.113
5. Iftikhar PM, Uddin MF, Ali F, Arastu AH, Khan J, Munawar M, et al. The top most-cited and influential published articles in atrial fibrillation from 1900 to 2019. Am J Cardiol. (2020) 125:420–6. doi: 10.1016/j.amjcard.2019.10.053
6. Beshyah WS, Beshyah SA. Bibliometric analysis of the literature on Ramadan fasting and diabetes in the past three decades (1989-2018). Diabetes Res Clin Pract. (2019) 151:313–22. doi: 10.1016/j.diabres.2019.03.023
7. Müller AM, Ansari P, Ebrahim NA, Khoo S. Physical activity and aging research: a bibliometric analysis. J Aging Phys Activ. (2016) 24:476–83. doi: 10.1123/japa.2015-0188
8. Chen X, Xie H, Wang FL, Liu Z, Xu J, Hao T. A bibliometric analysis of natural language processing in medical research. BMC Med Inform Decis Mak. (2018) 18:14. doi: 10.1186/s12911-018-0594-x
9. Gal D, Thijs B, Glänzel W, Sipido KR. Hot topics and trends in cardiovascular research. Eur Heart J. (2019) 40:2363–74. doi: 10.1093/eurheartj/ehz282
10. Li C, Liu Z, Shi R. A bibliometric analysis of 14,822 researches on myocardial reperfusion injury by machine learning. Int J Environ Res Public Health. (2021) 18:8231. doi: 10.3390/ijerph18158231
11. Aria M, Cuccurullo C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. (2017) 11:959–75. doi: 10.1016/j.joi.2017.08.007
12. Wang K, Feng C, Li M, Pei Q, Li Y, Zhu H, et al. A bibliometric analysis of 23,492 publications on rectal cancer by machine learning: basic medical research is needed. Therap Adv Gastroenterol. (2020) 13:320843838. doi: 10.1177/1756284820934594
13. Feng C, Wu Y, Gao L, Guo X, Wang Z, Xing B. Publication landscape analysis on gliomas: how much has been done in the past 25 years? Front Oncol. (2019) 9:1463. doi: 10.3389/fonc.2019.01463
14. Dozmorov MG. GitHub statistics as a measure of the impact of open-source bioinformatics software. Front Bioeng Biotechnol. (2018) 6:198. doi: 10.3389/fbioe.2018.00198
15. Jacomy M, Venturini T, Heymann S, Bastian M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS One. (2014) 9:e98679. doi: 10.1371/journal.pone.0098679
16. Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. (1961) 25:585–621. doi: 10.1016/0014-4827(61)90192-6
17. Hu C, Zhang X, Teng T, Ma ZG, Tang QZ. Cellular senescence in cardiovascular diseases: a systematic review. Aging Dis. (2022) 13:103–28.
18. Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. (2020) 16:263–75. doi: 10.1038/s41574-020-0335-y
19. Saez-Atienzar S, Masliah E. Cellular senescence and Alzheimer disease: the egg and the chicken scenario. Nat Rev Neurosci. (2020) 21:433–44. doi: 10.1038/s41583-020-0325-z
20. Li Y, Lerman LO. Cellular senescence: a new player in kidney injury. Hypertension. (2020) 76:1069–75. doi: 10.1161/HYPERTENSIONAHA.120.14594
21. Amaya-Montoya M, Pérez-Londoño A, Guatibonza-García V, Vargas-Villanueva A, Mendivil CO. Cellular senescence as a therapeutic target for age-related diseases: a review. Adv Ther. (2020) 37:1407–24. doi: 10.1007/s12325-020-01287-0
22. Zhao F, Shi B, Liu R, Zhou W, Shi D, Zhang J. Theme trends and knowledge structure on choroidal neovascularization: a quantitative and co-word analysis. BMC Ophthalmol. (2018) 18:86. doi: 10.1186/s12886-018-0752-z
23. Sun Y, Coppé J, Lam EWF. Cellular senescence: the sought or the unwanted? Trends Mol Med. (2018) 24:871–85.
24. González-Gualda E, Baker AG, Fruk L, Muñoz-Espín D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. (2021) 288:56–80.
25. Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. (2018) 28:436–53.
26. Park J, Shin DW. Senotherapeutics and their molecular mechanism for improving aging. Biomol Ther. (2022) 30:490–500.
27. Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. (2018) 9:5435.
28. Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. (2021) 12:81. doi: 10.1186/s13287-020-02128-1
29. Akbar AN. The convergence of senescence and nutrient sensing during lymphocyte ageing. Clin Exp Immunol. (2017) 187:4–5. doi: 10.1111/cei.12876
30. Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. (2017) 21:21–8. doi: 10.1016/j.ebiom.2017.04.013
31. Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. (2015) 14:644–58. doi: 10.1111/acel.12344
32. Salerno N, Marino F, Scalise M, Salerno L, Molinaro C, Filardo A, et al. Pharmacological clearance of senescent cells improves cardiac remodeling and function after myocardial infarction in female aged mice. Mech Ageing Dev. (2022) 208:111740. doi: 10.1016/j.mad.2022.111740
33. Palmer AK, Gustafson B, Kirkland JL, Smith U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. (2019) 62:1835–41. doi: 10.1007/s00125-019-4934-x
34. Marino F, Scalise M, Salerno N, Salerno L, Molinaro C, Cappetta D, et al. Diabetes-induced cellular senescence and senescence-associated secretory phenotype impair cardiac regeneration and function independently of age. Diabetes. (2022) 71:1081–98. doi: 10.2337/db21-0536
35. Moiseeva O, Deschênes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell. (2013) 12:489–98. doi: 10.1111/acel.12075
36. Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. (2020) 32:15–30. doi: 10.1016/j.cmet.2020.04.001
37. Nelson SJ, Schulman J. Orthopaedic literature and MeSH. Clin Orthop Relat Res. (2010) 468:2621–6. doi: 10.1007/s11999-010-1387-4
38. Liu J, Wang L, Wang Z, Liu J. Roles of telomere biology in cell senescence, replicative and chronological ageing. Cells (Basel, Switzerland). (2019) 8:54.
39. Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, et al. Programmed cell senescence during mammalian embryonic development. Cell. (2013) 155:1104–18. doi: 10.1016/j.cell.2013.10.019
40. Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. (2013) 155:1119–30. doi: 10.1016/j.cell.2013.10.041
41. Childs BG, Durik M, Baker DJ, Deursen JMAV. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. (2015) 21:1424–35. doi: 10.1038/nm.4000
42. Gurãu F, Baldoni S, Prattichizzo F, Espinosa E, Amenta F, Procopio AD, et al. Anti-senescence compounds: a potential nutraceutical approach to healthy aging. Ageing Res Rev. (2018) 46:14–31. doi: 10.1016/j.arr.2018.05.001
43. Perez-Mancera PA, Young AR, Narita M. Inside and out: the activities of senescence in cancer. Nat Rev Cancer. (2014) 14:547–58.
44. Xu Y, Li N, Xiang R, Sun P. Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem Sci (Amsterdam Regular Ed). (2014) 39:268–76. doi: 10.1016/j.tibs.2014.04.004
45. Maes OC, An J, Sarojini H, Wang E. Murine microRNAs implicated in liver functions and aging process. Mech Ageing Dev. (2008) 129:534–41. doi: 10.1016/j.mad.2008.05.004
Keywords: cellular senescence, bibliometric analysis, LDA analysis, machine learning, MeSH term
Citation: Li C, Liu Z and Shi R (2023) A comprehensive overview of cellular senescence from 1990 to 2021: A machine learning-based bibliometric analysis. Front. Med. 10:1072359. doi: 10.3389/fmed.2023.1072359
Received: 17 October 2022; Accepted: 06 January 2023;
Published: 19 January 2023.
Edited by:
Tzvi Dwolatzky, Technion – Israel Institute of Technology, IsraelReviewed by:
Nanqu Huang, First People’s Hospital of Zunyi, ChinaEleonora Cianflone, Magna Græcia University, Italy
Copyright © 2023 Li, Liu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoya Liu,  bGl1emhhb3lhQGNzdS5lZHUuY24=; Ruizheng Shi,
bGl1emhhb3lhQGNzdS5lZHUuY24=; Ruizheng Shi,  eHlzaGlydWl6aGVuZ0Bjc3UuZWR1LmNu
eHlzaGlydWl6aGVuZ0Bjc3UuZWR1LmNu
 Chan Li
Chan Li Zhaoya Liu
Zhaoya Liu Ruizheng Shi
Ruizheng Shi