- Department of Reproductive Medicine, Affiliated Shenzhen Maternity and Child Healthcare Hospital, Southern Medical University, Shenzhen, China
Objective: To compare the pregnancy outcome after fresh embryo transfer between GnRH antagonist and GnRH agonist regimens in patients with thin endometrium.
Methods: This retrospective study included all fresh embryo transfers following GnRH agonist or GnRH antagonist protocols in patients with thin endometrium from 2016 to 2021. The thin endometrium was defined as an endometrial thickness of 7.5 mm or less on the triggering day. Multivariant regression analysis was applied to assess the association of GnRH agonist or GnRH antagonist regimen with live birth following fresh embryo transfer in patients with thin endometrium.
Results: A total of 69 and 192 cases were, respectively, included in the GnRH antagonist or GnRH agonist group. The stimulation duration was significantly longer by the GnRH agonist protocol than the GnRH antagonist protocol (11.2 ± 2.1 vs. 9.1 ± 1.9 days, P = 0.002). The rates of clinical pregnancy or live birth were significantly lower in the GnRH antagonist group compared to the GnRH agonist group (26.1 vs. 47.9%, P = 0.027; 17.4 vs. 40.1%, P = 0.01, respectively). Multivariable regression analysis demonstrated that GnRH agonist regimen was related to higher live birth rate compared with GnRH agonist protocol [adjusted OR: 2.6, 95% confidence intervals (CI): 1.3–5.3]. No significant difference in miscarriage rate and the neonatal outcome was present between the two protocols.
Conclusion: Our findings suggest that GnRH agonist protocol results in a higher rate of live birth after fresh embryo transfer than GnRH antagonist protocol in patients with thin endometrium.
Introduction
The establishment of pregnancy depends on good quality embryos, endometrial receptivity and the synchronization of these two factors. Endometrial receptivity plays a pivotal role in embryo implantation and pregnancy. Various indicators including morphology and omics data are used to evaluate the endometrial receptivity. In daily practice, endometrial thickness functions as a simple and non-invasive sign for endometrial receptivity before embryo transfer. Thin endometrial thickness occurs in 2.4–8.5% of patients undergoing assisted reproductive technology and increases the adversity of pregnancy outcome (1, 2), though the uniform definition of thin endometrium is lacking. Thin endometrium usually refers to an endometrial thickness of less than 8 mm on the trigger day of fresh cycle or the progesterone addition day of frozen embryo thawing cycle (3–11).
Several studies reported that thin endometrium is associated with miscarriage, ectopic pregnancy, preterm birth and low birth weight (4, 12–17). Endometrium thickness on the day of human chorionic gonadotropin (hCG) trigger is well recognized as a predictor for live birth after fresh embryo transfer. Physicians usually choose not to proceed with the treatment cycle, counsel cycle cancelation, and subsequent frozen embryo transfer (FET) with patients with thin endometrium. However, the endometrial thickness may not be improved in subsequent FET cycles, leading to a longer treatment duration compared to fresh embryo transfer. In addition, little is known on factors associated with the pregnancy outcome after fresh embryo transfer in patients with thin endometrial thickness. Song et al. reported that fresh embryo transfer subsequent to GnRH agonist prolonged protocol yielded a higher live birth rate than the short-acting GnRH agonist long regimen (36.5 vs. 20.8%, respectively) (18), raising the hypothesis that controlled ovarian stimulation protocols might affect the pregnancy outcome following fresh embryo transfer in patients with thin endometrium. The two main protocols for controlled ovarian stimulation are GnRH agonist and GnRH antagonist regimens. Therefore, this study aims to compare the pregnancy outcome after fresh embryo transfer between GnRH antagonist and GnRH agonist regimens in patients with thin endometrium.
Materials and methods
Study design and patients
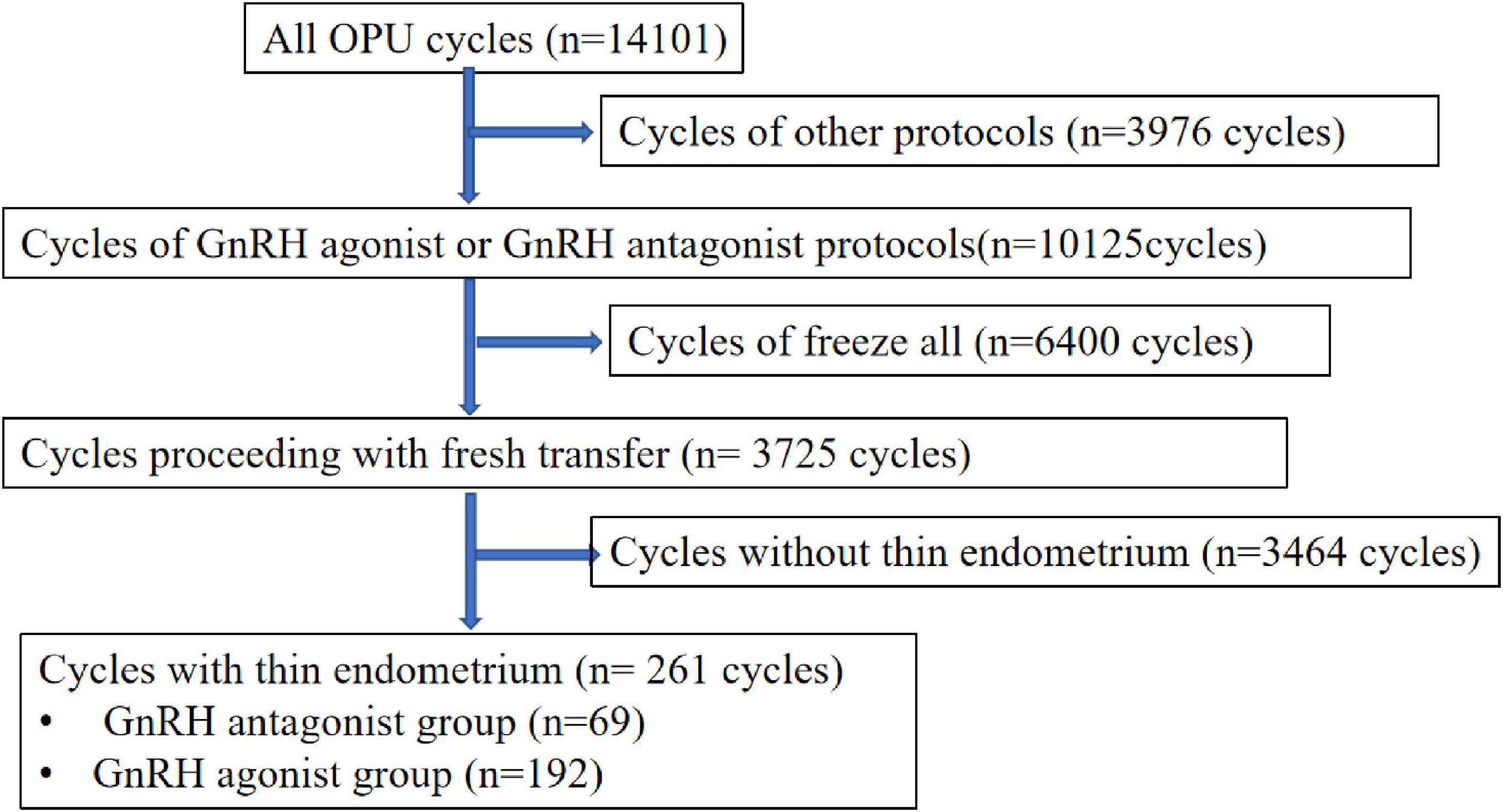
This retrospective study performed at the department of reproductive medicine, Shenzhen Maternity and Child Healthcare Hospital from January 2016 to December 2021. All patients who underwent in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) treatment and received fresh embryo transfer were eligible for this study. The inclusion criteria of the study were as follows: first, controlled ovarian hyper-stimulation are GnRH antagonist protocol or GnRH agonist protocol; second, age between 20 and 40 years; third, the first ovarian stimulation cycle; forth, fresh embryo transfer cycles; fifth, the number of embryos transferred ≤2. The exclusion criteria included: (1) other ovarian stimulation regimens; (2) stimulation cycles for pre-implantation genetic diagnosis; (3) free-all cycles. The flow chart of patient inclusion in the study is shown in Figure 1. Patients receiving hysteroscopy surgery for intrauterine adhesion was not excluded. In this study, the exposure measure was a thin endometrium on the triggering day. Since uniform definition of thin endometrium is lacking, recognized criteria for thin endometrium ranges from 6 to 8 mm (3, 4, 7, 11). In this study, we used 7.5 mm as a cut-off for the diagnosis of thin endometrium (16). The endometrial thickness was calculated as the maximal distance from one endometrial–myometrial interface to the other one in the midsagittal plane by ultrasonography.
The Medical Ethics Committee of Shenzhen Maternity and Child Healthcare reviewed and approved this study (SFYLS [2020] 067).
Controlled ovarian stimulation (COH) regimens
The option of COH stimulation regimen was based on anti-Müllerian hormone (AMH) test, antral follicle count (AFC), age, body weight and physician’s preference. The procedure of GnRH agonist and antagonist regimens was described as below. In GnRH agonist regimen, long-acting GnRH agonist for down-regulation of pituitary gland was injected at a dosage of 1.0–1.8 mg in the mid-luteal phase. The down-regulation of pituitary gland was assessed 14 days after administration of long-acting GnRH agonist. The criteria for successful down-regulation of pituitary gland were present as follicle diameter <8 mm, serum estradiol level <50 pg/mL, luteinizing hormone (LH) <5 IU/L and endometrium <5 mm. In case of reaching down regulation, injection of recombinant FSH (rhFSH) started with a dosage from 75 to 300 IU. In GnRH antagonist protocol, injection of rhFSH at a dosage of 75 to 300 IU was initiated on the day 2 to 4 of the menstrual cycle. The GnRH antagonist at a daily dosage of 250 mg was added when the dominant follicle was larger than 11–12 mm. In case of the diameter of two or more follicles larger than 18 mm, hCG triggering was administrated at a dose of 4,000 to 10,000 IU. Oocyte aspiration was performed at 35–38 h after hCG injection. Transfer of Cleavage-stage embryo or blastocyst proceeded on the 3rd or 5th days after fertilization. Embryo quality was evaluated according to the Istanbul consensus workshop on embryo assessment (19). In brief, the cleavage embryos on day 3 was assessed based on blastomere symmetry and fragmentation and described as good, fair or poor. Cleavage embryos scored as “good” with a blastomere count more than 7 were regarded as high-quality embryos. Blastocysts on day 5 or 6 were evaluated according to the trophectoderm and the inner cell mass (ICM), the degree of expansion of the blastocyst cavity and the status of the trophectoderm breakings out of the zona pellucida. All embryos transferred were at least good quality (Grade B). Luteal support was initiated on the day of oocyte aspiration with utility of Dydrogesterone (10 mg tid) and P suppository (Cyclogest, 400 mg bid) or 8% Crinone gel (90 mg qd). If pregnant, luteal support continued to 10 weeks of gestation.
Follow up of infertility outcomes
In this study, hospital-registry data were used to retrieve infertility outcomes. The first serum hCG test for pregnancy confirmation was scheduled 14 days after fresh embryo transfer. If pregnant, patients usually received ultrasound examination around 30 days after fresh embryo transfer. The primary outcome measure was the live birth rate. Other infertility outcomes were also recorded, including biochemical pregnancy, clinical pregnancy, miscarriage, gestational age at birth and birth weight.
Statistical analysis
Categorical data were expressed as frequency and percentage. Continuous variables were displayed as mean standard deviation (SD). We applied Shapiro–Wilk test to evaluate the normality of continuous variables. Student’s t-test or Kruskal-Wallis test was used to compare continuous variables. We applied Chi-square test or Fisher’s exact test to analyze the categorical data. Multivariable logistic regression model was built to evaluate the independent association of stimulation regimens with live birth adjusting for potential confounding factors. Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were calculated. IBM SPSS Statistics 22.0 (IBM Corporation, Armonk, NY, USA) was used to perform statistical analyses. Statistical significance was considered when a P-value was < 0.05.
Results
A total of 14,101 women underwent IVF or ICS treatment at our center during the study period. After applying the exclusion criteria, 69 women who underwent a first GnRH antagonist protocol and 192 women who underwent a GnRH agonist protocol were included, as shown in the flow chart in Figure 1.
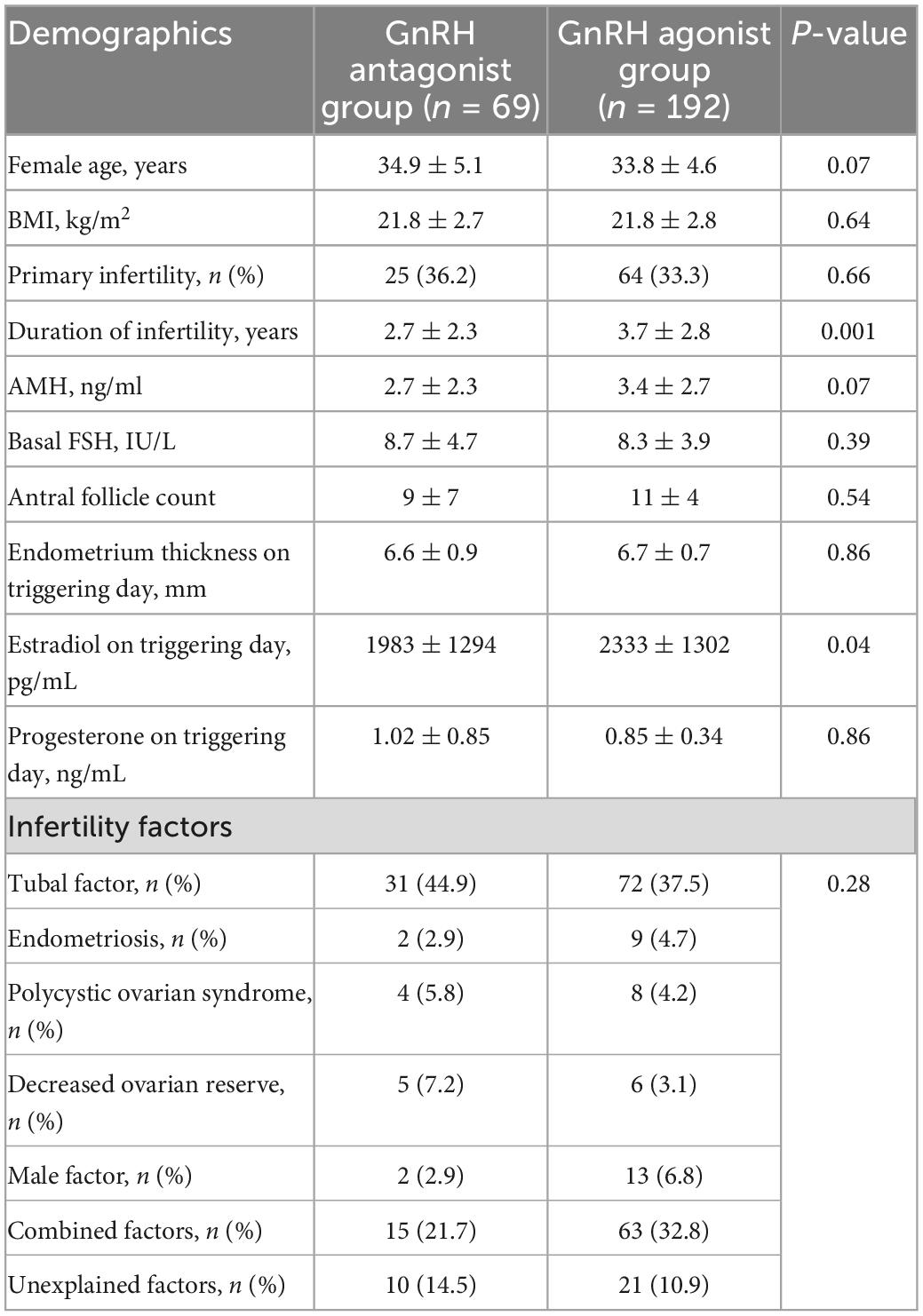
Baseline characteristics in the two groups were presented in Table 1, including maternal age, Body mass index (BMI), the rate of primary infertility, infertility duration, AMH, basal FSH, AFC, estradiol and progesterone level on the hCG trigger day (pg/ml), endometrium thickness on triggering day and infertility cause. There was no statistically significant difference in clinical characteristics between the two groups.
Outcomes of controlled ovarian hyper-stimulation
Table 2 shows ovarian stimulation procedures of the two protocols. The GnRH agonist protocol group had significantly higher duration of stimulation (11.2 vs. 9.1 days, P = 0.002), the number of oocytes retrieved (9.5 vs. 7.0, P = 0.001) and the Number of MII oocytes (8.5 vs. 6.2, P = 0.001) than the GnRH antagonist protocol group. While the GnRH agonist protocol group yielded a higher estradiol level (2333 vs. 1983 pg/mL, P = 0.04) on the hCG day than the GnRH antagonist protocol group. The GnRH antagonist protocol group yielded a higher total gonadotropin dose (2690 vs. 1802 pg/mL, P = 0.001) than the GnRH agonist protocol group. Each patient was transferred with one or two good quality embryos only and the rate of one embryo transferred was 21.7% in the GnRH antagonist protocol group was higher than the GnRH agonist protocol group (9.9%). Other results including the number of embryos available for transfer, the rate of cleavage-stage embryo transferred, and LH level on the hCG day were also similar.
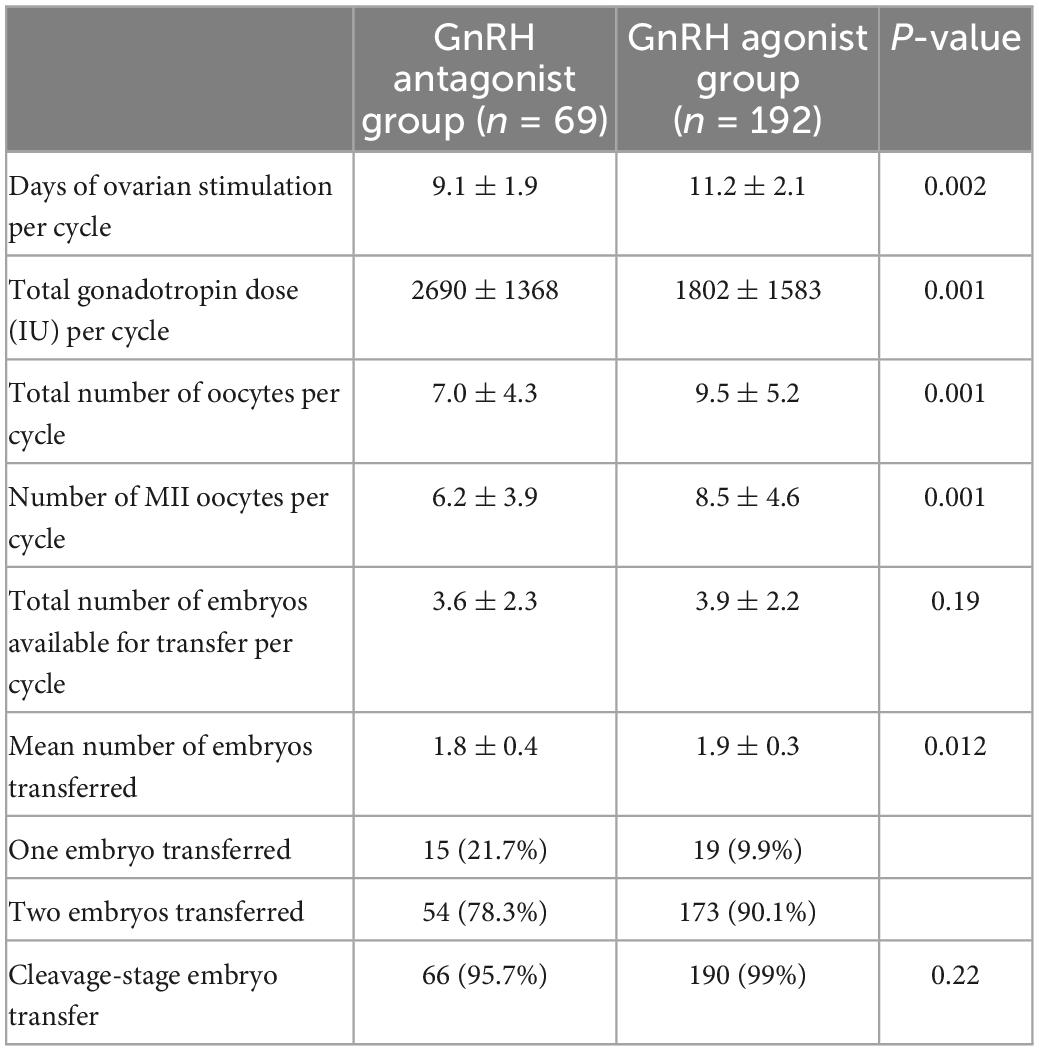
Table 2. Comparison of ovary stimulation and embryo transfer between GnRH antagonist group and GnRH agonist group.
Pregnancy outcomes
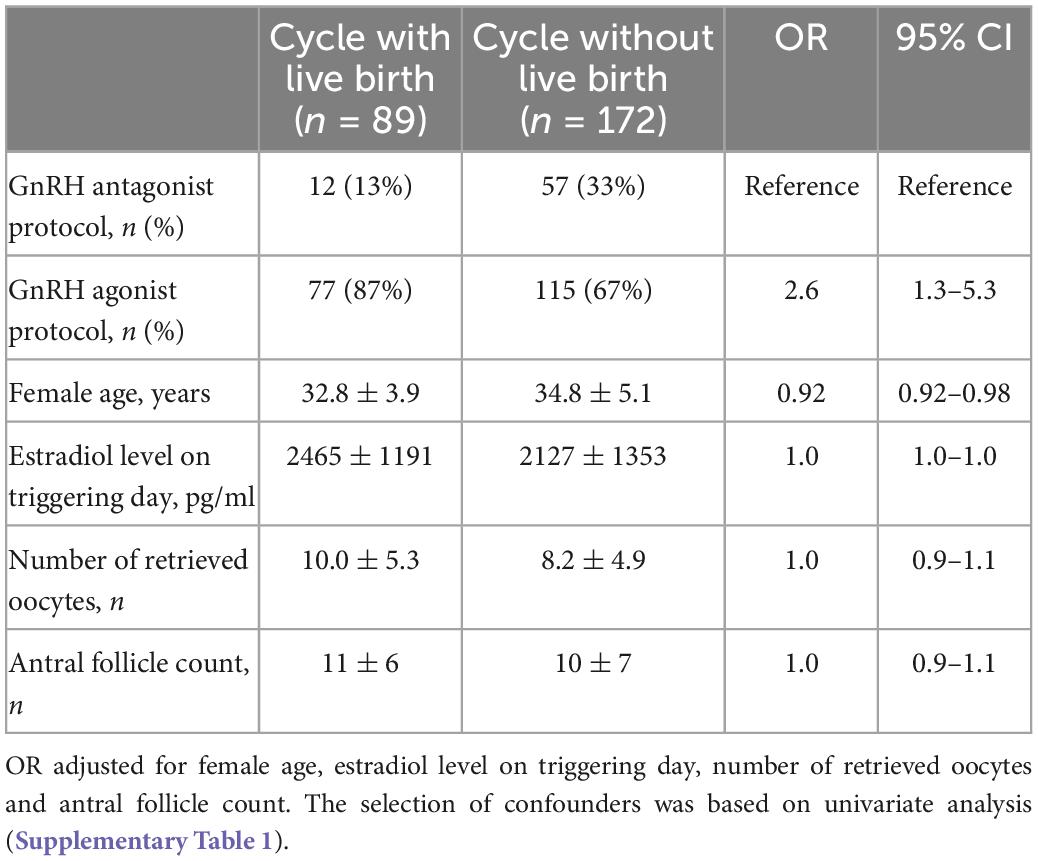
The live birth rate was 17.4% (12/69) in the GnRH antagonist protocol group and 40.1% (77/192) in the GnRH agonist protocol group, P = 0.001 (Table 3). The clinical pregnancy rate of the latter group were also significantly higher than that of the former group (47.9% vs. 26.1%, P = 0.002). There were no significant differences in the miscarriage rate, gestational age at birth and birth weight between the two protocols (P > 0.05). In multivariant regression analysis, applying GnRH agonist protocol contributes to a significantly higher live birth after fresh embryo transfer in patients with thin endometrium (OR 2.5, 95% CI 1.23–5.15, Table 4).
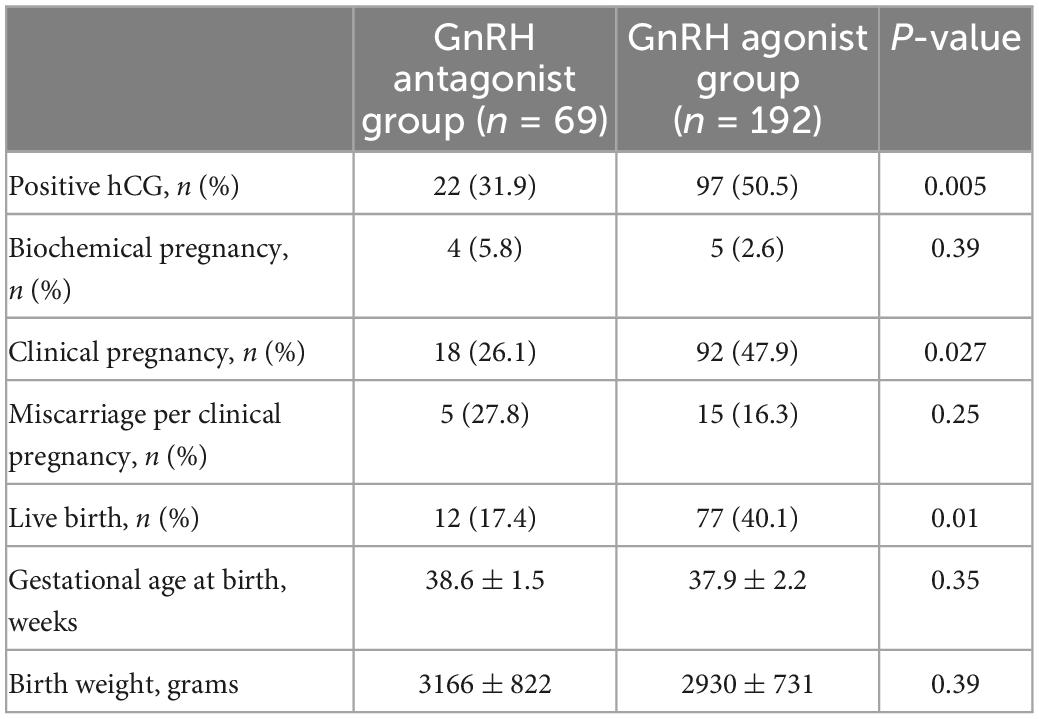
Table 3. Pregnancy outcome after embryo transfer by stimulation protocols in patients with thin endometrium.
Discussion
Sufficient endometrial thickness is a known key factor for embryo implantation. While how to manage patients with thin endometrium remains a challenge for physicians. It has been suggested that control ovarian stimulation protocols were associated with pregnancy outcomes. Our findings showed that compared with GnRH antagonist, the GnRH agonist protocol is associated with higher rates of positive HCG (31.9 vs. 50.5%), clinical pregnancy (26.1 vs. 47.9%) and live birth (17.4 vs. 40.1%) in patients with thin endometrium. After adjusting for potential confounders, GnRH agonist protocol independently improved the live birth rate (OR, 2.6, 95% CI 1.3–5.3).
The thin endometrium is a great challenge for infertility treatment and decreases the live birth rate after fresh embryo transfer. Although several studies were comparing the clinical outcomes of different control ovarian stimulation protocols (20, 21), few focused on patients with thin endometrium. A single-center retrospective cohort study in 2020 was conducted to compare the live birth rate and clinical pregnancy rate of 148 GnRH-a prolonged protocol and 154 the short GnRH-a long protocol in 302 patients with endometrium <8 mm who received fresh embryo transfer. The results showed that the clinical outcome of the GnRH-a prolonged protocol was better than that of the short GnRH-a long protocol in patients with endometrium <8 mm (18). In a 2021 study by Amir et al., IVF/ICSI patients undergoing fresh transplantation were divided into GnRH-a luteal long protocol (n = 3104) and GnRH antagonist protocol (n = 1527) groups (22). According to the endometrial thickness on the trigger day, they were divided into group of endometrium thickness ≤7 mm and group of endometrium thickness from 7 to 10 mm. After propensity score matching, the clinical outcomes of the GnRH antagonist protocol and the GnRH-a luteal long protocol were comparable in clinical pregnancy, live birth, and abortion rates (22). This discrepancy may be due to the application of long or short-acting agonist, the different study population (mean age of around 34 vs. 30 years) and various causes for infertility.
It is unclear why the GnRH agonist protocol yields a higher live birth rate after fresh embryo transfer than the GnRH antagonist protocol. The endometrial receptivity rather than thickness may be the key point. Several studies argue that a long-acting GnRH agonist regimen in the follicular phase may increase pregnancy outcomes by improving both endometrial receptivity and thickness (18, 23). A recent study substantiated that GnRH agonist improves endometrial receptivity by directly regulating the expression of related enzymes and cytokines (24). Further investigation unraveled that administration of higher doses of long-acting GnRH agonist enhances the expression of endometrial receptivity-related genes such as HOXA10, MEIS1, and LIF (18, 22, 25).
Our study has several limitations. First, given the retrospective nature of this study, endometrial thickness was not considered for the application of controlled ovarian stimulation protocol. Second, there was a considerable variation in the number of patients in each group, which may compromise the accuracy of statistics. Furthermore, the etiology for thin endometrium is incompletely determined and may be heterogenous in these patients. Whether the association exists of causes for thin endometrium with pregnancy outcome remains to be elucidated.
In conclusion, our findings suggest that the GnRH agonist protocol results in a higher rate of live birth after fresh embryo transfer compared to the GnRH antagonist protocol in patients with thin endometrium, indicating the GnRH agonist protocol might be more suitable in these patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Center for Reproductive Medicine of Shenzhen Maternity and Child Healthcare Hospital, Affiliated to Southern Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
DZ, RX, and XL contributed to the study design, data analysis, and manuscript preparation. DZ and RX handled the patient recruitment and data collection. All authors read and approved the final manuscript, made a substantial, direct, and intellectual output to the work, and approved it for publication.
Funding
This project was funded by the Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ20200109150410232, JCYJ20190809165601673, and JCYJ20210324131200002) and Shenzhen Maternity and Child Healthcare Hospital Science Foundation (Grant Number: FYA2017007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1071014/full#supplementary-material
Abbreviations
AMH, anti-Müllerian hormone; BMI, body mass index; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; hCG, human chorionic gonadotropin.
References
1. Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. (2014) 20:530–41.
2. Ribeiro VC, Santos-Ribeiro S, De Munck N, Drakopoulos P, Polyzos NP, Schutyser V, et al. Should we continue to measure endometrial thickness in modern-day medicine? The effect on live birth rates and birth weight. Reprod Biomed Online. (2018) 36:416–26. doi: 10.1016/j.rbmo.2017.12.016
3. Mahajan N, Sharma S. The endometrium in assisted reproductive technology: How thin is thin? J Hum Reprod Sci. (2016) 9: 3–8.
4. Yuan X, Saravelos SH, Wang Q, Xu Y, Li TC, Zhou C. Endometrial thickness as a predictor of pregnancy outcomes in 10787 fresh IVF-ICSI cycles. Reprod Biomed Online. (2016) 33:197–205. doi: 10.1016/j.rbmo.2016.05.002
5. El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, et al. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fert Steril. (2008) 89:832–9.
6. Kumbak B, Erden HF, Tosun S, Akbas H, Ulug U, Bahçeci M. Outcome of assisted reproduction treatment in patients with endometrial thickness less than 7 mm. Reprod Biomed Online. (2009) 18:79–84.
7. Bozdag G, Esinler I, Yarali H. The impact of endometrial thickness and texture on intracytoplasmic sperm injection outcome. J Reprod Med. (2009) 54:303–11.
8. Okohue JE, Onuh SO, Ebeigbe P, Shaibu I, Wada I, Ikimalo JI, et al. The effect of endometrial thickness on in vitro fertilization (IVF)-embryo transfer/intracytoplasmic sperm injection (ICSI) outcome. Afr J Reprod Health. (2009) 13:113–21.
9. Acharya S, Yasmin E, Balen AH. The use of a combination of pentoxifylline and tocopherol in women with a thin endometrium undergoing assisted conception therapies–a report of 20 cases. Hum Fert. (2009) 12:198–203. doi: 10.3109/14647270903377178
10. Papanikolaou EG, Pados G, Grimbizis G, Bili E, Kyriazi L, Polyzos NP, et al. GnRH-agonist versus GnRH-antagonist IVF cycles: is the reproductive outcome affected by the incidence of progesterone elevation on the day of HCG triggering? A randomized prospective study. Hum Reprod. (2012) 27:1822–8. doi: 10.1093/humrep/des066
11. Jimenez PT, Schon SB, Odem RR, Ratts VS, Jungheim ES. A retrospective cross-sectional study: fresh cycle endometrial thickness is a sensitive predictor of inadequate endometrial thickness in frozen embryo transfer cycles. Reprod Biol Endocrinol. (2013) 11:35. doi: 10.1186/1477-7827-11-35
12. Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. (2018) 33:1883–8. doi: 10.1093/humrep/dey281
13. Moffat R, Beutler S, Schötzau A, De Geyter M, De Geyter C. Endometrial thickness influences neonatal birth weight in pregnancies with obstetric complications achieved after fresh IVF-ICSI cycles. Arch Gynecol Obstetr. (2017) 296:115–22. doi: 10.1007/s00404-017-4411-z
14. von Wolff M, Fäh M, Roumet M, Mitter V, Stute P, Griesinger G, et al. Thin endometrium is also associated with lower clinical pregnancy rate in unstimulated menstrual cycles: a study based on natural cycle IVF. Front Endocrinol. (2018) 9:776. doi: 10.3389/fendo.2018.00776
15. Guo Z, Xu X, Zhang L, Zhang L, Yan L, Ma J. Endometrial thickness is associated with incidence of small-for-gestational-age infants in fresh in vitro fertilization-intracytoplasmic sperm injection and embryo transfer cycles. Fert Steril. (2020) 113:745–52. doi: 10.1016/j.fertnstert.2019.12.014
16. Oron G, Hiersch L, Rona S, Prag-Rosenberg R, Sapir O, Tuttnauer-Hamburger M, et al. Endometrial thickness of less than 7.5 mm is associated with obstetric complications in fresh IVF cycles: a retrospective cohort study. Reprod Biomed Online. (2018) 37:341–8. doi: 10.1016/j.rbmo.2018.05.013
17. Zhang J, Liu H, Mao X, Chen Q, Si J, Fan Y, et al. Effect of endometrial thickness on birthweight in frozen embryo transfer cycles: an analysis including 6181 singleton newborns. Hum Reprod. (2019) 34:1707–15. doi: 10.1093/humrep/dez103
18. Song J, Duan C, Cai W, Wu W, Lv H, Xu J. Comparison of GnRH-a Prolonged Protocol and Short GnRH-a Long Protocol in Patients with Thin Endometrium for Assisted Reproduction: A Retrospective Cohort Study. Drug Design Dev Ther. (2020) 14:3673–82. doi: 10.2147/DDDT.S270519
19. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. (2011) 26:1270–83.
20. Yang J, Zhang X, Ding X, Wang Y, Huang G, Ye H. Cumulative live birth rates between GnRH-agonist long and GnRH-antagonist protocol in one ART cycle when all embryos transferred: real-word data of 18,853 women from China. Reprod Biol Endocrinol. (2021) 19:124. doi: 10.1186/s12958-021-00814-0
21. Orvieto R, Patrizio P. GnRH agonist versus GnRH antagonist in ovarian stimulation: an ongoing debate. Reprod Biomed Online. (2013) 26:4–8.
22. Amir W, Micha B, Ariel H, Liat LG, Jehoshua D, Adrian S. Predicting factors for endometrial thickness during treatment with assisted reproductive technology. Fert Steril. (2007) 87:799–804.
23. Li G, Wu Y, Niu W, Xu J, Hu L, Shi H, et al. Analysis of the number of euploid embryos in preimplantation genetic testing cycles with early-follicular phase long-acting gonadotropin-releasing hormone agonist long protocol. Front Endocrinol. (2020) 11:424. doi: 10.3389/fendo.2020.00424
24. Ruan HC, Zhu XM, Luo Q, Liu AX, Qian YL, Zhou CY, et al. Ovarian stimulation with GnRH agonist, but not GnRH antagonist, partially restores the expression of endometrial integrin beta3 and leukaemia-inhibitory factor and improves uterine receptivity in mice. Hum Reprod. (2006) 21: 2521–9.
Keywords: pregnancy outcome, endometrial thickness, GnRH antagonist, GnRH, ovarian stimulation
Citation: Zhao D, Xie R and Li X (2023) Comparison of pregnancy outcome after fresh embryo transfer between GnRH antagonist and GnRH agonist regimens in patients with thin endometrium. Front. Med. 10:1071014. doi: 10.3389/fmed.2023.1071014
Received: 15 October 2022; Accepted: 04 January 2023;
Published: 19 January 2023.
Edited by:
Ali Çetin, University of Health Sciences (Turkey), TürkiyeReviewed by:
Nazan Yurtcu, Sivas Cumhuriyet University Faculty of Medicine, TürkiyeKarina Sequeira, Shady Grove Fertility Center, United States
Copyright © 2023 Zhao, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuemei Li,  bHhteXpmQDEyNi5jb20=
bHhteXpmQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Depeng Zhao
Depeng Zhao Rui Xie†
Rui Xie†