- 1Department of Respiratory and Critical Care Medicine, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai, China
- 2Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 3Department of Respiratory and Critical Care Medicine, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Jiangsu, China
Background: The high-resolution computed tomography (HRCT) score is an important component of the severity and prognosis score of pulmonary alveolar proteinosis (SPSP). However, the HRCT score in SPSP only considers the extent of opacity, which is insufficient.
Methods: We retrospectively evaluated HRCT scores for 231 patients with autoimmune pulmonary alveolar proteinosis (APAP) from three centers of the China Alliance for Rare Diseases. The SPSPII was created based on the overall density and extent, incorporating the SPSP. The severity of APAP patients was assessed using disease severity scores (DSS), SPSP, and SPSPII to determine the strengths and weaknesses of the different assessment methods. We then prospectively applied the SPSPII to patients before treatment, and the curative effect was assessed after 3 months.
Results: The HRCT overall density and extent scores in our retrospective analysis were higher than the extent scores in all patients and every original extent score severity group, as well as higher related to arterial partial oxygen pressure (PaO2) than extent scores. The mild patients accounted for 61.9% based on DSS 1–2, 20.3% based on SPSP 1–3, and 20.8% based on SPSPII 1–3. Based on SPSP or SPSPII, the number of severe patients deteriorating was higher in the mild and moderate groups. When applied prospectively, arterial PaO2 differed between any two SPSPII severity groups. The alveolar-arterial gradient in PaO2 (P[A-a]O2), % predicted carbon monoxide diffusing capacity of the lung (DLCO), and HRCT score were higher in the severe group than in the mild and moderate groups. After diagnosis, mild patients received symptomatic treatment, moderate patients received pure whole lung lavage (WLL) or granulocyte-macrophage colony-stimulating factor (GM-CSF) therapy, and severe patients received WLL and GM-CSF therapy. Importantly, the SPSPII in mild and severe groups were lower than baseline after 3 months.
Conclusion: The HRCT density and extent scores of patients with APAP were better than the extent score. The SPSPII score system based on smoking status, symptoms, PaO2, predicted DLCO, and overall HRCT score was better than DSS and SPSP for assessing the severity and efficacy and predicting the prognosis.
Trial registration:
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare lung syndrome characterized by the intra-alveolar accumulation of surfactant lipids and proteins. PAP is primarily divided into three groups: congenital PAP, secondary PAP, and autoimmune PAP (APAP) (1). The prevalence of APAP is 0.1 per 100,000 of the population and accounts for ~90% of all PAP cases (1, 2). Granulocyte-macrophage colony-stimulating factor (GM-CSF) antibody levels significantly increase in the serum and bronchoalveolar lavage fluid (BALF) of APAP patients (3) and have a high affinity for GM-CSF, decreasing its activity (4).
In a previous study, 7% of PAP patients went into spontaneous remission, indicating that not all patients require whole lung lavage (WLL) (5). In 2008, a disease severity score (DSS) was first proposed to assess PAP severity, divided into five grades based on symptoms and arterial partial pressure of oxygen (PaO2) (6). In 2016, our team proposed the severity and prognosis score for PAP (SPSP) based on smoking status, symptoms, PaO2, high-resolution computed tomography (HRCT) score, and the carbon monoxide diffusing capacity of the lung (DLCO) (7). In the SPSP, the HRCT score is the area based on the percentage extent of lung opacity in the corresponding lung region, not uninvolving the density of lesions. In 2017, Tokura et al. (8) suggested that increased parenchymal opacity be evaluated based on its density and extent. Therefore, updating the SPSP based on the density and extent of opacity with imaging systems is appropriate.
Methods
Study population
APAP patients aged between 18 and 80 years were enrolled at three hospitals in China: Shanghai Pulmonary Hospital, Peking Union Medical College Hospital, and Nanjing Drum Tower Hospital of China Alliance for Rare Diseases. This study was divided into two parts. In the first part, the data from 231 patients were collected and retrospectively cross-sectionally analyzed between January 2010 and December 2019. All patients completed at least a 6-month follow-up. The prognosis was assessed as improving, stable, or deteriorating by the physician based on the reviewed chest HRCT, many of which were not reviewed at the original hospital, and specific follow-up HRCT scores after treatment were not accessed. In the second part, 36 newly diagnosed patients were enrolled in a prospective study according to the newest severity assessment criteria between January 2020 to October 2021. They were provided with appropriate treatment and a 3 month follow-up. A total of 31 patients completed treatment and followed-up, and five patients withdrew during follow-up. This study was approved by the Ethics Committees of Peking Union Medical College Hospital (JS-2639), Shanghai Pulmonary Hospital (K19-133), and Nanjing Drum Tower Hospital (2019–106-1) and registered on the Clinical Trials database (ID: NCT04516577).
The eligibility criteria were selected according to Ben-Dov et al. (9): (1) histopathologic findings of specimens obtained by open or transbronchial lung biopsy confirmed by testing for amorphous periodic acid-schiff positive granules, (2) a typical milk-like BALF appearance with lamellar bodies visible on electron microscopy, (3) ground-glass opacity or a crazy-paving pattern on HRCT, and (4) a positive serum GM-CSF antibody test indicating an elevated serum GM-CSF antibody level. The retrospective patients were diagnosed with APAP by clinical synthetic judgment based on featured HRCT with typical pathology and BALF evidence (n = 86), with pathology evidence (n = 38), or with BALF evidence (n = 107). In the prospective study, APAP diagnosis was based on typical pathology and BALF.
Interview questionnaire
A standardized protocol was used to obtain informed consent from each participant during a medical visit. The interview questionnaire used included questions on the following topics: anthropometric information (i.e., age and sex), smoking status (e.g., smoker or never smoked), and clinical manifestation (e.g., the onset of symptoms, time of symptom onset, and symptoms).
Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and DLCO data were presented as percentages of predicted values (% predicted). Arterial blood measurements were performed on samples obtained while the patients were breathing room air at rest in the supine position, including PaO2, and alveolar–arterial gradient in PaO2 (P[A-a] O2).
Severity assessment of patients
Based on the recommendations of Inoue et al. (6), DSS categories included: 1 = asymptomatic and PaO2 ≥ 70 mm Hg; 2 = symptomatic and PaO2 ≥ 70 mm Hg; 3 = 60 ≤ PaO2 < 70 mm Hg; 4 = 50 ≤ PaO2 < 60 mm Hg; 5 = PaO2 < 50 mm Hg. The patients were divided into mild (DSS 1–2), moderate (DSS 3), and severe (DSS 4–5) groups according to Leth et al. (9).
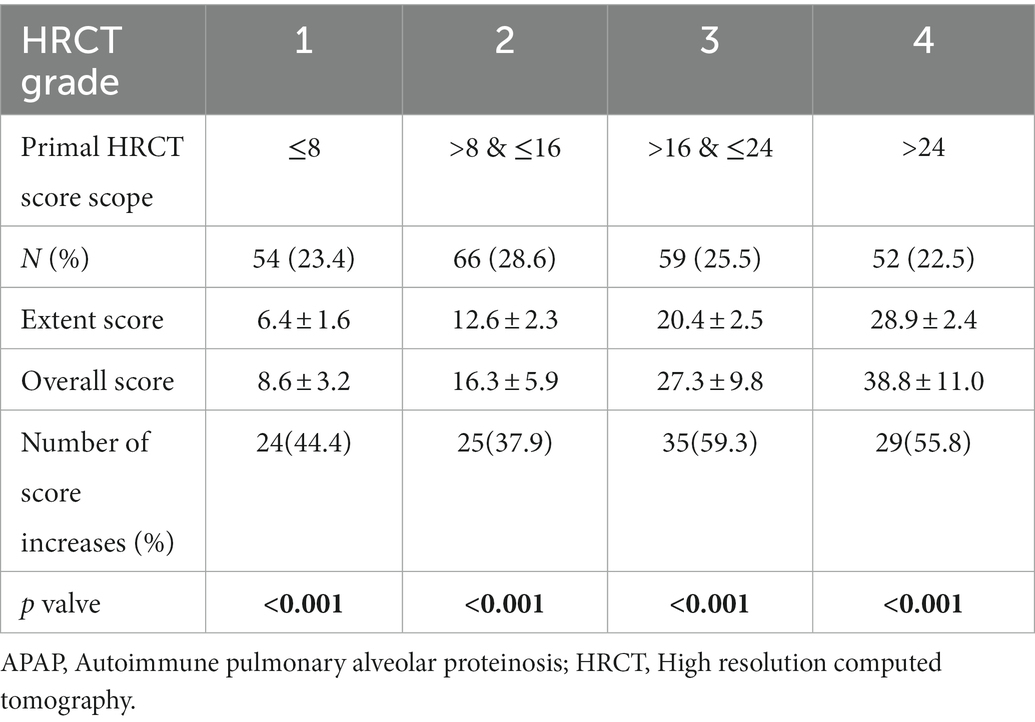
Chest HRCT were examined and interpreted independently by two chest physicians using imaging systems. The mean values obtained from the two readers were used for analysis. We selected the HRCT grades in four representative regions: the aortic arch, the tracheal carina, the convergence of the left and right inferior lung veins, and above the diaphragm. The extent score of lung opacity was estimated using a five-point scale: 0 = no opacity; 1 = opacity involving ≤25% of a region of hemithorax; 2 = 26–50%; 3 = 51–75%; 4 = >75%. The average density score of lung opacity in four representative regions was estimated using a three-point scale: 1 = density of opacity ≤ − 400; 2 = > −400 and ≤ −100; 3 = > −100. The overall score was updated based on the density score multiplied by the extent score and included a six-point scale: 0 = no opacity; 1 = ≤10; 2 = 11–20; 3 = 21–30; 4 = 31–40; 5 = >40.
The original SPSP included smoking statues (0 = never smoker; 1 = smoker); symptoms (0 = No; 1 = Yes); PaO2 (0 = ≥80 mm Hg; 1 = ≥60 mmHg and < 80 mm Hg; 2 = <60 mm Hg); HRCT extent score of lung opacity (1 = ≤8; 2 = >8 and ≤ 16; 3= >16 and ≤ 24; 4= >24); and % predicted DLCO (0 = ≥80%; 1 = ≥60 and < 80%; 2 = <0%) (7). SPSP was updated to SPSPII based on the overall HRCT chest score. Patients were divided into mild (SPSP or SPSPII 1–3), moderate (SPSP or SPSPII 4–6) and severe (SPSP or SPSPII ≥7).
Statistics
In the retrospective study, patient HRCT was evaluated based on the extent score and overall score with the self-contrasted method. The severity was assessed by three methods (DSS, SPSP, and SPSPII) and divided into three levels (mild, moderate, and severe). Patient prognoses were classified as improved, stable, or deteriorated based on their follow-up results. In the prospective study, patients were assessed by the SPSPII and divided into three severity levels. The improvement group (SPSPII was decreased) and non-improvement group (SPSPII was not decreased) were according to the change of SPSPII between the baseline and after 3 months. The statistical software SPSS (v.19.0; IBM, Armonk, NY, USA) and GraphPad Prism (v.5; GraphPad Software, San Diego, CA, USA) were used for all statistical analyzes and graph plotting, respectively. The data were tabulated as means and standard deviations (SDs) for quantitative variables or as absolute numbers and percentages for qualitative variables. The Kolmogorov–Smirnov test was used to assess the distribution for each variable. In bivariate analyzes, the Student’s t-test for independent variables was used for normally distributed variables, and the Mann–Whitney U test was used for non-normally distributed variables. Qualitative variables were compared using the Chi-square test. All results with p < 0.05 were considered statistically significant.
Results
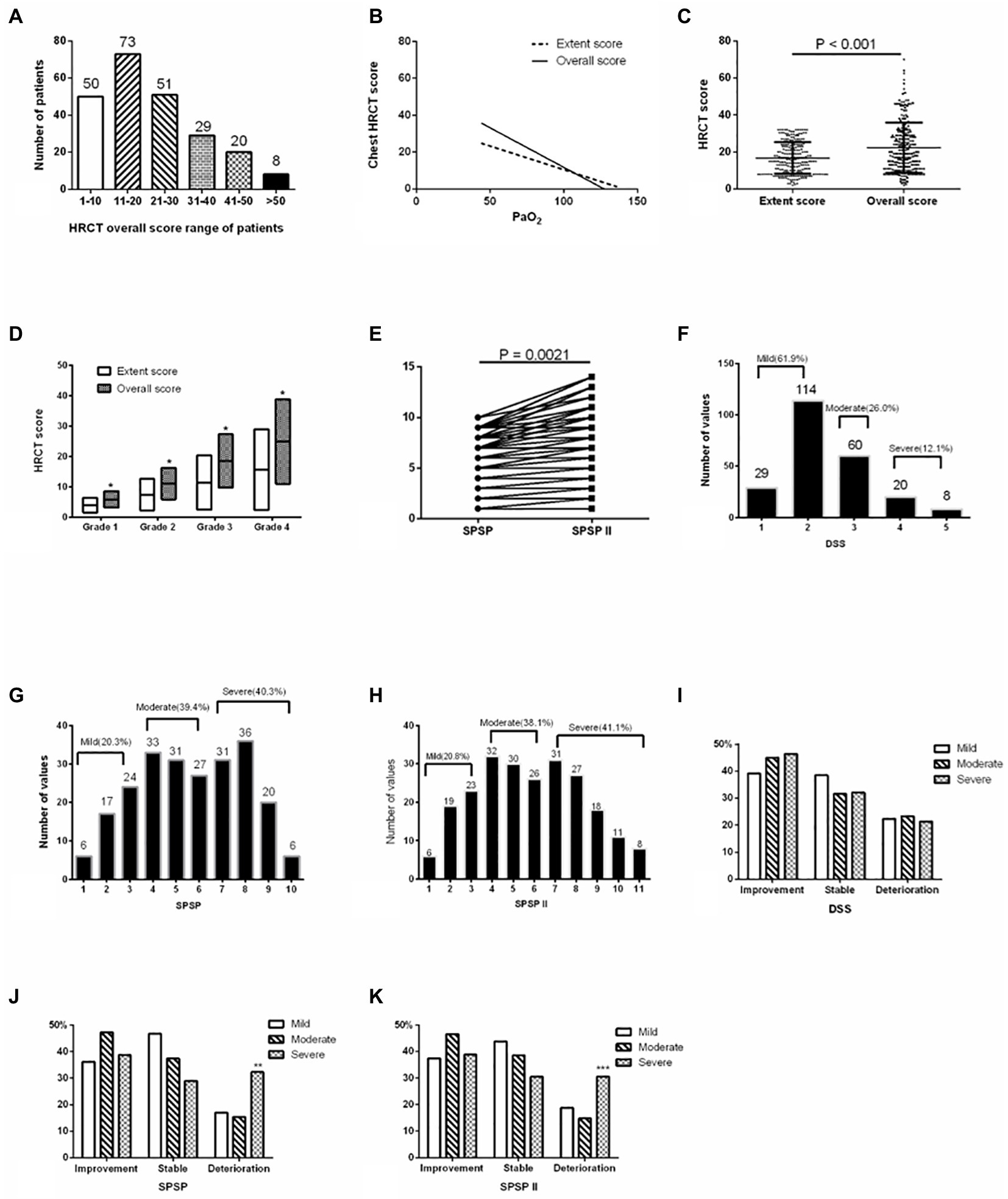
The overall HRCT score of patients in the retrospective study was shown in Figure 1A. This presented a negative relation to PaO2 (r = −0.4537, p < 0.001), and the coefficient was higher than that between the extent score of HRCT and PaO2 (r = −0.4366, p < 0.001; Figure 1B). The overall score of HRCT was higher than the extent score in all patients and every original extent score severity group (all p < 0.001; Figures 1C,D; Table 1). Updated SPSP (SPSPII) scores based on the overall score were shown in Table 2; these were higher than the SPSP scores (Figure 1E; p = 0.0021).
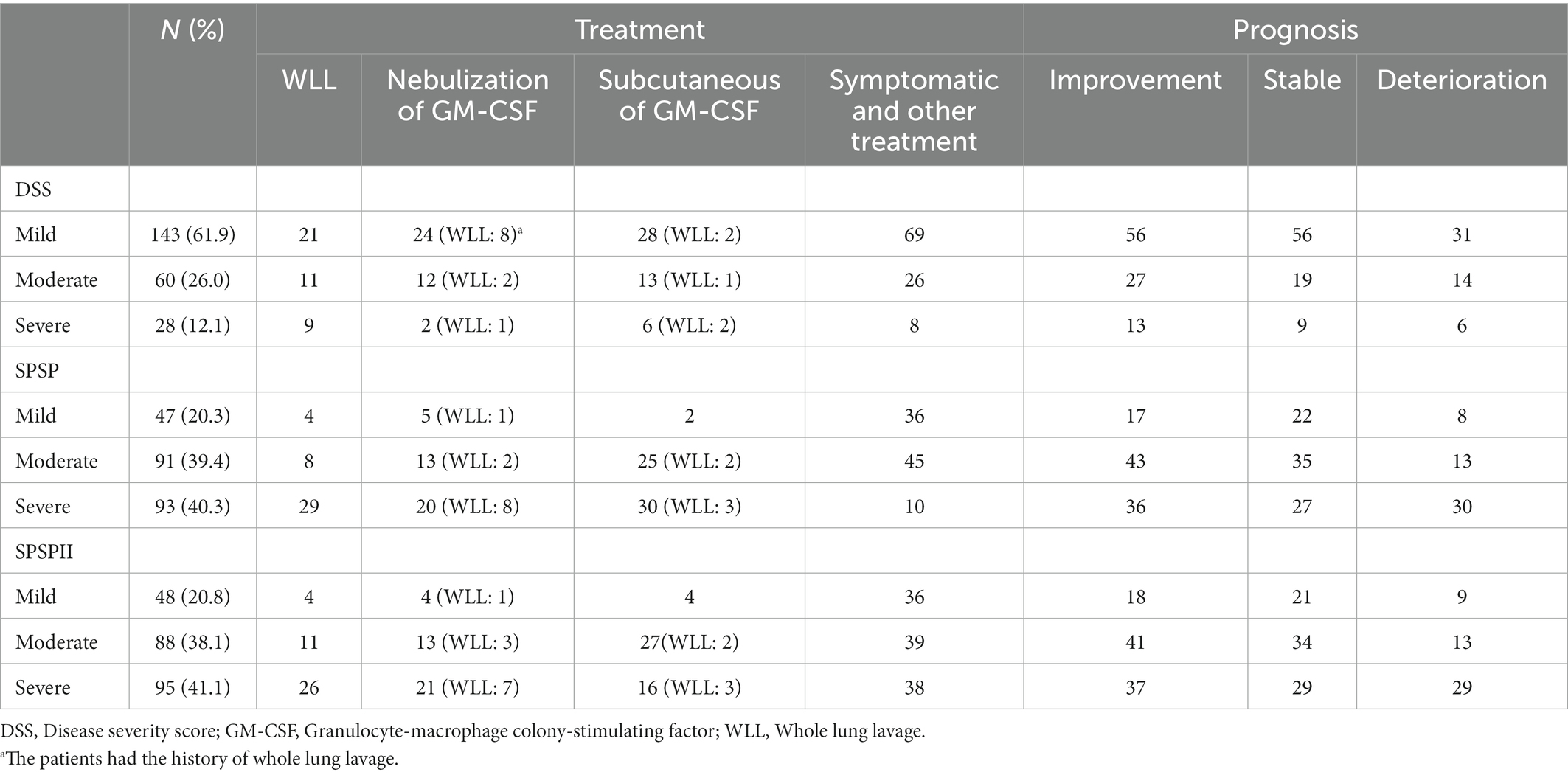
Based on DSS, the proportions of the different severities were 61.9% (DSS1, 29, 12.6%; DSS2, 114, 49.4%; mild), 26.0% (DSS3, moderate), and 12.1% (DSS4-5, severe; Figure 1F). Based on SPSP and SPSPII, the proportions of the different severity groups were shown in Figures 1G,H. The specific treatment and follow-up outcomes of patients were shown in Table 3. Based on DSS, the patient prognosis did not differ among severity groups (Figure 1I). However, based on SPSP or SPSPII, the proportion of patients deteriorating in the severe group was higher than in the mild and moderate groups (Figures 1J,K).
In the prospective study, the number of patients in the different SPSPII-based severity groups was 10 (mild), 11 (moderate), and 10 (severe). No apparent differences existed in age, sex, smoking history, onset age, or time of symptom onset among severity groups (all p > 0.05). Moderate and severe patients presented more symptoms than mild patients (p = 0.046; Figure 2A). However, PaO2, P[A-a] O2, % predicted DLCO, and overall HRCT score among different severity groups were shown in Figures 2B–E.
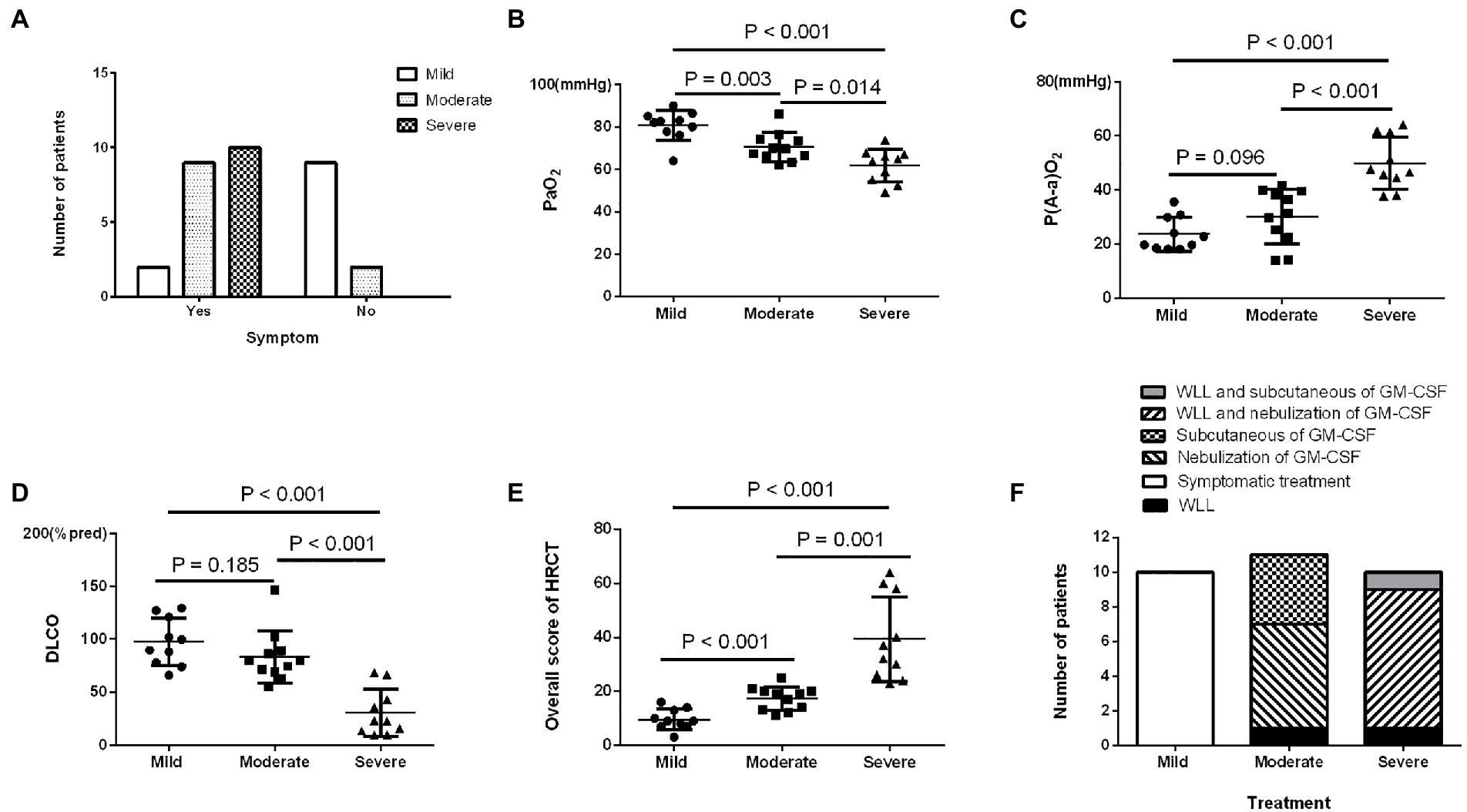
Figure 2. The characteristics and prognosis of patients with different SPSPII-based severities in the prospective study. (A) Symptoms. (B) PaO2. (C) P(A-a) O2. (D) DLCO. (E) Overall HRCT score. (F) The treatment options of patients with different severities.
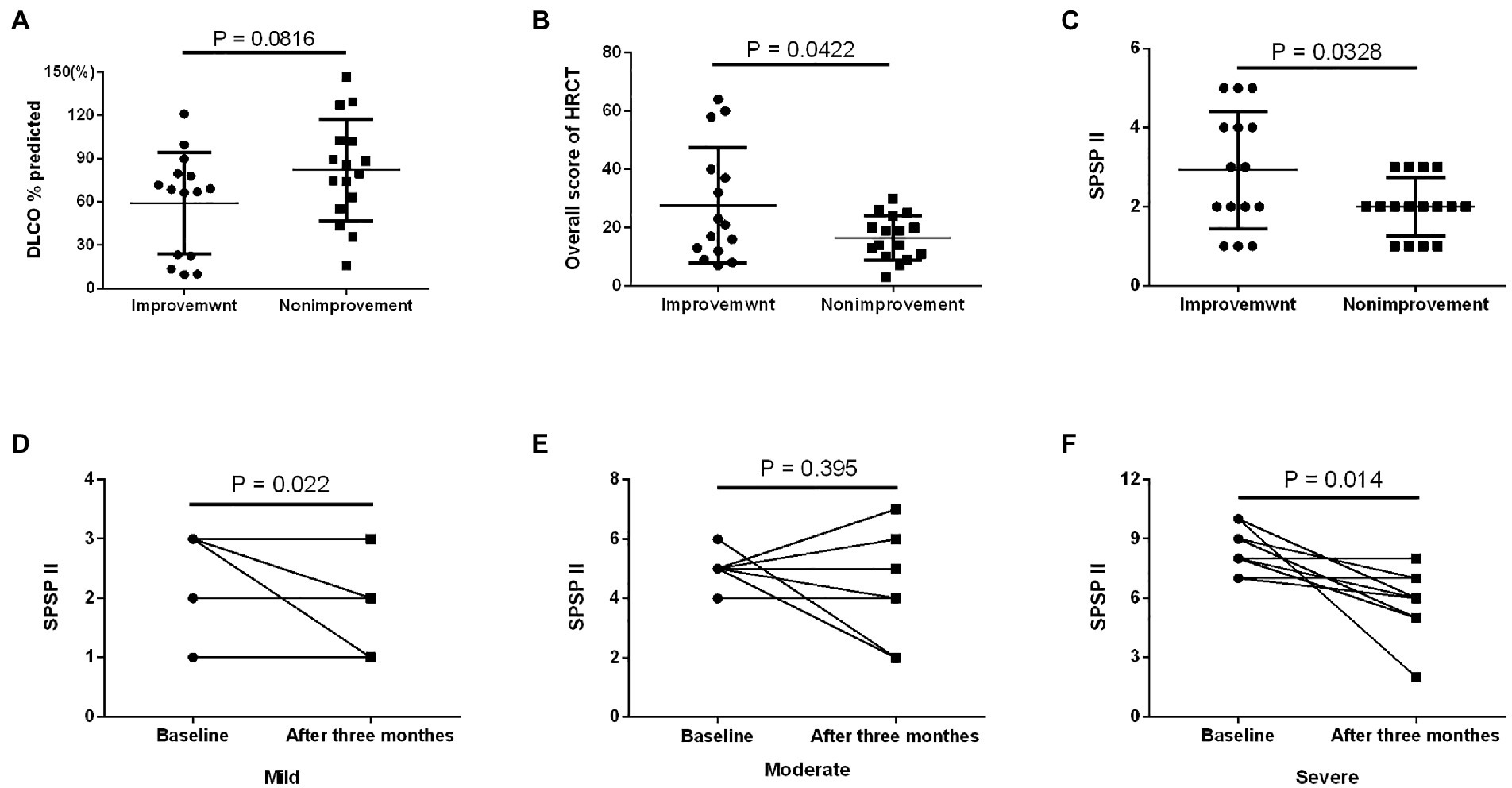
Based on the SPSPII, the patients in different severities received different treatment: symptomatic treatment in mild patients, WLL or GM-CSF therapy based on their willingness and consent in moderate patients, WLL and GM-CSF therapy in severe patients (Figure 2F). The SPSPII of all patients after 3 months (4.0 ± 2.2) was lower than at baseline (5.2 ± 2.5; p = 0.002). According to the change of SPSPII between baseline and after 3 months, these patients were divided into two groups: improvement group (SPSPII decreasing, 15) and non-improvement group (stable, 2; SPSPII increasing, 14). The sex, age, smoking history, symptoms, onset of symptoms, time of symptom onset, PaO2 and P[A-a] O2 were similar between improvement group and non-improvement group (all p > 0.1). The % predicted DLCO, overall score of HRCT and SPSPII between baseline and after 3 months were shown in Figures 3A–C. The change of SPSPII in different severities at baseline and after 3 months were shown in Figures 3D–F.
Discussion
APAP is an autoimmune disease of the lungs, (3) and similar to sarcoidosis, some patients experience spontaneous remission, (10, 11) and do not require WLL therapy. Inoue et al. (6) proposed the DSS to assess PAP severity in patients based on two criteria: symptoms and PaO2. DSS was simple, but relatively limited in assessing the efficacy and prognosis in patients (7). In 2016, we proposed SPSP to evaluate the severity and predict patient prognosis based on smoking status, symptoms, PaO2, HRCT extent score, and % predicted DLCO to some extent (7). In SPSP, the HRCT score was the percentage extent of lung opacity in the corresponding lung region, not uninvolving the density of lesions. However, while the extent of opacity was not significantly improved, density decreased in the HRCT of some PAP patients during follow-up during actual medical procedures. In this study, the overall score was based on the density score multiplied by the extent score, and presented higher correlation to PaO2 than simple extent score. Based on the overall HRCT score, SPSPII may be superior to the SPSP to evaluate the efficacy of patients before and after treatment. In addition, SPSPII was more beneficial to predict the prognosis of patients than DSS, as well as a better way to assess patient severity and prognosis.
Symptoms were subjective based on how the patient feels and may reflect severity to some extent. Previous studies have shown that some APAP patients were asymptomatic, (6, 12, 13) which may reflect the tolerance of patients with mild disease. PaO2 was a more objective indicator for assessing the severity of diffuse lung disease. In a previous study, the PaO2 of smokers was lower than nonsmokers in the general population (14). PAP patients with a smoking history had lower PaO2 than patients who had never smoked, (7) and the proportion of current smokers was significantly higher in the high DSS (DSS3-5) group than in the low DSS (DSS1-2) group (15). For PAP patients, the most common abnormality in pulmonary function tests was a decrease in % predicted DLCO, (10) which had a stronger correlation with PaO2 than FVC and FEV1 (7).
The degree of lesion abnormality on HRCT scans can be evaluated by visual assessment or objective quantification (16, 17). Ground-glass opacities and symmetric diffuse bilateral lung involvement were the predominant HRCT presentation of PAP (18, 19). In our previous study, the extent score of lung opacity was an SPSP component and negatively correlated with PaO2 (7). In 2017, Tokura et al. (8) suggested that increased parenchymal opacity be evaluated based on its density and extent. The visual score has been proposed to assess the severity of PAP patients based on evaluating the intensity and extent of opacities in HRCT (8, 20). However, this visual score was subjective and not completely accurate. Therefore, in this study, imaging systems were used to evaluate the intensity and extent of the opacities independently by two chest physicians, providing greater accuracy than the visual score.
The DSS 1–2 patients should be advised to regularly reassessment of symptoms, arterial blood gas analyzes, lung function tests, and chest X-rays by Leth et al. (9). A commonality (PaO2 ≥ 70 mm Hg) existed between DSS 1 and DSS 2. DSS 2 patients were considered mildly to moderately affected by Leth et al. (9). In this study, the DSS 2 patients accounted for 49.4%. What percentage of patients would further worsen and require treatment in the future was not clear. If mild patients include DSS1-2 and accounted for 61.9%, most patients would experience disease progression and require further treatment. Therefore, DSS was relatively simple and limited in clinical assessment of patient severity and choosing appropriate therapeutic schedule. When using SPSP or SPSPII, mild patients accounted for a little over 20%, indicating that nearly 80% of PAP patients will require treatment, this situation was more consistent with the actual treatment of patients with PAP. Deterioration was more likely to occur in severe patients than in mild and moderate patients classified based on SPSP or SPSPII. Therefore, SPSP and SPSPII appear superior to DSS for assessing the PAP severity and predicting patient prognosis.
Between improvement and non-improvement group at baseline and 3 months later, the patients in improvement group presented higher overall score of HRCT and SPSPII. This result may be because most patients with a high SPSPII received appropriate treatment, resulting in illness improvement. In the prospective study, mild patients were not given WLL or GM-CSF therapy, but their prognosis did not deteriorate. Ten moderate patients received GM-CSF therapy, and the SPSPII of more than 70% of patients was not decreased. This result may be related to finite-duration treatment with GM-CSF therapy. In previous studies, after 6 months of treatment, regardless of the treatment (subcutaneous injection or nebulized inhalation), GM-CSF showed significant improvement in % predicted DLCO and P[A-a] O2 (21, 22). Severe patients received WLL and GM-CSF therapy, and their condition was improved or stable.
This study was primarily limited in two aspects: there was no uniform standard on the treatment of patients in the retrospective study, and the number of cases was limited and the follow-up time was shorter in the prospective study. The retrospective study results indicated that SPSPII was the superior predictor, and the prospective study results largely accorded with this. Nevertheless, further studies of the SPSPII with larger sample sizes are required to confirm our findings.
Conclusion
The density and extent HRCT score of APAP patients was superior to the extent score. SPSPII, based on smoking status, symptoms, PaO2, % predicted DLCO, and the overall HRCT score, was better at assessing the severity and efficacy and predicting prognosis than DSS and SPSP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was, respectively, approved by the Ethics Committee of Peking Union Medical College Hospital, Shanghai Pulmonary Hospital and Nanjing Drum Tower Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
J-WB, K-FX and J-FX made substantial contributions to the concept and design of the work. J-WB, J-NH and SYS conducted statistical analysis. AG, H-WL, X-LS, S-YG, SL, K-BC and X-LT made substantial contributions to acquisition of data. J-WB and J-FX drafted the initial manuscript. K-FX and Y-LX reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported in part by grants from the Advanced and Appropriate Technology Promotion Project of Shanghai Municipal Health Commission (2019SY055) and Prevention and Treatment of Common and Frequent Diseases of National Key Research and Development Program Special project (2021YFC2500700).
Acknowledgments
We thank our great patients and their families for their trust and attendance in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1058001/full#supplementary-material
References
1.Salvaterra, E, and Campo, I. Pulmonary alveolar proteinosis: from classification to therapy. Breathe (Sheff). (2020) 16:200018. doi: 10.1183/20734735.0018-2020
2.Trapnell, BC, Nakata, K, Bonella, F, Campo, I, Griese, M, Hamilton, J, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primers. (2019) 5:16. doi: 10.1038/s41572-019-0066-3
3.Ataya, A, Knight, V, Carey, BC, Lee, E, Tarling, EJ, and Wang, T. The role of GM-CSF autoantibodies in infection and autoimmune pulmonary alveolar Proteinosis: a concise review. Front Immunol. (2021) 12:752856. doi: 10.3389/fimmu.2021.752856
4.Ben-Dov, I, and Segel, MJ. Autoimmune pulmonary alveolar proteinosis: clinical course and diagnostic criteria. Autoimmun Rev. (2014) 13:513–7. doi: 10.1016/j.autrev.2014.01.046
5.Campo, I, Mariani, F, Rodi, G, Paracchini, E, Tsana, E, Piloni, D, et al. Assessment and management of pulmonary alveolar proteinosis in a reference center. Orphanet J Rare Dis. (2013) 8:40. doi: 10.1186/1750-1172-8-40
6.Inoue, Y, Trapnell, BC, Tazawa, R, Arai, T, Takada, T, Hizawa, N, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. (2008) 177:752–62. doi: 10.1164/rccm.200708-1271OC
7.Bai, J, Xu, J, Yang, W, Gao, B, Cao, W, Liang, S, et al. A new scale to assess the severity and prognosis of pulmonary alveolar Proteinosis. Can Respir J. (2016) 2016:3412836–8. doi: 10.1155/2016/3412836
8.Tokura, S, Akira, M, Okuma, T, Tazawa, R, Arai, T, Sugimoto, C, et al. A Semiquantitative computed tomographic grading system for evaluating therapeutic response in pulmonary alveolar Proteinosis. Ann Am Thorac Soc. (2017) 14:1403–11. doi: 10.1513/AnnalsATS.201607-574OC
9.Leth, S, Bendstrup, E, Vestergaard, H, and Hilberg, O. Autoimmune pulmonary alveolar proteinosis: treatment options in year 2013. Respirology. (2013) 18:82–91. doi: 10.1111/j.1440-1843.2012.02274.x
10.Seymour, JF, and Presneill, JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. (2002) 166:215–35. doi: 10.1164/rccm.2109105
11.Fijołek, J, Wiatr, E, Radzikowska, E, Bestry, I, Langfort, R, Polubiec-Kownacka, M, et al. Pulmonary alveolar proteinosis during a 30-year observation. Diagn Treatment Pneumonol Alergol Pol. (2014) 82:206–17. doi: 10.5603/PiAP.2014.0028
12.Bai, J, Li, H, Shi, J, Xu, J, Li, X, Cao, W, et al. Biochemical index and immunological function in the peripheral blood of patients with idiopathic pulmonary alveolar proteinosis. Biomed Rep. (2013) 1:405–9. doi: 10.3892/br.2013.66
13.Kojima, K, Kato, K, Fukazawa, T, Morita, I, Takigawa, N, Monobe, Y, et al. A case of autoimmune pulmonary alveolar proteinosis appearing as a localized ground-glass opacity. Jpn J Radiol. (2014) 32:657–60. doi: 10.1007/s11604-014-0348-3
14.Ouattara, S, Keita, M, Tuo, N, Dah, C, Siransy, EA, and Bogui, P. Effet du tabagisme sur la PaO2, au repos et au cours d'un effort physique modéré Effect of smoking on PaO2 at rest and during moderate exercise. Dakar Med. (2002) 47:90–5.
15.Hwang, JA, Song, JH, Kim, JH, Chung, MP, Kim, DS, Song, JW, et al. Clinical significance of cigarette smoking and dust exposure in pulmonary alveolar proteinosis: a Korean national survey. BMC Pulm Med. (2017) 17:147. doi: 10.1186/s12890-017-0493-4
16.Fell, CD, Martinez, FJ, Liu, LX, Murray, S, Han, MK, Kazerooni, EA, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. (2010) 181:832–7. doi: 10.1164/rccm.200906-0959OC
17.Jacob, J, Bartholmai, BJ, Rajagopalan, S, Kokosi, M, Nair, A, Karwoski, R, et al. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J. (2017) 49:1601011. doi: 10.1183/13993003.01011-2016
18.Mehrian, P, Homayounfar, N, Karimi, MA, and Jafarzadeh, H. Features of idiopathic pulmonary alveolar proteinosis in high resolution computed tomography. Pol J Radiol. (2014) 79:65–9. doi: 10.12659/PJR.890218
19.Ishii, H, Trapnell, BC, Tazawa, R, Inoue, Y, Akira, M, Kogure, Y, et al. Comparative study of high-resolution CT findings between autoimmune and secondary pulmonary alveolar proteinosis. Chest. (2009) 136:1348–55. doi: 10.1378/chest.09-0097
20.Lee, KN, Levin, DL, Webb, WR, Chen, D, Storto, ML, and Golden, JA. Pulmonary alveolar proteinosis: high-resolution CT, chest radiographic, and functional correlations. Chest. (1997) 111:989–95. doi: 10.1378/chest.111.4.989
21.Trapnell, BC, Inoue, Y, Bonella, F, Morgan, C, Jouneau, S, Bendstrup, E, et al. Inhaled Molgramostim therapy in autoimmune pulmonary alveolar Proteinosis. N Engl J Med. (2020) 383:1635–44. doi: 10.1056/NEJMoa1913590
Keywords: autoimmune pulmonary alveolar proteinosis, disease severity score, granulocyte-macrophage colony-stimulating factor, severity and prognosis score, whole lung lavage
Citation: Bai J-W, Huang J-n, Shi S-y, Ge A, Lu H-w, Sun X-l, Gu S-y, Liang S, Cheng K-b, Tian X-l, Xiao Y-l, Xu K-f and Xu J-F (2023) Updated severity and prognosis score of pulmonary alveolar proteinosis: A multi-center cohort study in China. Front. Med. 10:1058001. doi: 10.3389/fmed.2023.1058001
Edited by:
Dawei Yang, Fudan University, ChinaCopyright © 2023 Bai, Huang, Shi, Ge, Lu, Sun, Gu, Liang, Cheng, Tian, Xiao, Xu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-long Xiao, ✉ eW9uZ2xvbmcxMWFAMTYzLmNvbQ==; Kai-feng Xu, ✉ eHVrZkBwdW1jaC5jbg==; Jin-Fu Xu, ✉ amZ4dUB0b25namkuZWR1LmNu
†These authors have contributed equally to this work
 Jiu-Wu Bai
Jiu-Wu Bai Jian-nan Huang
Jian-nan Huang Shen-yun Shi
Shen-yun Shi Ai Ge1†
Ai Ge1† Hai-wen Lu
Hai-wen Lu Xiao-li Sun
Xiao-li Sun Shuo Liang
Shuo Liang Ke-bin Cheng
Ke-bin Cheng Xin-lun Tian
Xin-lun Tian Yong-long Xiao
Yong-long Xiao Kai-feng Xu
Kai-feng Xu Jin-Fu Xu
Jin-Fu Xu