- Department of Nephrology, Chinese PLA General Hospital, Chinese PLA Institute of Nephrology, State Key Laboratory of Kidney Diseases, National Clinical Research Center of Kidney Diseases, Chinese PLA General Hospital, Beijing, China
Acute kidney injury (AKI) is a serious clinical comorbidity with clear short-term and long-term prognostic implications for inpatients. The diversity of risk factors for AKI has been recognized in previous studies, and a series of predictive models have been developed using traditional statistical methods in conjunction with its preventability, but they have failed to meet the expectations in limited clinical applications, the rapid spread of electronic health records and artificial intelligence machine learning technology has brought new hope for the construction of AKI prediction models. In this article, we systematically review the definition and classification of machine learning methods, modeling ideas and evaluation methods, and the characteristics and current status of modeling studies. According to the modeling objectives, we subdivided them into critical care medical setting models, all medical environment models, special surgery models, special disease models, and special nephrotoxin exposure models. As the first review article to comprehensively summarize and analyze machine learning prediction models for AKI, we aim to objectively describe the advantages and disadvantages of machine learning approaches to modeling, and help other researchers more quickly and intuitively understand the current status of modeling research, inspire ideas and learn from experience, so as to guide and stimulate more research and more in-depth exploration in the future, which will ultimately provide greater help to improve the overall status of AKI diagnosis and treatment.
1. Introduction
AKI is a clinical emergency associated with a variety of acute and chronic comorbidities; even mild AKI may lead to chronic kidney disease, and severe or recurrent events may lead to end-stage renal disease and are strongly associated with an increased risk of death and impaired quality of life (1). With the increasing incidence and mortality of AKI worldwide, clinicians are paying increasing attention to AKI (2).
It is well established that AKI usually occurs in susceptible populations with high-risk factors or following certain specific medical procedures, and several previous studies have obtained more consistent conclusions on the evaluation of risk factors by analyzing the static and dynamic characteristics of a large number of AKI patients (3–8). Based on the preventability of AKI and the ease of diagnosis, increasing research is focused on the early diagnosis of AKI, with the aim of early assessment of inpatients to determine their risk stratification, dynamic adjustment of treatment protocols, replacement of potentially dangerous medical orders, and interventions to avoid or reduce potential kidney injury and ultimately to achieve inpatient renal protection by reducing the incidence of AKI, avoiding a series of subsequent adverse events.
The construction of predictive models for AKI has been an outstanding achievement for nephrologists in the 21st century, and over the past 10–20 years, predictive models have been reported, especially in recent years, the electronic medical record information systems of different medical institutions around the world have gradually improved, the use of electronic health records to build models has become another breakthrough in this field, and since the introduction of artificial intelligence, it has provided more methods for efficient utilization of massive medical data in the age of big data, making mining massive information and training models based on machine learning algorithms become a frontier hotspot. It is exciting to see that the technology has not disappointed in terms of medical decision-making behavior; at least in current studies, such models can predict AKI with an AUC of 0.80 or more, and in some studies, the AUC even reached 0.93. In this report, we set the search keywords as “machine learning” and “acute kidney injury” through the pubmed database, we screened the relevant literature by reviewing the abstract information, after carefully reading the included literature, we focus on reviewing recent advances in the application of machine learning systems to predict AKI risk, providing evidence and summarizing ideas for subsequent studies to be conducted.
2. Concept of machine learning
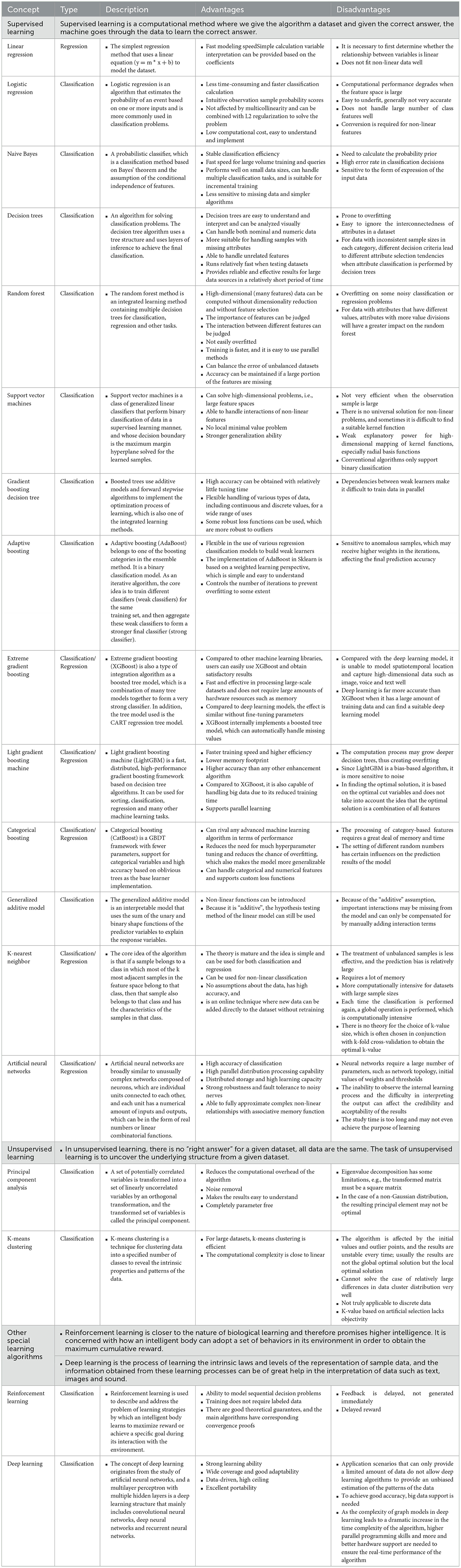
Machine learning is a major branch of AI technology, defined as the study of algorithms that use computer systems to learn from sample data and past experience (9). The popular machine learning algorithms include supervised learning and unsupervised learning, among which supervised learning includes decision trees, support vector machines, naive Bayes, k-nearest neighbor, logistic regression, random forests, gradient boosting trees, generalized additive models, artificial neural networks, and integrated tree models; unsupervised learning includes principal component analysis and k-mean classification. Among them, neural networks are a new addition to the field, and the most representative, deep learning, is considered to be the closest to the original goal of AI, solving many complex pattern recognition challenges. Other techniques include fully connected neural networks, convolutional neural networks (CNNs), recurrent neural networks (RNNs), generative adversarial networks (GANs), and deep reinforcement learning (10–12) (Table 1).
3. Machine learning modeling ideas
The process of machine learning modeling is continuous and consists of the following steps based on summarized research experiences. Step 1: Data acquisition. The research team defines the data concept according to the modeling objectives and accesses the authorized data, including structured data or unstructured data, using different data repositories from multiple medical institutions. Even though EMR data are now standardized through coding systems, this process is still tedious and time-consuming. Step 2: Data preparation. The collected data must be cleaned, managed and organized into a more computable format. This includes the labeling of supervised learning data, the transformation of categorical values, and the processing of outliers, missing values, and extremely imbalanced data. Step 3: Feature selection. A feature is the “column name” of the data, such as drug n, respiratory rate, or age. The model development process relies on feature selection from a large feature base, including LASSO or the Breiman and Cutler random forest method, and this process is repeated until the researcher is satisfied with the model performance. Step 4: Model training. Based on a training dataset of fixed ratio classification according to the model type, preselected features are used for iterative training. This process can be performed by replacing features and model configurations to continuously optimize model hyperparameters, fine-tune model performance, and reduce prediction errors. Step 5: Model validation. This step includes two substeps of internal and external verification. The preferred test method is k-fold cross-validation, where internal validation can identify data overfitting or underfitting during model training, while external validation can check the model performance under real conditions. Step 6: Model trial. The model is employed in a health care environment with new data to test its predictive feasibility and reliability in routine clinical work and clinician acceptability. Step 7: Model evaluation and interpretation. Model evaluation is an important process to judge the usability and reliability of models and provides key metrics for parallel comparisons among models. The evaluation of machine learning prediction models is similar to that of diagnostic experiments in that the main reference index is the area under the receiver operating characteristic curve (AUROC). In addition, other indices are often cited as auxiliary evaluations in studies, including accuracy, recall, precision, sensitivity, specificity, positive predictive value, negative predictive value, F1-value, etc. Effective model interpretation can help clinicians better understand the results of the model output and the relationship between variables. At present, the SHAP method based on the algorithmic game theory is widely used. which can interpret the prediction results from both global and local perspectives, and is proved to be more consistent with human intuition than the existing methods. Step 8: Model monitoring and updating. After the model is formally used, each step of its operation is monitored to ensure the expected use and prediction accuracy while continuously updating and optimizing the model performance through new data performance. It is important to note that the above processes and steps are not necessarily continuous and unidirectional, allowing for feedback during the process, with the exception of the deep learning process (Figure 1).
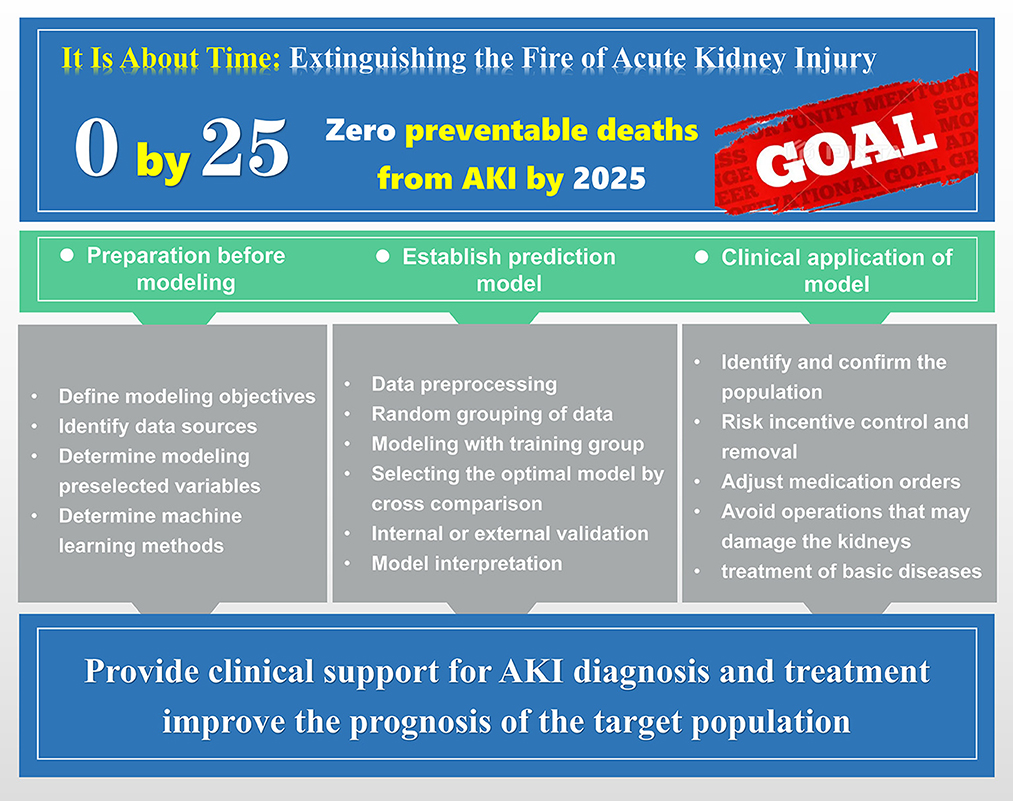
Figure 1. The modeling idea and flow chart of machine learning prediction model. Including the preparation work before modeling, the specific process of model training, and the applicability of the final model to the patient population.
4. Machine learning prediction model for AKI
4.1. AKI prediction model for critical care settings
In 2015, the International Society of Nephrology proposed the “AKI 0 by 25” initiative to achieve zero deaths from AKI by 2025, and the ICU, as the most affected area with high morbidity and mortality from AKI, is the greatest obstacle to achieving this goal (13). Against this background, there is a general consensus to identify the risk of AKI among critically ill patients early and to take a more proactive role in AKI prevention and management, it is worth mentioning that these measures are highly time dependent and time sensitive.
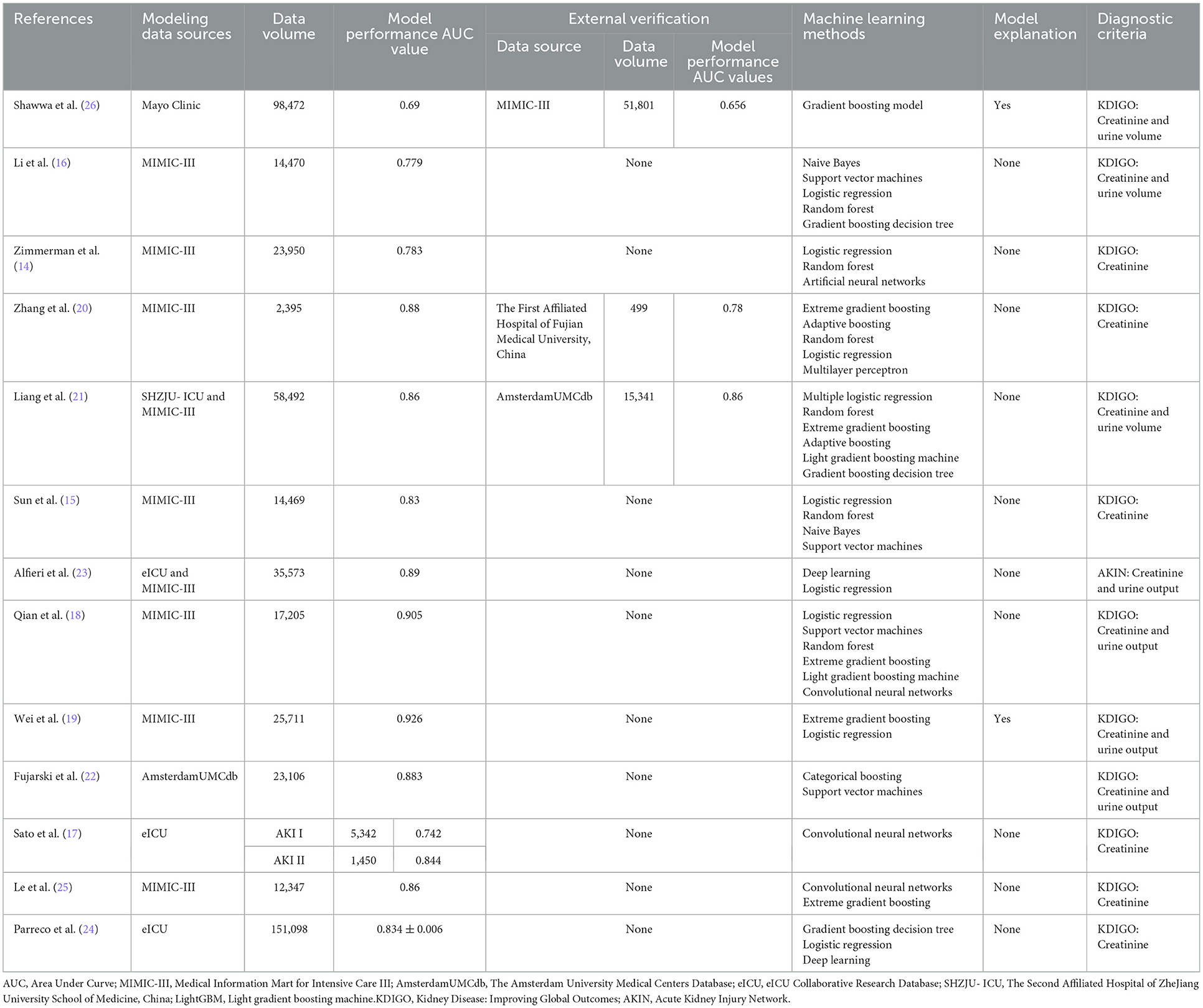
In recent years, there has been an abundance of research on machine learning prediction models for AKI for critically ill patients, and there are several features of this type of research. First, the choice of model training data was mostly focused on three publicly available databases, MIMIC-III, AmsterdamUMCdb and eICU (14–25). The use of local databases is not common, and only information from the Mayo Clinic and SHZJU-ICU can be retrieved (21, 26, 27). Public databases are highly integrated and easy to access, but it is inevitable that some of the missing information affects the authenticity of the data; for example, the variables with a large proportion of missing values in the MIMIC-III database include the lowest albumin level (74.1%), the highest bilirubin level (67.2%), the highest lactate level (55.8%), the highest C-reactive protein level (99.0%), the highest aspartate aminotransferase level (66.8%), the highest pH level (36.6%) and the lowest base excess level (64.8%) (14). Moreover, these studies mainly included European and American populations, and the generalizability of the models is doubtful for Asian populations. In addition, the differences in data extraction criteria settings or data preprocessing methods lead to a certain degree of incomparability among studies from the same database sources. Second, of the 14 studies we retrieved, only 3 studies conducted external validation of the model (20, 21, 26). While this is crucial for model generalization and relies on external validation for optimization of model hyperparameters, which is well understood by researchers, there are various constraints to external validation, including non-uniformity of case data formats across centers, self-protection of some medical structures for data security, or difficulty in matching predictions from other centers due to the complexity of the model. Third, all models were able to achieve the above moderate discrimination of AKI events with AUC values ranging from 0.69 to 0.926, incorporating both traditional modeling methods, such as logistic regression, in individual studies (14–16, 18, 20–24). In some studies that also incorporated traditional modeling methods, such as logistic regression, or compared with physicians' subjective diagnoses, machine learning modeling was also simultaneously superior. Fourth, interpretability is very important in the medical field, and a medically assisted diagnostic system must be understandable and interpretable; ideally, it should be able to explain the complete logic of providing corresponding decisions to all relevant parties to gain the trust of physicians, but the process of achieving model interpretation in the construction of predictive models regarding AKI for critically ill patients is rare, which somehow has led to a disconnect between modeling and analysis, forgoing additional analysis of AKI characteristics and risk factors and wasting the potential for the effective use of large amounts of information (19, 22, 26). Fifth, while variable screening for modeling is necessary and critical, the inclusion of static and dynamic variables reflects different modeling objectives. Most models include both at the same time so that patients with high dimensional variables can reference foundation conditions, such as demographic information and the basic state of complications. It is also possible to take into account objective indicators such as vital signs and laboratory tests in real time and to dynamically track the risk trajectory, which can be combined with the gradual or sudden onset of AKI to better predict AKI reliably. On the other hand, 57% of patients meet AKI criteria on the first day of ICU admission, according to the study results (27). Therefore, some studies have included only prehospital data, including basic information and admission diagnosis, to achieve early prediction of early-onset AKI (Table 2).
4.2. AKI prediction model for all care settings
Although it is very beneficial to build predictive models for critically ill patients, the risk of AKI among general inpatients should not be taken lightly. Since the beginning of machine learning modeling research, researchers have been diligently trying to build a predictive model for the entire inpatient population to achieve large predictive coverage once and for all to maximize the benefits of research. Subsequent studies have proven that these ideas are feasible, and the results were also reliable.
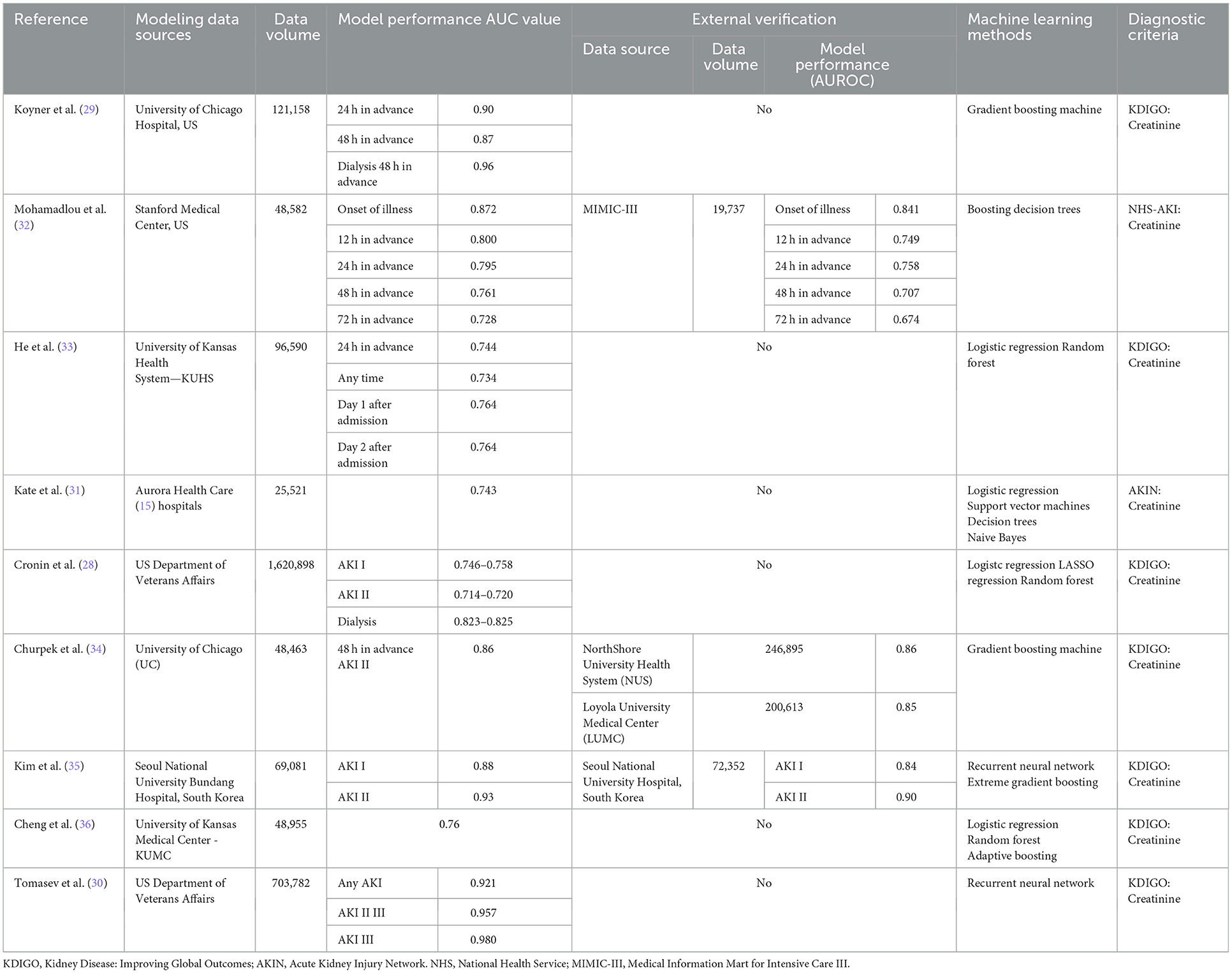
At present, this kind of model is mainly divided into two types, namely, “one-time” prediction models and real-time prediction Models. In 2015, Cronin et al. (28) included more than 1.6 million hospitalization data points of veterans for model training, and the results suggested that the AUC values of the three models for predicting AKI during 1–3 periods were 0.746–0.785, 0.714–0.720, and 0.823–0.825, respectively, but surprisingly, the traditional logistic regression and LASSO regression models performed slightly better than the random forest model, this may be due to the variable selection function of LASSO regression model, which can reduce overfitting by eliminating unimportant interfering variables, thus effectively improving model performance. In the 2018 study by Koyner et al. (29), which included ICU, general ward and emergency patients, care instruments such as the Morse fall scale were included in the analysis of variables for the first time, and the study focused on reflecting longitudinal data over time using a discrete time survival analysis framework, with the final gradient boosting model predicting AUC values of 0.9 and 0.87 for AKI 24 and 48 h in advance, respectively. In addition, the authors explored the creation of an algorithmic model that did not include changes in creatinine, suggesting that excluding this factor did not affect the model's ability to differentiate AKI independent of baseline renal function levels. In 2019, Tomasev et al.'s (30) team conducted a collaborative study with Deepmind, a Google company, whose inclusion of source data was consistent with earlier studies by Cronin et al. (28) with similarly large amounts of data (more than 700,000 adult case data, about 6 billion data points and 600,000 record features) and whose model developed using deep learning recurrent neural networks predicted AUC values over 0.9 for AKI events 48 h in advance, this algorithms has been proved to be very suitable for processing high-frequency time series data. In addition, researchers used ablation analysis to identify many factors related to the risk of AKI, which may explain why it was difficult for researchers to analyze this risk in the past.
Some studies have also restricted the age of the included subjects; for example, Kate et al. (31) developed a model for older adults over 60 years of age with a high incidence of AKI, in which four methods were utilized to model both AKI prediction and detection analysis, and the results suggested that the AUC values for both were 0.621–0.644 and 0.692–0.743, respectively, indicating that the detection of AKI is easier than the prediction. In addition, comorbidities were found to be more significant in predicting AKI in ablation experiments, including previous history of AKI and respiratory failure (Table 3).
Building continuous real-time prediction models is also a goal pursued by researchers, and in a study by Kim et al. (35) in 2021, a continuous prediction model was developed for general inpatients based on various dynamic and static clinical features using recurrent neural network algorithms, in order to achieve real-time prediction of AKI risk. Its applicability to support clinical decision-making was demonstrated by external validation, with internal and external validation AUC values of 0.88 and 0.84 for any stage of AKI and 0.93 and 0.90 for patients with stage 2 AKI or higher, respectively, both of which were better than XGBoost model, again proving the effectiveness of recurrent neural network algorithm in processing sequence data (Table 3).
The applicability of models that are constructed based on all inpatients to critically ill patients, such as ICU inpatients, is an important criterion for evaluating the performance of the model and one of the objectives for the construction of this kind of model. In 2018, a study by Mohamadlou et al. (32) that relied on Stanford local data modeled using the boosting decision tree method set and externally validated with the MIMIC-III database suggested that the model showed higher prediction values across different datasets in different windows (at AKI onset and 12, 24, 48, and 72 h before onset) and showed higher AUC values than SOFA scores. In another study by Churpek et al. (34) in which the above Koyner et al. (29) model was simplified by reducing the number of preselected variables, followed by a large multicenter study of nearly 500,000 inpatients in three health systems and six hospitals, the AUC value for predicting at least stage 2 AKI within 48 h was 0.86 in the internal validation cohort, while in the two external validation cohorts, the values were 0.85 and 0.86 (Table 3).
Some researchers have doubts about several modeling strategies mentioned above, mainly in the setting of the time range of data collection and using this to conduct a multiclinical perspective study in the hope of reflecting the clinical application of the models more realistically. He et al. (33) focused on answering this question in their study in 2019, which evaluated the performance of the models in differentiating AKI by setting different prediction time windows, and found that the AUC values for all models ranged from 0.720 to 0.764, which first confirmed the advantages of the strategy based on machine learning methods for modeling, and that the best model performance was achieved by predicting AKI one day in advance, which is precisely the most commonly used modeling strategy today. A similar study by Cheng et al. (36) was conducted to address the same two questions: how to predict the development of AKI in hospitalized patients early and accurately, and how to assess whether preadmission data enhanced model performance. The study used different data collection time windows for multiple datasets with three kinds of modeling methods for comparison. The results suggested that the AUC values of the random forest model with the best performance that predicted AKI 1–3 days in advance were 0.765, 0.733, and 0.709, respectively, and that the AUC values did not change significantly after adding preadmission data compared with post-admission data only (Table 3).
4.3. Special surgery-related AKI prediction model
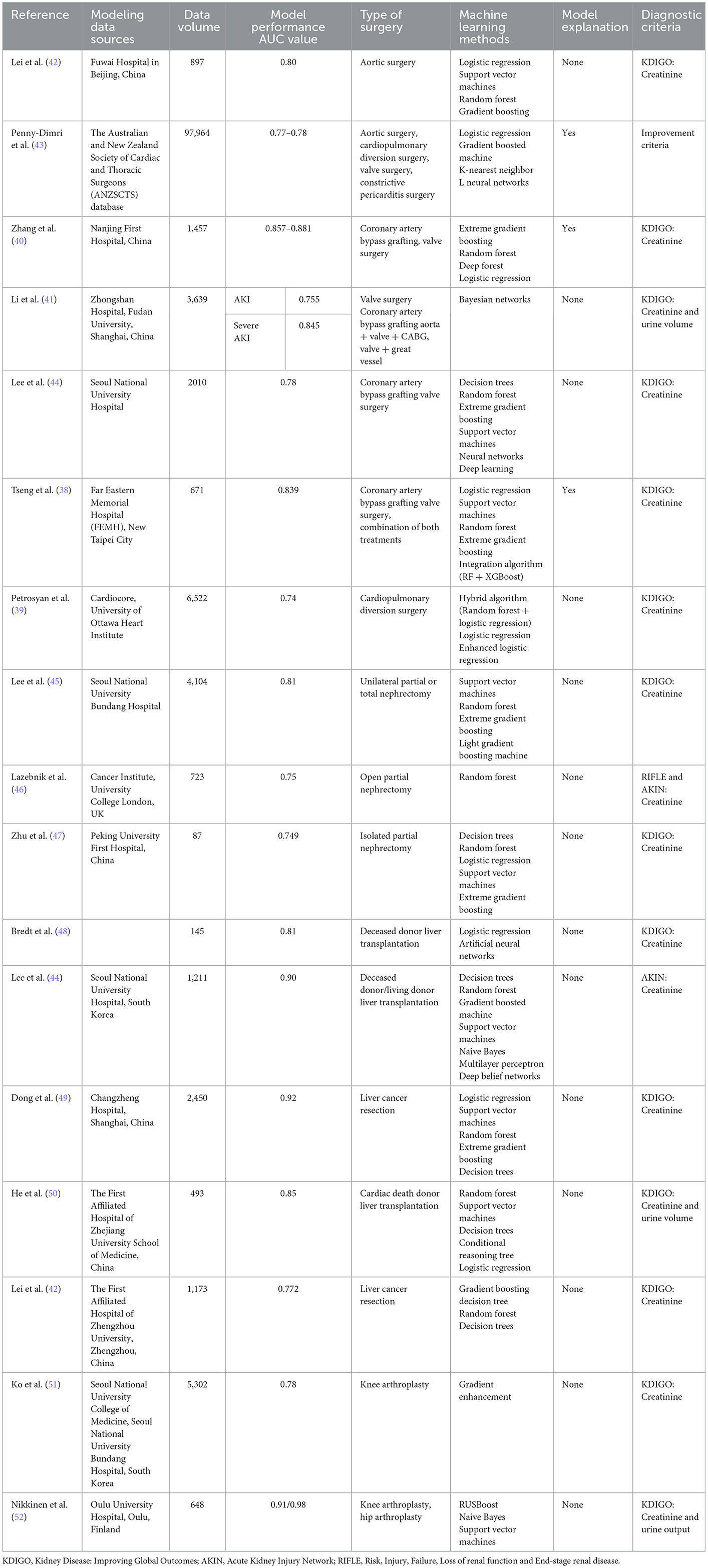
Given this background, there is an increasing amount of research on cardiac surgery-associated AKI (CSA-AKI) machine learning prediction models, which are able to capture AKI signals flexibly and effectively compared with traditional risk scoring methods while revealing more clearly the complex interconnections between CSA-AKI and its associated factors and are more applicable to the multifactorial pathogenic hypothesis of CSA-AKI. In the first CSA-AKI machine learning modeling study carried out by Lee et al. (37) in 2018, multiple machine learning approaches had stronger predictive efficacy than the previous eight risk scoring models (risk scoring system AUC values clustered at 0.55), and their poor performance may be due to the low number of predictors and the lack of intraoperative non-linear variables, such as transfusion volume or hemodynamic changes, which often represent acute intraoperative responses. Regarding the analysis of the contribution of variables at different stages of the perioperative period, in the study by Tseng et al. (38) in 2020, the importance matrix and SHAP summary plots of the random forest were used to provide double the evidence; that is, more than half of the top 20 important features were intraoperative features, which meant that intraoperative variables were the main influence on early renal function decline after cardiac surgery, which proved the value of intraoperative data. These data reflect the intraoperative acute physiological reactions associated with the prediction of CSA-AKI, whereas prediction models reported in previous studies have placed more emphasis on preoperative status. However, this has also been questioned by studies such as the most recent study by Petrosyan et al. (39) in 2022, in which a hybrid machine learning approach was used for the first time to derive and validate a model to predict CSA-AKI of any stage using only preoperative variables, which not only had outstanding performance compared to traditional logistic regression and other single machine learning approaches to modeling but was also able to adapt to the correlation between multiple variables and prevent overfitting of the data. In the study by Zhang et al. (40) in 2022, the types of procedures included were consistent with Lee and Tseng's study, both including patients undergoing coronary artery bypass grafting and valve replacement, and the modeling methods also overlapped; however, the modeling variables included preoperative, intraoperative, and early post-operative variables, such as early post-operative intubation, PaO2/FiO2 ratio, hemoglobin, serum potassium, and lactate dehydrogenase, and the AUC values of the models of these various machine learning methods were 0.857–0.881. Similarly, in another study conducted by Li et al. (41) in 2020, patients undergoing cardiac aortic surgery were added to the training set, and the variables included preoperative and intraoperative factors as well as post-operative central venous pressure, resulting in an AUC value of 0.845 for the prediction of severe AKI by the Bayes network model (Table 4).
The incidence of CSA-AKI also varies by type of cardiac surgery, and it is currently believed that cardiac major vascular surgery leads to a higher rate due to extracorporeal circulation. For example, Lei et al. (42) reported in their study on CSA-AKI modeling of patients undergoing major vascular surgery in 2020 that up to 72.6% of patients in the training cohort developed varying degrees of CSA-AKI. Based on this cohort modeling, the random forest method also achieved an AUC value of 0.8, much higher than logistic regression and other models. In the Penny-Dimri et al. (43) 2021 study, not only was the data volume of the training cohort large but the included objects were also more abundant, except for aortic surgery, valve surgery and cardiopulmonary bypass surgery, and included some patients with constrictive pericarditis surgery. Based on gradient boosting, k-nearest neighbor and neural network methods, the AUC values of multiple models ranged from 0.77 to 0.78, although they were lower than in previous study results; considering the heterogeneity of the training objects, there is even more reason to believe that the models are generalizable. The introduction of Shapley values for model interpretation is rare and critical in all studies, as it more intuitively illustrates the details of the contribution of the model variables and is more likely to gain the confidence of clinicians (Table 4).
Over the past few decades, liver transplantation has become the treatment of choice for patients with end-stage liver disease (ESLD), and advances in organ preservation, surgical approaches, anesthetic techniques, and immunosuppressive therapy have substantially improved expected outcomes, but recent data still report a 4–94% incidence of AKI after liver transplantation from both living and deceased donors, with 8–17% of patients requiring renal replacement therapy (53). In the few studies currently available, the authors used multiple machine learning methods for modeling comparisons, with optimal AUC values of 0.81, 0.85, and 0.90 in a single study, which were all superior to the values of logistic regression models for same-group comparisons (44, 48, 50). However, due to differences in modeling ideas, there are also differences in the categories of preselected variables and variable contribution characteristics of models, as in the latest study by Luis Cesar Bredt et al. (48) in 2022, where the main included variables in the modeling study with an artificial neural network approach were CKD, MELD score, intraoperative arterial hypotension, massive blood transfusion and extended criteria donor. In the study by Lee et al. (44) in 2018, it was proven that the XGBoost model had the best performance, and the top five variables that were ultimately included were cold ischemia time, mean venous oxygen saturation, mean cardiac index, urine output, and preoperative blood glucose. Both of the abovementioned models were dominated by preoperative and intraoperative variables, but there was almost no overlap in the comparison of variable importance. On the one hand, this may be related to the modeling sample size and functional adaptability of different machine learning algorithms; on the other hand, it may be associated with the source of the liver donor, such as in the latter study of simultaneous living and deceased donor transplantation patients. Certainly, current modeling studies are not interested in post-operative data to achieve early prediction and to prevent implications for post-operative interventions and prevention, even though some studies have confirmed that certain post-operative factors are equally risk factors for AKI, including immunosuppressive drugs, infectious agents, antibiotics, sepsis, long-term hypotension, and the use of radiographic contrast agents (54) (Table 4).
Hepatectomy is one of the most important treatments for primary liver cancer, and the incidence of AKI after hepatectomy (LSA-AKI) has been reported to be 0.9–17.9% (53), but AKI events are often underestimated in clinical practice. Many studies have investigated the risk factors associated with LSA-AKI, and several classical AKI scoring systems, such as the Kalisvaart score and Park score, have been established; however, due to the multifactorial nature of LSA-AKI, such risk scores are inefficient in predicting the occurrence of AKI (55, 56). There are few studies on machine learning modeling of LSA-AKI. In the study by Dong et al. (49), clinical data from 2,450 patients were retrospectively analyzed to compare the model performance of multiple machine learning algorithms, and by incorporating preoperative and intraoperative data, including age, cholesterol, time of surgery, serum creatinine and platelet count, the random forest model had an AUC value of 0.92. Another study by Lei et al. (57) also included preoperative and intraoperative data, and the results suggested that the decision tree model performed even better, with an AUC of 0.722. Unlike a previous study, this study also confirmed that tumor size was a significant predictor. Although the results of these two studies are different, the model performance of the latter study is significantly inferior to that of the former by parallel comparison, and the machine learning model performs significantly better than the traditional logistic regression model. However, the current study still has room for improvement; borrowing from Dong et al. (49), the model development should be more focused on the combination of novel biomarkers and model interpretation and should also consider the accuracy and recall rate to ensure model reliability (Table 4).
Previous studies have focused on long-term changes in renal function after nephrectomy, and models for predicting post-operative AKI are rare, especially in modeling studies using machine learning methods. In 2021, in a study by Lee et al. (45), a variety of machine learning methods were used for modeling comparison, and the results showed that the LightGBM model had the best predictive performance, with an AUC value of 0.81, much higher than that of the logistic regression model. Similarly, in a study by Lazebnik et al. (46) in 2022, the random forest model also showed better predictive performance with an AUC value of 0.75. However, in another study performed in 2020 by Zhu et al. (47), by incorporating preoperative, intraoperative and post-operative variables and using multiple methods to learn and train information on 87 patients, the results showed that the best performing XGBoost model had an AUC value of 0.749, which was lower than that of the classical logistic regression model of 0.826. It may be difficult to conduct parallel comparisons among the only three studies at present because of the degree of variation in the between-group design; for example, there was a certain proportion of RN patients among the 4,104 patients included in the first study (45), whereas the third study included all patients with isolated kidney (47). In addition, in a single study, differences in operator and surgical approaches (manual or robotic, laparoscopic or open) may also lead to confounding bias, making the accurate prediction of outcomes complex and difficult. However, there is relative consistency in the evidence of similar studies, including the inclusion analysis of modeling variables more in favor of type of surgery, male sex, tumor size, age, operation time, intraoperative ischemia time, and renal score, which is generally consistent with the results of previous risk factor studies. Additionally, the evaluation of the efficacy of the machine learning methods was positive, even though the results of the third study were slightly different. However, the reasons for this were related to the small amount of modeling data, which did not ensure that the machine learning method could fully exploit the information to establish the relationship between variables (47). In conclusion, similar to other types of AKI, the application of large-scale data information and machine learning algorithms for the prediction of AKI after nephrectomy has been shown to be feasible, even though none of the findings can be directly generalized across centers (Table 4).
Although there has been little previous interest in AKI after orthopedic surgery, studies have suggested that the incidence of AKI after undergoing hip and knee arthroplasty ranges from 0.5 to 24.0% (58–60), therefore post-arthroplasty AKI can be considered another example of a clinical prediction that could benefit from machine learning. In 2022, Ko et al. (51) used gradient boosting modeling to obtain internally and externally validated AUC values of 0.78 and 0.89 in a modeling study of patients undergoing total knee arthroplasty. In the same year, Nikkinen et al. (52) included patients undergoing knee and hip replacement and compared the models based on creatinine and urine volume, respectively, and the results suggested that the AUC values of the two models were 0.91 and 0.98, respectively. Although there were differences in sample size, machine learning methods, and case characteristics between the two studies, both obtained acceptable results, while in terms of variable selection, both agreed that preoperative creatinine level, male sex, ASA classification, and age were important risk factors for AKI, and this result was largely consistent with previous studies. However, the greatest contribution of similar studies is likely to be the emphasis on post-operative AKI in orthopedic surgery and the introduction of machine learning methods to provide new ideas for AKI prediction studies after orthopedic surgery, especially arthroplasty (Table 4).
4.4. Specific disease-related AKI prediction models
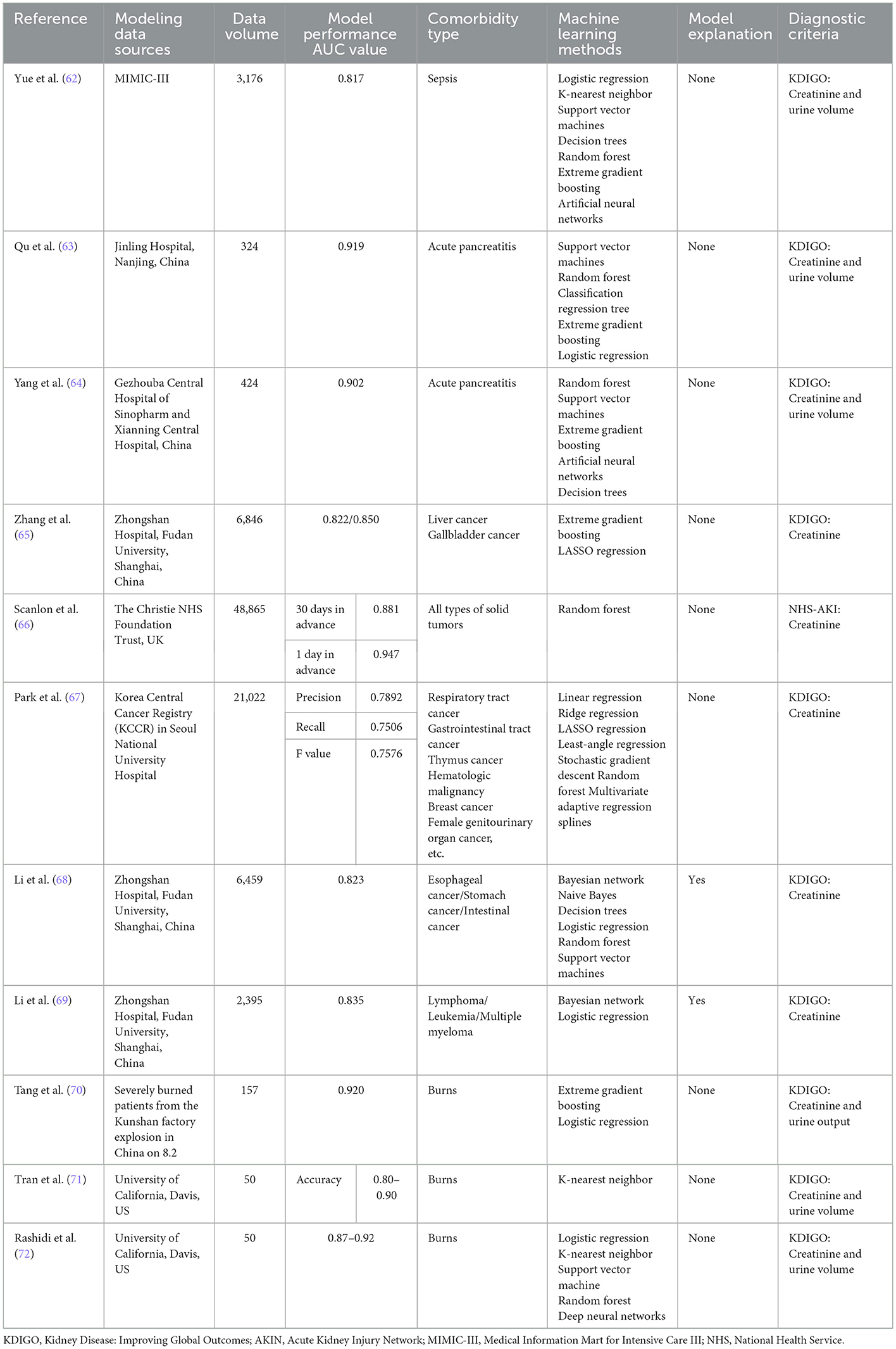
Sepsis is the most common cause of AKI in critically ill patients, and its incidence increases with the severity of sepsis, while mortality is significantly higher among SA-AKI patients than among non-AKI patients (61), therefore, identifying patients at risk for AKI is crucial for the management of patients with sepsis. Based on the complexity of the pathogenesis of SA-AKI, it is difficult to realize the early prediction of SA-AKI in clinical work. Currently, the performance of new biomarkers and some AKI scoring systems is not satisfactory. Similarly, the utilization of machine learning methods for SA-AKI is extremely rare, and there was only one relevant study by Yue et al. (62). In this study, data from 3,176 patients were included for model training, and the results showed that the AUC value of the XGBoost model was 0.817, which showed good predictive accuracy in terms of discrimination and calibration while outperforming previous scoring systems and traditional modeling methods. Even though the results do not equate to usefulness in clinical practice as stated by the authors, this study is the first of its kind in SA-AKI research, and while promising for the early prediction of SA-AKI, it is also worthwhile to attribute risk factors for morbidity by ranking the importance of variables such as urine volume, mechanical ventilation, body mass index, eGFR, lowest sCr, and minimum BUN. The study is also meaningful to future parallel studies. The focus remains on increasing the dimensionality of variables and screening for variable characteristics that contribute more to model performance, including comorbidities such as diabetes, hypertension and cardiovascular disease, information on the source of infection such as abdominal infection, the nature of pathogens such as Gram-negative bacteria, invasive operations such as mechanical ventilation, and pharmacological interventions such as diuretics and ACEIs/ARBs (Table 5).
AKI is a common complication in patients with severe burns, with a reported morbidity of ~40% and a mortality rate of 73–100% (73). There are few studies on burn-associated AKI modeling using classical machine learning methods, and the representative study was completed by Tang et al. (70) in 2018, which used the XGBoost method for model training and validation and compared it with the traditional logistic regression model. The results suggested that by including the APACHE II score, percentage of third-degree burn area, 24-h post-admission rehydration, sepsis, first 24-h urine volume, SOFA score, and 48-h post-admission rehydration, the AUC value of the model constructed by the machine learning algorithm was 0.92, which was significantly higher than that of the logistic regression model of 0.875, but the limitations of the study were also obvious. Because the included patients were survivors of a dust explosion accident, the diagnosis was mainly severe burns combined with inhalation injury, so the adaptability of external promotion could not be confirmed (Table 5).
Research on the construction of machine learning prediction models for AKI in burn patients has been equally pioneering, with a different emphasis and utilization of novel biomarkers than previous modeling ideas. In a study by Rashidi et al. (72) in 2020, it was first hypothesized that machine learning approaches could enhance the predictive potential of AKI biomarkers in critical care populations such as individuals with severe burns, and the results confirmed that when used in combination with other known biomarkers such as NT-probNP or sCr, machine learning could enhance the predictive ability and clinical sensitivity of NGAL, especially deep neural networks combined with NGAL; this would provide the best model sensitivity, specificity and AUC values, achieving a prediction 68.1 h in advance according to the KDIGO standard. More importantly, acceptable results have also been obtained for the external validation of diagnostically heterogeneous cohorts such as trauma patients. Another study conducted by Nam et al. (71) in 2019 published similar findings that the use of the k-nearest neighbor method increases the identification of AKI in burn patients based on sCr, NGAL, UOP, and NT-proBNP. In previous studies, there was concern that preexisting inflammation confounded the performance of biomarkers such as NGAL, thus limiting their role in AKI diagnosis, but machine learning methods can effectively overcome this problem by identifying complex diagnostic patterns masked by confounding factors, further proving and enhancing the performance of novel markers such as NGAL and providing hope for clinicians. In future research, the concept of modeling by this type of machine learning method may be given more attention, and the validation and utilization of other markers may bring us more surprises (Table 5).
AKI has long been considered a common and serious complication of acute pancreatitis (AP), with a prevalence of ~10–42% and a poor prognosis and mortality rate of 25–75% among patients with AP-AKI (74). In recent years, research on machine learning modeling of AP-AKI has been conducted. With the first relevant study conducted by Qu et al. (63) in 2020, after modeling by multiple methods, it was found that XGBoost achieved the best performance with the highest AUC value of 0.9193, in addition to excellent performance in terms of sensitivity and specificity, while screening based on modeled variables further complemented risk factors such as the APACHE II score and C-reactive protein and total bilirubin Levels. In addition, in recent years, there has been a deeper understanding of the possible mechanisms of AP-AKI, and an increasing number of basic experimental and clinical studies have shown that the inflammatory response plays a unique role in the pathophysiology of AKI. Based on this background, the modeling study by Yang et al. (64) in 2022 focused on the inclusion of cytokine variables, and the results suggested that by introducing C-reactive protein, platelet/lymphocyte percentages neutrophil/lymphocyte percentages, cystatin C and other inflammatory indicators, random forest and other learning methods achieved AUC values of 0.725–0.902, which not only proved the reliability of the model but also further verified the possible association of inflammatory responses with AP-AKI and simultaneously provided ideas and optimization directions for subsequent studies, namely, with the in-depth study of more biomarkers. Exploring the correlation between these biomarkers and the occurrence and development of AP-AKI by using machine learning or removing confounding factors and analyzing the connection between them and other inflammatory or non-inflammatory variables is very important for improving the treatment status of AP and strengthening the primary prevention of AP-AKI (Table 5).
AKI is a common complication in patients with malignancies, with an incidence of ~7.5–9.5% (75, 76). AKI associated with malignancy (MR-AKI) not only affects ongoing treatment but is also associated with lower tumor remission rates and higher mortality rates (77). MR-AKI is associated not only with advanced age and chronic comorbidities but also with tumor-specific factors such as malignant infiltration, tumor lysis syndrome, nephrotoxic drugs and contrast therapy (78, 79). In a study by Zhang et al. (65) in 2021, data from 6,846 patients with liver and gallbladder cancer were collected, and the XGBoost method was used for modeling. In internal validation, the AUC values of the liver cancer and gallbladder cancer models were 0.822 and 0.850, respectively. In the screening of modeling variables, it was found that both sCr and eGFR contributed more than 20% to the model gain, while liver cancer treatment was found to rank third in the ability of modeling predictors of liver cancer, which further supports the impact of partial hepatectomy or liver transplantation on AKI. In the study by Li et al. (68) in 2020, 6,459 participants with gastrointestinal cancers with a high incidence in the Chinese cancer spectrum, including esophageal, gastric and colon cancers, were recruited. Variable selection was first conducted by the GLASSO method to simplify the complexity of variables and avoid overfitting and misclassification, and then modeling was performed using various methods. Finally, the efficiency of the Bayesian network was proven to be the best, with AUC values of 0.823 and 0.790 in internal and external validation, respectively. Meanwhile, the Bayesian network gave explanations for the probabilistic dependencies among the modeled variables according to the characteristics of its own algorithms; however, this relationship did not represent a causal relationship. In addition, the study did not include recognized important clinical variables, such as infection and nephrotoxic drugs, which obscured the relationship between unknown variables and AKI to some extent. In the same year, Li et al. (69) also conducted a similar study on patients with hematologic neoplasms, covering lymphoma, leukemia, and multiple myeloma among 2,395 patients, again using the GLASSO method for variable selection and subsequently achieving an AUC value of 0.835 using the Bayesian network method. The shortcomings of the two studies are consistent, but the insights for the diagnosis and treatment of oncological diseases are profound, the models in the studies not only achieved clinical detection of MR-AKI earlier than the KDIGO criteria by machine learning methods but also further elaborated the advantages of the Bayesian network to visualize and graphically display and explain complex dependencies between variables, using solid tumors or hematologic tumors as representatives (Table 5).
The model type of the above three studies was classical, but similar studies have improved the model type. For example, Scanlon et al. (66) in 2020 only predicted the risk of AKI in the next 30 days based on the blood results of tumor patients, and the random forest method predicted AKI 30 days and 1 day earlier with AUC values of 0.881 and 0.947, respectively. Moreover, a prospective study found that ~60% of AKI cases can be detected 30 days before the onset of the disease. The study was characterized by an extended prediction range and the discarding of a large amount of non-therapeutic information to ensure that the model could be used in outpatient clinics. During the 7–28 day-chemotherapy cycle of tumor patients, clinicians have more opportunities to receive predictive alerts based on routine blood test results. Another study conducted by Park et al. (67) in 2018 developed a more applicable AKI prediction model, which relaxed the limitations of existing methods and made full use of irregular and heterogeneous data to learn and train the model. The study included a total dataset of 21,422 cancer patients. Multiple modeling methods were used to predict AKI events within 14 days with an accuracy of 0.7892, a recall rate of 0.7506, and an F value of 0.7576. The purpose of this study was to construct a machine learning model using non-intensive or irregular measurement results of non-ICU patients. First, the maximum SCr value within 14 days was predicted and then used to predict the occurrence and severity of AKI. This extended model can be applied to different clinical populations, enabling AKI prevention and better clinical decision-making for cancer patients in more diverse environments (Table 5).
4.5. Predictive models for AKI associated with specific nephrotoxin exposures
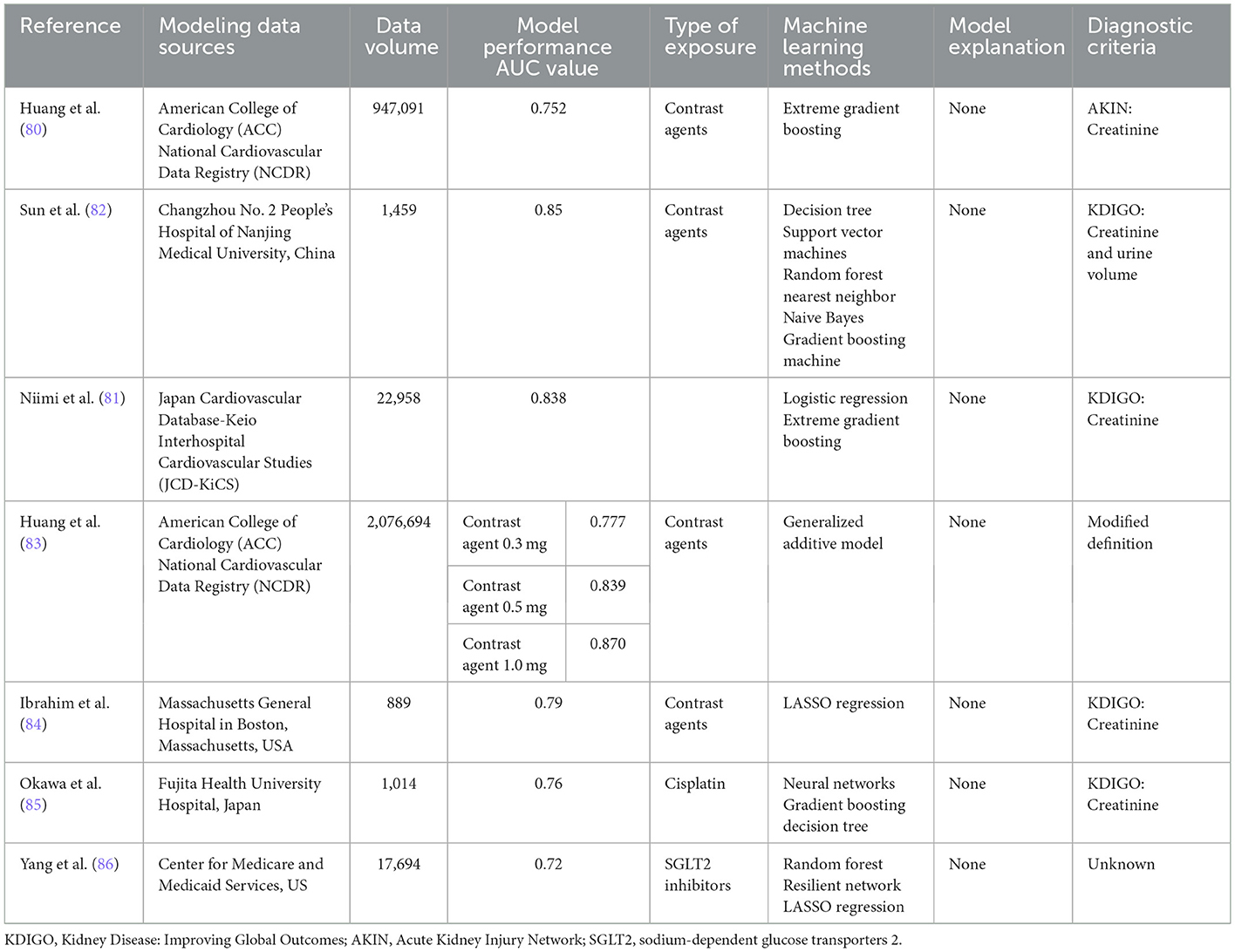
Contrast-related operations are currently an important approach to clinical diagnosis and treatment, including imaging enhancement examination, arteriovenous embolization, and cardiovascular intervention treatment, and AKI, one of its major comorbidities, has been receiving much attention, especially regarding the construction of machine learning prediction models for AKI associated with percutaneous coronary intervention (PCI). In 2018, Huang et al. (80) conducted the first machine learning modeling study, whose included case information was the original data developed by the NCDR-CathPCI scoring system, and multiple models were constructed and comparatively evaluated by using the same amount of data and original variables, different variable selection patterns, different candidate variable preprocessing strategies, and different modeling methods. The results suggested that the best model was constructed by using all available candidate variables in their original form, alignment-based variable selection, and XGBoost computational methods, with a wider prediction range and stronger risk stratification than the currently widely accepted NCDR-CathPCI scoring system; this comparison had a statistically significant difference, although the performance improvement was weak. In the latest study by Niimi et al. (81) in 2022, their modeling data sources were consistent with those of Huang et al. (80). They also used XGBoost and logistic regression methods to model separately. In addition to AKI events, bleeding and in-hospital mortality were also included in the endpoint results. The results suggested that the XGBoost model modestly improved the discrimination of AKI events with an AUC value of 0.84, which was significantly higher than that of the traditional logistic regression model, further supporting the conclusion of Huang et al. (80). However, the optimal modeling method is not fixed, and it is related to a variety of factors. For example, in a study by Sun et al. (82) in 2020 comparing the model performance of decision trees, support vector machines, and random forests, the results suggested that random forests eventually achieved an AUC value of 0.82 by using variables such as neutrophil percentage, age, and free triiodothyronine (Table 6).
In addition to these three classic machine learning approach modeling studies, some investigators have continued to expand their modeling ideas. For example, in the follow-up study by Huang et al. (83) in 2019, which aimed to develop a model to assess the relationship between the volume of contrast agents received by PCI patients and the risk of AKI, the results suggested that this correlation was non-linear and heterogeneous across patients with different baseline risks, which is difficult to achieve for traditional regression methods. With the introduction of machine learning methods, based on data from more than 1 million patients covering a wide range of baseline risks, the construction of this correlation was effectively completed using a generalized additive model by quantifying the contrast agent solvent during PCI in patients with different baseline risks to enable personalized treatment. In addition, the choice of candidate variables for modeling is not invariable; for example, all modeling studies have a bias toward static or dynamic variables. Ibrahim et al. (84) did not borrow the characteristics of the variables from the former study but focused on clinical and proteomic markers, including diabetes history, urea nitrogen/creatinine ratio, C-reactive protein and bone bridging protein, which were positively correlated with AKI risk, and CD5 and VII factors that were negatively correlated. The results of the machine learning model were equally reliable, with AUC values of 0.79–0.82 obtained after modeling with data from 889 patients. In conclusion, the current research on constructing a PCI-AKI prediction model based on machine learning methods is promising, but given the widespread implementation of PCI worldwide, contrast-induced AKI remains an inescapable clinical challenge; therefore, additional research is needed to develop a model that can achieve the widespread acceptance of the NCDR-CathPCI scoring system and can truly replace the system to achieve accurate and personalized treatment to reduce the medical and economic burdens on patients with cardiovascular disease (Table 6).
Cisplatin-based chemotherapy is the first-line treatment for solid tumors such as non-small cell lung cancer, but it can easily cause renal tubular damage during excretion, leading to cisplatin-related AKI (Cis-AKI) (87, 88). To prevent Cis-AKI, aggressive or short-term hydration therapy with magnesium supplementation is clinically recommended (89). However, the incidence of Cis-AKI remains high, so early detection and prediction of Cis-AKI is essential for the management of patients treated with cisplatin for chemotherapy. According to our database search, there is only one report published by Okawa in 2021 that introduced the efficacy of the Cis-AKI machine learning prediction model, which included 1,014 oncology patients receiving cisplatin as first-line chemotherapy and excluded cases treated with angiography or intra-arterial injection of cisplatin during chemotherapy. Two methods, a neural network and gradient boosting decision tree, were used to establish models by age group. The results suggested that the models showed the highest performance among patients aged ≥75 years with an AUC value of 0.78, while in the ranking of the contributions of model prediction variables, serum albumin level, body surface area and maximum daily dose of cisplatin contributed the most to the prediction of the model. Based on the specific values of the parameters, it was shown that high-dose cisplatin (100–120 mg/day) and hypoalbuminemia (1.30–3.10 mg/dL) were risk factors for Cis-AKI in all patients. The study, despite some shortcomings, is pioneering for oncology patients, especially those treated with nephrotoxic chemotherapeutic agents such as cisplatin, and the reduction in chemotherapy-related complications is extremely critical to improve the survival rate of oncology patients. It is believed that more similar studies will be conducted in the future, which will bring more benefits for the control of side effects from AKI-related drugs such as cisplatin or immune checkpoint inhibitors (85) (Table 6).
Sodium-glucose cotransporter two inhibitors (SGLT2s) are a new oral hypoglycemic agent with positive cardiovascular protective effects but controversial effects on renal function, and a meta-analysis concluded that SGLT2s not only reduce the progression of chronic kidney disease but also have a preventive effect on AKI. (90). However, several studies, including a warning from the US Food and Drug Administration (FDA) (https://www.fda.gov/Drugs/DrugSafety/ucm505860.htm), have concluded that SGLT2s can contribute to the development of AKI by affecting patients' blood volume and inducing renal medullary damage (91, 92). Therefore, Yang et al. (86) in 2022 used a machine learning approach to establish a model to predict the risk of AKI in diabetic patients receiving SGLT2s for the first time. Based on the source data of 17,649 patients' case information, the model used the random forest method and 14 preselected variables and had an AUC value of 0.72. This study is not only representative of the predictive analysis of rare and serious adverse events, but its risk factor analysis also provides new ideas for clinical decision optimization, such as the study's conclusion that diuretics contributed most to the model and, although it cannot be determined that it has the strongest correlation with AKI, it does illustrate the impact of drug combinations on AKI (Table 6).
5. Conclusion
In this review study, we systematically described the research status of machine learning prediction models for AKI, summarized the data characteristics, method characteristics and result characteristics of the existing models, and provided a relatively comprehensive field summary for peer research. however, it is inevitable that the methodological introduction of some studies is still not comprehensive enough, and a small number of non-English language articles are not included in the analysis, this may have left out some of the research results. In conclusion, AKI is a worldwide health problem, and its short-term and long-term adverse effects on hospitalized patients are very obvious. However, the current challenges facing the diagnosis and treatment of AKI are still huge, including timely detection and early prediction of AKI. Based on this, future research in machine learning predictive models is likewise well directed, except to overcome the above issues, increasing the use of novel biomarkers for model training, inviting more specialized scientific and technical teams for methodological assistance, and enabling the embedding of models with health care work systems will provide greater assistance in improving the overall diagnosis and treatment status of AKI.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Key Technological Innovation of Battle Field Internal Medicine Diseases Diagnosis and Treatment, Battle Field Internal Medicine of Construction of Key Military Disciplines, 13th Five Year Plan (No. A350109), Study on Risk Warning System of Senile Urinary Dysfunction, National Key Research and Development Program of China, 14th Five Year Plan (No. 2022YFC3602903), National Natural Science Foundation of China (82000631), Beijing Natural Science Foundation (7222169), and Young Elite Scientist Sponsorship Program by CAST (YESS20200400).
Acknowledgments
The authors thank our research team for their helpful comments in reviewing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pickkers P, Darmon M, Hoste E, Joannidis M, Legrand M, Ostermann M, et al. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med. (2021) 47:835–50. doi: 10.1007/s00134-021-06454-7
2. Adamczak M, Surma S, Wiecek A. Acute kidney injury in patients with COVID-19: Epidemiology, pathogenesis and treatment. Adv Clin Exp Med. (2022) 31:317–26. doi: 10.17219/acem/143542
3. Chang-Panesso M. Acute kidney injury and aging. Pediatr Nephrol. (2021) 36:2997–3006. doi: 10.1007/s00467-020-04849-0
4. Silverton NA, Lofgren LR, Hall IE, Stoddard GJ, Melendez NP, Van Tienderen M, et al. Non-invasive urine oxygen monitoring and the risk of acute kidney injury in cardiac surgery. Anesthesiology. (2021) 135:406–18. doi: 10.1097/ALN.0000000000003663
5. Chen JJ, Kuo G, Hung CC, Lin YF, Chen YC, Wu MJ, et al. Risk factors and prognosis assessment for acute kidney injury: The 2020 consensus of the Taiwan AKI Task Force. J Formos Med Assoc. (2021) 120:1424–33. doi: 10.1016/j.jfma.2021.02.013
6. Yamashita Y, Kawaguchi H, Yano T, Sakurai N, Shibata W, Oshima K, et al. Risk factors for acute kidney injury in vancomycin and piperacillin/tazobactam combination therapy: a retrospective study. J Infect Chemother. (2021) 27:1614–20. doi: 10.1016/j.jiac.2021.07.017
7. Alfano G, Ferrari A, Fontana F, Mori G, Magistroni R, Meschiari M, et al. Incidence, risk factors and outcome of acute kidney injury (AKI) in patients with COVID-19. Clin Exp Nephrol. (2021) 25:1203–14. doi: 10.1007/s10157-021-02092-x
8. Naser MN, Al-Ghatam R, Darwish AH, Alqahtani MM, Alahmadi HA, Mohamed KA, et al. Risk factors, predictions, and progression of acute kidney injury in hospitalized COVID-19 patients: an observational retrospective cohort study. PLoS ONE. (2021) 16:e0257253. doi: 10.1371/journal.pone.0257253
9. Mintz Y, Brodie R. Introduction to artificial intelligence in medicine. Minim Invasive Ther Allied Technol. (2019) 28:73–81. doi: 10.1080/13645706.2019.1575882
10. Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbel JP. Introduction to machine learning, neural networks, and deep learning. Transl Vis Sci Technol. (2020) 9:14. doi: 10.1167/tvst.9.2.14
11. Cote MP, Lubowitz JH, Brand JC, Rossi MJ. Artificial intelligence, machine learning, and medicine: a little background goes a long way toward understanding. Arthrosc J Arthrosc Relat Surg. (2021) 37:1699–702. doi: 10.1016/j.arthro.2021.04.022
12. Deshmukh R, Rathi P. Artificial intelligence in medicine. J Assoc Physicians India. (2022) 70:11–2.
13. Mehta RL, Burdmann EA, Cerdá J, Feehally J, Finkelstein F, García-García G, et al. Recognition and management of acute kidney injury in the international society of nephrology 0by25 global snapshot: a multinational cross-sectional study. Lancet. (2016) 387:2017–25. doi: 10.1016/S0140-6736(16)30240-9
14. Zimmerman LP, Reyfman PA, Smith ADR, Zeng Z, Kho A, Sanchez-Pinto LN, et al. Early prediction of acute kidney injury following ICU admission using a multivariate panel of physiological measurements. BMC Med Inform Decis Mak. (2019) 19:16. doi: 10.1186/s12911-019-0733-z
15. Sun M, Baron J, Dighe A, Szolovits P, Wunderink RG, Isakova T, et al. Early prediction of acute kidney injury in critical care setting using clinical notes and structured multivariate physiological measurements. Stud Health Technol Inform. (2019) 264:368–72.
16. Li Y, Yao L, Mao C, Srivastava A, Jiang X, Luo Y. “Early prediction of acute kidney injury in critical care setting using clinical notes,” In: 2018 IEEE international conference on bioinformatics and biomedicine (BIBM). Madrid, Spain: IEEE (2018). p. 683–6. doi: 10.1109/BIBM.2018.8621574
17. Sato N, Uchino E, Kojima R, Hiragi S, Yanagita M, Okuno Y. Prediction and visualization of acute kidney injury in intensive care unit using one-dimensional convolutional neural networks based on routinely collected data. Comput Math Methods Med. (2021) 206:106129. doi: 10.1016/j.cmpb.2021.106129
18. Qian Q, Sun H, Wu J, Wang J, Yang L. AKI prediction models in ICU: a comparative study. Int J Gen Med. (2020) 14:623–32. doi: 10.2196/preprints.18257
19. Wei C, Zhang L, Feng Y, Ma A, Kang Y. Machine learning model for predicting acute kidney injury progression in critically ill patients. BMC Med Inform Decis Mak. (2022) 22:17. doi: 10.1186/s12911-021-01740-2
20. Zhang X, Chen S, Lai K, Chen Z, Wan J, Xu Y. Machine learning for the prediction of acute kidney injury in critical care patients with acute cerebrovascular disease. Ren Fail. (2022) 44:43–53. doi: 10.1080/0886022X.2022.2036619
21. Liang Q, Xu Y, Zhou Y, Chen X, Chen J, Huang M. Severe acute kidney injury predicting model based on transcontinental databases: a single-centre prospective study. BMJ Open. (2022) 12:e054092. doi: 10.1136/bmjopen-2021-054092
22. Fujarski M, Porschen C, Plagwitz L, Brenner A, Ghoreishi N, Thoral P, et al. Prediction of acute kidney injury in the intensive care unit: preliminary findings in a European open access database. Stud Health Technol Inform. (2022) 294:139–40. doi: 10.3233/SHTI220419
23. Alfieri F, Ancona A, Tripepi G, Crosetto D, Randazzo V, Paviglianiti A, et al. A deep-learning model to continuously predict severe acute kidney injury based on urine output changes in critically ill patients. J Nephrol. (2021) 34:1875–86. doi: 10.1007/s40620-021-01046-6
24. Parreco J, Soe-Lin H, Parks JJ, Byerly S, Chatoor M, Buicko JL, et al. Comparing machine learning algorithms for predicting acute kidney injury. Am Surg. (2019) 85:725–9. doi: 10.1177/000313481908500731
25. Le S, Allen A, Calvert J, Palevsky PM, Braden G, Patel S, et al. Convolutional neural network model for intensive care unit acute kidney injury prediction. Kidney Int Rep. (2021) 6:1289–98. doi: 10.1016/j.ekir.2021.02.031
26. Shawwa K, Ghosh E, Lanius S, Schwager E, Eshelman L, Kashani KB. Predicting acute kidney injury in critically ill patients using comorbid conditions utilizing machine learning. Clin Kidney J. (2020) 14:1428–35. doi: 10.1093/ckj/sfaa145
27. Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41:1411–23. doi: 10.1007/s00134-015-3934-7
28. Cronin RM, VanHouten JP, Siew ED, Eden SK, Fihn SD, Nielson CD, et al. National veterans health administration inpatient risk stratification models for hospital-acquired acute kidney injury. J Am Med Inform Assoc. (2015) 22:1054–71. doi: 10.1093/jamia/ocv051
29. Koyner JL, Carey KA, Edelson DP, Churpek MM. The development of a machine learning inpatient acute kidney injury prediction model. Crit Care Med. (2018) 46:1070–7. doi: 10.1097/CCM.0000000000003123
30. Tomašev N, Glorot X, Rae JW, Zielinski M, Askham H, Saraiva A, et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature. (2019) 572:116–9. doi: 10.1038/s41586-019-1390-1
31. Kate RJ, Perez RM, Mazumdar D, Pasupathy KS, Nilakantan V. Prediction and detection models for acute kidney injury in hospitalized older adults. BMC Med Inform Decis Mak. (2016) 16:39. doi: 10.1186/s12911-016-0277-4
32. Mohamadlou H, Lynn-Palevsky A, Barton C, Chettipally U, Shieh L, Calvert J, et al. Prediction of acute kidney injury with a machine learning algorithm using electronic health record data. Can J Kidney Health Dis. (2018) 5:205435811877632. doi: 10.1177/2054358118776326
33. He J, Hu Y, Zhang X, Wu L, Waitman LR, Liu M. Multi-perspective predictive modeling for acute kidney injury in general hospital populations using electronic medical records. JAMIA Open. (2018) 2:115–22. doi: 10.1093/jamiaopen/ooy043
34. Churpek MM, Carey KA, Edelson DP, Singh T, Astor BC, Gilbert ER, et al. Internal and external validation of a machine learning risk score for acute kidney injury. JAMA Netw Open. (2020) 3:e2012892. doi: 10.1001/jamanetworkopen.2020.12892
35. Kim K, Yang H, Yi J, Son HE Ryu JY, Kim YC, et al. Real-time clinical decision support based on recurrent neural networks for in-hospital acute kidney injury: external validation and model interpretation. J Med Internet Res. (2021) 23:e24120. doi: 10.2196/24120
36. Cheng P, Waitman LR, Hu Y, Liu M. Predicting inpatient acute kidney injury over different time horizons: how early and accurate? AMIA Annu Symp Proc. (2017) 2017:565–74.
37. Lee HC, Yoon HK, Nam K, Cho YJ, Kim TK, Kim WH, et al. Derivation and validation of machine learning approaches to predict acute kidney injury after cardiac surgery. J Clin Med. (2018) 7:322. doi: 10.3390/jcm7100322
38. Tseng PY, Chen YT, Wang CH, Chiu KM, Peng YS, Hsu SP, et al. Prediction of the development of acute kidney injury following cardiac surgery by machine learning. Crit Care. (2021) 25:478. doi: 10.1186/s13054-020-03179-9
39. Petrosyan Y, Mesana TG, Sun LY. Prediction of acute kidney injury risk after cardiac surgery: using a hybrid machine learning algorithm. BMC Med Inform Decis Mak. (2022) 22:137. doi: 10.1186/s12911-022-01859-w
40. Zhang H, Wang Z, Tang Y, Chen X, You D, Wu Y, et al. Prediction of acute kidney injury after cardiac surgery: model development using a Chinese electronic health record dataset. J Transl Med. (2022) 20:166. doi: 10.1186/s12967-022-03351-5
41. Li Y, Xu J, Wang Y, Zhang Y, Jiang W, Shen B, et al. A novel machine learning algorithm, bayesian networks model, to predict the high-risk patients with cardiac surgery-associated acute kidney injury. Clin Cardiol. (2020) 43:752–61. doi: 10.1002/clc.23377
42. Lei G, Wang G, Zhang C, Chen Y, Yang X. Using machine learning to predict acute kidney injury after aortic arch surgery. J Cardiothorac Vasc Anesth. (2020) 34:3321–8. doi: 10.1053/j.jvca.2020.06.007
43. Penny-Dimri JC, Bergmeir C, Reid CM, Williams-Spence J, Cochrane AD, Smith JA. Machine learning algorithms for predicting and risk profiling of cardiac surgery-associated acute kidney injury. Semin Thorac Cardiovasc Surg. (2021) 33:735–45. doi: 10.1053/j.semtcvs.2020.09.028
44. Lee HC, Yoon SB, Yang SM, Kim WH Ryu HG, Jung CW, et al. Prediction of acute kidney injury after liver transplantation: machine learning approaches vs. logistic regression model. J Clin Med. (2018) 7:428. doi: 10.3390/jcm7110428
45. Lee Y, Ryu J, Kang MW, Seo KH, Kim J, Suh J, et al. Machine learning-based prediction of acute kidney injury after nephrectomy in patients with renal cell carcinoma. Sci Rep. (2021) 11:15704. doi: 10.1038/s41598-021-95019-1
46. Lazebnik T, Bahouth Z, Bunimovich-Mendrazitsky S, Halachmi S. Predicting acute kidney injury following open partial nephrectomy treatment using SAT-pruned explainable machine learning model. BMC Med Inform Decis Mak. (2022) 22:133. doi: 10.1186/s12911-022-01877-8
47. Zhu K, Song H, Zhang Z, Ma B, Bao X, Zhang Q, et al. Acute kidney injury in solitary kidney patients after partial nephrectomy: incidence, risk factors and prediction. Transl Androl Urol. (2020) 9:1232–43. doi: 10.21037/tau.2020.03.45
48. Bredt LC, Peres LAB, Risso M, Barros LCA. Risk factors and prediction of acute kidney injury after liver transplantation: logistic regression and artificial neural network approaches. World J Hepatol. (2022) 14:570–82. doi: 10.4254/wjh.v14.i3.570
49. Dong JF, Xue Q, Chen T, Zhao YY, Fu H, Guo WY, et al. Machine learning approach to predict acute kidney injury after liver surgery. World J Clin Cases. (2021) 9:11255–64. doi: 10.12998/wjcc.v9.i36.11255
50. He ZL, Zhou JB, Liu ZK, Dong SY, Zhang YT, Shen T, et al. Application of machine learning models for predicting acute kidney injury following donation after cardiac death liver transplantation. Hepatobiliary Pancreat Dis Int. (2021) 20:222–31. doi: 10.1016/j.hbpd.2021.02.001
51. Ko S, Jo C, Chang CB, Lee YS, Moon YW, Youm JW, et al. A web-based machine-learning algorithm predicting post-operative acute kidney injury after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. (2020) 30:545–54. doi: 10.1007/s00167-020-06258-0
52. Nikkinen O, Kolehmainen T, Aaltonen T, Jämsä E, Alahuhta S, Vakkala M. Developing a supervised machine learning model for predicting perioperative acute kidney injury in arthroplasty patients. Comput Biol Med. (2022) 144:105351. doi: 10.1016/j.compbiomed.2022.105351
53. Caragata R, Wyssusek KH, Kruger P. Acute kidney injury following liver transplantation: a systematic review of published predictive models. Anaesth Intensive Care. (2016) 44:251–61. doi: 10.1177/0310057X1604400212
54. Barreto AG, Daher EF, Silva Junior GB, Garcia JH, Magalhães CB, Lima JM, et al. Risk factors for acute kidney injury and 30-day mortality after liver transplantation. Ann Hepatol. (2015) 14:688–94. doi: 10.1016/S1665-2681(19)30763-X
55. Kalisvaart M, Schlegel A, Umbro I, de Haan JE, Polak WG, IJzermans JN, et al. The AKI prediction score: a new prediction model for acute kidney injury after liver transplantation. HPB. (2019) 21:1707–17. doi: 10.1016/j.hpb.2019.04.008
56. Park MH, Shim HS, Kim WH, Kim HJ, Kim DJ, Lee SH, et al. Clinical risk scoring models for prediction of acute kidney injury after living donor liver transplantation: a retrospective observational study. PLoS ONE. (2015) 10:e0136230. doi: 10.1371/journal.pone.0136230
57. Lei L, Wang Y, Xue Q, Tong J, Zhou CM, Yang JJ, et al. comparative study of machine learning algorithms for predicting acute kidney injury after liver cancer resection. PeerJ. (2020) 8:e8583. doi: 10.7717/peerj.8583
58. Porter CJ, Moppett IK, Juurlink I, Nightingale J, Moran CG, Devonald MAJ. Acute and chronic kidney disease in elderly patients with hip fracture: prevalence, risk factors and outcome with development and validation of a risk prediction model for acute kidney injury. BMC Nephrol. (2017) 18:20. doi: 10.1186/s12882-017-0437-5
59. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. (2014) 7:546–51. doi: 10.1093/ckj/sfu108
60. Jämsä P, Jämsen E, Lyytikäinen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop. (2017) 88:370–6. doi: 10.1080/17453674.2017.1301743
61. Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. (2019) 96:1083–99. doi: 10.1016/j.kint.2019.05.026
62. Yue S, Li S, Huang X, Liu J, Hou X, Zhao Y, et al. Machine learning for the prediction of acute kidney injury in patients with sepsis. J Transl Med. (2022) 20:215. doi: 10.1186/s12967-022-03364-0
63. Qu C, Gao L, Yu XQ, Wei M, Fang GQ, He J, et al. Machine learning models of acute kidney injury prediction in acute pancreatitis patients. Gastroenterol Res Pract. (2020) 2020:3431290. doi: 10.1155/2020/3431290
64. Yang Y, Xiao W, Liu X, Zhang Y, Jin X, Li X. Machine learning-assisted ensemble analysis for the prediction of acute pancreatitis with acute kidney injury. Int J Gen Med. (2022) 15:5061–72. doi: 10.2147/IJGM.S361330
65. Zhang Y, Wang Y, Xu J, Zhu B, Chen X, Ding X, et al. Comparison of prediction models for acute kidney injury among patients with hepatobiliary malignancies based on XGBoost and LASSO-logistic algorithms. Int J Gen Med. (2021) 14:1325–35. doi: 10.2147/IJGM.S302795
66. Scanlon LA, O'Hara C, Garbett A, Barker-Hewitt M, Barriuso J. Developing an agnostic risk prediction model for early AKI detection in cancer patients. Cancers. (2021) 13:4182. doi: 10.3390/cancers13164182
67. Park N, Kang E, Park M, Lee H, Kang HG, Yoon HJ, et al. Predicting acute kidney injury in cancer patients using heterogeneous and irregular data. PLoS ONE. (2018) 13:e0199839. doi: 10.1371/journal.pone.0199839
68. Li Y, Chen X, Shen Z, Wang Y, Hu J, Zhang Y, et al. Prediction models for acute kidney injury in patients with gastrointestinal cancers: a real-world study based on bayesian networks. Ren Fail. (2020) 42:869–76. doi: 10.1080/0886022X.2020.1810068
69. Li Y, Chen X, Wang Y, Hu J, Shen Z, Ding X. Application of group LASSO regression based bayesian networks in risk factors exploration and disease prediction for acute kidney injury in hospitalized patients with hematologic malignancies. BMC Nephrol. (2020) 21:162. doi: 10.1186/s12882-020-01786-w
70. Tang CQ Li JQ, Xu DY, Liu XB, Hou WJ Lyu KY, et al. Comparison of machine learning method and logistic regression model in prediction of acute kidney injury in severely burned patients. Zhonghua Shao Shang Za Zhi. (2018) 34:343–8.
71. Tran NK, Sen S, Palmieri TL, Lima K, Falwell S, Wajda J, et al. Artificial intelligence and machine learning for predicting acute kidney injury in severely burned patients: a proof of concept. Burns. (2019) 45:1350–8. doi: 10.1016/j.burns.2019.03.021
72. Rashidi HH, Sen S, Palmieri TL, Blackmon T, Wajda J, Tran NK. Early recognition of burn- and trauma-related acute kidney injury: a pilot comparison of machine learning techniques. Sci Rep. (2020) 10:205. doi: 10.1038/s41598-019-57083-6
73. Wu G, Xiao Y, Wang C, Hong X, Sun Y, Ma B, et al. Risk factors for acute kidney injury in patients with burn injury. J Burn Care Res. (2017) 38:271–82. doi: 10.1097/BCR.0000000000000438
74. Scurt FG, Bose K, Canbay A, Mertens PR, Chatzikyrkou C. Pankreatitisbedingte akute nierenschädigung (AP-AKI): definition, pathophysiologie, diagnostik und therapie. Z Gastroenterol. (2020) 58:1241–66. doi: 10.1055/a-1255-3413
75. Cheng Y, Nie S, Li L, Li Y, Liu D, Xiong M, et al. Epidemiology and outcomes of acute kidney injury in hospitalized cancer patients in China. Int J Cancer. (2019) 144:2644–50. doi: 10.1002/ijc.31993
76. Kitchlu A, McArthur E, Amir E, Booth CM, Sutradhar R, Majeed H, et al. Acute kidney injury in patients receiving systemic treatment for cancer: a population-based cohort study. J Natl Cancer Inst. (2018) 111:727–36. doi: 10.1093/jnci/djy167
77. Canet E, Zafrani L, Lambert J, Thieblemont C, Galicier L, Schnell D, et al. Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: impact on remission and survival. PLoS ONE. (2013) 8:e55870. doi: 10.1371/journal.pone.0055870
78. Małyszko J, Kozlowski L, Kozłowska K, Małyszko M, Małyszko J. Cancer and the kidney: dangereoux liasons or price paid for the progress in medicine? Oncotarget. (2017) 8:66601–19. doi: 10.18632/oncotarget.18094
79. Rosner MH, Capasso G, Perazella MA. Acute kidney injury and electrolyte disorders in the critically ill patient with cancer. Curr Opin Crit Care. (2017) 23:475–83. doi: 10.1097/MCC.0000000000000450
80. Huang C, Murugiah K, Mahajan S, Li SX, Dhruva SS, Haimovich JS, et al. Enhancing the prediction of acute kidney injury risk after percutaneous coronary intervention using machine learning techniques: a retrospective cohort study. PLoS Med. (2018) 15:e1002703. doi: 10.1371/journal.pmed.1002703
81. Niimi N, Shiraishi Y, Sawano M, Ikemura N, Inohara T, Ueda I, et al. Machine learning models for prediction of adverse events after percutaneous coronary intervention. Sci Rep. (2022) 12:6262. doi: 10.1038/s41598-022-10346-1
82. Sun L, Zhu W, Chen X, Jiang J, Ji Y, Liu N, et al. Machine learning to predict contrast-induced acute kidney injury in patients with acute myocardial infarction. Front Med. (2020) 7:592007. doi: 10.3389/fmed.2020.592007
83. Huang C, Li SX, Mahajan S, Testani JM, Wilson FP, Mena CI, et al. Development and validation of a model for predicting the risk of acute kidney injury associated with contrast volume levels during percutaneous coronary intervention. JAMA Netw Open. (2019) 2:e1916021. doi: 10.1001/jamanetworkopen.2019.16021
84. Ibrahim NE, McCarthy CP, Shrestha S, Gaggin HK, Mukai R, Magaret CA, et al. A clinical, proteomics, and artificial intelligence-driven model to predict acute kidney injury in patients undergoing coronary angiography. Clin Cardiol. (2019) 42:292–8. doi: 10.1002/clc.23143
85. Okawa T, Mizuno T, Hanabusa S, Ikeda T, Mizokami F, Koseki T, et al. Prediction model of acute kidney injury induced by cisplatin in older adults using a machine learning algorithm. PLoS ONE. (2022) 17:e0262021. doi: 10.1371/journal.pone.0262021
86. Yang L, Gabriel N, Hernandez I, Vouri SM, Kimmel SE, Bian J, et al. Identifying patients at risk of acute kidney injury among medicare beneficiaries with type 2 diabetes initiating SGLT2 inhibitors: a machine learning approach. Front Pharmacol. (2022) 13:834743. doi: 10.3389/fphar.2022.834743
87. Williams RM, Shah J, Mercer E, Tian HS, Thompson V, Cheung JM, et al. Kidney-targeted redox scavenger therapy prevents cisplatin-induced acute kidney injury. Front Pharmacol. (2022) 12:790913. doi: 10.3389/fphar.2021.790913
88. Wei H, Gou W, Gao J, Ning H, Song Y, Li D, et al. Novel PHD2/HDACs hybrid inhibitors protect against cisplatin-induced acute kidney injury. Eur J Med Chem. (2022) 230:114115. doi: 10.1016/j.ejmech.2022.114115
89. Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci. (2019) 20:3011. doi: 10.3390/ijms20123011
90. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. (2019) 393:31–9. doi: 10.1016/S0140-6736(18)32590-X
91. Chen G, Li X, Cui Q, Zhou Y, Zhao B, Mei D, et al. Acute kidney injury following SGLT2 inhibitors among diabetic patients: a pharmacovigilance study. Int Urol Nephrol. (2022). doi: 10.1007/s11255-022-03211-7
Keywords: AKI, inpatient, artificial intelligence, machine learning, predictive model
Citation: Yu X, Ji YW, Huang MJ and Feng Z (2023) Machine learning for acute kidney injury: Changing the traditional disease prediction mode. Front. Med. 10:1050255. doi: 10.3389/fmed.2023.1050255
Received: 21 September 2022; Accepted: 17 January 2023;
Published: 03 February 2023.
Edited by:
Thierry Hauet, University of Poitiers, FranceReviewed by:
Yue Gu, Henan Provincial People's Hospital, ChinaTomasz Porazko, Opole University, Poland
Copyright © 2023 Yu, Ji, Huang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Feng,  emhlemhlXzQwMjVAMTI2LmNvbQ==; Mengjie Huang,
emhlemhlXzQwMjVAMTI2LmNvbQ==; Mengjie Huang,  aHVhbmdtZW5namllMzAxQDE2My5jb20=
aHVhbmdtZW5namllMzAxQDE2My5jb20=
 Xiang Yu
Xiang Yu Yuwei Ji
Yuwei Ji Mengjie Huang
Mengjie Huang Zhe Feng
Zhe Feng