
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 07 February 2023
Sec. Dermatology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1049477
This article is part of the Research Topic Case Reports in Dermatology View all 23 articles
Non-specific lipid transfer proteins (nsLTPs) as the primary sensitizer in plant-food allergic patients used to be seen primarily in the Mediterranean area. However, more recently, increasing numbers of clinically relevant sensitizations are being observed in Northern Europe. We herein report an unusual case of a woman who developed an anaphylactic reaction during a meal including a variety of different foods ranging from fruits and nuts to oats, wheat, and salmon. Allergy diagnostics showed no Bet v 1 sensitization but an nsLTP-mediated food allergy. Despite the much more prominent birch food syndrome in Central and Northern Europe, LTPs should be considered disease-causing agents, especially for patients developing severe reactions after consuming LTP-containing foods.
Non-specific lipid transfer proteins (nsLTPs) are small, highly structurally stable proteins found in various plant foods and pollen. Their structure contains four disulfide bonds that are responsible for their resistance to thermal processing as well as gastrointestinal digestion (1). These characteristics provide them with the ability to induce primary gastrointestinal sensitization. LTPs as a major cause of food allergy have so far been mainly recognized in the Mediterranean area, while reports of LTP-mediated food allergy in Northern Europe have been rare (2). In this area, the birch food syndrome is the dominant cause of allergic reactions to various plant foods, whereas LTPs as the cause of pollen-associated or primary food allergy still seem to be rare (2). Patients with birch food syndrome sometimes simultaneously present with co-sensitization to LTPs like Pru p 3 (peach), Cor a 8 (hazelnut), Mal d 3 (apple), and others, but these cases mostly show mild allergic reactions (3). In contrast to that, LTP-mediated food allergy often presents with much more severe manifestations (4). Patients with LTP syndrome experience reactions to multiple plant foods due to the wide distribution of these allergens in plant sources (5). The severity of reactions additionally increases with the number of LTP sensitizations (2). In some cases, the manifestation of symptoms in allergic patients can depend on the presence of a cofactor, such as exercise (6). These cofactors potentially amplify allergic
reactions by decreasing the amount of allergen needed to induce reactions in patients with lower allergen sensitization (7). Currently, the understanding of the pathomechanism behind this phenomenon remains to be fully understood. One proposed mechanism behind exercise-induced anaphylaxis involves changes in the mucosal permeability resulting in increased allergen exposure (7). We herein describe the case of a woman who developed two separate allergic reactions after consumption of plant foods, the first one presenting as anaphylaxis and the second one as an oral allergy syndrome. In the case of the anaphylactic reaction, the cofactor exercise was present before consumption. Allergy diagnostics revealed evidence of an LTP-mediated food allergy.
A 37-year-old woman presented to the emergency department with an anaphylactic shock including burning and tingling of her tongue, emesis and diarrhea, generalized urticaria, facial angioedema progressing to dizziness, and difficulties in swallowing and breathing due to swelling of her throat. Her past medical history included lactose intolerance and sensitization to house dust mites, and she also experienced non-allergy related diseases including Hashimoto’s thyroiditis and autoimmune uveitis. The patient neither suffered from allergic rhinitis, asthma, or atopic eczema nor did she take any medication regularly. This anaphylactic reaction happened during a buffet breakfast with a multitude of allergen sources including salmon, pine nuts, walnuts, sesame, wheat, oats, mustard, honey, orange juice, melon, apple, grapes, grapefruit, blood orange, and coffee. Thorough clinical history revealed that the patient underwent physical exercise 60 min before her breakfast. She remained incident-free for more than one and a half years until she presented a second time to a primary care physician with an intense tingling of the tongue after consumption of an apple crumble and coffee (Figure 1). No other LTP-containing foods were consumed in parallel. Almost all the ingredients of the apple crumble she had been eating daily, besides the apple itself, which is why the apple was considered suspicious of being the allergy trigger. This time physical activity as a cofactor was excluded; however, menses and the intake of non-steroidal antiphlogistics are probable. Medical history further revealed that the patient had experienced itchy eyes, an erythematous itchy reaction at her neck, and swelling of her lips after the consumption of peach many years ago, which was her very first allergic reaction. The consumption of walnut and hazelnut was accompanied by oral allergy syndrome, and in the case of hazelnut (hazelnut flour in a bread roll), the eyes were itchy and the lips were swollen in addition to oral allergy syndrome.
Diagnostic assessment: skin prick testing revealed sensitization to peach (3 mm) and hazelnut (5 mm). Herring, hen’s egg extract, crab extract, peanut extract, walnut, almond, cow’s milk, soya milk, wheat flour, lupine flour, raw apple, raw carrot, raw celery, and raw tomato were negative. The positive control (histamine) reaction was 5 mm. The concentration of specific IgE-antibodies to different allergen extracts and single allergens was determined by Immuno Solid-phase Allergen Chip (ImmunoCAP; Phadia; Uppsala, Sweden). Total serum IgE was slightly elevated (110 kU/L; normal <100 kU/L). Specific IgE (sIgE) to allergen extracts was positive for pine nut, lupine seed, mugwort, olive tree, Dermatophagoides farinae, and D. pteronyssinus, and negative (<0.01 kU/L) for wheat and mustard. Specific IgE to single allergens was positive in descending concentration for the nsLTPs of peach (rPru p 3), apple (rMal d 3), mugwort (n Art v 3), walnut (rJug r 3), rAra h 9, wheat (rTri a 14), hazelnut (rCor a 8), and the olive tree (r Ole e 7). These results are shown in Table 1. The detailed medical history led to the suspicion of an LTP-mediated reaction to food. This suspicion was confirmed by in vitro allergy diagnostics showing no IgE reaction toward birch pollen [Bet v 1, Bet v 2 (profilin), and Bet v 4 (polcalcin)] but positive reactions to multiple lipid transfer proteins (Table 1). In addition to specific IgE detection, a basophil activation test (BAT) with the lipid transfer proteins from lupine (Lupinus luteus, originates from the research group Prof. Jappe) (Figure 2A) and from wheat, peanut, peach, and hazelnut (Indoor Biotechnologies Ltd., Cardiff, UK) (Figure 2B) was performed, which revealed a positive result for peach, lupine, and peanut in descending order and was negative for wheat and hazelnut. The LTPs from apple and pollen were not included as they were not available for BAT. There were no IgE reactions in ImmunoCAP to the peanut storage proteins Ara h 1–3 and Ara h 8, the Bet v 1-homolog in peanut, so the reaction to lupine LTP is most probably a cross-reactivity between LTPs. With sIgE of five or more LTPs, reactions are usually severe, leading to the diagnosis of LTP syndrome in this patient. The patient was advised to avoid the consumption of apples in any form and was equipped with an emergency set consisting of an adrenaline auto-injector, glucocorticosteroids, and antihistamines. There has been no re-presentation since.
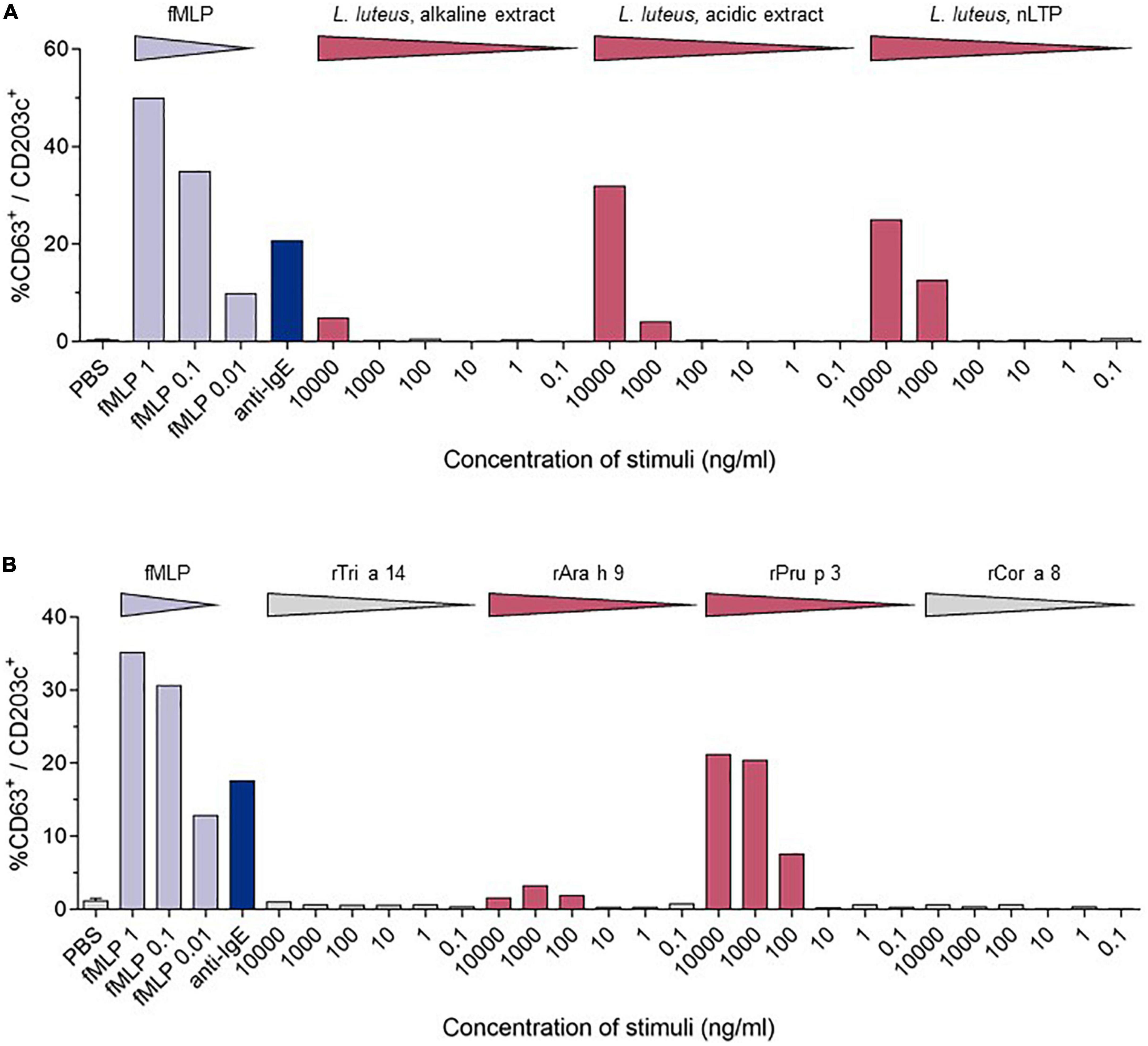
Figure 2. Comparison of the stimulation-induced basophil activation levels. Basophil activation was measured by the percentages of CD63 positive basophils (CD203c+/FcϵRIα+). PBS—phosphate-buffered saline, fmIP- formyl-, methionyl-leucyl phenylalanine. (A) Results of the BAT for LTPs from Lupinus luteus [acidic extract; alkaline extract; nLup l 3 (LTP)] (10). (B) BAT results from wheat (rTri a 14), peanut (rAra h 9), peach (rPru p3), and hazelnut (rCor a 8).
Outside the Mediterranean area, LTP-mediated allergic reactions remain a rare diagnosis, and a full understanding of these geographical differences is still to be developed. Northern European countries have a higher load of birch pollen in comparison to Mediterranean countries. This high load of birch pollen with the dominance of Bet v 1 as a major allergen and primary sensitizer is suspected to decrease the probability of primary sensitizations to lipid transfer proteins in pollen, leading to mostly mild allergic reactions in LTP-sensitized Northern European patients (8). In contrast to that, LTP-mediated allergy can provoke various and more severe symptoms, including urticaria, nausea, diarrhea, angioedema, dizziness and even swelling of the throat, dyspnea, as well as cardiovascular disruptions. In our case, LTP-mediated anaphylaxis was induced by the consumption of multiple LTP-containing foods with preceding physical activity as a cofactor. The absence of typical birch pollen allergy symptoms like allergic rhinitis combined with the missing Bet v 1 sensitization led to the exclusion of the diagnosis of a birch pollen food syndrome. Our patient showed a strong sensitization to peach (rPru p 3), apple (rMal d 3), a moderate sensitization to walnut (rJug r 3) and wheat (rTri a 14) nsLTPs, as well as a low sensitization to the hazelnut nsLTP (rCor a 8). For peach, walnut, and hazelnut, the patient reported previous mild allergic reactions, which according to ImmunoCAP-results are not based on Bet v 1 or panallergens like profilins and polcalcins, but LTPs. The skin prick test and IgE-antibody assays can only detect sensitization and do not provide proof of allergy (9). The basophil activation test mimics the allergic reactions in vitro, and after optimization, discriminates between peanut-allergic and -sensitized individuals (9). For this patient, the BAT was performed using lupine, which is a legume like peanut, and the lipid transfer protein from yellow lupine seeds (nLup l 3), which was first identified and purified by Jappe et al. (10), and was, therefore, the original part of the “tool box” of the research group. After having obtained a positive reaction in BAT as proof-of-principle, additional commercially available LTPs (rTri a 14, rAra h 9, rPru p 3, and rCor a 8) were used in BAT. Although lupine seeds or lupine products were not knowingly a part of the buffet breakfast and the cross-reactive legume peanut is consumed daily without symptoms, this result together with the strong reaction to Pru p 3 and additional weaker reaction to Ara h 9 supports the diagnosis “LTP syndrome” in which patients are polysensitized to multiple LTPs from different food sources (1). During the anaphylactic event, the patient consumed multiple LTP-containing foods. None of them were identified as a new food source for the patient, which is why it is unlikely that anaphylaxis was induced by a single newly introduced allergen/allergen source. We propose it to be more likely that physical activity might have decreased the threshold of allergy induction. That, in combination with a high load of consumed LTP-containing foods, has potentially led to the severity of her reaction. This hypothesis of the LTP amount consumed as a risk factor in our patient is supported by the fact that the patient has not suffered a comparable allergic reaction before. The hypothesis is also supported by a recently published study on 67 Spanish patients with LTP-related anaphylaxis, 55/67 with anaphylaxis and a total of 134 anaphylactic reactions and 12/67 with anaphylactic shock and a total of 16 reactions (11). Another aspect in question is the route of sensitization. Possible routes include sensitization through cutaneous, gastrointestinal, and inhalant exposure (1). Asero reported peach-induced contact urticaria showing an association with nsLTPs (12). Primary gastrointestinal sensitization is a common cause of food allergy. For Pru p 3, Tordesillas et al. showed that it crosses the gastric barrier, which could be a reason for primary sensitization via the gastric route (13). Reactions to plant food sources can also occur via the inhalant route. One example is the birch-food syndrome describing cross-reactivity to vegetables and fruits containing Bet v 1-like proteins. Other inhalant allergen sources contain nsLTPs, which could lead to sensitization to nsLTPs via the inhalant route (14). Pollen nsLTPs like Ole e 7 from the olive tree, Art v 3 from mugwort, and Pla a 3 from the plane tree share partial cross-reactivity with Rosaceae nsLTPs and could be responsible for primary sensitization (15). As mugwort is especially common also in Northern Europe, we tested for the respective specific IgE-antibodies. The patient was indeed sensitized to the mugwort nsLTP nArt v 3. The patient originates from Northern Germany. There were no journeys to the Mediterranean area longer than 2 weeks. As the IgE concentrations to inhalant allergen sources and their LTPs were considerably lower than for peach and apple LTPs, we assume that the sensitization has most probably occurred via the ingestion of peach (see the first allergic reaction she has ever experienced.). This assumption is supported by the dominant sensitization to rPru p 3 in ImmunoCAP and the strong reaction to rPru p 3 in the BAT.
An LTP-mediated allergy can provoke potentially life-threatening allergic reactions. Despite the much more prominent birch food syndrome in Central and Northern Europe, LTP allergens should be considered disease-causing agents and included in allergy diagnostic tests, especially for patients who experience severe reactions after consuming LTP-containing foods.
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
EA wrote the manuscript and designed the Figure 1. TW planned, measured and analyzed BAT experiments and data, and designed the Figure 2. JB measured and analyzed BAT experiment and data. UJ diagnosed the patient, wrote and obtained ethical approval, wrote and revised the manuscript, and wrote the Table 1. All authors approved the final version of the manuscript.
We wish to express our gratitude to Daniel Rosero for his excellent technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Skypala IJ, Asero R, Barber D, Cecchi L, Diaz Perales A, Hoffmann-Sommergruber K, et al. Non-specific lipid-transfer proteins: allergen structure and function, cross-reactivity, sensitization, and epidemiology. Clin Transl Allergy. (2021) 11:e12010. doi: 10.1002/clt2.12010
2. Scheurer S, van Ree R, Vieths S. The role of lipid transfer proteins as food and pollen allergens outside the Mediterranean area. Curr Allergy Asthma Rep. (2021) 21:7. doi: 10.1007/s11882-020-00982-w
3. Petersen A, Kleine-Tebbe J, Scheurer S. Stable plant food allergens I: lipid-transfer-proteins. In: J Kleine-Tebbe, T Jakob editors. Molecular Allergy Diagnostics: Innovation for a Better Patient Management. Cham: Springer International Publishing (2017). p. 60–2.
4. Gomez F, Bogas G, Gonzalez M, Campo P, Salas M, Diaz-Perales A, et al. The clinical and immunological effects of Pru p 3 sublingual immunotherapy on peach and peanut allergy in patients with systemic reactions. Clin Exp Allergy. (2017) 47:339–50. doi: 10.1111/cea.12901
5. Mothes-Luksch N, Raith M, Stingl G, Focke-Tejkl M, Razzazi-Fazeli E, Zieglmayer R, et al. Pru p 3, a marker allergen for lipid transfer protein sensitization also in Central Europe. Allergy. (2017) 72:1415–8. doi: 10.1111/all.13151
6. Wölbing F, Fischer J, Köberle M, Kaesler S, Biedermann T. About the role and underlying mechanisms of cofactors in anaphylaxis. Allergy. (2013) 68:1085–92.
7. Poziomkowska-Gęsicka I, Kostrzewska M, Kurek M. Comorbidities and cofactors of anaphylaxis in patients with moderate to severe anaphylaxis. Analysis of data from the anaphylaxis registry for West Pomerania Province, Poland. Int J Environ Res Public Health. (2021) 18:333. doi: 10.3390/ijerph18010333
8. Fernández-Rivas M, Bolhaar S, González-Mancebo E, Asero R, van Leeuwen A, Bohle B, et al. Apple allergy across Europe: how allergen sensitization profiles determine the clinical expression of allergies to plant foods. J Allergy Clin Immunol. (2006) 118:481–8. doi: 10.1016/j.jaci.2006.05.012
9. Behrends J, Schwager C, Hein M, Scholzen T, Kull S, Jappe U. Innovative robust basophil activation test using a novel gating strategy reliably diagnosing allergy with full automation. Allergy. (2021) 76:3776–88. doi: 10.1111/all.14900
10. Jappe U, Karstedt A, Warneke D, Hellmig S, Böttger M, Riffelmann FW, et al. Identification and purification of novel low-molecular-weight lupine allergens as components for personalized diagnostics. Nutrients. (2021) 13:409. doi: 10.3390/nu13020409
11. Casas-Saucedo R, de la Cruz C, Araujo-Sánchez G, Gelis S, Jimenez T, Riggioni S, et al. Risk factors in severe anaphylaxis: which matters the most, food or cofactors? Investig Allergol Clin Immunol. (2022) 32:282–90.
12. Asero R. Peach-induced contact urticaria is associated with lipid transfer protein sensitization. Int Arch Allergy Immunol. (2010) 154:345–8. doi: 10.1159/000321827
13. Tordesillas L, Gómez-Casado C, Garrido-Arandia M, Murua-García A, Palacín A, Varela J, et al. Transport of Pru p 3 across gastrointestinal epithelium – An essential step towards the induction of food allergy? Clin Exp Allergy. (2013) 43:1374–83. doi: 10.1111/cea.12202
14. García-Sellés FJ, Díaz-Perales A, Sánchez-Monge R, Alcántara M, Lombardero M, Barber D, et al. Patterns of reactivity to lipid transfer proteins of plant foods and artemisia pollen: an in vivo study. Int Arch Allergy Immunol. (2002) 128:115–22. doi: 10.1159/000059401
Keywords: non-specific lipid transfer protein, LTP, LTP syndrome, food allergy, basophil activation test, exercise-induced anaphylaxis
Citation: Albert E, Walsemann T, Behrends J and Jappe U (2023) Lipid transfer protein syndrome in a Northern European patient: An unusual case report. Front. Med. 10:1049477. doi: 10.3389/fmed.2023.1049477
Received: 20 September 2022; Accepted: 03 January 2023;
Published: 07 February 2023.
Edited by:
Takashi Hashimoto, Osaka City University, JapanReviewed by:
José Antonio Cañas, Universidad de Málaga, SpainCopyright © 2023 Albert, Walsemann, Behrends and Jappe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: U. Jappe,  dWphcHBlQGZ6LWJvcnN0ZWwuZGU=
dWphcHBlQGZ6LWJvcnN0ZWwuZGU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.