
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med., 10 March 2023
Sec. Rheumatology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1046420
This article is part of the Research TopicNew Insights in Sarcoidosis: from Bench to BedsideView all 8 articles
 Svetlana Kašiković Lečić1,2†
Svetlana Kašiković Lečić1,2† Jovan Javorac1,2*†
Jovan Javorac1,2*† Aleksandra Lovrenski2,3
Aleksandra Lovrenski2,3 Jelena Đokić2
Jelena Đokić2 Danica Sazdanić Velikić2,4
Danica Sazdanić Velikić2,4 Dejan Živanović5,6
Dejan Živanović5,6Sarcoidosis is a multi-systemic disease of unknown etiology that is characterized by the formation of non-necrotizing and non-caseating granulomas in affected organs. Sarcoidosis granulomas can form in any organ, but the lungs and intrathoracic lymph nodes are the most commonly affected. Thyroid involvement in sarcoidosis is very rare, with prevalence estimates of 1–4.5% in case series of autopsied patients with systemic sarcoidosis. It is extremely rare for sarcoidosis to occur solely in the thyroid gland, but it is usually associated with the involvement of other organs, primarily the lungs and lymph nodes. Typical manifestations are diffuse goiter and solitary or multiple thyroid nodules. Thyroid function remains intact in the majority of cases, but sometimes it can result in hypothyroidism or hyperthyroidism. The diagnosis can be made after fine needle aspiration cytology, but usually it is diagnosed as an incidental finding while analyzing thyroidectomy tissue or during autopsy. Oral steroids are the cornerstone of thyroid sarcoidosis management, along with specific endocrinological treatment on some occasions. Given that we found only 71 reports of thyroid sarcoidosis available in the literature, we created and analyzed a cohort of 24 patients with thyroid sarcoidosis who were described in the literature in the 21st century and reported two additional cases of thyroid sarcoidosis.
Sarcoidosis is a chronic systemic disease of an unknown etiology that is pathologically characterized by the formation of non-caseating and non-necrotizing epithelioid cell granulomas in affected organs or tissues (1). The proposed pathophysiologic mechanism involves an unidentified, most likely infectious antigen that causes an autoimmune reaction in genetically predisposed individuals (2). Sarcoidosis typically affects patients in their 40s and 50s, with women being slightly more affected, particularly in Afro-Americans, where the female-to-male ratio is 2:1 (3).
Sarcoidosis granulomas can develop in any organ, but the lungs and intrathoracic lymph nodes are the most commonly affected (around 90% of patients) (4). Other frequently involved organs include skin (around 50% of cases), liver (up to 80% in autopsy series reports), eyes (10–50%), spleen (6–16%) and peripheral lymph nodes (about 30%). Cardiac (3–39%) and neurosarcoidosis (3–10%) are uncommon but potentially fatal manifestations of sarcoidosis (5). In only 8% of cases, extrapulmonary sarcoidosis is not accompanied by pulmonary sarcoidosis (6), so it is expected that the majority of patients will have some type of pulmonary involvement during the course of the disease.
Involvement of the thyroid gland by sarcoidosis is very rare. In post-mortem studies of patients with previously diagnosed systemic sarcoidosis, the thyroid gland was affected in up to 4.5% (7). Usually, the extrathyroidal manifestation of the disease precedes the diagnosis of thyroid involvement. Some rare cases of sarcoidosis limited to the thyroid gland can also be found in the literature (8).
The aim of this paper was to conduct a review of the literature on the subject as well as to report two additional cases of our patients with thyroid sarcoidosis.
A 53-year-old Caucasian female was admitted to a referral pulmonary institute after the chest X-ray showed infiltrations in the lungs and enlarged hilus on both sides. The chest X-ray was performed as a preoperative assessment for the scheduled thyroidectomy due to the euthyroid nodular goiter. The patient experienced a gradual enlargement of the thyroid over the past 4–5 months that caused difficulties with breathing and swallowing. Among the other complaints, she cited a dry cough in the last 2 months. She started treatment with antihypertensives 2 months earlier, while denying having any other comorbid conditions. The patient reported a positive family history of malignant lung and cardiovascular diseases, while her psycho-social history was unremarkable. Physical examination revealed an enlarged right lobe of the thyroid gland, while other findings were not abnormal.
Blood laboratory analysis at the admission revealed elevated values of serum angiotensin-converting enzyme (ACE) and serum calcium, while other biochemical parameters, including the value of calcium in 24-h urine, were within reference range (Table 1). High-resolution computed tomography (HRCT) of the chest revealed numerous bilateral micronodular lesions in the pulmonary parenchyma and mediastinal and hilar lymphadenopathy (Figures 1A–C). An inhomogeneous, predominantly hypodense abnormality in the enlarged right thyroid lobe, 30×28 mm in diameter, was also observed (Figure 1D). No nodular changes were visible inside the left lobe. Pulmonary function tests and gas exchange during rest and exercise were both preserved. A bronchoscopy was performed. Histopathological examination confirmed non-necrotizing granulomatous inflammation of pulmonary parenchyma and lymph glands (Figures 2A,B). The ultrasound examination of the upper abdomen and the ophthalmological examination did not find any evidence of sarcoidosis extension. Tuberculosis was excluded since acid-fast staining of the patient’s sputum and lung biopsies revealed no acid-fast bacilli, while cultures on Mycobacterium tuberculosis and other mycobacteria later yielded negative results. Other causes of granulomatous inflammation, such as fungal infection, were excluded as well, since sputum and biopsies’ fungal cultures grew negative, while pathohistological staining of lung biopsies was negative for fungal infection. Following that, steroid therapy was administered, with a 40 mg/day (0.5 mg/kg) starting dose of oral prednisone. After 3 months of therapy, there was a radiological improvement on patient’s chest X-ray, with ACE levels in the reference range, while only minor changes in the size of the thyroid gland were observed. Considering that, a video-assisted lobectomy of the right thyroid lobe was performed shortly after. Histopathological examination of the thyroid tissue revealed non-necrotizing granulomas consistent with sarcoidosis (Figures 2C,D). Substitution therapy with 75 mcg/day oral levothyroxine was prescribed, along with the slow dose tapering of oral steroids over the next 9 months, when oral steroids were stopped (Figure 3). After that, the patient was asymptomatic, without visible changes on chest X-ray and no biochemical evidence of the disease’s activity. After being attentively monitored for the following 12 months, the patient stopped showing up for her scheduled checkups.
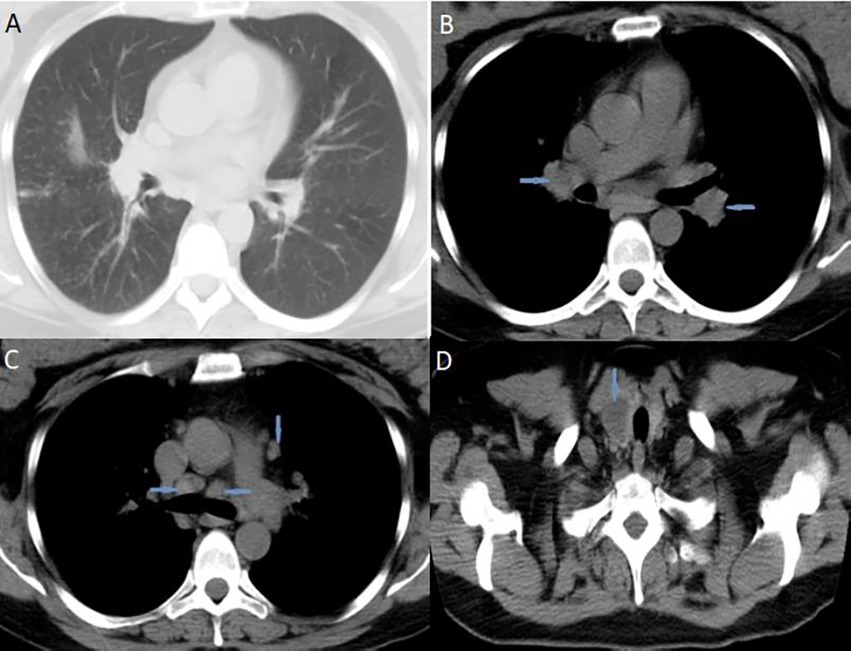
Figure 1. High-resolution computed tomography (HRCT) of the chest suggested pulmonary sarcoidosis: (A) micronodular lesions in pulmonary parenchyma; (B) hilar lymphadenopathy (blue arrows); (C) mediastinal lymphadenopathy (blue arrows). CT scans of the neck (D) indicated nodular mass inside the right thyroid lobe (blue arrow). HRCT, high-resolution computed tomography; CT, computed tomography.
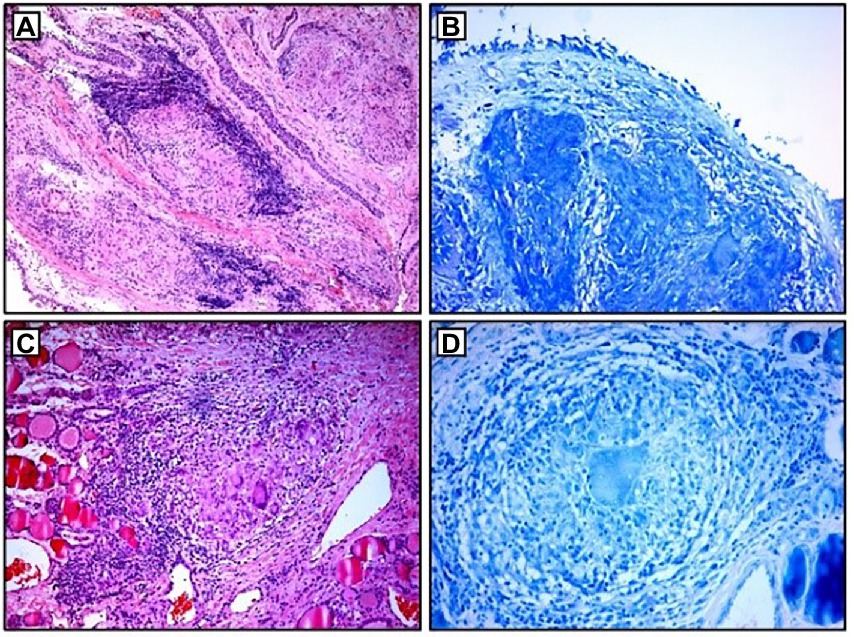
Figure 2. Histopathological examination: (A,B) non-necrotizing granulomas in bronchial mucosa and acid-fast stain showing absence of AFB; (C,D) thyroid tissue with non-necrotizing granulomas and acid-fast stain showing the absence of AFB. AFB, acid-fast bacilli.
A 68-year-old Caucasian female was admitted to a referral pulmonary institute for additional testing for suspected sarcoidosis. She had been treated with drugs for hyperthyroidism for about 35 years. However, around 12 months prior to the admission, she developed weakness, malaise, and a feeling of heart palpitations. An endocrinologist evaluated the patient multiple times. Since the hormone values (T3, T4, and TSH) were still irregular even after the prescribed therapy, she was treated surgically three months before the hospitalization. A total thyroidectomy was performed and definitive pathohistological findings revealed a polynodous goiter with numerous diffuse noncaseating granulomas whose morphology, number, and localization were typical of thyroid sarcoidosis (Figures 4C,D). Once the diagnosis of thyroid sarcoidosis was suspected, the patient was referred to a pulmonologist for further evaluation. At admission, she denied having any other comorbidity except thyroidectomy-associated hypothyroidism, which was managed with oral levothyroxine 50 mcg daily. She reported a positive family history of pulmonary tuberculosis. The psycho-social history as well as the physical examination were unremarkable.
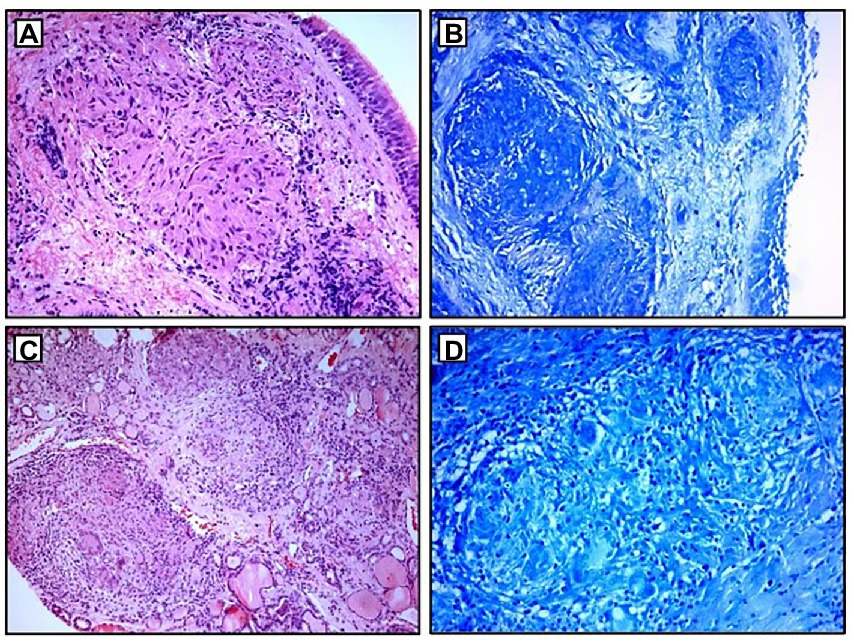
Figure 4. Histopathological examination: (A,B) non-necrotizing granulomas in bronchial mucosa and acid-fast stain showing absence of AFB; (C,D) thyroid tissue with non-necrotizing granulomas and acid-fast stain showing the absence of AFB. AFB, acid-fast bacilli.
Blood laboratory findings at the admission revealed elevated values of serum ACE, while other biochemical parameters, including the levels of serum calcium and calcium in 24-h urine, were within reference range (Table 1). Chest X-ray and HRCT scans were without lesions in the lung parenchyma and lymphadenopathy. Pulmonary function tests and gas exchange at rest and during exertion were preserved. Histopathological examination of transbronchial and bronchial biopsies revealed non-necrotizing granulomas consistent with sarcoidosis (Figures 4A,B). The ultrasound examination of the upper abdomen as well as the ophthalmological examination did not reveal any sarcoidosis extension. Considering family history, a thorough assessment of pulmonary tuberculosis was done, and it was excluded since acid-fast staining of the patient’s sputum and lung biopsies showed the absence of acid-fast bacilli, while cultures of Mycobacterium tuberculosis and other mycobacteria later on grew negative. The patient also had a negative result on the QuantiFERON TB-Gold In-Tube test. Other causes of granulomatous inflammation, such as fungal infection, were excluded as well, since fungal cultures of sputum and lung biopsies grew negative, while staining of lung biopsies was negative for fungal infection. During the cardiac evaluation, a heart rhythm disorder was recorded (left bundle branch block and first-degree atrioventricular block). Positron emission tomography/computed tomography was performed due to the suspicion of cardiac sarcoidosis; however, only bilateral hilar accumulation of radiopharmaceutical was observed. Once this diagnostic algorithm was finished, we decided not to administer steroids treatment, and the patient was discharged with thyroid substitution therapy (oral levothyroxine 50 mcg per day), while the cardiologist recommended conservative therapeutic approach of heart rhythm disorder. At the follow-up pulmonology visit 3 months later, the patient denied having any respiratory symptoms; the chest X-ray revealed no pathological alternations; and the serum ACE levels were now within the reference range. Without changing the treatment, she was followed every 3 months for the next 12 months until she stopped coming to scheduled controls.
Patients agreed to our treatment plan and were thankful for the assistance provided. Since the patients stopped visiting our pulmonary institute after a certain period of follow-up, we contacted them by phone at the time of writing the study and received information that both patients are being monitored by endocrinologists, and periodically have check-ups with local pulmonologists, without symptoms characteristic of sarcoidosis.
Thyroid involvement in sarcoidosis is very rare, with literature data estimating its prevalence of 1–4% in case series of autopsied patients with systemic sarcoidosis (9), while it is extremely rarely diagnosed in vivo (2). Thyroid sarcoidosis most commonly manifests clinically as gradual enlargement of the gland, which can be asymptomatic, cause pain in the gland, or cause compressive symptoms such as difficulty breathing and swallowing (9). The most frequently reported constitutional symptom is fatigue (10). Physical examination may reveal a diffusely and homogeneously enlarged thyroid, multinodular goiter, or cold solitary thyroid nodules, with or without cervical lymphadenopathy (9, 11).
Sarcoidosis is a disease that has been previously associated with the development of a variety of autoimmune and non-autoimmune endocrine disorders (12). Even though the majority of patients with sarcoidosis do not have an associated functional thyroid disorder (10), in about 2–20% of cases, sarcoidosis may be associated with either hypothyroidism or hyperthyroidism (2, 13). A significantly higher prevalence of clinical hypothyroidism and Graves’ disease was found in female patients (14). Similarities in the pathogenesis of Graves’ disease and sarcoidosis may explain the prevalence of thyroid autoimmunity in sarcoidosis patients, even when the thyroid is not involved (13). Simultaneous Graves’ disease and thyroid sarcoidosis may result in an unusual or suboptimal clinical response of Graves’ disease to various treatment modalities, such as antithyroid drug therapy and/or radioactive iodine therapy (7, 13). Hashimoto’s thyroiditis may also coexist with sarcoidosis, with several studies identifying it as the most common autoimmune disease associated with sarcoidosis (15). The association between Hashimoto’s thyroiditis and sarcoidosis could be due to increased thyroid-specific T-cell activation caused by increased expression of Th1/Th17 cells in both diseases (16). Non-necrotizing granulomas infiltration of the gland can also cause thyroid dysfunction and the development of hypothyroidism (7). Considering the above, clinicians should properly functionally evaluate certain sarcoidosis patients for thyroid disease, particularly determine thyroid antibodies for autoimmune thyroid diseases.
As with any other localization, the clinical manifestations of the disease, radiological findings, and histopathological evidence of non-caseating granulomas in the absence of any underlying microorganisms or particles must be considered for the diagnosis of thyroid sarcoidosis (17). Laboratory tests may be helpful and should begin with the measurement of serum thyroid hormones. The serum ACE, serum amyloid A (SAA), serum lysozyme, chitotriosidase, soluble interleukin-2 receptor (sIL2-R), and other biochemical markers may reflect the activity of sarcoidosis (18). Ultrasound is the preferred imaging modality for thyroid nodules. The typical ultrasonographic findings of thyroid sarcoidosis are scattered nodular shadows, 1–3 cm in diameter with irregular hypoechoic areas that reflect granuloma formation (8). Thyroid scintigraphy/radionuclide thyroid scan should be performed in patients presenting with a low serum TSH (19). The ultrasound-guided fine needle aspiration cytology (FNAc) is the preferred and the most commonly used method for evaluating thyroid changes seen on ultrasonography (20). This method has been shown to be very useful for detecting thyroid cancer, with high specificity and sensitivity. The false-negative rate of a benign interpretation is 0–3% (10). However, the diagnosis of thyroid sarcoidosis can be missed by FNAc, with findings indicating sarcoidosis malignancy or other non-sarcoid disorders (11). Potential explanations for this include the possibility of not obtaining a sample of granulomatous inflammation due to its uneven distribution in the gland, reactive follicular cells mimicking papillary structures, or misinterpreting condensed colloid as psammoma bodies (9, 11). Core needle biopsy (CNB) could be a useful diagnostic tool in cases of inconclusive FNAc results, with higher specificity and sensitivity than FNAc, avoiding misdiagnosis and overtreatment (9). In a retrospective study of nodules with two or more prior nondiagnostic FNAs, CNB was diagnostic in 86% compared with 29% for repeat FNAc (10). However, there are studies where CNB did not show superior diagnostic performance compared to FNAc for diagnosing thyroid nodules (21). In the majority of cases, thyroid sarcoidosis is diagnosed as an incidental finding through pathohistological examination of the surgically removed thyroid, which can be done due to glandular hyperplasia causing compressive symptoms, inability to regulate hormonal status despite therapy, due to previously suspected primary thyroid malignancy, or during autopsy (7).
The histopathological hallmark of non-caseating granulomas is non-specific to sarcoidosis. Due to similar clinical and ultrasonographic manifestations, the possibility of cervical lymph node involvement, and the low specificity of FNAc, the differential diagnosis between thyroid sarcoidosis and primary thyroid carcinoma is very challenging (9, 10). The possibility of coexistence of both thyroid sarcoidosis and thyroid carcinomas should also be contemplated (22). Although very rare, other granulomatous diseases should be considered when patients present with a neck mass and cervical lymphadenopathy, such as tuberculosis, atypical mycobacterial infections, brucellosis, fungal infections, subacute (DeQuervain’s) granulomatous thyroiditis, foreign body reactions, or non-Hodgkin’s lymphoma (7, 11, 23, 24).
Most patients with thyroid sarcoidosis do not require immunosuppressive therapy if the gland is not enlarged enough to cause compressive symptoms, if there are no functional disorders, or if there are no other organs affected (10). However, as isolated sarcoidosis of the thyroid is extremely rare (25), a large number of patients will present with multisystem sarcoidosis, necessitating the introduction of appropriate therapy. Systemic corticosteroids are the first-line treatment for sarcoidosis (26). Steroids have been shown to be effective in reducing the size of sarcoidosis masses in the thyroid (8), enough that surgical removal of the gland may be avoided in some cases. Second-line and third-line treatment, such as immunosuppressive and biological agents, should be considered for patients with corticosteroid-refractory disease, intolerable side effects, or corticosteroid toxicity (27). In cases of the presence of symptoms, especially if they are resistant to the applied therapy, or if there is a concern for malignancy, thyroidectomy is recommended (7), while the associated functional disorders are treated with appropriate therapy (10). The majority of nodules are asymptomatic, and with only 5 to 10% of nodules being malignant, the decision to operate is made on therapeutic or diagnostic grounds (28, 29). In addition, cases of spontaneous decreases in the size of thyroid granulomas have also been described in the literature (8).
The first report of thyroid sarcoidosis originates from an autopsy report in 1938 (30), with several case reports available after that. In a 2017 study by Okuma et al. (8), only 65 cases of systemic sarcoidosis with thyroid involvement were reported. Relying on that fact, using the selected key words “thyroid” and “sarcoidosis,” we reviewed the literature databases PubMed and Google Scholar over the next 6 years (January 1, 2017– June 1, 2022) and discovered an additional six case reports, bringing the total number of thyroid sarcoidosis reports in the available literature to 71. Due to the scarcity of data on this subject, we created a cohort of patients with diagnosed thyroid sarcoidosis who were described in the literature between January 1, 2000, and June 1, 2022. A total of 20 studies (2, 4, 7–11, 13, 17, 23, 25, 31–39), all of which were designed as case reports, and 24 patients with thyroid sarcoidosis were analyzed (Supplementary Table S1). Patients’ basic socio-demographic characteristics (gender, age), clinical manifestations of the disease, thyroid functional status, diagnostic procedure of thyroid sarcoidosis, possible extra-thyroid localizations of the disease, implemented treatment, and disease outcome were all collected.
Female patients were found to be the most represented in our sample, accounting for 83.3% of all patients. The sample’s average age was 50.2 years, ranging from 23 to 74. The majority of patients (45.8%) reported thyroid enlargement as the first clinical manifestation of the disease, as well as hyperthyroid symptoms and signs such as weight loss, heat intolerance, excessive sweating, flushing, palpitations, tachycardia, and tremors (in 29.2% of respondents). Constitutional symptoms like generalized weakness and malaise, as well as shortness of breath (dyspnea) and difficulty swallowing (dysphagia), were almost equally prevalent in our sample (20.8, 20.8, and 16.7%, respectively). The thyroid function analysis revealed that the majority of patients with thyroid sarcoidosis (63.6%) were euthyroid, 36.4% had laboratory signs of hyperthyroidism, and none had hypothyroidism. Antithyroid antibodies (thyroglobulin antibodies, thyroperoxidase, thyroid-stimulating-receptor antibodies) were reported for approximately half of the patients (13/24), and they were positive in 23% of cases. Initial thyroid ultrasound results were available for 17 of 24 patients. The majority of them (76.5%) presented with single or multiple thyroid nodules, with 46.1% also having a diffusely enlarged thyroid. In 11.7% of cases, heterogeneous thyroid tissue or a diffusely enlarged thyroid without nodules was found. In 14/24 patients, fine needle aspiration cytology (FNAc) was used as the initial diagnostic method to determine the etiology of ultrasound-observed pathological changes in the thyroid. Only 21.4% of cases had a FNAc finding of thyroid sarcoidosis, while in the rest of the cases it indicated primary thyroid malignancy (21.4%), inflammatory changes in the gland (14.3%), or other benign cytological characteristics of thyroid tissue (42.8%). A core needle biopsy (CNB) was performed on 3 of the 24 patients and was successful in detecting non-caseating granulomas each time (100%). However, in the majority of the patients (17/24), thyroid sarcoidosis was diagnosed following thyroidectomy. Only one patient (4.2%) had isolated thyroid sarcoidosis with no involvement of other organs. The remaining patients had multi-systemic sarcoidosis, with the lymph nodes of the chest (78.3%), lungs (47.8%), lymph nodes of the neck (34.8%), skin (17.4%), and liver (13%) being the most commonly affected. The majority of patients were treated endocrinologically with antithyroid drugs, radioactive iodine, or thyroidectomy, with oral steroids being used in 29.2% of cases. There was follow-up data available for 14/24 patients, with a mean follow-up of approximately 2 years and no progression of systemic sarcoidosis observed after treatment. The results of the analysis are also shown tabularly in Table 2.
In conclusion, sarcoidosis involving the thyroid gland is a rare entity and may pose a diagnostic challenge. In the vast majority of cases, sarcoid involvement of the thyroid remains clinically insignificant. In clinically significant thyroid sarcoidosis, abnormalities in respect to gland morphology (i.e., nodules, goiter, thyroiditis) and function (hypo- and hyperthyroidism) may be isolated or combined. In patients with suspected thyroid sarcoidosis, thorough diagnosis and observation are necessary. The diagnosis may only be confirmed if known causes of granulomas and, in particular, sarcoid reactions are excluded. Often, particularly due to the diagnostic difficulties, surgery is indicated.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SKL and JJ drafted the case report and performed the review of the literature. AL obtained the histopathological figures and descriptions. JĐ obtained the radiological figures and descriptions. DSV and DŽ contributed to the revising of the manuscript and language modifying. All authors contributed to the article and approved the submitted version.
We thank the patients who contributed to this report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1046420/full#supplementary-material
ACE, angiotensin-converting enzyme; TSH, thyroid-stimulating hormone; T4, thyroxine; T3, triiodothyronine; fT4, free thyroxine; fT3, free triiodothyronine; CNB, core needle biopsy; FNAc, fine needle aspiration cytology; FNAB, fine needle aspiration biopsy; US, ultrasonography; CT, computed tomography; HRCT, high-resolution computed tomography; LN, lymph nodes; TG-Ab, thyroglobulin antibodies; TPO-Ab, thyroperoxidase; TS-Ab, TSH-receptor antibodies.
1. Kašiković Lečić, S, Lovrenski, A, Đokić, J, Popović, M, Javorac, J, Milenković, A, et al. Does miliary sarcoidosis really exist? A case report and review of the literature. Eur Rev Med Pharmacol Sci. (2022) 26:5098–102. doi: 10.26355/eurrev_202207_29296
2. Kmieć, P, Lewandowska, M, Dubaniewicz, A, Mizan-Gross, K, Antolak, A, Wołyniak, B, et al. Two cases of thyroid sarcoidosis presentation as painful, recurrent goiter in patients with Graves' disease. Arq Bras Endocrinol Metabol. (2012) 56:209–14. doi: 10.1590/s0004-27302012000300010
3. Ungprasert, P, Ryu, JH, and Matteson, EL. Clinical manifestations, diagnosis, and treatment of sarcoidosis. Mayo Clin Proc Innov Qual Outcomes. (2019) 3:358–75. doi: 10.1016/j.mayocpiqo.2019.04.006
4. Quertermous, BP, Kavuri, S, and Walsh, DW. An old disease with a new twist: CNS and thyroid sarcoidosis presenting as subacute dementia. Clin Case Rep. (2021) 9:e04829. doi: 10.1002/ccr3.4829
5. Sève, P, Pacheco, Y, Durupt, F, Jamilloux, Y, Gerfaud-Valentin, M, Isaac, S, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. (2021) 10:766. doi: 10.3390/cells10040766
6. James, WE, Koutroumpakis, E, Saha, B, Nathani, A, Saavedra, L, Yucel, RM, et al. Clinical features of extrapulmonary sarcoidosis without lung involvement. Chest. (2018) 154:349–56. doi: 10.1016/j.chest.2018.02.003
7. Manchanda, A, Patel, S, Jiang, JJ, and Babu, AR. Thyroid: an unusual hideout for sarcoidosis. Endocr Pract. (2013) 19:e40–3. doi: 10.4158/EP12131.CR
8. Okuma, H, Hashimoto, K, Wang, X, Ohkiba, N, Murooka, N, Akizuki, N, et al. Systemic sarcoidosis with thyroid involvement. Intern Med. (2017) 56:2181–6. doi: 10.2169/internalmedicine.8324-16
9. Katsamakas, M, Tzitzili, E, Boudina, M, Kiziridou, A, Valeri, R, Zafeiriou, G, et al. Thyroid sarcoidosis: a rare entity in the differential diagnosis of thyroid cancer. Endocrinol Diabetes Metab Case Rep. (2021) 2021:21–0095. doi: 10.1530/EDM-21-0095
10. Bangolo, A, Bukasa Kakamba, J, Atoot, A, Jurri, M, Mahmoud, A, Lo, AO, et al. A rare case of systemic sarcoidosis in a middle-aged female with a solitary thyroid nodule. Case Rep Endocrinol. (2021) 2021:5231703–4. doi: 10.1155/2021/5231703
11. Hoang, TD, Mai, VQ, Clyde, PW, Glister, BC, and Shakir, MK. Multinodular goiter as the initial presentation of systemic sarcoidosis: limitation of fine-needle biopsy. Respir Care. (2011) 56:1029–32. doi: 10.4187/respcare.01000
12. Gaddam, M, Ojinnaka, U, Ahmed, Z, Kannan, A, Quadir, H, Hakobyan, K, et al. Sarcoidosis: various presentations, coexisting diseases and malignancies. Cureus. (2021) 13:e16967. doi: 10.7759/cureus.16967
13. Yanamandra, U, Kotwal, N, Menon, A, and Nair, V. Resistant thyrotoxicosis: a case of sarcoidosis of thyroid. Indian J Endocrinol Metab. (2013) 17:332–5. doi: 10.4103/2230-8210.109662
14. Fazzi, P, Fallahi, P, and Ferrari, SM. Sarcoidosis and thyroid autoimmunity. Front Endocrinol (Lausanne). (2017) 8:177. doi: 10.3389/fendo.2017.00177
15. Shi, TY, Wen, XH, Shi, XH, Meng, J, and Lu, YW. Associations between sarcoidosis, autoimmune diseases, and autoantibodies: a single-center retrospective study in China. Clin Exp Med. (2022) 22:277–83. doi: 10.1007/s10238-021-00737-5
16. Semiz, H, Yalcin, M, and Kobak, S. Coexistence of sarcoidosis and Hashimoto thyroiditis. Reumatismo. (2018) 70:106–10. doi: 10.4081/reumatismo.2018.1017
17. Alsahwi, N, Blavo, D, and Karanchi, H. Systemic sarcoidosis with hypercalcaemia, hypothalamic-pituitary dysfunction and thyroid involvement. BMJ Case Rep. (2016) 2016:bcr2016216696. doi: 10.1136/bcr-2016-216696
18. Mousapasandi, A, Herbert, C, and Thomas, P. Potential use of biomarkers for the clinical evaluation of sarcoidosis. J Investig Med. (2021) 69:804–13. doi: 10.1136/jim-2020-001659
19. Larici, AR, Glaudemans, AW, Del Ciello, A, Slart, RH, Calandriello, L, and Gheysens, O. Radiological and nuclear medicine imaging of sarcoidosis. Q J Nucl Med Mol Imaging. (2018) 62:14–33. doi: 10.23736/S1824-4785.17.03046-1
20. Tamhane, S, and Gharib, H. Thyroid nodule update on diagnosis and management. Clin Diabetes Endocrinol. (2016) 2:17. doi: 10.1186/s40842-016-0035-7
21. Shin, I, Kim, EK, Moon, HJ, Yoon, JH, Park, VY, Lee, SE, et al. Core-needle biopsy does not show superior diagnostic performance to fine-needle aspiration for diagnosing thyroid nodules. Yonsei Med J. (2020) 61:161–8. doi: 10.3349/ymj.2020.61.2.161
22. Wenter, V, Albert, NL, Ahmaddy, F, Unterrainer, M, Hornung, J, Ilhan, H, et al. The diagnostic challenge of coexistent sarcoidosis and thyroid cancer - a retrospective study. BMC Cancer. (2021) 21:139. doi: 10.1186/s12885-020-07745-w
23. Ozkan, Z, Oncel, M, Kurt, N, Kargi, AB, Ozdemir, N, Kaptanoglu, L, et al. Sarcoidosis presenting as cold thyroid nodules: report of two cases. Surg Today. (2005) 35:770–3. doi: 10.1007/s00595-005-3004-9
24. Trivedi, DP, Bhagat, R, Nakanishi, Y, Wang, A, Moroz, K, and Falk, NK. Granulomatous thyroiditis: a case report and literature review. Ann Clin Lab Sci. (2017) 47:620–4.29066492.
25. Cabibi, D, Di Vita, G, La Spada, E, Tripodo, C, Patti, R, and Montalto, G. Thyroid sarcoidosis as a unique localization. Thyroid. (2006) 16:1175–7. doi: 10.1089/thy.2006.16.1175
26. Baughman, RP, Valeyre, D, Korsten, P, Mathioudakis, AG, Wuyts, WA, Wells, A, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. (2021) 58:2004079. doi: 10.1183/13993003.04079-2020
27. Qiao, X, He, S, Altawil, A, Wang, QY, Kang, J, Li, WY, et al. Multisystemic sarcoidosis presenting with leg ulcers, pancytopenia, and polyserositis was successfully treated with glucocorticoids: a case report and literature review. Front Med (Lausanne). (2022) 8:803852. doi: 10.3389/fmed.2021.803852
28. Papini, E, Guglielmi, R, Bianchini, A, Crescenzi, A, Taccogna, S, Nardi, F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. (2002) 87:1941–6. doi: 10.1210/jcem.87.5.8504
29. Nam-Goong, IS, Kim, HY, Gong, G, Lee, HK, Hong, SJ, Kim, WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol. (2004) 60:21–8. doi: 10.1046/j.1365-2265.2003.01912.x
30. Spencer, J, and Warren, S. Boeck's sarcoid: report of a case with clinical diagnosis confirmed at autopsy. Arch Intern Med. (1938) 62:285–8. doi: 10.1001/archinte.1938.00180130106007
31. Wadhwa, P, and Vaideeswar, P. Hepatic, splenic and thyroidal nodular sarcoidosis. Indian J Pathol Microbiol. (2017) 60:608–9. doi: 10.4103/IJPM.IJPM_242_17
32. Balasanthiran, A, Sandler, B, Amonoo-Kuofi, K, Swamy, R, Kaniyur, S, and Kaplan, F. Sarcoid granulomas in the parathyroid gland – a case of dual pathology: hypercalcaemia due to a parathyroid adenoma and coexistent sarcoidosis with granulomas located within the parathyroid adenoma and thyroid gland. Endocr J. (2010) 57:603–7. doi: 10.1507/endocrj.k10e-028
33. Rodriguez, MC, Rani, D, and Faas, FH. Unusual clinical course of Graves' thyrotoxicosis and concomitant sarcoidosis: case report and review of literature. Endocr Pract. (2007) 13:159–63. doi: 10.4158/EP.13.2.159
34. Bruins, NA, Oswald, JE, Morreau, H, Kievit, J, Pavel, S, and Smelt, AH. Papillary thyroid carcinoma in a patient with sarcoidosis treated with minocycline. Neth J Med. (2007) 65:185–7.17519514.
35. Papi, G, Briganti, F, Artioli, F, Cavazza, A, Carapezzi, C, Roggeri, A, et al. Sarcoidosis of the thyroid gland associated with hyperthyroidism: review of the literature and report of two peculiar cases. J Endocrinol Investig. (2006) 29:834–9. doi: 10.1007/BF03347380
36. Gentilucci, UV, Picardi, A, Manfiini, S, D'Avola, D, Costantino, S, and Pozzilli, P. Granulomatous thyroiditis: an unexpected finding leading to the diagnosis of sarcoidosis. Acta Biomed. (2004) 75:69–73.15315090.
37. Saydam, L, Bozkurt, MK, Kutluay, L, and Ozçelik, T. Sarcoidosis of the thyroid gland initially diagnosed as malignancy. Otolaryngol Head Neck Surg. (2003) 129:154–6. doi: 10.1016/s0194-5998(03)00484-4
38. Yarman, S, Kahraman, H, Tanakol, R, and Kapran, Y. Concomitant association of thyroid sarcoidosis and Graves' disease. Horm Res. (2003) 59:43–6. doi: 10.1159/000067937
Keywords: sarcoidosis, thyroid, prevalence, diagnosis, treatment
Citation: Kašiković Lečić S, Javorac J, Lovrenski A, Đokić J, Sazdanić Velikić D and Živanović D (2023) Case report: Thyroid sarcoidosis as a rare localization of the disease: Report of two cases and review of the literature. Front. Med. 10:1046420. doi: 10.3389/fmed.2023.1046420
Received: 16 September 2022; Accepted: 20 February 2023;
Published: 10 March 2023.
Edited by:
Carmel Stock, Imperial College London, United KingdomReviewed by:
Prasanta Padhan, Kalinga Institute of Medical Sciences (KIMS), IndiaCopyright © 2023 Kašiković Lečić, Javorac, Lovrenski, Đokić, Sazdanić Velikić and Živanović. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jovan Javorac, am92YW4uamF2b3JhY0BtZi51bnMuYWMucnM=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.