- 1Department of Clinical Laboratory, Medical University of Plovdiv, University Hospital “St. George”, Plovdiv, Bulgaria
- 2Research Institute at Medical University of Plovdiv, Plovdiv, Bulgaria
- 3Department of Psychology, University of Plovdiv Paisii Hilendarski, Plovdiv, Bulgaria
- 4Department of Infection Diseases, Parasitology and Tropical Medicine, Medical University of Plovdiv, University Hospital “St. George”, Plovdiv, Bulgaria
Background: During the COVID-19 pandemic, mental health disorders and level of stress show a major increase compared to before the pandemic. Coronavirus-related stress is recently the leading cause of negative impacts on global mental health. Thus, maintaining positive mental health is as important as maintaining physical health during COVID-19. The aim of this study was to analyze salivary mental stress biomarkers as cortisol, alpha-amylase, and chromogranin A in hospitalized patients with COVID-19 to compare their potential relationship with stress symptoms.
Material and methods: A total of 80 adult hospitalized patients with moderate COVID-19 disease and a control group (n = 80) randomly selected were conducted as participants. Saliva cortisol (sCort), saliva alpha-amylase (sAA), and saliva and chromogranin A (sCgA) were determined by the ELISA method (Bio Vendor, USA). Symptoms of stress were measured with a stress symptom checklist (SSCL).
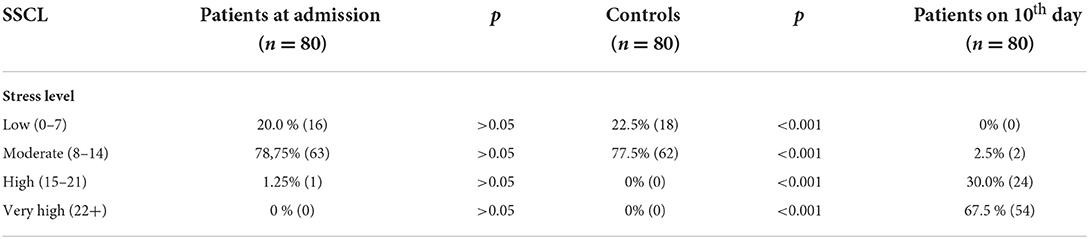
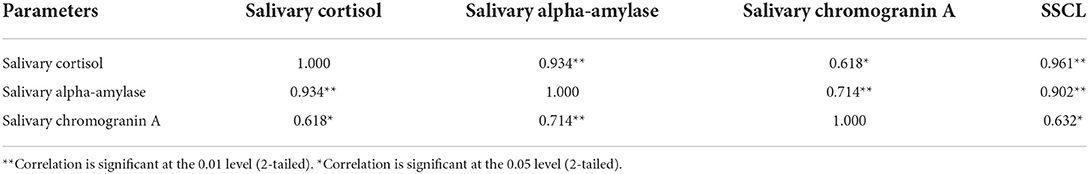
Results: The patients group presented significantly higher levels of sCort, sAA, and sCgA compared with the control group. The correlation analysis showed a positive correlation with strong strength between sCort and sAA (r = 0.934, p < 0.01), as well as sAA with sCgA (r = 0.714, p < 0.01). A moderate positive correlation was found between sCort with sCgA (r = 0.618, p < 0.05). Based on their stress scores from the SSCL the patients were associated with high stress level (30.00%) and very high stress levels (67.5%). In terms of the controls, all the participants showed a low to moderate stress level. We found significant positive correlation between levels of stress and salivary biomarkers.
Conclusion: Data from our study demonstrated that salivary biomarkers are promising tools of exploring COVID-19 related stress.
Introduction
The stress response triggers a series of psychological, immunological, and biochemical responses that directly affect human health and wellbeing. The COVID-19 pandemic contributes considerably to increasing the number of mental health disorders related to anxiety, depression, distress, and aggressive behavior. Studies show significant levels of stress, anxiety, burnout, fear, and frustration during the pandemic, compared with studies before the pandemic (1–6). Meanwhile, COVID-19 may cause neurological and psychiatric disorders such as stroke, dementia, Parkinson's disease, schizophrenia, cognitive disorders, and bipolar disorders (7). Therefore, people suffering from chronic health diseases are more vulnerable to SARS-CoV-2 infection and they are at higher risk for adverse mental health outcomes (8–10).
Stressful life situations have a negative impact on mental and physical health and lead to serious psychological problems. Such changes in daily life caused by COVID-19 have the potential to increase stress levels with a wide range of psychosocial health problems such as various psychodynamic and physiological dysfunctions (11–14) which occur not only to COVID-19 patients, but also to their family members (15). In this regard, the assessment of the level of stress on the psychological health in patients with coronavirus disease is becoming an essential topic of research in developing effective, reliable, and valid tools for stress assessment.
In recent years, researchers concentrated attention on the evaluation of different proteins in saliva secreted by healthy people and patients with various diseases during response to acute mental stress. Such studies have centered on cortisol, alpha-amylase, chromogranin A, and immunoglobulin A as salivary biomarkers of stress, anxiety, or depressive disorders (16–22). These proteins can be analyzed in other biological specimens, but saliva samples can reflect real time levels of biomarkers with a wide range of their concentration during stress-related disease (23).
Among other salivary stress biomarkers, saliva cortisol (sCort) is most frequently used as a “gold standard marker of stress” (24, 25). The concentration of cortisol in saliva is proportional to the plasma level and it can directly indicate the activity of the hypothalamic-pituitary adrenal axis (HPA) (26).
Alpha-amylase and chromogranin A are other biomarkers representative of activation of the sympathetic-adrenal-medullary (SAM) system which are easily detected in saliva (22, 27). Saliva alpha-amylase (sAA), a digestive enzyme produced and excreted from norepinephrine-responsive salivary gland cells (28), is generally used as a biomarker of psychophysiological stress (27, 29). In recent years, sAA activity has emerged as a valid and reliable marker of sympathetic activation in stress research (28–31).
Chromogranin A (CgA) is the transmembrane glycoprotein belonging to the granin family. It is stored and released with catecholamines into the circulation from secretory vesicles of neurons and endocrine cells upon sympathetic stimulation (32). After being discovered, CgA was initially widely accepted as a biomarker for neuro-endocrine tumors with different primary localization (33, 34). Previous research shows salivary CgA (sCgA) is a sensitive and quantitative index of the activity of the SAM like sA-A (35–37). A variety of studies on stress suggest that elevated levels of sCgA are used as markers of psychological stress (16, 24, 27, 28, 37, 38).
Even though many studies have scientifically proven the potential applicability of sCort, sAA, and sCgA as markers of both psychological and physical stress, data about their use as indicators of activation of the HPA/SAM system during stress response in COVID-19 are still limited. Therefore, more specific reliable studies are needed to assess the stress response not only because of the long-term nature of the virus, but also because of the large scale of the chronic stress associated with COVID-19.
The aim of this study was to analyze salivary mental stress biomarkers as cortisol, chromogranin A, and alpha-amylase in hospitalized patients with COVID-19 and to objectively assess the presence of stress levels using a stress symptom checklist (SSCL) test to compare the potential relationship with salivary biomarkers.
Materials and methods
Study design
Our study included 80 adult patients (age ≥ 20 years) with moderate COVID-19 symptoms, who were admitted to our hospital isolation wards between January 2022 and April 2022. The control group of 80 individuals was randomly selected and used as a control to verify the results. All patients with COVID-19 were confirmed by using real-time reverse transcriptase polymerase chain reaction (RT-PCR) assays from oropharyngeal swab specimens. The diagnosis and classification of COVID-19 were based on the Interim Guidance for Clinical Management of COVID-19 issued by the WHO (39). Patients with moderate disease were individuals who showed evidence of lower respiratory disease during clinical assessment or imaging, but no signs of severe pneumonia, including oxygen saturation (SpO2) ≥ 90% on room air at sea level. The exclusion criteria for study were primary axis disorders; psychiatric disorders or using psychotropic drugs—anti-depressants, sedatives, or hypnotics; cardiovascular disease, diabetes, cancer, stroke, metabolic, or endocrinological abnormalities.
The control group was composed of volunteers who gave a negative result for COVID-19 by RT–PCR assay and had not suffered from an infection in the last 6 months. All participants were given a document about the objectives and procedures of the study and informed consent was obtained. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local Institutional Ethical Board.
Instruments
Saliva specimens (SalivetteR, Sarstedt) were taken in the morning between 6 and 8 AM, within the first 24 h of hospital admission, and second salivary swab was collected on the 10th day of hospitalization. The samples were stored at −20°C until the time of the analysis, but for no longer than 2 months according to the manufacturer's instructions. The manners of withdrawal, processing, and storage of saliva samples were to follow the requirements and the recommendations given by the manufacturer to compensate for the factors of result variation and for standardization of the preanalytical stage.
Salivary stress biomarkers (sCort, sAA, sCgA), were determined using a competitive ELISA assay (BioVendor, USA) after it was validated locally. The methods show high precision; the results are consistent with the recommended minimal non-reproducibility (intra-assay CV < 10%; inter-assay CV < 12%) for ELISA, as given by the manufacturer.
Symptoms of stress were measured with a stress symptom checklist (SSCL) (40, 41). The SSCL is a valid instrument to measure the level of stress based on the number of symptoms that have occurred often enough. Therefore, the patients were examined twice to avoid the possible influence of previous stressful life situations. The SSCL consists of 52 items divided into the following two subscales: physical symptoms (27 items) and psychological symptoms (25 items). Mean values were calculated, and subscales were categorized into “low” (0–7 items checked), “moderate” (8–14 items checked), “high” (15–21 items checked) and “very high” (22+) degrees of stress using the cut-off values suggested by Bourne (40). Higher scores mean a higher level of stress. A total score of 10 on the two sub-scales indicates moderate stress for that person.
Statistical analysis
Collected data was analyzed using R software, version 4.2. Continuous variables were expressed as median and interquartile ranges (median ± IQR). Qualitative variables were presented as numbers (n) and percentage (%). Column proportions were compared using a two-tailed z-tests. The Shapiro-Wilk test was used to test the normal distribution of all continuous variables. For not-normal distributed variables, we use non-parametric tests (such as Wilcoxon signed rank—for paired observation and Wilcoxon rank sum test). The SSCL was described through the mean value and standard deviation (SD) and as an ordinal variable including the following stress levels: low (0–7), moderate (8–14), high (15–21) and very high (>22). Statistical inference is considered at the level p < 0.05.
Sample size and power analysis
The results of the minimum sample size −67 patients—were calculated to achieve a power of 80% and a level of significance of 5% (two sided), for detecting a mean of 7 points in SSCL differences between pairs, assuming the standard deviation of the differences to be 20. Assumptions are based on literature results validating the stress symptom checklist (SSCL).
Results
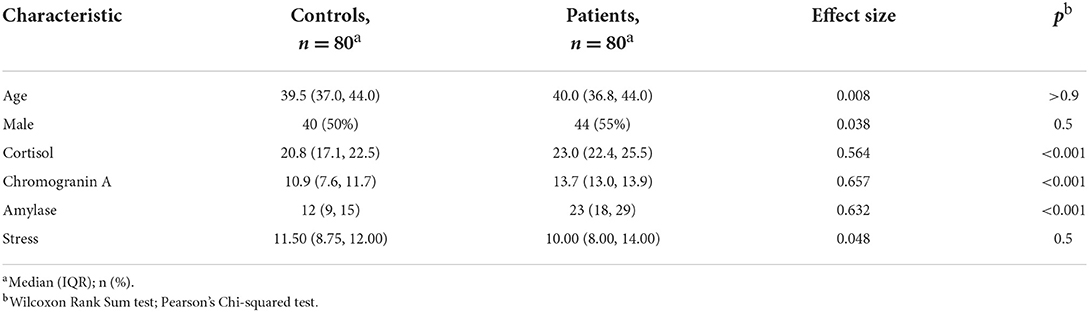
The studied patients group included 44 male (55%) and 36 female (45%) COVID-19 patients. The median age of the patients was 40 years (IQR 36.8–44). The control group consisted of 80 asymptomatic individuals, equally distributed by sex with the median age of 39.5 years (IQR = 37.0, 44.0). No statistical significance between patients' and controls' age, sex, and stress (SSCL) distribution were found. The Wilcoxon Rank Sum Test testing the difference in ranks between patients' and controls' chromogranin A levels, suggests that the effect is positive, statistically significant, and large (W = 768, p < 0.001; r = 0.657). Similar findings also occurred when the test was peformed for amylase (W = 861, p < 0.001; r = 0.632) and cortisol levels (W = 1,116, p < 0.001; r = 0.564) (Table 1).
In our study, we used the SSCL to measure the level of stress based on the number of symptoms that have occurred during hospitalization. Out of 25 psychological symptoms included in the SSCL, anxiety was the most common psychological symptom shared by 92.70% of the patients followed by constant worrying by 78.50%, restlessness 69%, frequent irritability 63.40%, and temper flare-ups 61%. Out of 27 physical symptoms in the SSCL, five showed frequencies over 70%: insomnia was reported by 98.50%, backaches by 79.00%, neck pain and tight muscles by 80.00%, muscle cramps by 78.00% and other pain by 72.00%. The remaining 22 physical symptoms were experienced by <50% of the patients and some of them had very low frequency, including: cold feet and hands (3.7%); diarrhea (3.7%); teeth grinding (1.2%); upset stomach (14.6%); and constipation (8.5%).
Based on their stress scores at admission to the hospital there were not significant differences in levels of stress between patients and healthy controls. According to their stress scores on the 10th day of hospitalization −2.5% of the patients were associated with moderate stress levels, 30.00% with high stress levels and 67.5% with very high stress levels. In terms of the controls, all the participants showed a low to moderate stress level (Table 2).
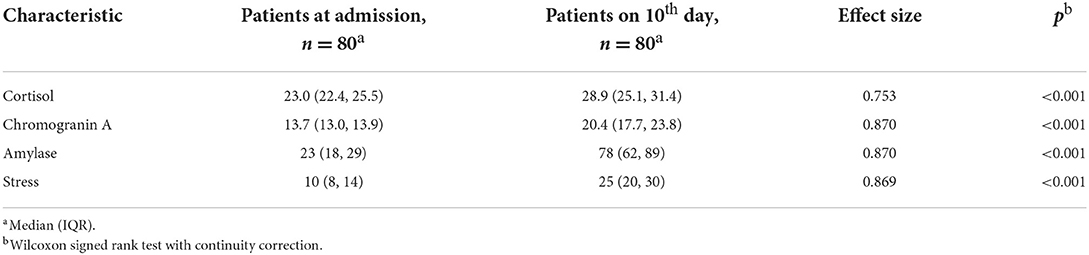
We analyzed the levels of cortisol, chromogranin A and alpha-amylase activity in saliva as potential biomarkers associated with stress during COVID-19. Comparing the patients in the first 24 h at admission and on the 10th day of hospitalization, a statistically significant increase in the levels of the studied substances was found (Table 3). The greatest effect size was found for chromogranin A (0.870, p < 0.001) and amylase (ES = 0.870, p < 0.001). There was also a significant increase in stress levels measured with the SSCL instrument (ES = 0.869, p < 0.001).
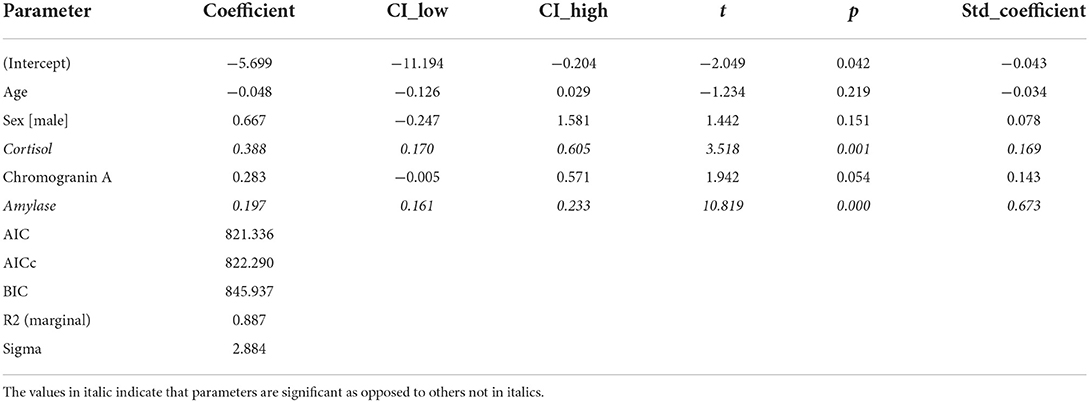
Regarding stress measured by an SSCL, we fitted a linear mixed model (estimated using REML and nloptwrap optimizer) to predict stress with the levels of cortisol, amylase, and chromogranin A. We controlled for sex and age by including them in the model. Standardized parameters were obtained by fitting the model on a standardized version of the dataset, and 95% Confidence Intervals (CIs) and p were computed using a Wald t-distribution approximation. The model included patient ID as a random effect in order to account for repeated design. The model's explanatory power related to the fixed effects alone (marginal R2) is 0.89. Within this model we estimated the effect of cortisol as statistically significant and positive [beta = 0.39, 95% CI (0.17, 0.61), t(152) = 3.52, p < 0.001; Std. beta = 0.17, 95% CI (0.07, 0.26)]. The effect of amylase also was statistically significant and positive [beta = 0.20, 95% CI (0.16, 0.23), t(152) = 10.82, p < 0.001; Std. beta = 0.67, 95% CI (0.55, 0.80)] (Table 4; Figure 1).
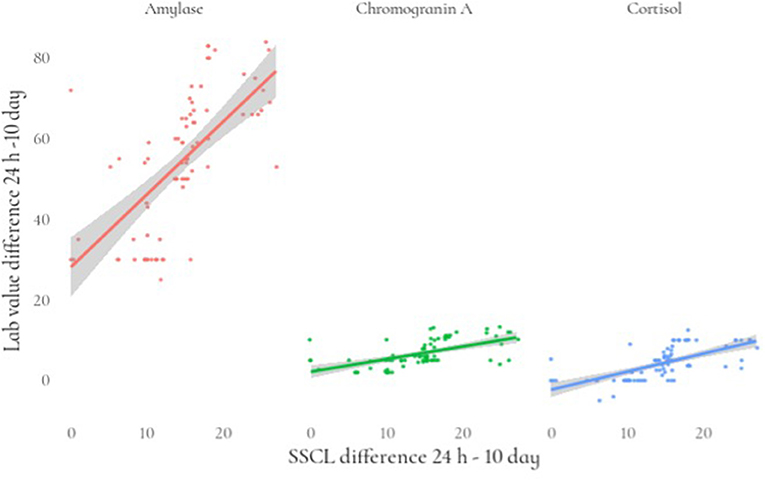
Figure 1. Relationship between the differences (24 h-10 day) in stress levels and lab value of stress biomarkers.
To determine the relationship between salivary biomarkers in COVID-19 patients, correlation analyses were performed with statistically significant differences between the two groups. A positive correlation with statistical significance was observed between sCort, sAA, and sCgA. The correlation analysis between the stress levels in the SSCL instrument and salivary biomarkers (sCort, sAA, sCgA) showed a significant positive correlation with strong strength between stress levels with sCort and sAA (r = 0.961 and r = 0.902, p < 0.01). A significant positive correlation of moderate strength was shown between levels of stress in SSCL and sCgA (r = 0.632, p < 0.05) (Table 5). Established correlations also matched in the control group.
Discussion
The present study on stress and its relationship with COVID-19 infection among hospitalized patients is the first study to examine salivary markers such as sCort, sAA, sCgA together with a psychological stress assessment tool. Our results show that patients with moderate disease have higher levels of salivary biomarkers compared to controls, both at hospital admission and on the 10th day of hospitalization.
Presently salivary cortisol and salivary alpha amylase are scientifically proven as biomarkers of stress and they are used as diagnostic markers not only in acute mental stress, but also in anxiety, depression and PSSD (16, 22, 23, 27). A substantial number of large-scale studies demonstrated that increased level of sCort, sAA, and sCgA indicated the activity of the HPA/SAM system in response to various stress models—psychological, physical, academic, post-traumatic, etc. (17, 28, 38, 42–45). Although sCort and sAA are generally used to indicate activation of the HPA/SAM system, there is more evidence that sCgA is mostly used as a SAM system marker (33, 43, 45).
Despite these studies reporting conflicting responses between sCort, sAA, and sCgA patterns, our results show a significant positive correlation between these three salivary biomarkers, which supports the idea that saliva-based biomarkers could be indicators for dysfunction of the HPA/SAM system during stressor response.
Moreover, in our study to assess potential biomarker variables associated with stress we used an SSCL as a valid, effective instrument measuring non-pathological stress levels in the general population (41). Our results demonstrated that patients have high to very high levels of stress, which are associated with a strong positive correlation with salivary biomarkers. We found no statistical dependence of stress level on age, gender, or diagnosis, despite data reported from global surveys where higher levels of stress were associated with younger age and female gender (9, 46). Rather, these differences may be due not so much to gender and age as to the influence of other environmental factors—socioeconomic, professional and/or family responsibilities, education, and others.
Based on the results of our study, we suggest that the analysis of salivary biomarkers in combination with psychological tools can be useful to achieving a more precise evaluation of the psychophysiological changes and side effects of the stress-induced metabolic abnormalities with increased risk of cardiovascular events, metabolic disease, and neuro-endocrine disease. Therefore, the researchers' attention in the last decade is focused on cardiovascular stress-induced risk produced respectively by the HPA axis and the SAM system. Recent studies propose some evidence that salivary biomarkers were associated with cardiovascular and other metabolic risk factors (47, 48).
Although there is evidence about stress in COVID-19, the extant literature search suffers from studies about the overall biological impact of stress measured by salivary stress biomarkers to examine their association with COVID-19-induced stress. From this perspective, prospective studies and meta-analysis on clinical usefulness of salivary stress biomarkers in COVID-19 should be done to approach key components of the impact of COVID-19 for the mental health.
The results of our study, however, can be interpreted with some limitations. First, it was a single-center, cross-sectional study and therefore it was not possible to analyze the causal relationships between variables. Further, the current study does not take comorbidities into account while estimating salivary stress markers, even though the group of patients was intentionally selected with a moderately severe form, as well as in middle age and history of mental disorders precisely to avoid the influence of comorbidities. Third, we also did not consider the hospital environment influence as an additional source of stress. Moreover, our findings cannot be extrapolated to patients who do not require hospitalization.
Nevertheless, the differences in the levels of salivary biomarkers as well as the correlations found are of high statistical significance compared to the control group and can be used possibly to identify patients with a severe form of disease and comorbidities exposed at risk of chronic stress or psychological problems.
Conclusion
In hospitalized COVID-19 patients, high levels of mental stress biomarkers sCort, sAA, and sCgA were correlated with high levels of stress symptoms and demonstrated to be a promising tool for good indicators of psychological stress in future stress response assessments during disease outcomes. Further data on the clinical usefulness to making treatment decisions is sparse and needs confirmatory studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Medical University of Plovdiv (Protocol N°1/25.01.2022 and date of approval P-314/02/02/2022). The patients/participants provided their written informed consent to participate in this study.
Author contributions
TD, YI, and OB designed and coordinated the field study and performed investigation and data curation. TD and YI conceptualized the experiments and performed software, formal analysis, writing, and review and editing. TD designed and performed laboratory assays. TD and OB project administration and funding acquisition. All authors reviewed and approved the final version of the manuscript.
Funding
This study was supported by Project COV-4/2021—Laboratory monitoring of prognostic biomarkers in hospitalized patients with COVID-19, contract R-2375/02.08.2021, Medical University of Plovdiv and Project COVID-19 HUB—Information, innovations and implementation of integrative research activities, contract KP-06-DK1/6/29.03.2021, National Science Foundation, Ministry of Education and Science.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pierce M, Hope H, Ford T, Hatch S, Hotopf M, John A, et al. Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry. (2020) 7:883–92. doi: 10.1016/S2215-0366(20)30308-4
2. Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. (2020) 3:19686. doi: 10.1001/jamanetworkopen.2020.19686
3. de Vroege L, van den Broek A. Substantial impact of COVID-19 on self-reported mental health of healthcare professionals in the Netherlands. Front Public Health. (2022) 9:796591. doi: 10.3389/fpubh.2021.796591
4. Zakeri MA, Rahiminezhad E, Salehi F, Ganjeh H, Dehghan M. Burnout, Anxiety, stress, and depression among Iranian nurses: before and during the first wave of the COVID-19 pandemic. Front Psychol. (2021) 12:789737. doi: 10.3389/fpsyg.2021.789737
5. McGinty EE, Presskreischer R, Han H, Barry CL. Psychological distress and loneliness reported by US adults in 2018 and April 2020. JAMA. (2020) 324:93–4. doi: 10.1001/jama.2020.9740
6. Breslau J, Finucane ML, Locker AR, Baird MD, Roth EA, Collins RL. A longitudinal study of psychological distress in the United States before and during the COVID-19 pandemic. Prev Med. (2021) 143:106362. doi: 10.1016/j.ypmed.2020.106362
7. LiuL, Ni SY., Yan W, Lu QD, Zhao YM, Xu YY, et al. Mental and neurological disorders and risk of COVID-19 susceptibility, illness severity and mortality: A systematic review, meta-analysis and call for action. EClin Med. (2021) 40:101111. doi: 10.1016/j.eclinm.2021.101111
8. Bueno-Notivol J, Gracia-García P, Olaya B, Lasheras I, López-Antón R, Santabárbara J. Prevalence of depression during the COVID-19 outbreak: a meta-analysis of community-based studies. Int J Clin Health Psychol. (2021) 21:100196. doi: 10.1016/j.ijchp.2020.07.007
9. Kowal M, Coll-Martín T, Ikizer G, Rasmussen J, Eichel K, Studzińska A, et al. Who is the most stressed during the COVID-19 pandemic? Data from 26 countries and areas. Appl Psychol Health Well Being. (2020) 12:946–66. doi: 10.1111/aphw.12234
10. Holman EA, Thompson RR, Garfin DR, Silver RC. The unfolding COVID-19 pandemic: a probability-based, nationally representative study of mental health in the United States. Sci Adv. (2020) 6:eabd5390. doi: 10.1126/sciadv.abd5390
11. Arslan G, Yildirim M, Tanhan A, Bulus M, Allen KA. Coronavirus stress, optimism-pessimism, psychological inflexibility, and psychological health: psychometric properties of the Coronavirus Stress Measure. Int J Mental Health Addict. (2020) 2020:1–17. doi: 10.1007/s11469-020-00337-6
12. Talaee N, Varahram M, Jamaati H, Salimi A, Attarchi M. Stress, and burnout in health care workers during COVID-19 pandemic: Validation of a questionnaire. Z Gesundh Wiss. (2020) 2020:1–6. doi: 10.1007/s10389-020-01313-z
13. Marčinko D, Jakovljević M, Jakšić N, Bjedov S, Mindoljević Drakulić A. The importance of psychodynamic approach during COVID-19 pandemic. Psychiatr Danub. (2020) 32:15–21. doi: 10.24869/psyd.2020.15
14. Vindegaard N, Benros MN. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun. (2020) 890:531–42. doi: 10.1016/j.bbi.2020.05.048
15. Duan L, Shao X, Wang Y, Huang Y, Miao J, Yang X, et al. An investigation of mental health status of children and adolescents in china during the outbreak of COVID-19. J Affect Disord. (2020) 275:112–8. doi: 10.1016/j.jad.2020.06.029
16. Chojnowska S, Ptaszyńska-Sarosiek I, Kepka A, Knaś M, Waszkiewicz N. Salivary biomarkers of stress, anxiety and depression. J Clin Med. (2021) 10:517. doi: 10.3390/jcm10030517
17. Hek K, Direk N, Newson RS, Hofman A, Hoogendijk WJG, Mulder CL, et al. Anxiety disorders and salivary cortisol levels in older adults: a population-based study. Psychoneuroendocrinology. (2013) 38:300–5. doi: 10.1016/j.psyneuen.2012.06.006
18. Engeland CG, Hugo FN, Hilgert JB, Nascimento GG, Celeste RK, Lim HJ, et al. Psychological distress and salivary secretory immunity. Brain Behav Immun. (2016) 52:11–7. doi: 10.1016/j.bbi.2015.08.017
19. Takatsuji K, Sugimoto Y, Ishizaki S, Ozaki Y, Matsuyama E, Yamaguchi Y. The effects of examination stresson salivary cortisol, immunoglobulin A, and chromogranin A in nursing students. Biomed Res. (2008) 29:221–4. doi: 10.2220/biomedres.29.221
20. Koh D, Ng V, Naing L. Alpha amylase as a salivary biomarker of acute stress of venepuncture from periodic medical examinations. Front Public Health. (2014) 2:121. doi: 10.3389/fpubh.2014.00121
21. Jafari A, Pouramir M, Shirzad A, Motallebnejad M, Bijani A, Moudi S, et al. Evaluation of salivary alpha amylase as a biomarker for dental anxiety. Iran J Psychiatr Behav Sci. (2018) 12:9350. doi: 10.5812/ijpbs.9350
22. Obayashi K. Salivary mental stress proteins. ClinChim Acta. (2013) 425:196–201. doi: 10.1016/j.cca.2013.07.028
23. Dhama K, Latheef SK, Dadar M, Samad HA, Munjal A, Khandia R, et al. Biomarkers in stress related diseases/disorders: diagnostic, prognostic, and therapeutic values. Front Mol Biosci. (2019) 6:91. doi: 10.3389/fmolb.2019.0009
24. Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. (2009) 34:163–71. doi: 10.1016/j.psyneuen.2008.10.026
25. Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiol Behav. (2007) 90:43–53. doi: 10.1016/j.physbeh.2006.08.025
26. Bozovic D, Racic M, Ivkovic N. Salivary cortisol levels as a biological marker of stress reaction. Med Arch. (2013) 67:374–7. doi: 10.5455/medarh.2013.67.374-377
27. Ali N, Nater UM. Salivary alpha-amylase as a biomarker of stress in behavioral medicine. Int J BehavMed. (2020) 27:337–42. doi: 10.1007/s12529-019-09843-x
28. Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. (2009) 34:486–96. doi: 10.1016/j.psyneuen.2009.01.014
29. Chatterton RT, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol. (1996) 16:433–48. doi: 10.1111/j.1475-097x.1996.tb00731.x
30. Marques AH, Silverman MN, Sternberg EM. Evaluation of stress systems by applying noninvasive methodologies: measurements of neuroimmune biomarkers in the sweat, heart rate variability and salivary cortisol. Neuroimmunomodulation. (2010) 17:205–8. doi: 10.1159/000258725 PMID:20134204
31. Schumacher S, Kirschbaum C, Fydrich T, Ströhle A. Is salivary alpha-amylase an indicator of autonomic nervous system dysregulations in mental disorders? A review of preliminary findings and the interactions with cortisol. Psychoneuroendocrinology. (2013) 38:729–43. doi: 10.1016/j.psyneuen.2013.02.003
32. Kanno T, Asada N, Yanase H, Iwanaga T, Ozaki T, Nishikawa Y, et al. Salivary secretion of highly concentrated chromogranin a in response to noradrenaline and acetylcholine in isolated and perfused rat submandibular glands. Exp Physiol. (1999) 84:1073–83. doi: 10.1111/j.1469-445X.1999.01907.x
33. Turner AI, Smyth N, Hall SJ, Torres SJ, Hussein M, Jayasinghe SU, et al. Psychological stress reactivity and future health and disease outcomes: a systematic review of prospective evidence. Psychoneuroendocrinology. (2020) 114:104599. doi: 10.1016/j.psyneuen.2020.104599
34. Mahata SK, Corti A. Chromogranin A and its fragments in cardiovascular, immune metabolic, and cancer regulation. Ann N Y Acad Sci. (2019) 1455:34–58. doi: 10.1111/nyas.14249
35. Dimsdale JE, O'Connor DT, Ziegler M, Mills P. Chromogranin A correlates with norepinephrine release rate. Life Sci. (1992) 51:519–25. doi: 10.1016/0024-3205(92)90029-o
36. Nakane H, Asami O, Yamada Y, Harada T, Matsui N, Kanno T, et al. Salivary chromogranin A as an index of psychosomatic stress response. Biomed Res. (1998) 19:401–6. doi: 10.2220/biomedres.19.401
37. Valdiglesias V, Maseda A, Lorenzo-López L, Pásaro E, Millán-Calenti JC, Laffon B. Is salivary chromogranin A a valid psychological stress biomarker during sensory stimulation in people with advanced dementia? J Alzheimers Dis. (2017) 55:1509–17. doi: 10.3233/JAD-160893
38. Diaz MM, Bocanegra OL, Teixeira RR, Soares SS, Espindola FS. Response of salivary markers of autonomic activity to elite competition. Int J Sports Med. (2012) 33:763–8. doi: 10.1055/s-0032-1304638
39. World Health Organization. Living Guidance for Clinical Management of COVID-19. (2021). Available online at: https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf (accessed on July 17, 2022).
40. Bourne EJ. The Anxiety and Phobia Workbook, 3rd edition. Oakland, CA: New Harbinger Publications (2000).
41. Schlebusch L. The development of a stress symptom checklist. S Afr J Psychol. (2004) 34:327–49. doi: 10.1177/008124630403400301
42. Yamaguchi M, Hanawa N, Yoshida H. Evaluation of a novel monitor for the sympathetic nervous system using salivary amylase activity. Trans Jpn Soc Med Biol Eng. (2007) 45:161–8.
43. Chennaoui M, Bougard C, Drogou C, Langrume C, Miller C, Gomez-Merino D, et al. Stress biomarkers, mood states, sleep during a major competition: “success” and “failure” athlete's profile of high-level swimmers. Front Physiol. (2016) 7:e94. doi: 10.3389/fphys.2016.00094
44. Yonekura T, Takeda K, Shetty V, Yamaguchi M. Relationship between salivary cortisol and depression in adolescent survivors of a major natural disaster. J Physiol Sci. (2014) 64:261–7. doi: 10.1007/s12576-014-0315-x
45. Tammayan M, Jantaratnotai N, Pachimsawat P. Differential responses of salivary cortisol, amylase, and chromogranin A to academic stress. PLoS ONE. (2021) 16:e025672. doi: 10.1371/journal.pone.0256172
46. Tharp DT, Parks-Stamm EJ, Kitces M, Lurtz M. Gender differences in COVID-19-related stress and relationships with life satisfaction among financial advisors. Financ Plan Rev. (2021) 4:e1129. doi: 10.1002/cfp2.1129
47. Kogawa EM, Grisi DC, Falcão DP, Amorim IA, Rezende TM, da Silva IC, et al. Impact of glycemic control on oral health status in type 2 diabetes individuals and its association with salivary and plasma levels of chromogranin A. Arch Oral Biol. (2016) 62:10–9. doi: 10.1016/j.archoralbio.2015.11.005
Keywords: COVID-19, mental stress, salivary biomarkers, saliva cortisol, saliva alpha-amylase, saliva and chromogranin A
Citation: Deneva T, Ianakiev Y and Boykinova O (2022) Salivary mental stress biomarkers in COVID-19 patients. Front. Med. 9:999215. doi: 10.3389/fmed.2022.999215
Received: 22 July 2022; Accepted: 09 September 2022;
Published: 02 November 2022.
Edited by:
Maria Contaldo, University of Campania L. Vanvitelli, ItalyReviewed by:
Mustafa Amin, Universitas Sumatera Utara, IndonesiaRafał Gerymski, University of Opole, Poland
Copyright © 2022 Deneva, Ianakiev and Boykinova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tanya Deneva, dGFueWEuZGVuZXZhQG11LXBsb3ZkaXYuYmc=
 Tanya Deneva
Tanya Deneva Youri Ianakiev3
Youri Ianakiev3