- 1Division of Nephrology, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan
- 2School of Medicine, Tzu Chi University, Hualien, Taiwan
- 3Department of Physiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 4TMU Research Center of Urology and Kidney, Taipei Medical University, Taipei, Taiwan
- 5Division of Nephrology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 6Division of Nephrology, Department of Internal Medicine, Hsin Kuo Min Hospital, Taipei Medical University, Taoyuan City, Taiwan
- 7Division of Nephrology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
- 8Health Data Analytics and Statistics Center, Office of Data Science, Taipei Medical University, Taipei, Taiwan
- 9Institute of Health and Welfare Policy, College of Medicince, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 10Graduate Institute of Data Science, College of Management, Taipei Medical University, Taipei, Taiwan
- 11Division of Nephrology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan
Whether valacyclovir-associated neurotoxicity (VAN) occurs more frequently in patients with end-stage renal disease (ESRD) on dialysis is unknown. This is the first population-based study to examine the risk of VAN associated with ESRD patients on dialysis. Among 2,284,800 patients diagnosed as having herpes zoster from 2002 to 2016, patients with ESRD on dialysis and individuals with normal renal function were enrolled in this study. Following propensity score matching, we compared the risk of altered mental status between valacyclovir users and non-users in the ESRD and normal renal function cohorts over a 30-day follow-up period. In the ESRD cohort, the incidence of altered mental status was 1.68 and 0.52 per 1,000 person-day in valacyclovir users and non-users, respectively, with an adjusted hazard ratio (HR) of 3.22 (95% confidence interval [CI]: 2.04–4.99, P < 0.001). The incidence of altered mental status of valacyclovir users on hemodialysis (HD) and peritoneal dialysis (PD) was higher than that of non-users. The adjusted HR was 3.20 (95% CI: 1.98–5.15, P < 0.001) for those on HD and 3.44 (95% CI: 1.13–10.49, P = 0.030) for those with PD. However, altered mental status was not observed in patients on HD receiving ≤500 mg of valacyclovir three times per week or in those on PD receiving ≤500 mg of valacyclovir per day. The findings demonstrate that adjusting the valacyclovir dosage and monitoring VAN in patients with HD and PD who have herpes zoster is crucial.
Introduction
Varicella-zoster virus (VZV) causes a primary infection known as chickenpox or varicella and remains dormant in peripheral neurons. VZV is reactivated in the host at an older age or when their body is in an immunosuppressed state. VZV reactivation causes herpes zoster, which is characterized by painful and cutaneous eruptions with a dermatomal distribution. One study indicated that patients with renal failure who may be immunosuppressed had a higher risk of herpes zoster than did the general population (1).
Herpes zoster is usually treated with antiviral therapy and analgesics. Indications of antiviral treatment include older age, severe pain or rash, face or eye involvement, other complications of herpes zoster, or a weakened immune system (2). Antiviral agents, including acyclovir, valacyclovir, and famciclovir are used in the treatment of acute herpes zoster infection. Valacyclovir is preferred in clinical practice because it entails less frequent dosing compared with acyclovir and lower costs compared with famciclovir (2). In one study, valacyclovir was confirmed to alleviate the pain related to herpes zoster; moreover, it had a similar safety profile to that of acyclovir (3).
Because valacyclovir is excreted through the kidneys, patients with impaired renal function might be the most susceptible to valacyclovir-associated neurotoxicity (VAN). Several case reports have documented the occurrence of VAN in patients with end-stage renal disease (ESRD) who are on dialysis, including hemodialysis (HD) and peritoneal dialysis (PD) (4–11). To the best of our knowledge, the risk of VAN in a population-based study, including the general population and patients on dialysis, has rarely been investigated. Therefore, we estimated herein the incidence of significant neurotoxicity (altered mental status) after valacyclovir use among patients with ESRD on HD and PD and among individuals with normal renal function. Moreover, we examined the association between the risk of VAN and valacyclovir use in both cohorts.
Materials and methods
Data source
Data were retrieved from the National Health Insurance (NHI) Research Database (NHIRD), Taiwan’s population-based medical claims database. Established in 1995, the NHI program covers more than 99% of the Taiwanese population. The NHIRD contains medical information such as inpatient and outpatient visits, disease diagnosis, and medication prescriptions. One of the largest medical databases worldwide, it has been used in numerous observational studies (12–19). Data from 2000 to 2016 were analyzed in this study. In the NHIRD, diagnoses before 2015 are based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and those made after 2016 are based on the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Data on prescribed medications are classified according to the Anatomical Therapeutic Chemical classification system. The accuracy of the diagnoses and treatment rationale is routinely reviewed by the NHI Administration. All personally identifiable information was encrypted before the data were released. Therefore, the Institutional Review Board (IRB) of the Taipei Tzu Chi Hospital (IRB number: 03-W02-091) waived the need for informed consent.
Study design and cohort
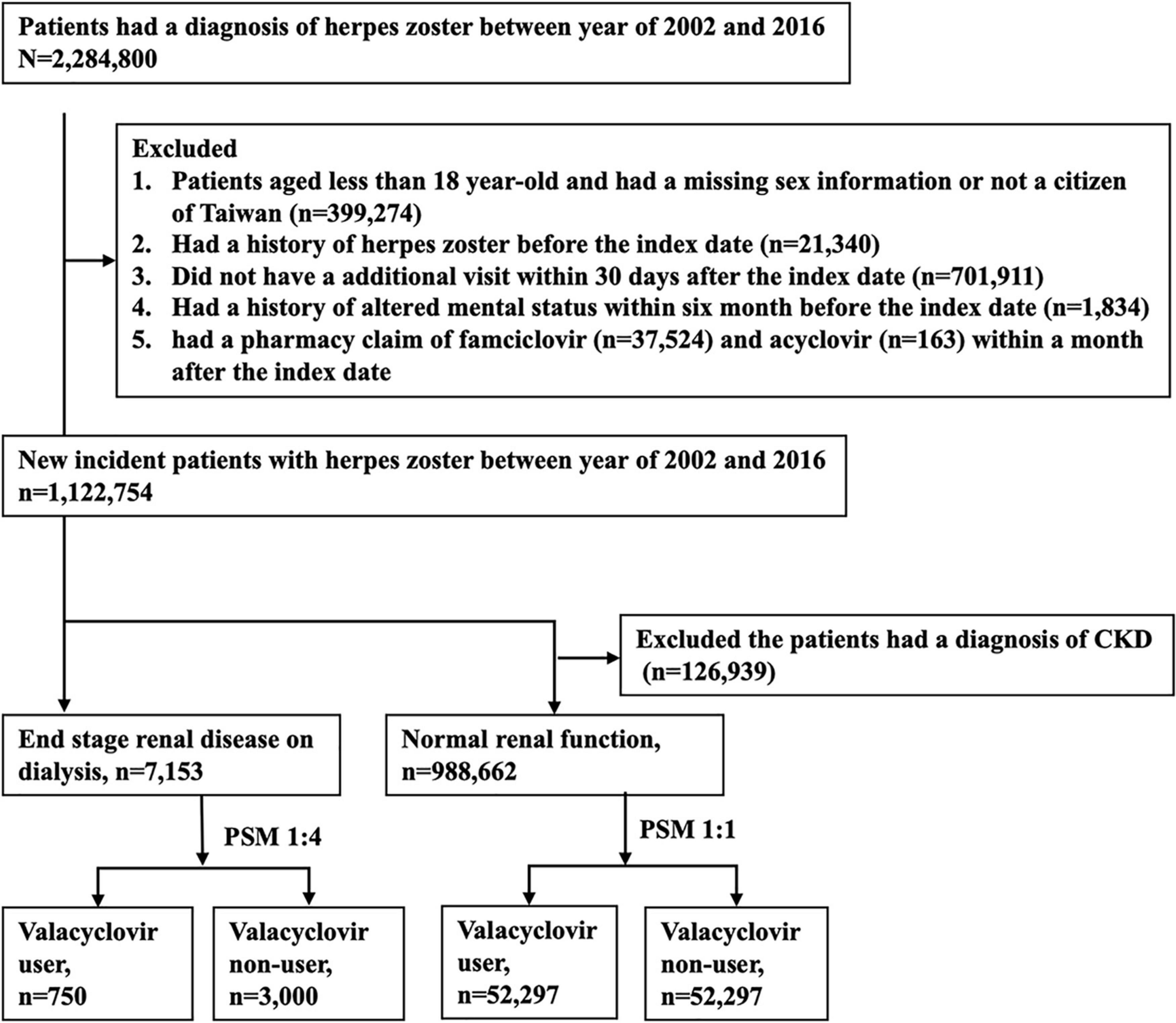
This study adopted a population-based retrospective design. Figure 1 displays the participant selection process. Patients with a diagnostic claim for herpes zoster (ICD-9-CM: 053.x or ICD-10-CM: B02) between 2002 and 2016 were first included, and the date of the first diagnosis was defined as the index date. We excluded patients who (1) were aged younger than 18 years, (2) had missing sex information or were not Taiwanese citizens, (3) had a history of herpes zoster before the index date, (4) did not make follow-up medical visits in the 30-day period following the index date, or (5) had a history of altered mental status in the 6-month period before the index date. In addition, patients prescribed famciclovir or acyclovir in the 30-day period following the index date were excluded. According to NHI reimbursement regulations, indications of valacyclovir for herpes zoster in Taiwan include face, eye, or genital involvement and an immunocompromised status.
Patients who had continuously received dialysis for more than 3 months before the index date were classified into the ESRD cohort. Individuals with no diagnosis of chronic kidney disease (CKD) before the index date were classified into the normal renal function cohort. We adopted the propensity score matching (PSM) approach to select two groups of patients with similar baseline characteristics but different valacyclovir use status in each cohort. PSM is commonly applied in epidemiologic observational studies to minimize sample selection bias (20–22). Valacyclovir users and non-users were matched at a ratio of 1:4 in the ESRD cohort and at a ratio of 1:1 in the normal renal function cohort. A propensity score was estimated on the basis of variables associated with altered mental status, including age; sex; and a history of diabetic mellitus, coronary artery disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, liver cirrhosis, or cancer (Table 1). We also used the Charlson comorbidity index (CCI) as a proxy to adjust for the severity of previous or coexisting conditions. The diagnostic codes are listed in Supplementary Table 1.
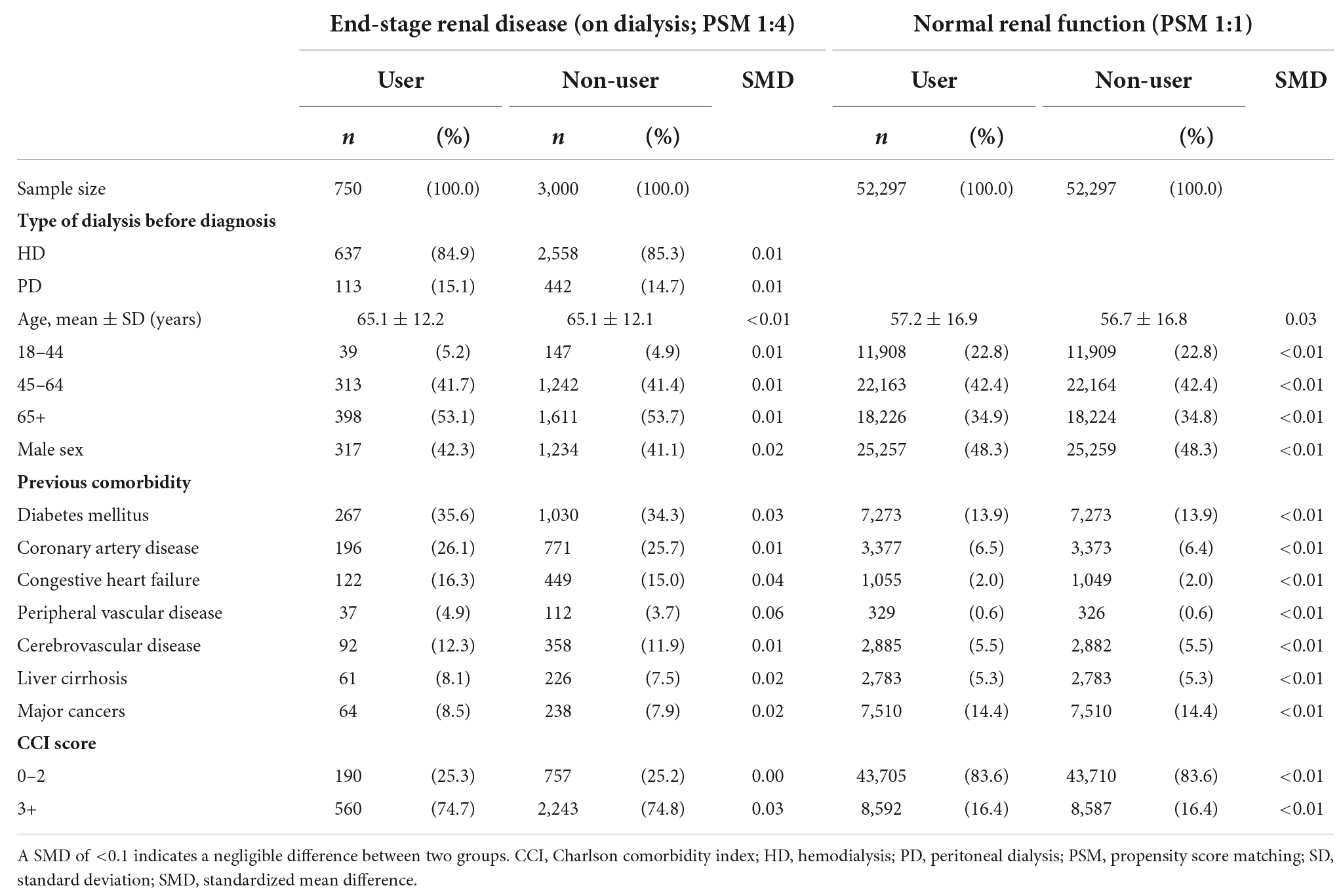
Table 1. Baseline characteristics of patients with herpes zoster receiving or not receiving valacyclovir after propensity score matching.
Study outcome
The main study outcome was a diagnostic claim for altered mental status in the 30-day period after the index date. We applied a 6-month washout period to ensure that altered mental status constituted a new episode. Altered mental status was diagnosed according to ICD-9-CM codes (Supplementary Table 1), which were used in a previous study (23).
Recommended dosage of valacyclovir in patients with hemodialysis and peritoneal dialysis
Because valacyclovir is excreted through the kidneys, patients with ESRD on dialysis, including HD and PD, might be vulnerable to adverse effects. In patients with normal renal function, the recommended dosage is 1,000 mg of valacyclovir by mouth three times daily for 7 days. One case review indicated that the dosage was reduced to 500 mg/day for patients with HD. However, three patients on HD treated with this reduced dosage developed VAN (4). Furukubo et al. proposed the administration of 500 mg of valacyclovir after every HD session for patients with herpes zoster; this is equivalent to a dosage of 1,500 mg per week (4). Therefore, we evaluated the risk, according to the following dosage categories in patients on HD: overdose (>500 mg/day), recommended dosage A (>1,500 mg/week and ≤500 mg/day), and recommended dosage B (500 mg three times/week). Moreover, we evaluated the risk according to the following dosage categories in patients on PD according to a previous report: overdose (>500 mg/day) and recommended dosage (≤500 mg/day) (10).
Statistical analysis
Potential confounders at baseline were compared between valacyclovir users and non-users by using the standardized mean difference (SMD) in both cohorts. A SMD of ≤0.1 denotes a negligible imbalance in the potential confounders between the two study groups (21). We employed the Cox proportional hazard model to estimate the risk of the study outcome. Thus, we present the Kaplan–Meier curve and the hazard ratio (HR) for the risk of altered mental status in valacyclovir users compared with non-users. All analyses were performed using SAS/STAT software, Version 9.4 of the SAS System for Unix (SAS Institute Inc., Cary, NC, USA) and STATA 15 software (StataCorp LP, College Station, TX, USA). A two-sided P-value of <0.05 was considered significant.
Results
Patient characteristics
Among the 2,284,800 patients who had received a diagnosis of herpes zoster between 2002 and 2016, 1,122,754 met the eligibility criteria. The final sample comprised 7,153 patients with ESRD on dialysis and 998,662 patients with normal renal function. Following PSM, we enrolled 750 valacyclovir users and 3,000 valacyclovir non-users in the ESRD cohort and 52,297 valacyclovir users and 52,297 valacyclovir non-users in the normal renal function cohort (Figure 1). Baseline characteristics of the patients are presented in Table 1. In the ESRD cohort, approximately 85% of patients on dialysis were in HD. Compared with patients with the normal renal function cohort, the ESRD cohort had a higher mean age, lower percentage of male patients, and higher comorbidity and CCI scores, except for cancer.
Valacyclovir-associated neurotoxicity risk
A Kaplan–Meier failure curve demonstrated that valacyclovir users in the ESRD cohort had a higher risk of developing an altered mental status within 30 days after receiving a diagnosis of herpes zoster than did valacyclovir non-users in the same cohort (Figure 2A). However, no significant difference in the risk of altered mental status was observed between valacyclovir users and non-users in the normal renal function cohort (Figure 2B).
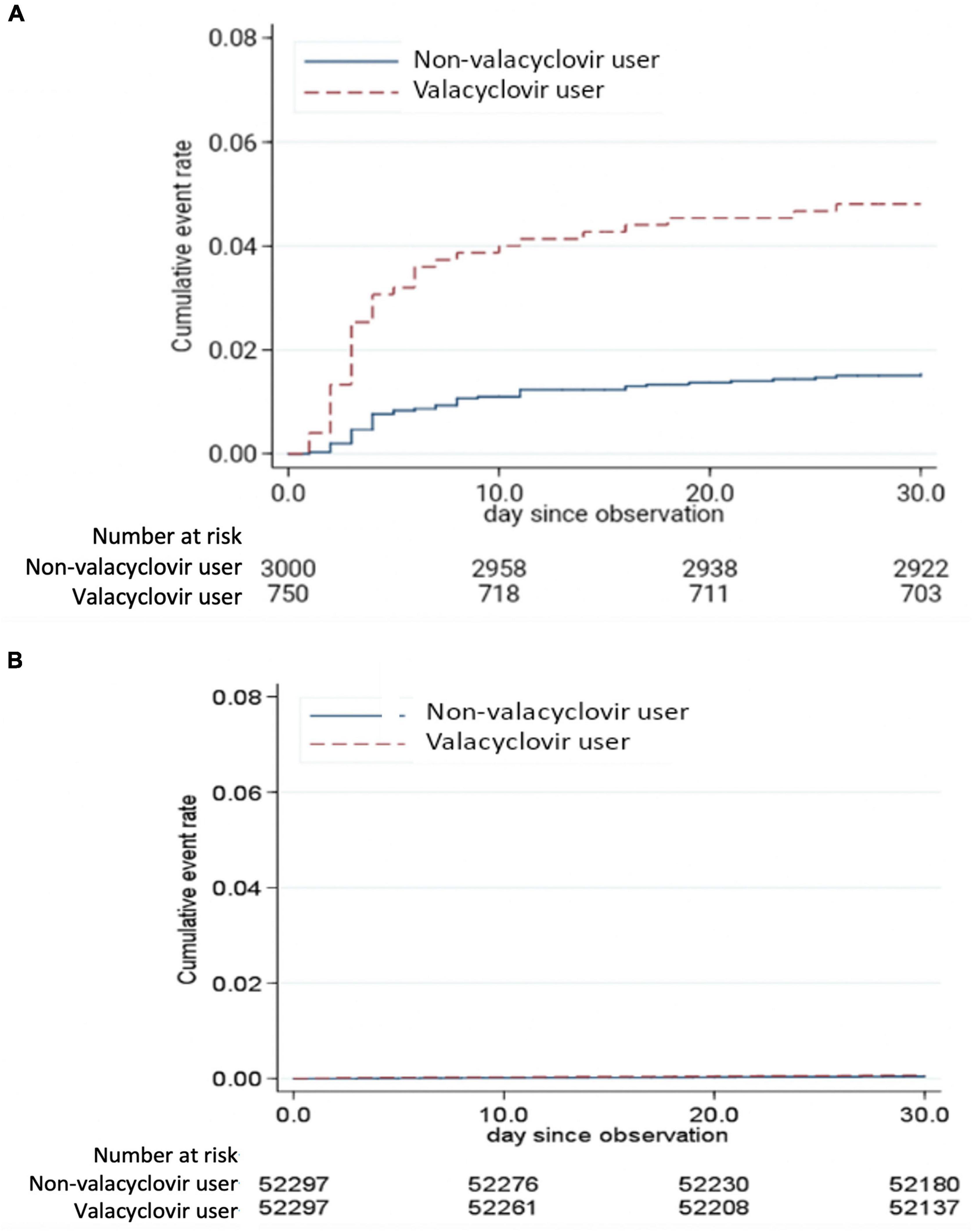
Figure 2. Kaplan–Meier failure curve of altered mental status in the end-stage renal disease cohort (A) and normal renal function cohort (B) over a 30-day follow-up period.
The incidence (per 1,000 person-day) and the risk of altered mental status in patients who did or did not use valacyclovir within the 30-day follow-up period are presented in Table 2. The incidence of altered mental status in users and non-users in the ESRD cohort was 1.68 and 0.52 per 1,000 person-days, respectively (adjusted HR, 3.22; 95% confidence interval [CI]: 2.08–4.99, P < 0.001). These results were consistent between the patients with HD and those with PD. The incidence of altered mental status in users and non-users among patients on HD was 1.64 and 0.52 per 1,000 person-days, respectively (adjusted HR, 3.20; 95% CI: 1.98–5.15, P < 0.001). The incidence of altered mental status in users and non-users among the patients with PD was 1.85 and 0.53 per 1,000 person-days, respectively (adjusted HR, 3.44; 95% CI: 1.13–10.49, P = 0.030). The incidence of altered mental status was extremely low among patients with normal renal function, with no association between valacyclovir use and altered mental status.
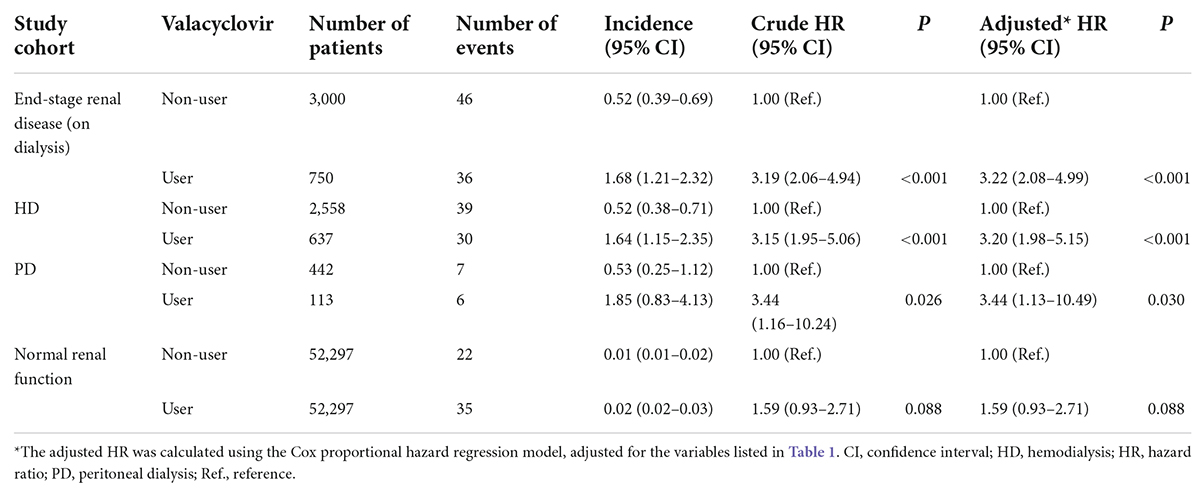
Table 2. Incidence (per 1,000 person-days) and risk of altered mental status among valacyclovir users and non-users over a 30-day period.
Subgroup analysis
The results of the subgroup analysis of patients in the ESRD cohort who were prescribed valacyclovir medication are shown in Table 3. Among the patients on HD, 60.0% were given an overdose of valacyclovir; among those with PD, 54.9% did. Thus, most cases of VAN involved a significantly higher dosage of valacyclovir than recommended. In patients on HD, the incidence of altered mental status was 1.84 and 1.51 per 1,000 person-days in those who were given an overdose (>500 mg/day) and those who were given the recommended dosage A (>1,500 mg/week and ≤500 mg/day), respectively. However, mental status was not altered in patients on HD who were given the recommended dosage B (500 mg three times/week). In patients with PD, the incidence of altered mental status was 3.51 per 1,000 person-days in those who were given an overdose (>500 mg/day). However, mental status was not altered in patients with PD who were given the recommended dosage (≤500 mg/day).
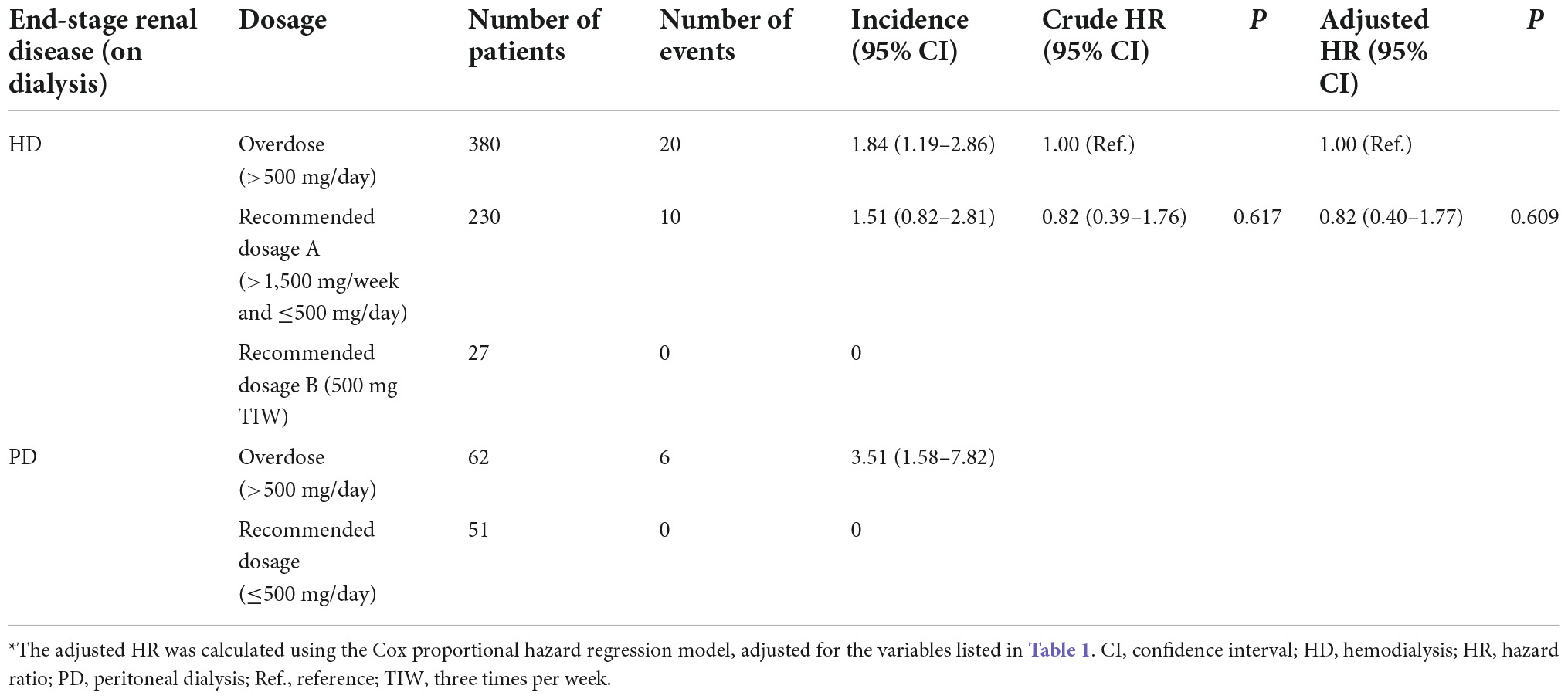
Table 3. Incidence (per 1,000 person-days) and risk of altered mental status among patients with end-stage renal disease on dialysis receiving different dosages of valacyclovir over a 30-day period.
Discussion
This is the first nationwide population-based study to demonstrate that valacyclovir might increase the risk of altered mental status in patients on HD and PD. More than 50% of patients on HD or PD were given an overdose of valacyclovir. Additionally, most cases of VAN occurred in patients on dialysis being given a significantly higher dosage of valacyclovir than that recommended.
Valacyclovir is an antiviral medication used for treating herpes zoster and herpes simplex infections (24). In multiple case reports, most cases of VAN occurred in patients with impaired renal function (4, 8–11, 25). Orally administered valacyclovir is a prodrug that is converted to acyclovir in the intestine and liver. The bioavailability of valacyclovir per intravenous infusion is 54% vs. approximately 20% per oral dose of acyclovir (26). In patients with normal renal function, valacyclovir is excreted in the urine after 2.5 to 3.3 h, mainly as acyclovir (89%). Neurotoxicity is generally seen with serum acyclovir >3.38 μg/ml although some individuals might be susceptible at lower levels. In a previous study (27) of chronic renal failure patients who receiving 2.5 mg/kg acyclovir as a 1-h infusion, the mean peak acyclovir plasma level at the end of infusion was 8.4 ± 5.4 μg/ml and the level dropped to 0.7 ± 0.3 μg/ml at approximately 48 h. In ESRD patients (28), a large amount of acyclovir is metabolized to 9-carboxymethoxymethylguanine (CMMG), likely through the action of alcohol dehydrogenase and aldehyde dehydrogenase. Hellden et al. (29) showed that serum concentrations of CMMG are consistently increased with VAN in ESRD patients.
Patients who develop VAN might present diverse symptoms, such as confusion, consciousness change, ataxia, or dysarthria. A review article noted that symptoms of disturbance of consciousness (85.0%) and hallucination (45.0%) have the highest incidence in this regard (4). These symptoms of neurotoxicity usually appear within the first 24 to 72 h of treatment. The pathogenesis of VAN is not well established. VAN may be associated with acyclovir primary metabolite, CMMG. In one study, CMMG was detected only in the cerebrospinal fluid (CSF) of patients with VAN (28). CMMG in the CSF might lead to cellular dysfunction in the brain and subsequent neurotoxicity. VAN is a clinical diagnosis and is usually challenging to distinguish from acute encephalitis associated with herpes zoster. If a CSF study confirms encephalitis, patients receive intravenous acyclovir standard treatment.
Reversing VAN requires the discontinuation of the drug involved and emergent HD; in one study, serum valacyclovir levels decreased from 7.4 mcg/mL (average 2–4 mcg/L) to 1.6 mcg/mL after 4 h of HD, thus alleviating VAN. After secondary HD, the serum valacyclovir level further decreased to 0.83 mcg/L without a subsequent rebound (6). Studies have reported that PD could not accelerate recovery from neurotoxicity, indicating that the clearance of valacyclovir through PD was insufficient. However, a recent report observed considerable neurological improvement within a day of intense dialyzate exchanges through PD (9). In this report, when the CAPD dose was increased from four to six exchanges (five exchanges of 2 L of 2.5% dextrose plus one exchange of 2 L of icodextrin), hallucinations were alleviated after 24 h (9). Based on these findings, the intensification of PD therapy could be considered for patients with mild VAN.
For the treatment of herpes zoster in adults, 1,000 mg of valacyclovir three times a day for 7 days is suggested. However, clinicians recommend that this dosage be reduced to 500 mg/day for patients with HD. Patients on valacyclovir might exhibit serum drug accumulation between dialysis sessions. Therefore, Furukubo et al. (4) proposed the administration of 500 mg of valacyclovir after every HD session. In our study, altered mental status was observed in some patients given the recommended dosage A (>1,500 mg/week and ≤500 mg/day). However, mental status was not altered in patients on HD who were given the recommended dosage B 500 mg three times per week. These results suggest that this dosage is suitable for reducing the risk of VAN in patients with HD.
Although PD has a lower valacyclovir clearance rate than HD, PD can enable continuous drug excretion, thereby reducing drug toxicity. Case reports noted that most cases of VAN occurred in patients with renal failure who were given an overdose of valacyclovir (4). In the present study, all VAN cases were reported in patients with PD who were given an overdose of valacyclovir. Therefore, we recommended a dosage of ≤500 mg of valacyclovir per day to lower the risk of VAN in patients on PD.
The risk of valacyclovir overdose–induced VAN in patients with ESRD is preventable through software support systems. For example, an electronic support system can be used to send warnings against overdose to physicians prescribing valacyclovir. Moreover, herpes zoster vaccination could lower the occurrence of herpes zoster in patients with ESRD (30) and also provide protection against VAN.
The current study has several limitations and reimbursement claims data; no laboratory data were collected or examined to classify the patients according to renal function. We employed several strategies to improve the accuracy in cohort grouping; however, we were unable to determine the risk of VAN in patients with mild-to-moderate CKD. Moreover, the accuracy of diagnostic coding might have affected the results. We confirmed the diagnosis of a herpes zoster infection if the patients had at least two diagnostic claims, and we applied a 6-month washout period to ensure that altered mental status was a new episode. Furthermore, because only the data of Taiwanese patients were used, the results might not be generalizable to other populations.
Conclusion
Our study found that the risk of VAN in patients with HD and PD is significantly higher than that in patients with normal renal function, but this problem is frequently overlooked. Adjusting the valacyclovir dosage according to the recommended value and monitoring adverse effects are crucial for preventing VAN in patients with ESRD who are on dialysis and have herpes zoster.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All personally identifiable information was encrypted before the data were released. Therefore, the Institutional Review Board (IRB) of the Taipei Tzu Chi Hospital (IRB number: 03-W02-091) waived the need for informed consent. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Y-CW, S-HJ, C-HL, L-NC, and T-CF designed the study. Y-CW, C-LC, L-YC, L-NC, and T-CF conducted the study. Y-CW, L-NC, and T-CF analyzed the data, performed the statistical analysis, and wrote and edited the manuscript. T-CF takes primary responsibility for the final content of the manuscript. All authors contributed to conceived the study, have read and approved the final manuscript.
Funding
This work was supported by Taipei Medical University Hospital, Taipei Medical University (108TMU-TMUH-12 and 110TMU-TMUH-17) and Taipei Medical University (DP2-111-21121-01-O-07-02). The sponsor had no role in the study design, data collection and analysis, publication decision, or manuscript preparation.
Acknowledgments
We thank the Bureau of Health Promotion, Department of Health, and National Health Research Institutes of Taiwan for providing access to the NHIRD. The interpretations and conclusions contained herein do not represent those of these entities. We appreciate the assistance of the staff members at the Health and Clinical Data Research Center of the College of Public Health, Taipei Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.997379/full#supplementary-material
References
1. Kuo CC, Lee CT, Lee IM, Ho SC, Yang CY. Risk of herpes zoster in patients treated with long-term hemodialysis: a matched cohort study. Am J Kidney Dis. (2012). 59:428–33. doi: 10.1053/j.ajkd.2011.10.049
2. Cohen JI. Clinical practice: herpes zoster. N Engl J Med. (2013) 369:255–63. doi: 10.1056/NEJMcp1302674
3. Schuster AK, Harder BC, Schlichtenbrede FC, Jarczok MN, Tesarz J. Valacyclovir versus acyclovir for the treatment of herpes zoster ophthalmicus in immunocompetent patients. Cochrane Database Syst Rev. (2016) 11:CD011503. doi: 10.1002/14651858.CD011503.pub2
4. Asahi T, Tsutsui M, Wakasugi M, Tange D, Takahashi C, Tokui K, et al. Valacyclovir neurotoxicity: clinical experience and review of the literature. Eur J Neurol. (2009) 16:457–60. doi: 10.1111/j.1468-1331.2008.02527.x
5. Izzedine H, Mercadal L, Aymard G, Launay-Vacher V, Martinez V, Issad B, et al. Neurotoxicity of valacyclovir in peritoneal dialysis: a pharmacokinetic study. Am J Nephrol. (2001) 21:162–4. doi: 10.1159/000046241
6. Kambhampati G, Pakkivenkata U, Kazory A. Valacyclovir neurotoxicity can be effectively managed by hemodialysis. Eur J Neurol. (2011) 18:e33. doi: 10.1111/j.1468-1331.2010.03250.x
7. Linssen-Schuurmans CD, van Kan EJ, Feith GW, Uges DR. Neurotoxicity caused by valacyclovir in a patient on hemodialysis. Ther Drug Monit. (1998) 20:385–6. doi: 10.1097/00007691-199808000-00005
8. Okada T, Nakao T, Matsumoto H, Nagaoka Y, Iwasawa H, Nanri K, et al. Valacyclovir neurotoxicity in a patient with end-stage renal disease treated with continuous ambulatory peritoneal dialysis. Clin Nephrol. (2002) 58:168–70. doi: 10.5414/cnp58168
9. Pipili C, Pantelias K, Deda E, Tsiamalos P, Kostis E, Grapsa E. Intensification of peritoneal dialysis improves valacyclovir neurotoxicity. Ren Fail. (2013) 35:289–90. doi: 10.3109/0886022X.2012.743914
10. Prasad B, McIsaac M, Toppings J. Valacyclovir-associated neurotoxicity treated with intensification of peritoneal dialysis. BMJ Case Rep. (2017) 2017:bcr2017220678. doi: 10.1136/bcr-2017-220678
11. Singh NP, Shah HR, Aggarwal N, Jha LK, Behura S. Valacyclovir associated neurotoxicity in a patient on dialysis. Indian J Nephrol. (2014) 24:128–9. doi: 10.4103/0971-4065.127915
12. Chien LN, Chou CL, Chen HH, Kao CC, Lin YC, Wu YL, et al. Association between stroke risk and metformin use in hemodialysis patients with diabetes mellitus: a nested case-control study. J Am Heart Assoc. (2017) 6:e007611. doi: 10.1161/JAHA.117.007611
13. Chou CL, Hsieh TC, Chen JS, Fang TC. Sudden sensorineural hearing loss in hemodialysis patients could be a marker of pathogenic progression in the mortality and atherosclerotic events: a national cohort study. Otol Neurotol. (2018) 39:1241–9. doi: 10.1097/MAO.0000000000001967
14. Chou CL, Hsieh TC, Chen JS, Fang TC. Risks of all-cause mortality and major kidney events in patients with new-onset primary open-angle glaucoma: a nationwide long-term cohort study in Taiwan. BMJ Open. (2018) 8:e021270. doi: 10.1136/bmjopen-2017-021270
15. Chou CL, Hsieh TC, Wang CH, Hung TH, Lai YH, Chen YY, et al. Long-term outcomes of dialysis patients after coronary revascularization: a population-based cohort study in Taiwan. Arch Med Res. (2014) 45:188–94. doi: 10.1016/j.arcmed.2014.01.009
16. Kuo CH, Hsieh TC, Wang CH, Chou CL, Lai YH, Chen YY, et al. Increased risks of mortality and atherosclerotic complications in incident hemodialysis patients subsequently with bone fractures: a nationwide case-matched cohort study. PLoS One. (2015) 10:e0121705. doi: 10.1371/journal.pone.0121705
17. Lin HF, Li YH, Wang CH, Chou CL, Kuo DJ, Fang TC. Increased risk of cancer in chronic dialysis patients: a population-based cohort study in Taiwan. Nephrol Dial Transplant. (2012) 27:1585–90. doi: 10.1093/ndt/gfr464
18. Wang YC, Hsieh TC, Chou CL, Wu JL, Fang TC. Risks of adverse events following coprescription of statins and calcium channel blockers: a nationwide population-based study. Medicine. (2016) 95:e2487. doi: 10.1097/MD.0000000000002487
19. Wang YC, Juan SH, Chou CL, Hsieh TC, Wu JL, Fang TC. Long-term effects of ketoanalogues on mortality and renal outcomes in advanced chronic kidney disease patients receiving a low-protein diet. Nutrients. (2020) 12:2708. doi: 10.3390/nu12092708
20. Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. (2008) 27:2037–49. doi: 10.1002/sim.3150
21. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
22. Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. (2005) 14:465–76. doi: 10.1002/pds.1062
23. Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol. (2018) 29:1970–8. doi: 10.1681/ASN.2018010096
24. Wu JJ, Brentjens MH, Torres G, Yeung-Yue K, Lee P, Tyring SK. Valacyclovir in the treatment of herpes simplex, herpes zoster, and other viral infections. J Cutan Med Surg. (2003) 7:372–81. doi: 10.1007/s10227-002-0140-3
25. He QE, Xia M, Ying GH, He XL, Chen JH, Yang Y. Severe mental disorders following anti-retroviral treatment in a patient on peritoneal dialysis: a case report and literature review. World J Clin Cases. (2019) 7:3329–34. doi: 10.12998/wjcc.v7.i20.3329
26. Garre B, Shebany K, Gryspeerdt A, Baert K, van der Meulen K, Nauwynck H, et al. Pharmacokinetics of acyclovir after intravenous infusion of acyclovir and after oral administration of acyclovir and its prodrug valacyclovir in healthy adult horses. Antimicrob Agents Chemother. (2007) 51:4308–14. doi: 10.1128/AAC.00116-07
27. Blum MR, Liao SH, de Miranda P. Overview of acyclovir pharmacokinetic disposition in adults and children. Am J Med. (1982) 73:186–92. doi: 10.1016/0002-9343(82)90088-2
28. Hellden A, Lycke J, Vander T, Svensson JO, Odar-Cederlof I, Stahle L. The aciclovir metabolite cmmg is detectable in the csf of subjects with neuropsychiatric symptoms during aciclovir and valaciclovir treatment. J Antimicrob Chemother. (2006) 57:945–9. doi: 10.1093/jac/dkl067
29. Hellden A, Odar-Cederlof I, Diener P, Barkholt L, Medin C, Svensson JO, et al. High serum concentrations of the acyclovir main metabolite 9-carboxymethoxymethylguanine in renal failure patients with acyclovir-related neuropsychiatric side effects: an observational study. Nephrol Dial Transplant. (2003) 18:1135–41. doi: 10.1093/ndt/gfg119
Keywords: valacyclovir, neurotoxicity, hemodialysis (HD), peritoneal dialysis, population-based study
Citation: Wang Y-C, Juan S-H, Li C-H, Chou C-L, Chen L-Y, Chien L-N and Fang T-C (2022) Valacyclovir-associated neurotoxicity among patients on hemodialysis and peritoneal dialysis: A nationwide population-based study. Front. Med. 9:997379. doi: 10.3389/fmed.2022.997379
Received: 22 July 2022; Accepted: 01 September 2022;
Published: 20 September 2022.
Edited by:
Te-Chun Shen, China Medical University, TaiwanReviewed by:
Yu-Juei Hsu, Tri-Service General Hospital, TaiwanKuo-Cheng Lu, Fu Jen Catholic University Hospital, Taiwan
Copyright © 2022 Wang, Juan, Li, Chou, Chen, Chien and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Te-Chao Fang, ZmFuZ3RlY2hhb0BnbWFpbC5jb20=; Li-Nien Chien, bGluaWVuLmNoaWVuQG55Y3UuZWR1LnR3
†These authors have contributed equally to this work and share first authors
 Yi-Chun Wang1,2†
Yi-Chun Wang1,2† Shu-Hui Juan
Shu-Hui Juan Chu-Lin Chou
Chu-Lin Chou Li-Nien Chien
Li-Nien Chien Te-Chao Fang
Te-Chao Fang