- 1Xiamen Branch, Zhongshan Hospital, Fudan University, Xiamen, China
- 2Zhongshan Hospital, Fudan University, Shanghai, China
Objective: The objective of the present study was to describe and analyze the clinical characteristics of nocardiosis.
Materials and methods: We described and analyzed the clinical characteristics of nocardiosis cases from two centers over the past 5 years from the following aspects: age and sex, Nocardia species, sites of Nocardia infection, test specimens, detection methods, concurrent pathogens, symptoms, imaging features, co-conditions, drug susceptibility tests, antibiotic therapy/duration, outcomes, and follow-up.
Results: The median age of the 19 cases was 64 years, with an interquartile range (IQR) of 56–68 years. Eight cases (42.1%) were immunocompromised [those who had been on corticosteroid use (62.5%), those who had used immunosuppressants (50.0%), or those who had suffered from chronic nephrosis (37.5%) or diabetes mellitus (DM) (25.0%)]. The plethora of comorbidities of these cases included diabetes (10.5%), chronic kidney disease (CDK) (15.8%), chronic lung disease (36.8%), and rheumatic diseases (10.5%). Cough and expectoration (73.7%) was the most common symptom of nocardiosis. The respiratory tract (89.5%) was the most common site of the clinical disease. Nearly half (9 cases, 47.3%) of these patients had concurrent infections. The most common Nocardia isolation site was the respiratory tract (73.7%). All patients were given antibiotic therapies, out of whom as many as 63.6% of patients were treated with two concurrent antimicrobial agents, 15.8% of patients were treated under monotherapy and 21.1% of patients were treated with three or more concurrent antimicrobial agents.
Conclusions: An uncommon life-threatening infection, nocardiosis, affects those patients with structural lung disease or immunosuppression. Although nocardiosis is capable of progressing into a serious and metastatic disease, early recognition and prompt treatment usually result in successful outcomes benefitting the patient.
Introduction
Nocardia is an aerobic Gram-positive filamentous bacterium that belongs to the actinomycetes group. Nocardia exists in soil, persists in decomposing vegetation and some other organic matter thrives in fresh water and subsists even in salt water (1). Nocardia is also known to give rise to opportunistic infections, referred to as nocardiosis. The infection is usually caused by exogenous inhalation or by a direct invasion of the injured skin. Pulmonary infection is often caused by inhaling broken hyphae. The individuals who are most vulnerable to Nocardia infection are patients who are immunocompromised. Nevertheless, patients with normal immunity accounted for one-third of all nocardiosis cases (2). Patients who have undergone organ transplantation, suffer from cancer, chronic nephrosis, or diabetes mellitus (DM) and those receiving long-term corticosteroids or immunosuppressants have an increased risk of acquiring Nocardia infection. The main clinical diseases afflicted with nocardiosis are pulmonary infection, sepsis, chronic bronchitis, brain abscess, skin abscess, and so on (3, 4). Half of the pulmonary nocardiosis cases are disseminated and also involve extrapulmonary infections, including pericardial, mediastinal, skin, subcutaneous tissue, and central nervous system (CNS) infections. Approximately 20% of the disseminated nocardiosis cases are entirely extrapulmonary diseases. Patients with normal immunity may primarily develop subcutaneous nocardiosis. The histopathological features of nocardiosis are abscess and obvious necrosis with neutrophil infiltration, which is usually surrounded by the granulation tissue, but extensive fibrosis or encapsulation is rare. More than 30 species of Nocardia have been reported to cause nocardiosis, and the most familiar species are Nocardia asteroides, Nocardia nova, Nocardia farcinica, etc. (5). Sulfonamides are still the first choice for the treatment of Nocardia. The combination of SMZ–TMP (sulfamethoxazole and trimethoprim) is equally effective or even more effective than SMZ alone, but the hematological toxicity of SMZ–TMP seems to be slightly higher.
Materials and methods
This two-center retrospective study evaluated the data from adult patients who presented with Nocardia and had received treatment at the Zhongshan Hospital, Fudan University, Shanghai (Shanghai General Hospital) or at the Zhongshan Hospital, Fudan University, Xiamen branch (Xiamen branch) between January 2017 and May 2022.
We collected information about the adult cases of patients with Nocardia by extracting the field “Nocardia” in the Hospital Information Systems (HISs) of two hospitals. The diagnosis was confirmed by a microbiologically positive result, and those patients who were suspected but proved to be microbiologically negative were excluded. Finally, we obtained 19 targeted cases. Fifteen of these cases were from the Shanghai General Hospital and four of them were from the Xiamen branch. We described and analyzed the clinical characteristics of the nocardiosis cases from the following aspects: age and sex, Nocardia species, site of Nocardia infection, test specimens, detection methods, concurrent pathogens, symptoms, imaging features, co-conditions, drug susceptibility tests, antibiotic therapy/duration, outcomes, and follow-up. We described and analyzed the clinical characteristics of the 19 cases in terms of the median and quartile because of their non-normal distribution.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Zhongshan Hospital, Fudan University, Shanghai, and Zhongshan Hospital, Fudan University, Xiamen branch. Written informed consent was obtained from the participants in our study.
Results
A two-center retrospective analysis
The demographic and baseline characteristics of the 19 cases are presented in Tables 1, 2. The median age of the 19 cases was 64 years, with an interquartile range (IQR) of 56–68 years. The age of the patients ranged from 15 to 80 years. Eleven out of 19 (57.9%) cases were men.
Cough and expectoration (73.7%) was the most common symptom of nocardiosis at the time of presentation, followed by local pain (36.7%), fever (26.3%), hemoptysis (21.1%), and dyspnea (15.8%). The respiratory tract was the most common site of the clinical disease, which affected 84.2% of these patients. In 14 of these patients, only the lung was infected. Extrapulmonary disease was also common, as 2 cases had evidence of bone/joint involvement, 3 cases had central nervous system (CNS) involvement, 1 case had skin and soft tissue involvement, 1 case had lumbar vertebra involvement, and 1 case had hand and wrist joint involvement.
The comorbidities of these cases included diabetes (10.5%), chronic kidney disease (CKD) (15.8%), chronic lung disease (36.8%), and rheumatic diseases (10.5%). Only six patients had no underlying disease. Chronic lung disease (7 cases) was the most common underlying disease, which included bronchiectasis (3 cases), chronic bronchitis with postoperative lung abscess (1 case), allergic bronchopulmonary aspergillosis (ABPA, 1 case), chronic obstructive pulmonary disease (COPD) with bronchiectasis (1 case), and idiopathic pulmonary fibrosis (IPF) with bronchiectasia and COPD (1 case). Two patients had a history of pulmonary tuberculosis. Oral corticosteroids were used by five patients (26.3%) and immunosuppressants were used by four patients (21.0%). As detailed in Table 2, one patient with Behcet's disease received mycophenolate mofetil (MMF) and azathioprine (AZA), while two patients with membranous nephropathy were treated with cyclophosphamide (CTX) or tacrolimus (FK506). The case of rheumatoid arthritis (RA) was treated with long-term oral hydroxychloroquine and iguratimod. All patients with COPD received inhaled corticosteroids (ICS), while ABPA was treated with oral corticosteroids.
Microbiology
The microbiological data of these 19 cases are presented in Table 3. More than half (N = 10, 52.6%) of the patients had concurrent infections with Nocardia at the time of presentation. Six out of ten patients had a concurrent bacterial infection (Klebsiella pneumoniae, Pseudomonas aeruginosa, Mycobacterium tuberculosis, Nontuberculous mycobacterium, and Stenotrophomonas maltophilia); one of the ten patients had a viral infection [cytomegalovirus (CMV) and Epstein–Barr virus (EBV)]; three out of ten patients had a fungal infection (Aspergillus species, Cryptococcus and Trichophyton); and one of the ten patients had an intestinal microsporidial infection. The intestinal microsporidial infection also affected the central nervous system (CNS). One case of non-tuberculous mycobacteria (NTM) infection disseminated to the skin, the hesoft tissues, the lungs, and the brain. The tuberculosis (TB) infection site was diagnosed with lumbar tuberculosis, and the Trichophyton infection site was the joint of the left hand. The remaining coinfected bacteria, fungi, viruses, NTM, etc., were all lung infections.
The most common site for the isolation of Nocardia species is the respiratory tract (73.7%), followed by local pus, cerebrospinal fluid (CSF), blood, the pulmonary biopsy tissue, the bone, and the hydrothorax. Invasive manipulation was the most common way to obtain microbial samples. The traditional diagnostic method of nocardiosis is bacterial culture. Metagenomic next-generation sequencing (mNGS) is a new approach that can detect the species of Nocardia. In our study, cultures in more than half of the cases (10/19) were unable to detect the Nocardia species. A total of 11.1% (N = 1) of the isolates in our cases were resistant to TMP–SMX, while no linezolid or any other drug resistance was described, as shown in Table 3.
Imaging features
All 19 patients underwent chest CT (computed tomography). Bronchiectasis (N = 7) and consolidation (N = 7) were the most common presentations of the chest CT findings, followed by masses (N = 5), cavities (N = 4), nodules (N = 3), infiltration (N = 3), interstitial effusion (N = 2), and pleural effusion (N = 2).
The chest CTs of two patients were normal for only bones/joints. In one case, spinal magnetic resonance imaging (MRI) showed an abscess and narrowing of the intervertebral space. The joint MRI showed bone destruction and joint swelling in the other case (as shown in Figure 1).
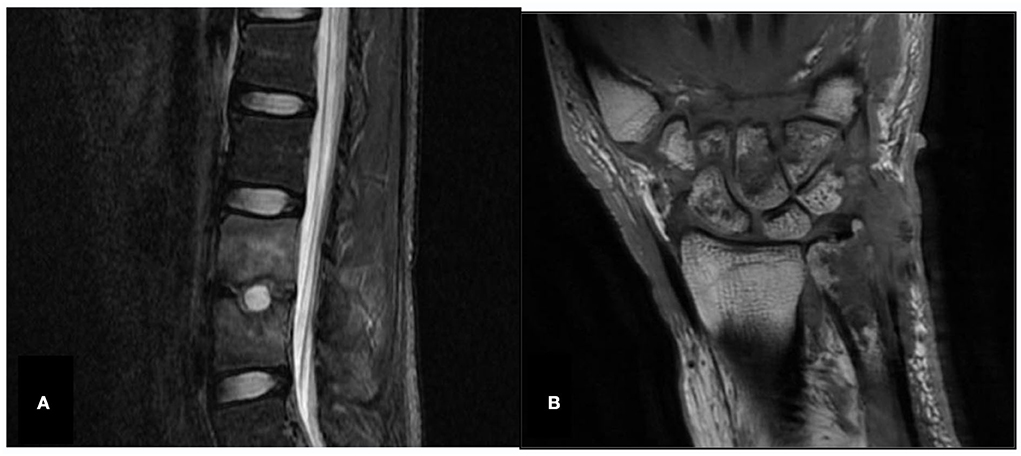
Figure 1. Spine and joint MRI (Case 10 and Case 18X). (A) Case 10, spinal magnetic resonance imaging (MRI), abnormal signals in the L3 and L4 vertebrae, abscess, narrowing intervertebral space and disappearance of the normal disc signal. (B) Case 18X, left wrist joint MRI, left distal ulna, multiple carpal and metacarpal bone destruction, left wrist joint soft tissue swelling.
We obtained CT images of cases 16X and 17X, whose imaging findings are shown in Figure 2. We compared the chest CT before and after 4 weeks of antibiotic treatment of case 16X in Figures 2A,B. In Figure 2A, the chest CT plain scan before antibiotic therapy showed local cystic and columnar dilatation of bronchi in both lungs. The surrounding lung tissue of the bronchi had multiple plaques and speckled images with high density. The adjacent pleura of the bronchi was thickened with multiple cystic lucid images in both lungs. In Figure 2B, the chest CT plain scan after 4 weeks of antibiotic therapy shows that some patchy and speckled lesions were absorbed, but the rest of the lung is similar to that before treatment.
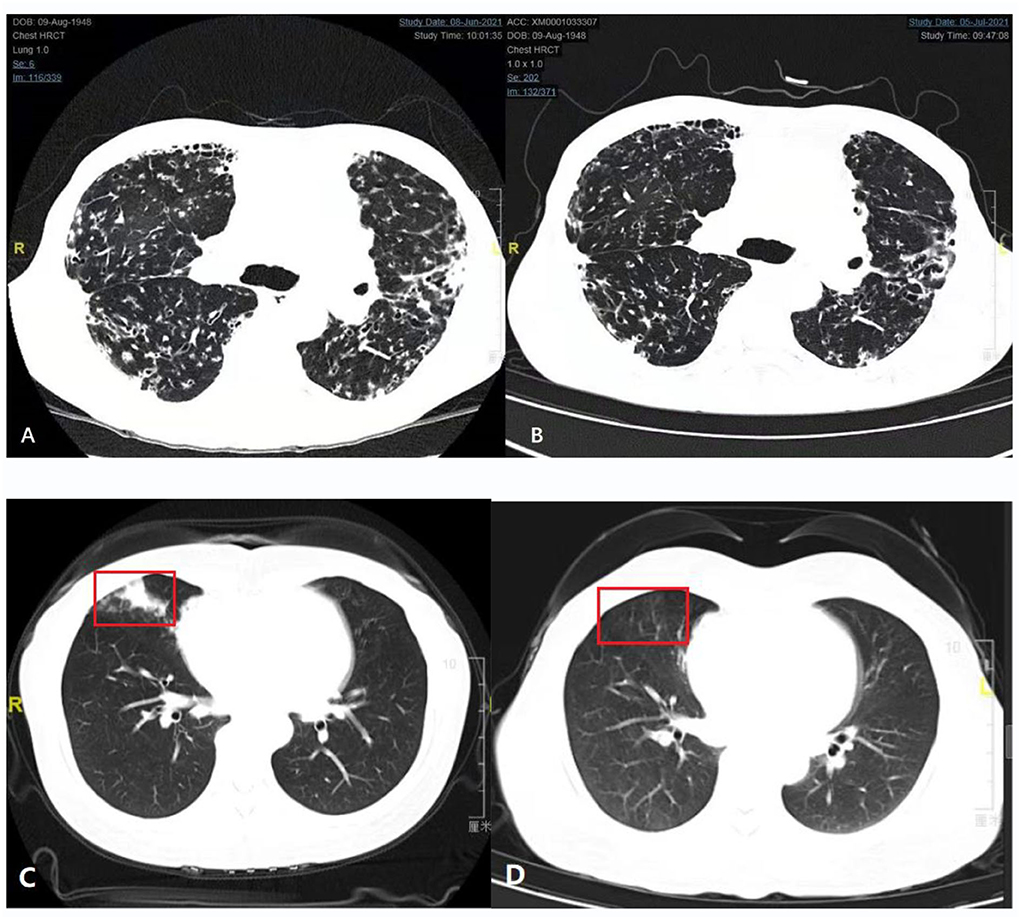
Figure 2. Comparison of chest computed tomography (CT) before and after treatment (Case 16X and Case 17X). (A,B) Comparison of chest computed tomography (CT) before and after 4 weeks of antibiotic treatment (Case 16X). (A) Plain chest CT scan before antibiotic therapy. (B) Plain chest CT scan after 1 month of antibiotic therapy. (C,D) Comparison of chest CT before and after 3 months of antibiotic treatment (Case 17X). (C) Plain chest CT scan before antibiotic therapy. The red box is a typical Nocardia infection site. (D) Plain chest CT scan after 3 months of antibiotic therapy. Inflammation of the red box had subsided.
We also compared the chest CT before and after 3 months of antibiotic treatment of case 17X in Figures 2C,D. Patient 17X was a 44-year-old woman who had no history of chronic lung disease or immunosuppression therapy. Before treatment, her lung CT showed consolidation of the middle lobe of the right lung, as shown in Figure 2C. After 3 months of antibiotic treatment, her CT scan of the chest revealed that the inflammation of the right lung had subsided, as shown in Figure 2D.
Treatment and outcome
All patients were treated with antibiotic therapies, and 3 patients (15.8%) were treated under monotherapy. The majority of patients were subjected to treatment with two or more concurrent antimicrobial agents (63.6 and 21.1%, respectively). The main therapeutic drugs were tetracycline or SMZ (N = 11, 57.9%), followed by linezolid or carbapenems (N = 6, 37.6%). Thirteen patients discontinued the therapy; 12 out of 13 cases were due to stable disease, while the remaining 1 case was due to drug intolerance. Two patients (case 6 and case 14) were still receiving medication at the end of the follow-up period because of fever or coughing from time to time. Another two patients (cases 17X and 18X) were newly diagnosed and under therapy and were treated for 7 and 8 months, respectively. The median duration of treatment was 18 months (IQR 13–30 months). The all-cause mortality of patients with Nocardia infection was 15.8%. After a median follow-up of 45 months (IQR 31–58 months), 3 (15.8%) patients died (case 1, case 9, and case 19X).
The patient in case 1 was an 80-year-old man who had a history of structural lung disease. He had concomitant NTM infection and Klebsiella pneumoniae. The patient had a history of silicosis for 13 years and chronic bronchitis for more than 10 years. He received medication for 6 months due to a deteriorating physical condition and advanced age. Then, he died of multiple organ failure (MOF) and severe infection 1 year after Nocardia diagnosis.
The patient in case 9 had suffered from nephrotic syndrome for 20 years and was not given correct treatment. He had a wide spectrum of infections involving the thorax, the lungs, and the central nervous system (CNS). His concomitant NTM infection remained difficult to control. After 18 months of treatment for his Nocardia infection, he died of severe infection and MOF.
The patient in case 19X was a 65-year-old man whose underlying diseases were diabetes mellitus (DM) and membranous nephropathy with long-term oral corticosteroids and tacrolimus (FK506). Fever with respiratory failure led to his admission to the intensive care unit (ICU). Bronchoscopy and alveolar lavage were performed to confirm the diagnosis of nocardiosis and cryptococcosis. Despite active antifungal and anti-Nocardia treatment, he finally died of complex complications, renal insufficiency, hypokalaemia and septic shock 3 months after the diagnosis of nocardiosis.
Discussion
This study is the largest nocardiosis case collection to date, with cases collected from two centers. Nocardia is a relatively rare opportunistic pathogen. Infection is caused by exogenous inhalation or by a direct invasion of the injured skin. The lung and skin are the most susceptible organs. Spine and joint infections are rare but not completely unknown. The characteristics of nocardiosis include abscess formation, extensive neutrophil infiltration, and significant necrosis (6). These pathological changes are similar to those of tuberculosis. People with immune deficiency are more susceptible.
The study of Weng et al. (7) showed that, when comparing the nocardiosis culture and next-generation sequencing (NGS) methods, the latter method can not only improve the detection rate of Nocardia but also greatly reduce the turnaround time. Nearly half of the patients in this study had Nocardia diagnosed by the mNGS method. The identification of the Nocardia species is important to guide treatment.
The drugs to treat Nocardia are sensitive to include TMP–SMZ, amikacin, linezolid, imipenem, and so on. Different Nocardia species have different drug susceptibility results. However, TMP–SMZ is the first choice for the treatment of Nocardia when the susceptibility test is positive. As its drug resistance rate to TMP–SMZ increased to 10.8%, imipenem and amikacin are good options (8). An in vitro study showed drug interactions between linezolid and amikacin, but a combination of these two agents should be avoided (9). Oral minocycline is well tolerated and can be used in patients with mild nocardiosis. For patients with severe diseases that cannot accept oral therapy or patients with SMZ-resistant strains, imipenem, ceftriaxone, cefotaxime, or amikacin are alternatives (10).
Oral therapy is the first choice for patients with no severe clinical diseases. Considering the resistance of Nocardia species to SMZ and minocycline, oral therapy, including linezolid, clarithromycin, amoxycillin clavulanate, or others, works well for the treatment of nocardiosis (10). Although linezolid is more commonly used to treat nocardiosis, several reports recommended amoxycillin clavulanate as an effective treatment when given in combination with other drugs (11–13). Clinicians should be aware of the higher rate of Nocardia resistance to TMP–SMZ and amikacin when treating these infections. Before treatment, drug sensitivity tests should be performed to develop a suitable therapeutic schedule.
According to published reports, the total duration of treatment (intravenous first, oral second) depends on the severity of the disease and the clinical and radiological response to therapy. The duration of intravenous antibiotic therapy should be 2–3 weeks for patients without CNS involvement and 3–6 weeks for those with CNS nocardiosis (14). Then, the patients should be switched to oral therapy when their clinical symptoms improve. Clinicians usually extend the duration of treatment to minimize the risk of disease recurrence. When infected with pulmonary or multifocal disseminated (non-CNS) Nocardia, the duration of treatment might extend by a duration of 6–12 months for patients with normal immunity. When the CNS is involved, the duration of treatment should be maintained for 12 months. At least 12 months of treatment are recommended for immunodeficient patients, regardless of the organ involved (15).
Magnetic resonance imaging (MRI) is an important method to determine the focus size and the infection situation in the brain, the spine, or the joint. MRI has a stronger soft tissue resolution than CT and is superior to CT in the visualization of brain, spinal cord, and intraarticular lesions. CT is an important method for the diagnosis of diseases, especially thoracic diseases. In the early stage of antibiotic treatment, if the clinical condition of patients with nocardiosis improves, a CT scan (4–6 weeks after initial drug administration) is helpful to evaluate the patient's response to antibiotic therapy. If the patient's clinical status does not improve, an early (2-week) CT scan is essential to evaluate the true state of the lesion and find the local cause of the negative results (16).
Limitations
This study has very few limitations. One limitation of this study is that it pertains to the small sample size, as Nocardia is a rarely encountered opportunistic pathogen and cases of nocardiosis are infrequent. Also, this two-center study could not reflect the overall clinical characteristics of different Nocardia species in China.
Conclusion
Nocardiosis is an uncommon life-threatening infection that affects patients suffering from structural lung disease or immunosuppression. Although nocardiosis is capable of progressing into a serious and metastatic disease, early recognition of the disease and prompt treatment measures given on time to the patient usually result in successful outcomes benefitting the patient.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhongshan Hospital, Fudan University, Shanghai and the Ethics Committee of Zhongshan Hospital, Fudan University, Xiamen. The patients provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW collected and processed data and wrote the introduction, materials and methods, and discussion. YX processed and analyzed data and wrote the results. ZC provided CT figures. WJ and XX provided consultation. YS collected data. YY provided idea. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from Science and Technology Guidance Project of Xiamen (3502Z20214ZD1079), Xiamen Government and Xiamen Medical and Health Guidance Project (3502Z20199037), Xiamen Government.
Acknowledgments
We thank the patients who participated in this study for their devotion of time to take part in our follow-up.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. (2012) 87:403–7. doi: 10.1016/j.mayocp.2011.11.016
2. Beaman BL. Nocardia species-host-parasite relationships. Clin Microbiol Rev. (1994) 7:213–64. doi: 10.1128/CMR.7.2.213
3. Kontoyiannis DP, Ruoff K, Hooper DC. Nocardia bacteremia. Report of 4 cases and review of the literature. Medicine. (1998) 77:255–67. doi: 10.1097/00005792-199807000-00004
4. Budzik JM, Hosseini M, Mackinnon Jr AC, Taxy JB. Disseminated Nocardia farcinica: literature review and fatal outcome in an immunocompetent patient. Surg Infect (Larchmt). (2012) 13:163–70. doi: 10.1089/sur.2011.012
5. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. (2006) 19:259–82. doi: 10.1128/CMR.19.2.259-282.2006
6. Huang L, Sun L, Yan Y. Characteristics of nocardiosis patients with different immune status from a Chinese tertiary general hospital during 8-year period: a STROBE-compliment observational study. Medicine (Baltimore). (2019) 98:e17913. doi: 10.1097/MD.0000000000017913
7. Weng SS, Zhang HY, Ai JW, Gao Y, Liu YY, Xu B, et al. Rapid detection of nocardia by next-generation sequencing. Front Cell Infect Microbiol. (2020) 10:13. doi: 10.3389/fcimb.2020.00013
8. Martinez Tomas R, Menendez Villanueva R, Reyes Calzada S, Santos Durantez M, Valles Tarazona JM, Modesto Alapont M. Pulmonary nocardiosis—risk factors and outcomes. Respirology. (2007) 12:394–400. doi: 10.1111/j.1440-1843.2007.01078.x
9. Tripodi MF, Durante-Mangoni E, Fortunato R, Cuccurullo S, Mikami Y, Farina C, et al. In vitro activity of multiple antibiotic combinations against Nocardia: relationship with a short-term treatment strategy in heart transplant recipients with pulmonary nocardiosis. Transpl Infect Dis. (2011) 13:335–43. doi: 10.1111/j.1399-3062.2010.00588.x
10. Minero MV, Marín M, Cercenado E, Rabadán PM, Bouza E. Muñoz P. Nocardiosis at the turn of the century. Medicine (Baltimore). (2009) 88:250–61. doi: 10.1097/MD.0b013e3181afa1c8
11. Arduino RC, Johnson PC, Miranda BG. Nocardiosis in renal transplant recipients undergoing immunosuppression with cyclosporine. Clin Infect Dis. (1993) 16:505–12. doi: 10.1093/clind/16.4.505
12. Javaly KE, Horowitz HW, Wormser GP. Nocardiosis in patients with human immunodeficiency virus infection. Report of 2 cases and review of the literature. Medicine. (1992) 71:128–38. doi: 10.1097/00005792-199205000-00003
13. Merigou D, Beylot-Barry M, Ly S, Doutre MS, Texier-Maugein J, Billes P, et al. Primary cutaneous Nocardia asteroides infection after heart transplantation. Dermatology. (1998) 196:246–7. doi: 10.1159/000017883
14. Lafont E, Conan PL, Rodriguez-Nava V, Lebeaux D. Invasive nocardiosis: disease presentation, diagnosis and treatment—old questions, new answers? Infect Drug Resist. (2020) 13:4601–13. doi: 10.2147/IDR.S249761
15. Lafont E, Conan PL, Rodriguez-Nava V, Lebeaux D. Nocardiosis. Clin Infect Dis. (1996) 22:891–905. doi: 10.1093/clinids/22.6.891
Keywords: Nocardia, nocardiosis, clinical characteristics, immunosuppressed, two-center retrospective study
Citation: Wang L, Xu Y, Chen Z, Jiang W, Xiao X, Shen Y and Ye Y (2022) Nocardiosis: A two-center analysis of clinical characteristics. Front. Med. 9:996442. doi: 10.3389/fmed.2022.996442
Received: 26 July 2022; Accepted: 25 October 2022;
Published: 16 November 2022.
Edited by:
Dipesh Dhakal, University of Florida, United StatesReviewed by:
Mansoor C. Abdulla, Sultan Qaboos Hospital, OmanYishan Lu, Guangdong Ocean University, China
Copyright © 2022 Wang, Xu, Chen, Jiang, Xiao, Shen and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Shen, c2hlbi55dW5AenMtaG9zcGl0YWwuc2guY24=; Yanrong Ye, eWV5YW5yb25nMTk5MEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Lumin Wang
Lumin Wang Yijiao Xu
Yijiao Xu Zhisheng Chen1
Zhisheng Chen1 Yanrong Ye
Yanrong Ye