
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 28 September 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.995019
This article is part of the Research TopicGluten: Yes, No, MaybeView all 6 articles
 Aurelio Seidita1†
Aurelio Seidita1† Pasquale Mansueto1†
Pasquale Mansueto1† Alessandra Giuliano2,3
Alessandra Giuliano2,3 Marta Chiavetta2,3
Marta Chiavetta2,3 Francesca Mandreucci2,3
Francesca Mandreucci2,3 Maurizio Soresi1
Maurizio Soresi1 Mattia Pistone1
Mattia Pistone1 Stella Compagnoni2,3
Stella Compagnoni2,3 Daniele Castellucci2,3
Daniele Castellucci2,3 Gianluca Bisso2,3
Gianluca Bisso2,3 Francesco Faraci2,3
Francesco Faraci2,3 Salvatore Maestri2,3
Salvatore Maestri2,3 Rosaria Disclafani4
Rosaria Disclafani4 Anna Sapone5
Anna Sapone5 Alessio Fasano5
Alessio Fasano5 Antonio Carroccio2,3*
Antonio Carroccio2,3*Background and aims: A wheat-free diet (WFD) represents the elective treatment for Non-celiac Wheat Sensitivity (NCWS) patients. Preliminary reports have shown a possible better tolerability of ancient grains in these subjects. The aim of this observational study was to evaluate the frequency of consumption of ancient grains and its correlation with clinical manifestations in NCWS patients.
Methods: 223 NCWS patients were recruited, and their consumption of ancient grains was monitored. Participants were first administered a modified version of the Pavia/Biagi questionnaire to investigate their adherence to “modern WFD.” The appearance/exacerbation of symptoms after ingestion of ancient grains was then assessed with WHO toxicity grading scale.
Results: 50.2% of the recruited patients reported consuming ancient grains before NCWS diagnosis; the diagnostic delay in this group was significantly higher than in non-consumers [median (range) 72 (6–612) vs. 60 months (3–684), P = 0.03] and these patients reported lower frequency of constipation (P = 0.04). Of the 107 patients with optimal adherence to modern WFD, 14 reported eating ancient wheat after NCWS diagnosis. Among them, 5 reported milder symptoms than those caused by modern wheat intake and 3 had an excellent tolerability without symptoms. Timilia/Tumminia variety was the most frequently used ancient grain.
Conclusions: NCWS patients who consume ancient grains may receive a late diagnosis due to the possible clinical benefit (tolerability) obtained with these grains. Even after diagnosis, 10% of the patients still consumed ancient grains and had mild or no symptoms. Further studies are required to define the pathophysiological mechanism behind their putative greater tolerability.
The consumption of gluten- and wheat-free foods, even without a diagnosis of celiac disease (CD) or wheat allergy (WA), has greatly increased in recent years. Indeed, more and more people are self-reporting intestinal symptoms [i.e., irritable bowel syndrome (IBS)-like symptoms, such as abdominal pain, diarrhea, constipation, abdominal bloating, meteorism] and extra-intestinal manifestations (headaches, dermatitis, fatigue, etc.) secondary to wheat ingestion and, at the same time, referring the clinical benefits obtained when avoiding gluten- and wheat-containing products. Starting from this self-reported condition, about 10 years ago a panel of experts defined a new gluten-related disease, which was labeled at first as “Non-celiac Gluten Sensitivity” (1), and, subsequently, “Non-celiac Wheat Sensitivity” (NCWS) (2).
In fact, NCWS is unlike CD, where there is a clear autoimmune condition characterized by a specific serological and histological profile triggered by gluten ingestion in genetically predisposed individuals (HLA-DQ2/DQ8 positivity and non-HLA genes) (3), and it has not yet been established whether or not gluten is the real trigger in NCWS. Some authors have also suggested a role for Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols (FODMAPs) (4, 5) while other studies have focused their attention on the activation of both innate and acquired immunity in inducing symptoms (6, 7). In this context, it has been highlighted that wheat contains Amylase-Trypsin Inhibitors (ATIs), proteins able to activate innate immunity via Toll-like receptor 4 (TLR4) on myeloid cells (8). The epidemiology of NCWS is difficult to estimate because of the absence of specific biological markers. However, in tertiary centers, its frequency seems 3/6-fold that of CD, and women aged between 20 and 50 years are most affected (F:M = 6:1) (9, 10).
The pathogenesis of IBS, on the other hand, is heterogeneous; traditionally, focus has been on abnormalities in motility, visceral sensation, brain-gut interaction, and psychosocial distress. More recently, alterations in gut immune activation, intestinal permeability, visceral hypersensitivity and the intestinal and colonic microbiota have been identified in some IBS patients (11–16).
Preliminary in vitro and in vivo data suggest that the consumption of ancient grain varieties could be better tolerated by NCWS and IBS patients (17–19), thus, making it plausible that other wheat components, different from gluten, may play a leading role in NCWS pathogenesis. However, it is necessary to underline that these ancient grains are typically consumed as whole grain products, thus it cannot be ruled out that the possible benefits could be due to this practice rather than to their intrinsic composition (20). Moreover, leavened breads made with ancient wheat are usually produced with natural yeast, which contains Lactobacilli that degrade ATIs (21).
The purpose of this study was to evaluate the frequency of consumption of ancient grains and its correlation with clinical manifestations in a population of NCWS patients diagnosed by double-blind placebo-controlled challenges (DBPCCs) with modern wheat.
Our observational study consisted of a retrospective and a prospective part. In the first, retrospective part, a large group of 230 patients with NCWS, diagnosed by DBPCC with modern wheat in 3 clinical departments of Internal Medicine (“P. Giaccone” University Hospital, Palermo, Italy, “V. Cervello” Hospital, Palermo, Italy, and “Giovanni Paolo II” Hospital, Sciacca, Italy), between January 2011 and December 2020, were included. All the data from our medical records were reviewed and collected in a digital database.
In the second, prospective part of the study, all the included NCWS patients were contacted by experienced physicians between November 2021 and April 2022, at a median time of 24 months after the first recruitment, and invited to answer a questionnaire to investigate their current adherence to the modern wheat-free diet (WFD), their well-being and health conditions, their consumption of ancient wheat (before and/or after NCWS diagnosis), and the kind of ancient wheat consumed.
Inclusion criteria for the NCWS patients were: (A) age between 18 and 65 years old; (B) reported intestinal and/or extra-intestinal wheat-related symptoms; (C) negative serum anti-tissue transglutaminase (anti-tTG), Immunoglobulin (Ig)A and IgG antibodies; (D) absence of duodenal villous atrophy, sampled in all patients with the DQ2 or DQ8 HLA haplotypes, and assessed when they had a minimum intake of 100 grams of pasta and/or bread per day for at least 45 days or when considered clinically appropriate; (E) exclusion of WA, diagnosed by negative skin prick tests and/or serum specific IgE for wheat, gluten and gliadin detection; (F) resolution of symptoms on a strict standard elimination diet (i.e., oligoantigenic diet, without wheat, cow’s milk, egg, tomato, chocolate, and other foods reported as causing symptoms by the patients themselves), lasting at least 4 weeks, and followed by the reappearance of the same symptoms after DBPCCs with modern wheat (for details of the elimination diet and challenge methods see Supplementary File 1); (G) follow-up duration longer than 12 months after the initial diagnosis, with at least 2 outpatient visits during this period; (H) complete medical records.
The exclusion criteria were: (A) self-exclusion of gluten/wheat from the diet and refusal to reintroduce it for diagnostic purposes before entering the study; (B) anti-endomysium antibody (EmA) positivity in the culture medium of duodenal mucosa samples (EMA-biopsy), even in the presence of a normal villus/crypt ratio in the mucosa; (C) incomplete clinical records; (D) pregnancy; (E) abuse of alcohol and/or drugs; (F) treatment with corticosteroids and/or NSAIDs in the 2 weeks prior to duodenal biopsies (when performed); (G) diagnosis of chronic inflammatory bowel disease or other organic pathologies affecting the digestive system; (H) coexisting infectious diseases.
For each patient, the following data were extracted from the medical records and then analyzed: gender, age at diagnosis, diagnostic delay, body mass index (BMI), IBS-like symptoms presence and subtypes (diarrhea, constipation or mixed bowel movements), dyspepsia, body weight loss (established by an at least 10% reduction in body weight in 6 months or less), presence and kind of extra-intestinal symptoms, presence of autoimmune diseases, coexistence of other atopic diseases (allergic rhino-conjunctivitis and/or allergic asthma and/or atopic dermatitis), and any other comorbidities.
Adherence to the modern WFD was assessed according to a modified version of the “Pavia” or “Biagi” score (22).
In detail, the following score was used: 0 = no adherence to the modern WFD; 1–2 = poor adherence to the modern WFD; 3–4 = “strict” adherence to the modern WFD (for details, see Supplementary File 2). The score was applied to the consumption/avoidance of modern wheat; the contemporary consumption of pasta or bakery products made with ancient wheat flour was not considered a “transgression.” Patients were then asked about the specific ancient wheat flours they consumed: Perciasacchi, Senatore Cappelli, Timilia/Tumminia, Russello, Khorasan (hereafter referred to as “Kamut®,” as it is the commercial name best known to our patients), spelt, or others. The first four above-mentioned wheat varieties are among the most frequently cultivated and consumed ancient varieties in Sicily. The frequency of ancient wheat flour consumption was graded as frequent (>3 times per week, 50 grams at least), moderate (1–2 times per week) or rare (<1 time per week). The clinical tolerability of ancient grains was assessed according to the World Health Organization toxicity grading scale (23, 24) and both intestinal (abdominal pain, altered bowel movements, nausea/vomiting, etc.) and extra-intestinal symptoms (headache, fatigue, myalgia, etc.) were investigated.
In detail, the following grading scale was used: 0 = absence of symptoms; 1 = mild symptoms; 2 = moderate symptoms, 3 = severe symptoms, and 4 = life threatening.
Data were expressed as mean ± standard deviation (SD) when the distribution was Gaussian, and Student’s t test was used to evaluate differences between group means. Comparisons between more than 2 groups were performed by ANOVA, followed by a post hoc analysis using the Bonferroni tests. Otherwise, data were expressed as median and range and analyzed with the Kruskal–Wallis and Mann–Whitney U tests. The χ2 test and Fisher’s exact test were used to compare the frequency values in the various population groups. The McNemar test for paired data was performed to analyze the frequency of changes in symptoms (IBS-like, dyspepsia and extra-intestinal) before and after modern WFD, according to adherence: “poor adherence” and “strict adherence.”
The study was registered on clinicaltrials.gov (registration number n. NCT03024775) and approved by the Ethics Committee of the AOUP “P. Giaccone” hospital of Palermo. Recruited patients gave their informed consent to the study.
Of the initial 230 NCWS patients, 4 were excluded due to the presence of other gastrointestinal diseases at the time of the questionnaire, and another 3 patients were excluded because they refused to answer the questionnaire. Thus, data from 223 patients were analyzed.
Table 1 shows the demographic and clinical features of the NCWS patients. Most of the patients were female (89.3%), aged between the third and fourth decade of life, and with a significant diagnostic delay following the onset of symptoms [median and range 60 (3–684) months].
When contacted, 82 (36.9%) patients were no longer following the modern WFD (Score 0), 34 (15.2%) had poor adherence to the modern WFD (Score 1–2), and 107 (48.0%) had a Score of 3–4, which represents a strict adherence to the modern WFD (Figure 1).
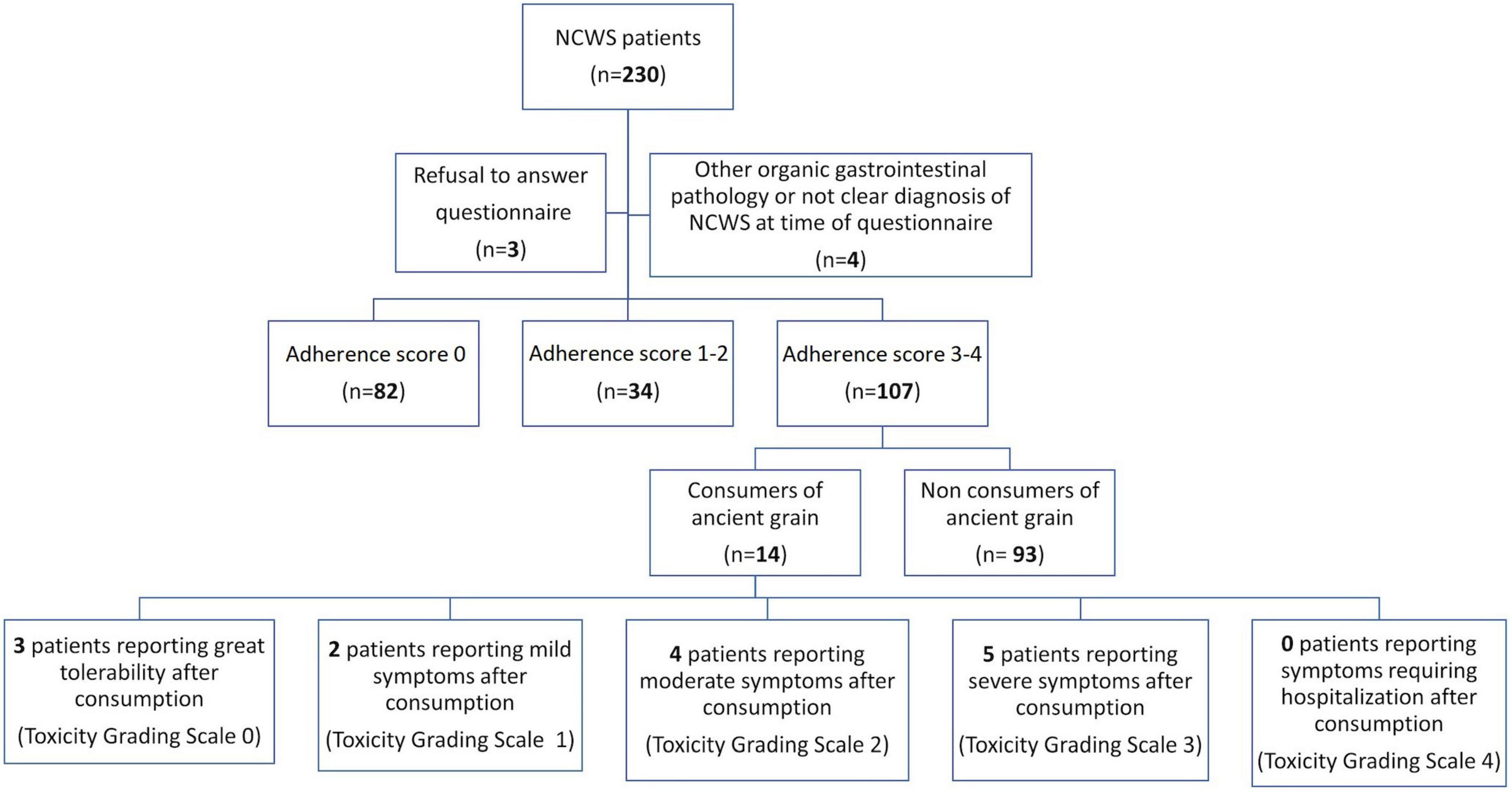
Figure 1. Flow-chart of the study, showing patient adherence to a modern wheat-free diet and consumption of ancient grains after Non-celiac Wheat Sensitivity (NCWS) diagnosis.
The patients were then interviewed about the persistence of symptoms, which was inversely correlated with the adherence score, and NCWS patients on a strict modern WFD reported a very high frequency of symptom disappearance. Of note, even poor adherence to the modern-WFD was able to improve both IBS-like (P < 0.02 vs. P < 0.0001, respectively, in poor-adherents and strict-adherents) and extraintestinal (P < 0.0005 vs. P < 0.0001, respectively, in poor-adherents and strict-adherents) symptoms in a part of the NCWS subjects, albeit with a lower significance than strict adherence; this was not proved for dyspepsia (P = 0.15 vs. P < 0.0001, respectively, in poor-adherents and strict-adherents).
Figure 2 shows the number of patients reporting IBS-like symptoms (Figure 2A), dyspepsia (Figure 2B) and extra-intestinal symptoms (Figure 2C) before and after the WFD, according to the degree of adherence to this diet. In the group reporting strict adherence to the modern WFD, the frequency of IBS-like symptom disappearance was significantly higher than in the NCWS subjects with no or poor adherence to the modern-WFD (87.0% vs. 5.8%; P < 0.0001), and this also applied to dyspepsia (89.7% vs. 0.0%; P < 0.0001) and extraintestinal symptoms (88.9% vs. 18.5%; P < 0.0001). Table 2 shows the demographic and clinical features of the 223 NCWS patients according to ancient grain consumption before NCWS diagnosis 112 (50.2%) patients reported habitual consumption of ancient grains before NCWS diagnosis. A higher diagnostic delay was observed in these patients compared to those who had never consumed ancient grain products [72 (6–612) months vs. 60 months (3–684), P = 0.03]; moreover, a higher frequency of constipation was observed in the patients who had never consumed ancient wheat (19.8% vs. 8.9%, P = 0.04). No other statistically significant differences were demonstrated.
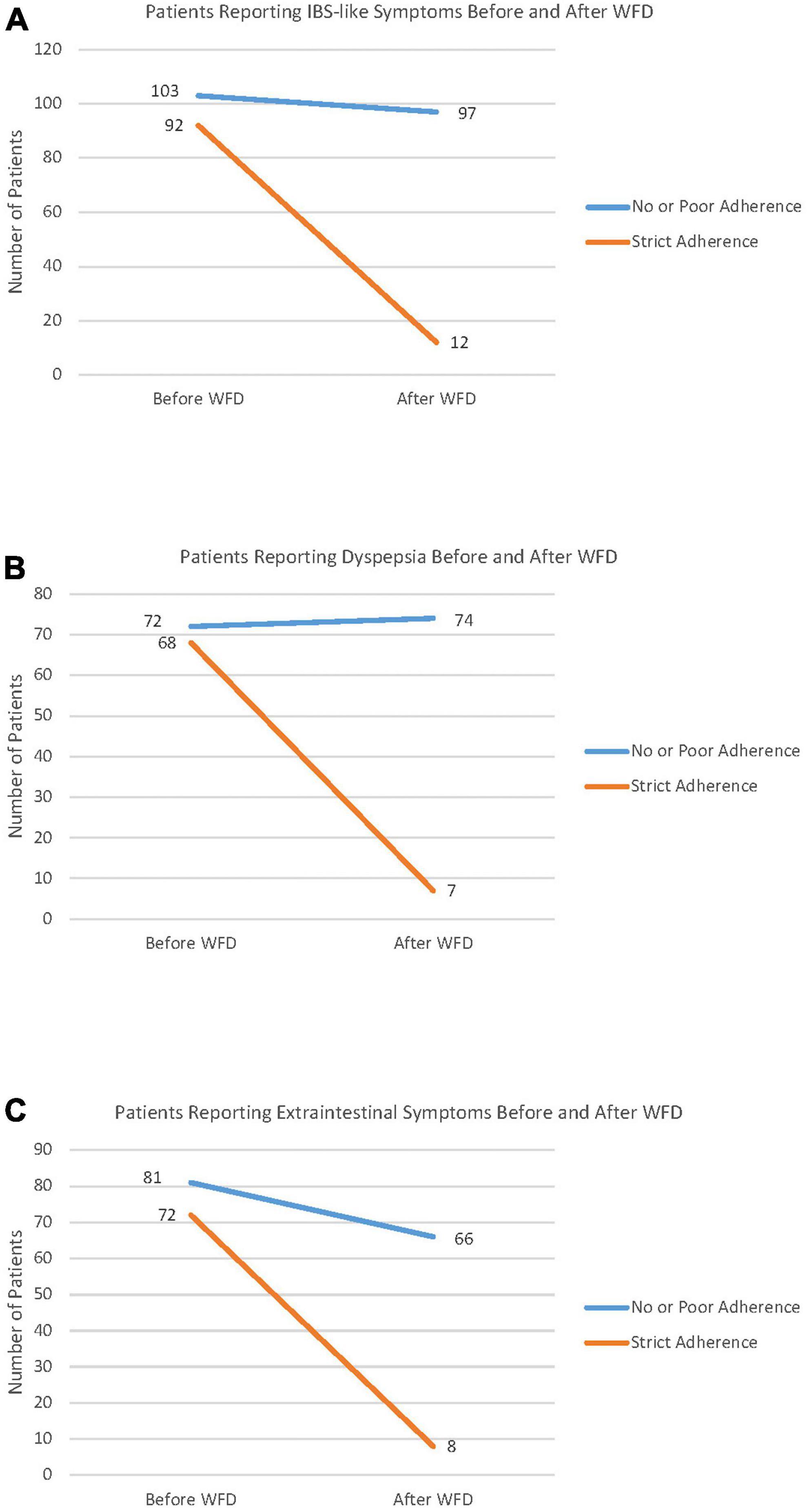
Figure 2. Symptom changes before and after the wheat-free diet (WFD) according to a modern WFD adherence score. Patients with an adherence score between 0 and 2 were classified as non- or poorly adherent to the wheat-free diet. Patients with an adherence score of 3–4 were considered as strictly adherent to the diet. Panel (A) shows the number of patients reporting Irritable Bowel Syndrome-like (IBS) symptoms, before and after the WFD. Panel (B) shows the number of patients reporting dyspepsia, before and after WFD. Panel (C) shows the number of patients reporting extraintestinal symptoms, before and after WFD.

Table 2. Demographic and clinical features of NCWS patients according to ancient grain consumption before NCWS diagnosis.
When contacted for the prospective study, in the subset of patients who declared a strict adherence to the modern WFD (107 subjects), 14 (13.1%) reported consuming ancient wheat at least once after NCWS diagnosis. Their individual data are shown in Table 3. Three patients reported excellent tolerability (toxicity grading scale 0), and grain consumption was in the form of whole grain products. Furthermore, 2 patients had mild symptoms (toxicity grading scale 1), 4 had moderate symptoms (toxicity grading scale 2), and 5 patients had severe symptoms (toxicity grading scale 3) after consuming products based on either refined or whole ancient grains. Among the 11 consumers of ancient wheat with consequent symptoms, 5 (45.5%) defined symptoms as more tolerable than those caused by eating modern grain products. Overall, the NCWS patients with adherence score 3–4 tried ancient grain products after a median time of 24 (range 1–48) months on a strict WFD. As regards the reason for consuming ancient wheats, patients reported their better palatability and lower cost compared to wheat-free products.
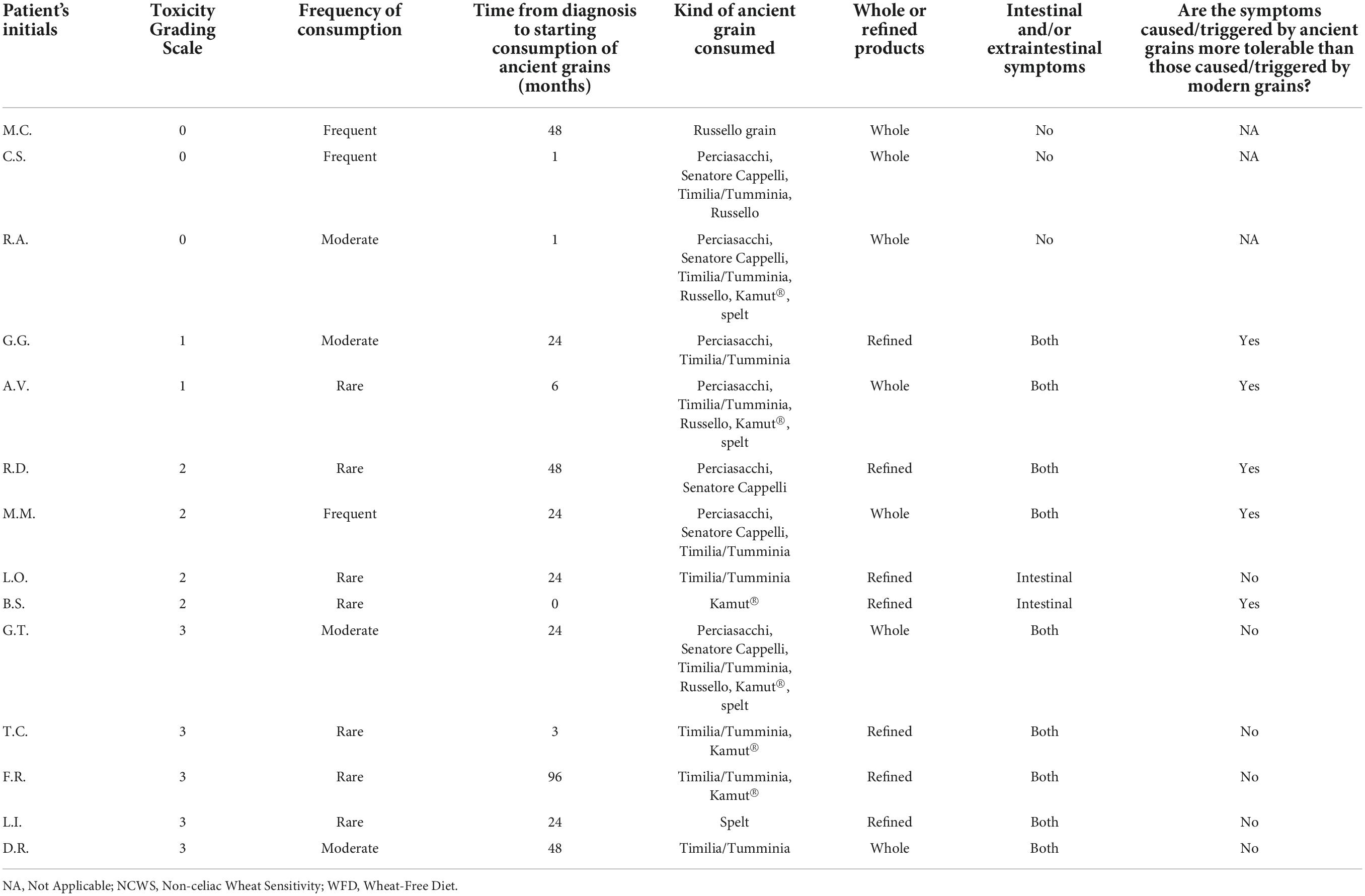
Table 3. Tolerability and consumption of ancient grains after NCWS diagnosis in 14 patients with strict adherence to modern WFD, according to toxicity grading scale order.
Finally, Figure 3 shows the kinds of ancient grains consumed by the NCWS patients with adherence score 3–4: Timilia/Tumminia (71.4%), Perciasacchi (50.0%), and Kamut® (42.9%) were the most frequent.
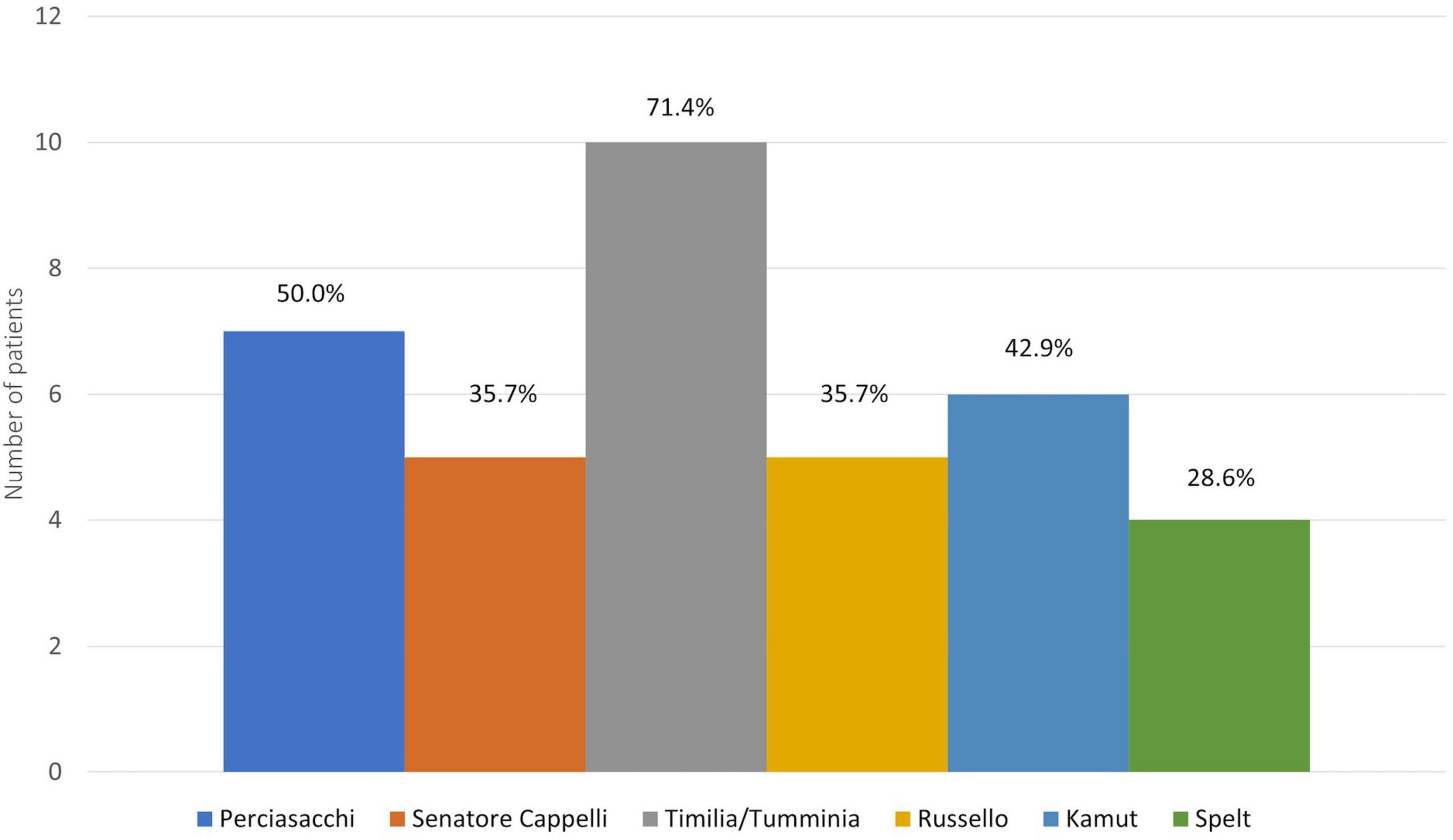
Figure 3. Kind of ancient grains consumed by patients with strict adherence to the modern wheat-free diet.
Treatment of NCWS essentially consists of prescribing a WFD, but due to the problematical nutritional, economic, social and psychological implications of adopting a classic WFD, there is growing interest in finding other viable options suitable for NCWS sufferers. For many people, and according to some preliminary scientific reports, the most plausible alternative would seem to be a diet that simply replaces “modern” with “ancient” grains (25). However, no studies to date have evaluated the real frequency of ancient wheat variety consumption in NCWS patients diagnosed by the rigorous DBPCC method, or the putative higher tolerability of these wheats in this wheat-related disease.
In our study, we evaluated 223 NCWS patients, diagnosed by DBPCC, to assess the frequency of a dietary intake of ancient grains, before and/or after diagnosis, and the possible effects on NCWS-related symptoms.
The demographic and clinical characteristics of the recruited NCWS patients are in agreement with existing reports in the scientific literature and with our own previous studies (26–32). Our patients showed a good, but not very high percentage of strict adherence to the modern WFD (48.0%), likely reflecting a strong self-perceived relationship between the recurrence of symptoms and the intake of modern wheat, and their attenuation or disappearance thanks to the elimination diet. As a matter of fact, all our patients with adherence score 3–4 reported a strong reduction or a complete disappearance of symptoms when following the modern WFD. It is interesting to note that in our study group even the patients with poor adherence to the diet (Score 1–2) reported a significant improvement in IBS-like and extra-intestinal symptoms in comparison to the period before the NCWS diagnosis. Nevertheless, about one third of all the patients had stopped following the WFD.
Regarding the consumption of ancient grains, 50.2% of our patients reported that they had habitually consumed ancient grains before NCWS diagnosis. A greater diagnostic delay was statistically more frequent in these subjects, a finding which could be the result of a putative clinical benefit obtained, which could have led patients to postpone the “diagnostic contact” with physicians. In addition, the lower frequency of constipation in these patients is interesting, and it could be linked to the very frequent use of ancient wheat flours in the form of whole grain products, thus with a higher fiber content compared to modern ones, which are much more frequently used refined. However, the consumption of additional vegetable fibers and water intake (which might influence bowel movements) was not investigated.
To assess the potential tolerability of ancient grains in NCWS patients after diagnosis, we evaluated only the recruited patients with adherence scores 3–4, thus eliminating any modern wheat intake effects. In this way, we selected only patients that had completely eliminated modern wheat from their diet and were, therefore, clinically completely well. In this subset of patients, the percentage of ancient grain consumers was 13.1% (14 patients). Despite being aware that they were breaking the WFD by consuming ancient grains, these patients reported that they had done so to evaluate their tolerability of the ancient grains because of the greater palatability they offered, and the lower cost compared to wheat-free products. The decision to try ancient grains was made after a median period of 24 months following NCWS diagnosis and in conditions of well-being. Only 3 patients reported excellent tolerability (toxicity grading scale 0). Among the patients who developed symptoms, 45.5% reported more tolerable symptoms than those secondary to modern wheat intake, and 36% consumed ancient grains more than once a week. As regards the various grain varieties consumed, we recorded a high consumption of Kamut® (42.9%) and of ancient Sicilian varieties, in particular Timilia/Tumminia (71.4%) and Perciasacchi (50.0%). In addition, all patients with a toxicity grading scale 0 ate wholegrain products, while 63.6% of patients with toxicity grading scale 1–3 consumed refined ones.
No definitive conclusions can be drawn from this study due to the small sample population of NCWS patients consuming ancient grains. Moreover, tolerability to the intake of ancient grains in these patients was variable, probably as a result of the different varieties of ancient grains consumed and the use of either whole grain or refined products. Nevertheless, the present study integrates findings already reported in the literature and confirms the need to further investigate the pathophysiology of NCWS, as it would appear to indicate that gluten is not the only “culprit.” Indeed, some data, mainly in vitro, suggest that a fair percentage of patients with NCWS could better tolerate certain varieties of ancient grains (17, 18). According to other authors, the symptoms in these patients might be linked not only to gluten but also to the presence of FODMAPs and/or ATIs in ingested wheat (2, 4, 5, 8, 33). It could be hypothesized that, the better tolerance to some ancient grains could be explained by the lower presence of FODMAPs and ATIs or other toxic peptides, compared to modern wheats (34–36). However, evidence in this regard seems to indicate that the amount of such components could be related to both the wheat genotype and the leavening methodology used (37, 38).
Our study has several limitations. First, the clinical data were collected at the time of diagnosis and used retrospectively. Thus, a selection bias must be considered, and our findings need to be confirmed in a larger and specifically designed prospective study. Second, the patients’ biochemical/immunological/histological response to the intake of ancient grains was not evaluated, and the self-reported consumption of ancient grains was not stratified in terms of quantity (grams). Consequently, we have no data to explain why only a certain percentage of NCWS subjects tolerated ancient wheat. Future studies should be planned to determine the biological basis of our results. Third, the NCWS patients who ate ancient wheat consumed different grains, very probably from different regional locations and grown in different seasons. Since the biochemical composition of grains is known to be influenced by these factors, we cannot extend the concept of a safe use of the tolerated grains quoted in our study without taking them into account. Moreover, it was not possible to assess any association between a specific symptom and the consumption of a certain variety of ancient wheat. Fourth, we did not investigate the reasons for non-adherence to the WFD in a percentage of the NCWS patients recruited to the study. Finally, we used a modified version of the adherence score to evaluate adherence to the “modern” WFD, which had not been previously validated for this specific purpose, but for all gluten-containing products in patients with CD.
However, the study does have some strengths: it is the first research study specifically aimed at evaluating the use of ancient grains and their clinical effects in NCWS patients. All the patients included were diagnosed by the DBPCC method, thus involving subjects identified by the current diagnostic gold standard.
In conclusion, our study shows a high frequency of consumption of ancient grains in NCWS patients before diagnosis, with a greater diagnostic delay and lower constipation frequency in this population, as well as a variable tolerability among the patients who consumed ancient grains after NCWS diagnosis. Further research is needed to define the pathophysiological mechanisms underlying their greater tolerability compared to modern grains in at least a subgroup of patients suffering from NCWS.
The datasets presented in this article are not readily available because the study presented here is a preliminary part of a larger project funded by the Italian Ministry of Health. Therefore, all, or part of, this data will be used to complete the main project. For this reason, the data cannot be provided. Requests to access the datasets should be directed to PM, cGFzcXVhbGUubWFuc3VldG9AdW5pcGEuaXQ=.
The studies involving human participants were reviewed and approved by Ethics Committee of the AOUP “P. Giaccone” Hospital of Palermo. The patients/participants provided their written informed consent to participate in this study.
ASe, AC, and PM had full access to all the data in the study and take full responsibility for the integrity of the data, the accuracy of the data analysis, and conceptualized and designed the study. PM, MP, AC, SC, DC, MC, GB, FF, SM, AG, and FM contributed to the acquisition of data. ASe, PM, AG, MS, MP, RD, SC, DC, MC, GB, FF, SM, AF, ASa, and AC analyzed and interpreted the data. ASe, AG, PM, MP, AC, ASa, and AF drafted the manuscript. AC, PM, ASe, AG, ASa, and AF contributed to critical revision of the manuscript for important intellectual content. MS contributed to statistical analysis. AC and PM obtained the funding. AC and AF supervised the study. All authors contributed to the article and approved the submitted version.
This study was funded by the Italian Health Ministry, Grant PE-2016-02363692 “Non-celiac gluten sensitivity (NCGS): Is gluten the true culprit? A clinical and immunological study on the tolerability of different wheat grains in NCGS patients.” The funder had no role in the study design, in the collection, analysis and interpretation of the data, in writing the manuscript nor in the decision to submit the manuscript for publication.
We would like to thank all the patients who agreed to participate in the study. We would also like to thank English native speaker Carole Greenall for revising the text.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.995019/full#supplementary-material
anti-Ttg, anti-tissue transglutaminase antibodies; ATI, amylase/trypsin Inhibitors; BMI, body mass index; CD, celiac disease; DBPCC, double-blind placebo-controlled challenges; EmA, anti-Endomysium antibodies; FODMAPs, fermentable oligosaccharides, disaccharides, monosaccharides and polyols; HLA, human leukocyte antigens; IBS, irritable bowel syndrome; Ig, Immunoglobulin; NCWS, non-celiac wheat sensitivity; SD, standard deviation; TLR4, toll-like receptor 4; WA, wheat allergy; WFD, wheat-free diet.
1. Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. (2021) 10:13. doi: 10.1186/1741-7015-10-13
2. Carroccio A, Rini G, Mansueto P. Non-celiac wheat sensitivity is a more appropriate label than non-celiac gluten sensitivity. Gastroenterology. (2014) 146:320–1. doi: 10.1053/j.gastro.2013.08.061
3. Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, Catassi C, et al. Celiac disease: a comprehensive current review. BMC Med. (2019) 17:142. doi: 10.1186/s12916-019-1380-z
4. Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. (2013) 145:320–8.e1-3. doi: 10.1053/j.gastro.2013.04.051
5. Skodje GI, Sarna VK, Minelle IH, Rolfsen KL, Muir JG, Gibson PR, et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. (2018) 154:529–39.e2. doi: 10.1053/j.gastro.2017.10.040
6. Brottveit M, Beitnes AC, Tollefsen S, Bratlie JE, Jahnsen FL, Johansen FE, et al. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol. (2013) 108:842–50. doi: 10.1038/ajg.2013.91
7. Schuppan D, Pickert G, Ashfaq-Khan M, Zevallos V. Non-celiac wheat sensitivity: differential diagnosis, triggers and implications. Best Pract Res Clin Gastroenterol. (2015) 29:469–76. doi: 10.1016/j.bpg.2015.04.002
8. Zevallos VF, Raker V, Tenzer S, Jimenez-Calvente C, Ashfaq-Khan M, Rüssel N, et al. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology. (2017) 152:1100–33.e12. doi: 10.1053/j.gastro.2016.12.006
9. Carroccio A, Giambalvo O, Blasca F, Iacobucci R, D’Alcamo A, Mansueto P. Self-reported non-celiac wheat sensitivity in high school students: demographic and clinical characteristics. Nutrients. (2017) 9:771. doi: 10.3390/nu9070771
10. Volta U, Bardella MT, Calabrò A, Troncone R, Corazza GR. Study group for non-celiac gluten sensitivity. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. (2014) 12:85. doi: 10.1186/1741-7015-12-85
11. Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, et al. Intestinal microbiota in functional bowel disorders: a Rome Foundation report. Gut. (2013) 62:159–76. doi: 10.1136/gutjnl-2012-302167
12. Dupont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. (2014) 39:1033–42. doi: 10.1111/apt.12728
13. Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. (2009) 146:41–6. doi: 10.1016/j.pain.2009.06.017
14. Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. (2014) 11:497–505. doi: 10.1038/nrgastro.2014.40
15. Farrè R, Tack J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. Am J Gastroenterol. (2013) 108:698–706.
16. Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. (2013) 108:707–17. doi: 10.1038/ajg.2013.96
17. Valerii MC, Ricci C, Spisni E, Di Silvestro R, De Fazio L, Cavazza E, et al. Responses of peripheral blood mononucleated cells from non-celiac gluten sensitive patients to various cereal sources. Food Chem. (2015) 176:167–74. doi: 10.1016/j.foodchem.2014.12.061
18. Alvisi P, De Fazio L, Valerii MC, Cavazza E, Salerno A, Lacorte D, et al. Responses of blood mononucleated cells and clinical outcome of non-celiac gluten sensitive pediatric patients to various cereal sources: a pilot study. Int J Food Sci Nutr. (2017) 68:1005–12. doi: 10.1080/09637486.2017.1315058
19. Sofi F, Whittaker A, Gori AM, Cesari F, Surrenti E, Abbate R, et al. Effect of Triticum turgidum subsp. Turanicum wheat on irritable bowel syndrome: a double-blinded Khorasan dietary intervention trial. Br J Nutr. (2014) 111:1992–9. doi: 10.1017/S000711451400018X
20. Hoevenaars F, Van der Kamp JW, Van den Brink W, Wopereis S. Next generation health claims based on resilience: the example of whole-grain wheat. Nutrients. (2020) 12:2945. doi: 10.3390/nu12102945
21. Caminero A, McCarville JL, Zevallos VF, Pigrau M, Yu XB, Jury J, et al. Lactobacilli degrade wheat amylase trypsin inhibitors to reduce intestinal dysfunction induced by immunogenic wheat proteins. Gastroenterology. (2019) 156:2266–80. doi: 10.1053/j.gastro.2019.02.028
22. Biagi F, Andrealli A, Bianchi PI, Marchese A, Klersy C, Corazza GR. A gluten-free diet score to evaluate dietary compliance in patients with coeliac disease. Br J Nutr. (2009) 102:882–7. doi: 10.1017/S0007114509301579
23. Zanini B, Petroboni B, Not T, Di Toro N, Villanacci V, Lanzarotto F, et al. Search for atoxic cereals: a single blind, cross-over study on the safety of a single dose of Triticum monococcum, in patients with celiac disease. BMC Gastroenterol. (2013) 13:92. doi: 10.1186/1471-230X-13-92
24. Edwards IR, Biriell C. Harmonisation in pharmacovigilance. Drug Saf. (1994) 10:93–102. doi: 10.2165/00002018-199410020-00001
25. Ianiro G, Rizzatti G, Napoli M, Matteo MV, Rinninella E, Mora V, et al. A durum wheat variety-based product is effective in reducing symptoms in patients with non-celiac gluten sensitivity, a double-blind randomized cross-over trial. Nutrients. (2019) 11:712. doi: 10.3390/nu11040712
26. Catassi C, Alaedini A, Bojarski C, Bonaz B, Bouma G, Carroccio A, et al. The overlapping area of non-celiac gluten sensitivity (NCGS) and wheat-sensitive irritable bowel syndrome (IBS): an update. Nutrients. (2017) 9:1268. doi: 10.3390/nu9111268
27. Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol. (2012) 107:1898–906. doi: 10.1038/ajg.2012.236
28. Catassi C, Elli L, Bonaz B, Bouma G, Carroccio A, Castillejo G, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the salerno experts’criteria. Nutrients. (2015) 7:4966–77. doi: 10.3390/nu7064966
29. Khan A, Suarez MG, Murray JA. Nonceliac gluten and wheat sensitivity. Clin Gastroenterol Hepatol. (2020) 18: doi: 10.1016/j.cgh.2019.04.009
30. Molina-Infante J, Carroccio A. Suspected nonceliac gluten sensitivity confirmed in few patients after gluten challenge in double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol. (2017) 15:339–48. doi: 10.1016/j.cgh.2016.08.007
31. Carroccio A, D’Alcamo A, Iacono G, Soresi M, Iacobucci R, Arini A, et al. Persistence of nonceliac wheat sensitivity, based on long-term follow-up. Gastroenterology. (2017) 153:56–8.e3. doi: 10.1053/j.gastro.2017.03.034
32. Carroccio A, Soresi M, Chiavetta M, La Blasca F, Compagnoni S, Giuliano A, et al. Frequency and clinical aspects of neurological and psychiatric symptoms in patients with non-celiac wheat sensitivity. Nutrients. (2021) 13:1971. doi: 10.3390/nu13061971
33. Priyanka P, Gayam S, Kupec JT. The role of a low fermentable oligosaccharides, disaccharides, monosaccharides, and polyol diet in nonceliac gluten sensitivity. Gastroenterol Res Pract. (2018) 2018:1561476. doi: 10.1155/2018/1561476
34. Geisslitz S, Longin CFH, Koehler P, Scherf KA. Comparative quantitative LC-MS/MS analysis of 13 amylase/trypsin inhibitors in ancient and modern Triticum species. Sci Rep. (2020) 10:14570. doi: 10.1038/s41598-020-71413-z
35. Ruisi P, Ingraffia R, Urso V, Giambalvo D, Alfonzo A, Corona O, et al. Influence of grain quality, semolinas and baker’s yeast on bread made from old landraces and modern genotypes of Sicilian durum wheat. Food Res Int. (2021) 140:110029. doi: 10.1016/j.foodres.2020.110029
36. Spisni E, Imbesi V, Giovanardi E, Petrocelli G, Alvisi P, Valerii MC. Differential physiological responses elicited by ancient and heritage wheat cultivars compared to modern ones. Nutrients. (2019) 11:2879. doi: 10.3390/nu11122879
37. Sievers S, Rohrbach A, Beyer K. Wheat-induced food allergy in childhood: ancient grains seem no way out. Eur J Nutr. (2020) 59:2693–707. doi: 10.1007/s00394-019-02116-z
Keywords: Non-celiac Wheat Sensitivity (NCWS), ancient grains, wheat free diet, wheat tolerability, Amylase-Trypsin Inhibitors (ATIs)
Citation: Seidita A, Mansueto P, Giuliano A, Chiavetta M, Mandreucci F, Soresi M, Pistone M, Compagnoni S, Castellucci D, Bisso G, Faraci F, Maestri S, Disclafani R, Sapone A, Fasano A and Carroccio A (2022) Potential tolerability of ancient grains in non-celiac wheat sensitivity patients: A preliminary evaluation. Front. Med. 9:995019. doi: 10.3389/fmed.2022.995019
Received: 15 July 2022; Accepted: 13 September 2022;
Published: 28 September 2022.
Edited by:
Stefano Guandalini, The University of Chicago, United StatesReviewed by:
Marco Silano, National Institute of Health (ISS), ItalyCopyright © 2022 Seidita, Mansueto, Giuliano, Chiavetta, Mandreucci, Soresi, Pistone, Compagnoni, Castellucci, Bisso, Faraci, Maestri, Disclafani, Sapone, Fasano and Carroccio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Carroccio, YWNhcnJvY2Npb0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.