
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 05 October 2022
Sec. Pathology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.993091
Bronchogenic cysts are congenital malformations caused by aberrant foregut budding. They major occur in the thorax, with subdiaphragmatic cases being uncommon. Here, we present a series of 19 patients diagnosed with subdiaphragmatic bronchogenic cysts histopathologically at a single institution in China from 2012 to 2021. A literature review was also conducted by searching the PubMed database using keywords related to “bronchogenic cysts” and “subdiaphragmatic,” yielding 107 cases. Taken together, the 126 cases had a median age of 41.0 years (interquartile range, 30.0–51.0 years) and 62 of them were male (49.2%). The cysts were most commonly detected in the left adrenal region (36.2%), followed by the pancreatic region (11.5%) and gastric cardia/lesser curvature of the stomach (9.2%). All patients except two underwent surgery for a definite diagnosis, symptom alleviation, and (or) malignancy prevention. Most patients recovered fast and were discharged from the hospital within 1 week after surgery, and the surgical complications were infrequent. The prognosis was generally favorable, as no recurrence was reported during the follow-up as long as 77 months.
Bronchogenic cysts are congenital cysts caused by aberrant budding of the primitive foregut or tracheobronchial tree. They usually locate in the thorax, especially in the mediastinum (1–3). However, they can be found in various atypical locations along the developmental pathway of the foregut, ranging from the pharynx to the spinal canal (4, 5). Bronchogenic cysts can occur infrequently below the diaphragm, with the majority located in the retroperitoneal space, particularly the left adrenal region. Subdiaphragmatic bronchogenic cysts (sBCs) can be asymptomatic, and thus are usually recognized occasionally by imaging analyses. Nevertheless, symptoms, such as fever, abdominal pain, and nausea, appear when the cysts become infected or expand sufficiently to compress nearby organs (6, 7). Due to their rarity and non-specific imaging presentation, sBCs are frequently misdiagnosed, and only histopathological examination can currently provide a definitive diagnosis. Additionally, though generally benign, malignant transformation was observed in several cases (8). Therefore, up to now, surgical resection has been the only therapeutic strategy to alleviate symptoms, establish a definitive diagnosis, and prevent malignant transformation in patients with sBCs.
Despite the fact that cases of sBCs have been previously reported (6, 7), the sample size was generally small, leading to an obscure understanding of the epidemiology, clinical presentation, diagnosis, and management strategy, et al. Here, we aim to provide a better comprehension of sBCs by presenting a series of 19 patients from a single institution as well as a review of related articles.
All cases of sBCs from 2012 to 2021 at Peking Union Medical College Hospital in China were retrospectively identified from the hospital information system. Only patients with histopathological diagnoses of sBCs were included in the analysis. Parameters, such as demographic characteristics, clinical presentation, imaging findings, surgical information, and histopathological examination, were extracted from electronic medical records. All patients were contacted by phone in May 2022 for information on recurrence and surgical complications.
For the literature review, PubMed was searched in May 2022 using the following keywords: “bronchogenic cyst*”, “bronchial cyst*,” “subdiaphragm*,” “retroperitoneal,” and “abdom*.” The detailed search strategy can be found in Supplementary Appendix 1. The inclusion criteria of studies were full-text English articles reporting patient(s) diagnosed as sBCs histopathologically with detailed demographic and clinical information. Unpaired t-test or Pearson’s chi-square test was employed when appropriate. All statistical analyses were performed using SPSS 22.0.
Nineteen patients (4 [21.1%] male; median age, 44.0 years [interquartile range, 35.0–46.5 years]) with histopathological diagnoses of subdiaphragmatic bronchogenic cysts from 2012 until 2021 were identified. Patient demographics are depicted in Table 1. Fourteen patients (73.7%) were asymptomatic and discovered the cyst by accident, either during a regular check-up (n = 12) or a radiographic examination done for unrelated indications (n = 2). Among the symptomatic patients (n = 5), two complained of flank pain, one of abdominal pain, one of coughing, and the other of acid reflux and abdominal distention. In all patients, the cyst was solitary with a median diameter of 5.1 cm (interquartile range [IQR]: 3.6-6.6 cm). The cysts were inclined to locate in the left abdomen (15/19, 78.9%) rather than the midline or right abdomen, the upper abdomen (16/19, 84.2%) instead of the lower abdomen, and the retroperitoneal space (16/19, 84.2%) compared with intra-abdomen. The left adrenal region (7/19, 36.8%) was the most common location, followed by the pancreatic region (3/19, 15.8%) and gastric cardia/lesser curvature of the stomach (3/19, 15.8%). On computed tomography (CT), the density of cysts ranged from low to slightly high. The number of cysts with low to slightly low, soft-tissue, and slightly high density on CT is eight, nine, and two, respectively. Only three patients underwent magnetic resonance imaging (MRI), and all of their cysts were hypointense on T1 weighted image. As for T2 weighted image, two of them were hyperintense while the other was hypointense. Twelve of 19 cysts (63.2%) showed no enhancement on CT or MRI. Ten patients received the B ultrasound examination, displaying an anechoic (n = 3), hypoechoic (n = 3), or mix-echoic (anechoic to hypoechoic) cyst (n = 4). Three of the cysts were multilocular with septa present. The representative appearance of the cysts on CT, MRI, and ultrasound is demonstrated in Figure 1. Due to their rarity, none of the patients were diagnosed with sBCs before the surgery.
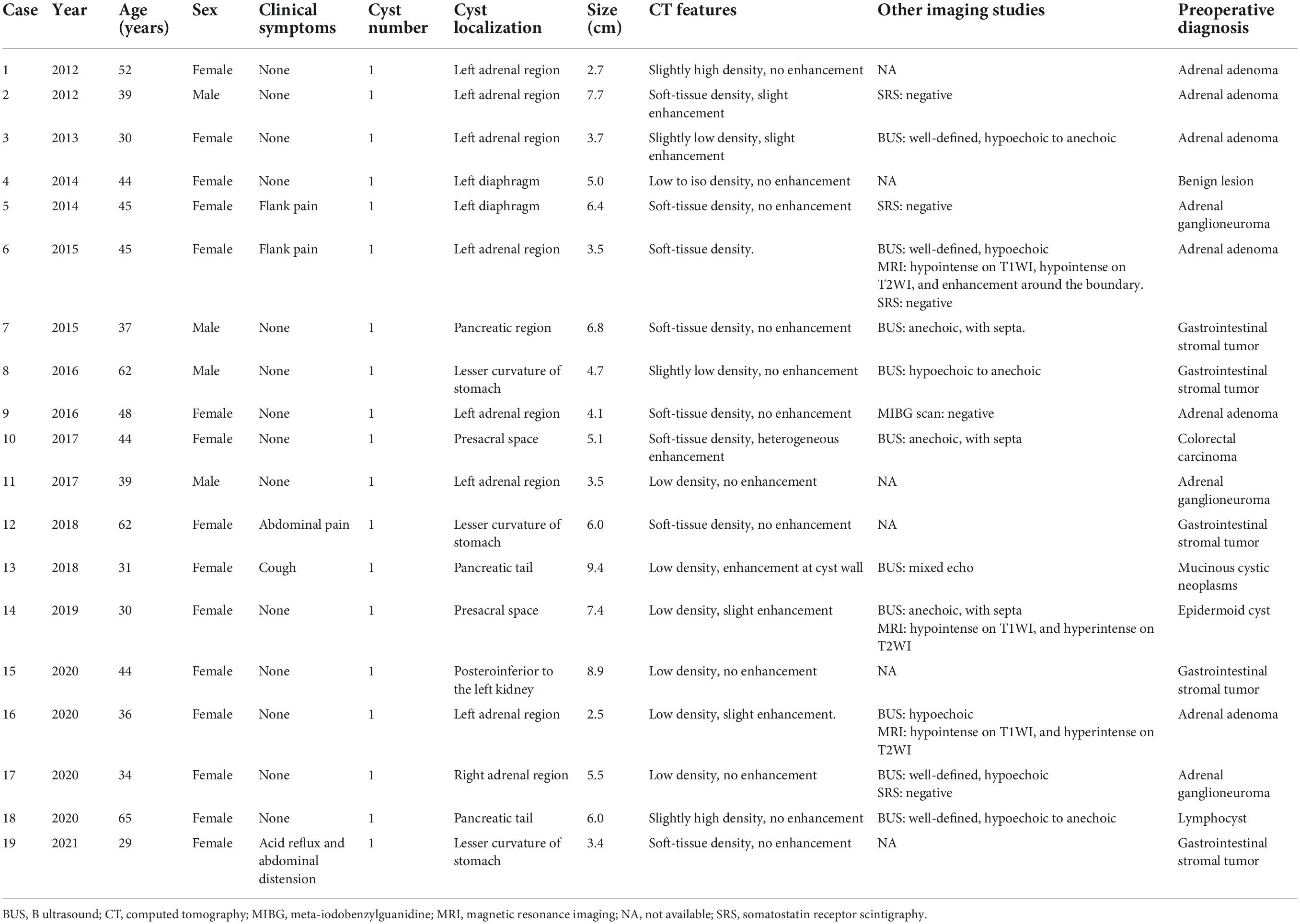
Table 1. Demographics, clinical symptoms, imaging features, and preoperative diagnosis of 19 patients with subdiaphragmatic bronchogenic cyst from our case series.
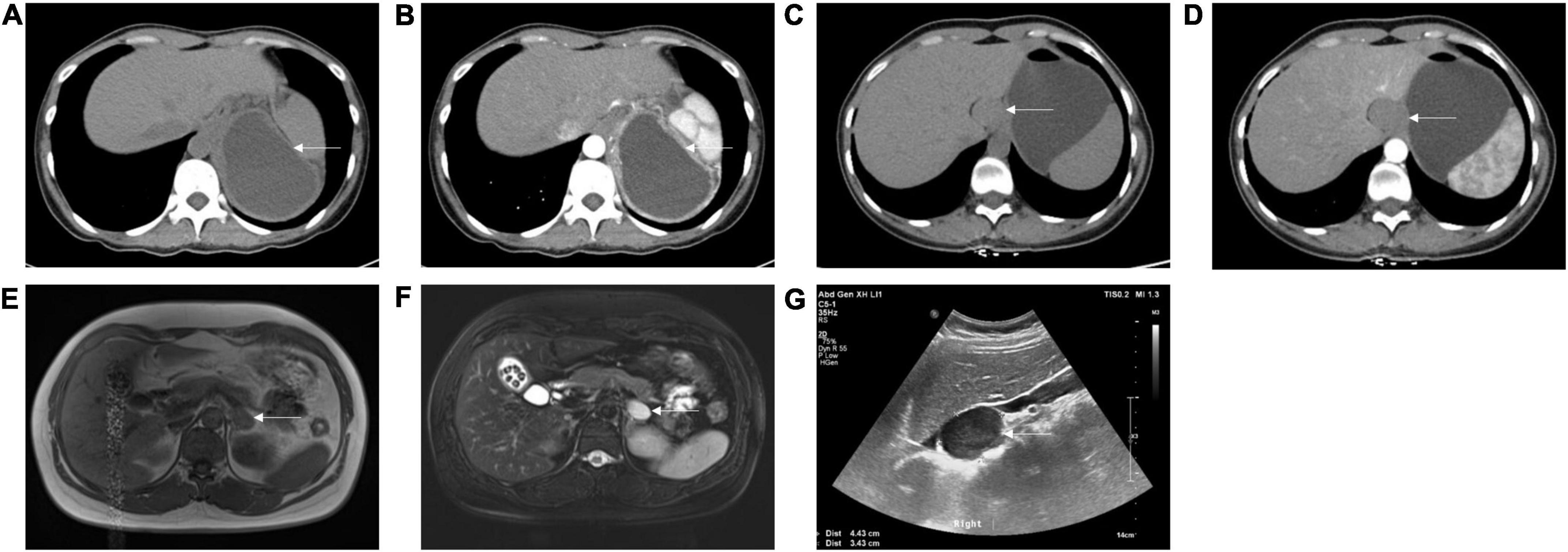
Figure 1. Appearance of subdiaphragmatic bronchogenic cysts on computed tomography (CT), magnetic resonance imaging (MRI), and B ultrasound. (A) Non-enhanced CT showed a low-density cystic lesion between the pancreatic tail, stomach, spleen, and diaphragm from case 13. (B) Enhanced CT showed slight enhancement of the cystic wall from case 13. (C) Non-enhanced CT showed a soft-tissue density lesion adjacent to the lesser curvature of the stomach from case 19. (D) Enhanced CT showed no enhancement of the lesion from case 19. (E) T1 weighted image on MRI showed a hypointense lesion at the left adrenal region from case 16. (F) T2 weighted image on MRI showed a hyperintense lesion at the left adrenal region from case 16. (G) B ultrasound showed a well-defined, hypoechoic lesion at the right adrenal region from case 17. Arrows indicate the cysts.
Laparoscopic surgery was conducted in most of the patients (16/19, 84.2%), while two underwent laparotomy and one underwent video-assisted thoracoscopic surgery (VATS; Table 2). The content of cysts was found to be either serous (1/13, 7.7%), mucoid (3/13, 23.1%), gelatinous (6/13, 46.2%), brittle (2/13, 15.4%), or mixed (1/13, 7.7%). Specifically, the gross appearance of the cyst from case 18 is depicted in Figure 2, with a cut surface demonstrating the gelatinous content within the cyst. Generally, most patients recovered fast after surgery and were discharged from the hospital within one week post-surgery. Two patients (cases 6 and 13) encountered perioperative complications and thus stayed for an extended period of time in the hospital. Case 6 had lymphatic leakage that improved with local drainage and a low-fat diet. Case 13 experienced gastroparesis and gastrointestinal decompression and acupuncture led to improvement.
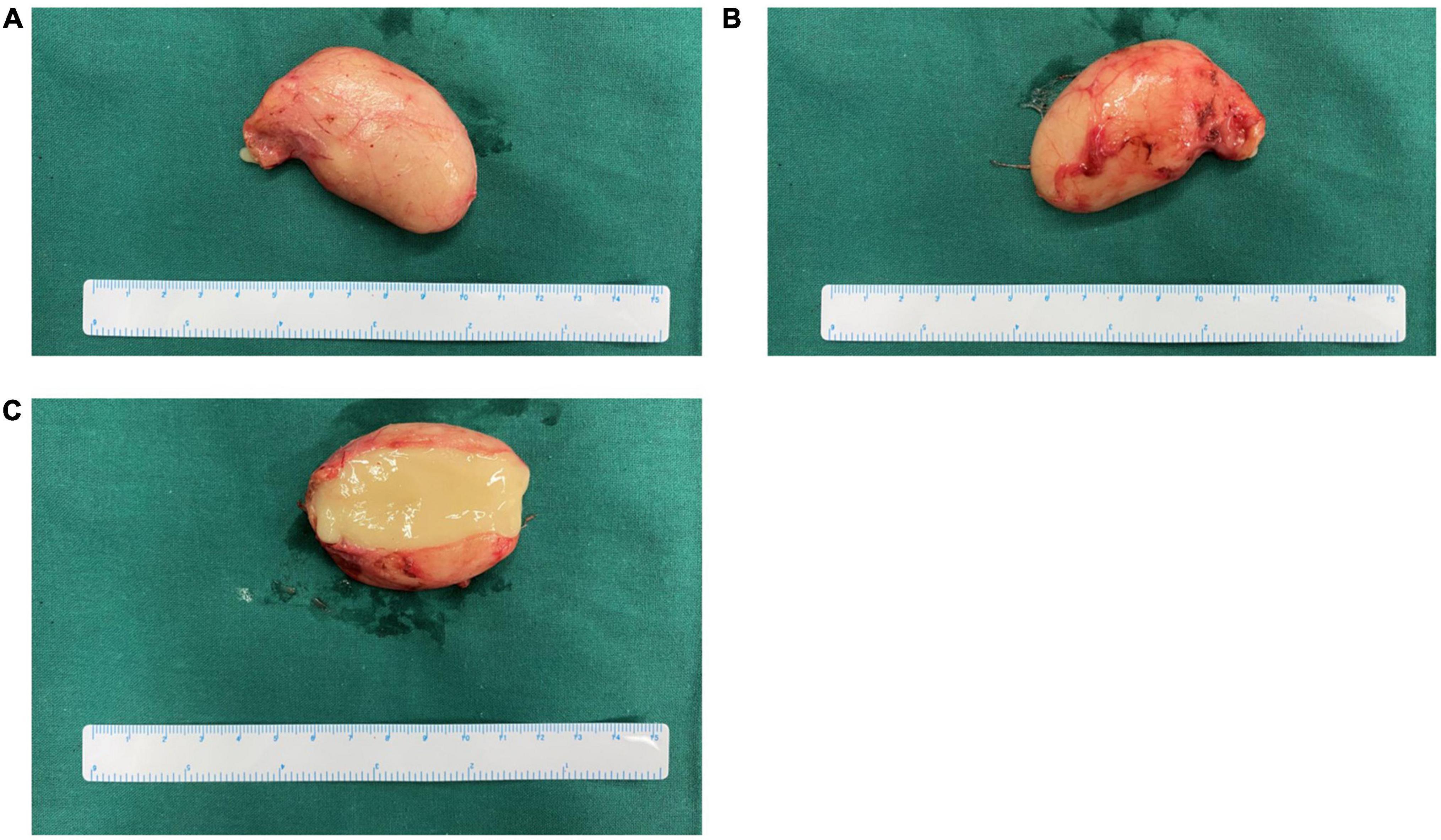
Figure 2. Gross appearance of the cyst from case 18 in our series. (A,B) Gross appearance from two sides, respectively. (C) Cross-section of cyst showed yellowish gelatinous content.
We attempted to contact all patients for information on recurrence and long-term complications in May, 2022, with 12 responses. Unfortunately, not every patient had regular imaging examinations after surgery, but they all claimed no signs of recurrence until their most recent imaging assessment, and the longest one was 77 months after surgery. As for long-term complications, two patients (cases 8 and 12) had incision hernias and both received surgery for repairment. Additionally, left renal artery stenosis and left renal atrophy were discovered 8 months after surgery in case 5 whose cyst was located in the left adrenal region. Later, this patient received a left nephrectomy.
194 records were retrieved initially after searching the PubMed using the strategy displayed in Supplementary Appendix 1. Among them, 49 were non-English, and the full text was not available for 21 English citations. Of the remaining 124 articles, 95 were identified to describe cases of sBCs and were included for further analysis (6, 7, 9–101). The detailed information of all included studies is displayed in Supplementary Table 1.
The 95 citations reported 107 cases (58 [54.2%] male) in total (Table 3). The median age of the cases was 41.0 years, with an IQR of 27.0–51.0 years. Specifically, the cysts could be identified as early as the prenatal stage (19, 64, 87), and were histopathologically diagnosed as sBCs later either through an autopsy after a spontaneous abortion or surgery after birth. The cases were reported from 24 countries around the world, and China is the one that described the most (25/107, 23.4%). Different from our series (5/19, 26.3%), more than half of the cases from the literature were symptomatic (59/103, 57.3%). Abdominal pain/flank pain was the most common symptom (35/103, 34.0%), and other symptoms included nausea, vomiting, and abdominal discomfort, et al.
sBC was solitary in 96.3% (103/107) cases. One case had two cysts in the left adrenal region (62), and one case had three cysts arising from the stomach (73). In one case, the cysts were found to be bilateral, affecting both the left and right adrenal regions (66). Moreover, multiple cysts could be identified on both sides of the diaphragm, as one patient had one cyst in the left adrenal region with three in the left lung (75). The median size of the 111 sBCs reported in the literature was 5.0 cm (IQR: 3.5–7.3 cm), and could be as large as 18.9 cm (30). It should be noticed that the sBCs might enlarge over time, as manifested in several studies (19, 25, 37, 38, 50). Similar to our series, the cysts reported in the literature were more likely to be detected in the left abdomen (85/111, 76.6%), upper abdomen (105/111, 94.6%), and retroperitoneal space (85/111, 76.6%). The three most frequent areas for sBCs were the left adrenal region (40/111, 36.0%), pancreatic region (12/111, 10.8%), and gastric cardia/lesser curvature of the stomach (9/111, 8.1%).
Generally speaking, patients with sBCs usually showed normal blood tests, though abnormalities could be seen seldomly. For instance, several patients had significantly elevated serum levels of carbohydrate antigen 19-9 (CA19-9), even reaching 4330.7 U/mL. In these cases, bronchial epithelial cells were positive for CA19-9 in the immunohistochemical study on surgical specimens and the serum levels returned to normal after surgery (27, 81, 98). Interestingly, one study illustrated that the sBC was able to uptake radioiodine in the single photon emission computed tomography (SPECT) examination (68). Whether this is universal awaits further evidence.
All patients except two underwent surgery in the literature. Laparoscopy was the most common surgical procedure for patients with sBCs (42/72, 58.3%), followed by laparotomy (23/72 31.9%). One of the two patients that did not receive surgery was diagnosed with sBC based on the pathohistological examination of the specimen gained from CT-guided biopsy (56). The patient was followed up for 3 years with a CT examination every 6 months, showing a stable lesion. The other patient was diagnosed through core biopsy guided by both fine needle aspirate (FNA) and endoscopic ultrasound (EUS), but the follow-up information was absent in the article (82). Though predominantly benign, malignant transformation has been demonstrated in two cases, one with well-differentiated papillary adenocarcinoma (17) and the other with intermediate-grade neuroendocrine tumor (89). The latter patient remained asymptomatic and had no recurrence for 14 months after surgery. In addition to this, the follow-up information was also provided for another 32 cases and the longest reached 49 months. There was no sign of recurrence in any of the cases, indicating a good prognosis of sBCs.
Bronchogenic cysts are primitive-foregut-derived congenital cystic abnormalities that usually occur in the thorax, particularly in the mediastinum (1–3). Infrequently, they can be found in subdiaphragmatic region, and only around 100 cases were reported in English up to now based on our database searching. Our case series reported here added another 19 cases.
As displayed in three articles, sBCs can be detected even during pregnancy (19, 64, 87), but most patients discovered the cyst(s) in their thirties and forties. As for sex distribution, our study and the literature produced different results. Females predominated in our study, while males had a slightly higher proportion in the literature review. One possible reason is that the sample size of our series is insufficient, while reporting bias of the articles is an alternative explanation. As our series and the literature illustrated, most of the cysts were solitary. Only on rare ocaasions were they reported to be multiple, either unilateral or bilateral, either on the same side or the both sides of diaphragm. The size of sBCs varied greatly, ranging from 1.5 to 18.9 cm based on available data from our cases and citations, but the diameter of the cysts was mostly between 3.0 and 7.0 cm (Supplementary Table 1). When it comes to the localization, our research demonstrated that the cysts were more likely to be found in the left abdomen, upper abdomen, and retroperitoneal space. Moreover, in addition to the previously reported left adrenal region and pancreatic region (7), our analysis revealed that gastric cardia/lesser curvature of the stomach was another predilection site for sBCs.
A large proportion of patients with sBCs were asymptomatic (73.7% in our series, and 42.7% in the literature review). Symptoms caused by sBCs were usually non-specific, mainly due to complications such as local compression and infection. For example, one patient with sBC in the left adrenal region had a 5-year history of hypertension (63). After the cyst was resected completely, his blood pressure returned to normal. This patient’s hypertension might be caused by a compression on the adrenal gland, kidney, or renal artery. In one of our cases (case 13), the cystic fluid was found to be turbid and smelly during the surgery. The culture of cystic fluid was positive for Streptococcus anginosus, providing direct evidence of intra-cyst infection. Patients with sBCs lacked specific symptoms, which was the same for laboratory findings. The tumor markers test did not aid in the diagnosis of sBCs, as nearly all patients tested negative for them. Seldomly, the epithelial cells lining the cyst were able to produce tumor markers like CA19-9, leading to a significantly elevated serum level (27, 81, 98). Additionally, it was also difficult to differentiate sBCs from other more common subdiaphragmatic cystic lesions based on imaging examinations. The density of sBCs on CT ranged from low to high, with either enhancement or no enhancement. It could be multilocular with septa or unilocular. In summary, sBCs could not be diagnosed preoperatively as they lack a distinct clinical presentation as well as laboratory and imaging findings. Furthermore, their rarity complicates the diagnosis.
Up to now, a histopathological examination is required for the diagnosis of sBCs. Biopsy guided by CT or EUS could provide the specimen before surgery. Several cases from the literature were diagnosed based on biopsy, and two of them did not receive surgery because the patients were asymptomatic (56, 82). In particular, the lesion of one patient remained stable during the 3-year follow-up (56). According to Berger-Richardson et al., core needle biopsy of retroperitoneal lesion had low rates of both early complications and needle tract seeding (102). Given that nearly 80% of sBCs were found in retroperitoneal space and they were generally benign, it might be feasible to perform a biopsy for a diagnosis and thus avoid surgery for some of the patients with retroperitoneal bronchogenic cysts. However, it should be noticed that the biopsy might be non-diagnostic due to the sampling issue (46). Furthermore, quite a few patients had symptoms that could only be alleviated by surgery. Additionally, though rare, malignant changes were detected in two cases and only affected a portion of the cyst in both cases (17, 89). Other clinical parameters, such as symptoms, cyst size, tumor marker levels, and imaging findings, did not help predict malignancy. Therefore, it was very likely that malignancy was missed due to the sampling issue of biopsy. According to our cases and the literature, patients usually recovered quickly after surgery, with a low rate of post-surgery complications. Because of the aforementioned factors, though biopsy might help with the diagnosis of sBCs, it could not yet replace surgery at present.
After the complete cyst(s) resection, patients with sBCs generally had a good prognosis. Forty-five (n = 12 in our series, and n = 33 in the literature) patients had no recurrence during the follow-up, and the longest one reached 77 months in our series. Particularly, one patient with malignant change reported in the literature was also free of recurrence for 14 months after surgery (89).
In conclusion, our series added another 19 cases to the list of rarely reported sBCs. Together with the 107 published cases, we demonstrated that the sBCs are predisposed to locate in the left adrenal region, pancreatic region, and gastric cardia/lesser curvature of the stomach. Patients with sBCs lack specific clinical symptoms, laboratory examinations, and imaging features. Surgery is still the best management strategy for patients for a definite diagnosis, symptom alleviation, and complication prevention. The prognosis is generally favorable after complete resection of the lesion.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The ethics committee waived the requirement of written informed consent for participation.
WL conceived the study. JX and XZ contributed to the case collection, discussion, literature review, and first manuscript draft. WL, HZ, TH, BL, and XH provided critical revisions. All authors contributed to the article and approved the submitted version.
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (2020YFF0305104).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.993091/full#supplementary-material
1. St-Georges R, Deslauriers J, Duranceau A, Vaillancourt R, Deschamps C, Beauchamp G, et al. Clinical spectrum of bronchogenic cysts of the mediastinum and lung in the adult. Ann Thorac Surg. (1991) 52:6–13. doi: 10.1016/0003-4975(91)91409-O
2. Aktoðğ S, Yuncu G, Halilçolar H, Ermete S, Buduneli T. Bronchogenic cysts: clinicopathological presentation and treatment. Eur Respir J. (1996) 9:2017–21. doi: 10.1183/09031936.96.09102017
3. Limaïem F, Ayadi-Kaddour A, Djilani H, Kilani T, El Mezni F. Pulmonary and mediastinal bronchogenic cysts: a clinicopathologic study of 33 cases. Lung. (2008) 186:55–61. doi: 10.1007/s00408-007-9056-4
4. Xu Y, Han F, Seng D, Jiang L, Wang S, Ni X, et al. A clinical analysis of pharyngeal bronchogenic cysts in the pharynx of children. Front Pediatr. (2021) 9:629009. doi: 10.3389/fped.2021.629009
5. Ma X, Li W, Niu C, Liang F, Guo L, Shakir TM, et al. Intraspinal bronchogenic cyst: series of case reports and literature review. J Spinal Cord Med. (2017) 40:141–6. doi: 10.1080/10790268.2017.1279816
6. Liang MK, Yee HT, Song JW, Marks JL. Subdiaphragmatic bronchogenic cysts: a comprehensive review of the literature. Am Surg. (2005) 71:1034–41. doi: 10.1177/000313480507101210
7. Yuan K, Shu M, Ma Y, Feng W, Ye J, Yuan Y. Ectopic bronchogenic cyst in the retroperitoneal region: a case report and literature review of adult patients. BMC Surg. (2021) 21:347. doi: 10.1186/s12893-021-01341-w
8. Kirmani B, Kirmani B, Sogliani F. Should asymptomatic bronchogenic cysts in adults be treated conservatively or with surgery? Interact Cardiovasc Thorac Surg. (2010) 11:649–59. doi: 10.1510/icvts.2010.233114
9. Sumiyoshi K, Shimizu S, Enjoji M, Iwashita A, Kawakami K. Bronchogenic cyst in the abdomen. Virchows Arch A Pathol Anat Histopathol. (1985) 408:93–8. doi: 10.1007/BF00739965
10. Braffman B, Keller R, Gendal ES, Finkel SI. Subdiaphragmatic bronchogenic cyst with gastric communication. Gastrointest Radiol. (1988) 13:309–11. doi: 10.1007/BF01889087
11. Swanson SJ III, Skoog SJ, Garcia V, Wahl RC. Pseudoadrenal mass: unusual presentation of bronchogenic cyst. J Pediatr Surg. (1991) 26:1401–3. doi: 10.1016/0022-3468(91)91046-2
12. Fischbach R, Benz-Bohm G, Berthold F, Eidt S, Schmidt R. Infradiaphragmatic bronchogenic cyst with high CT numbers in a boy with primitive neuroectodermal tumor. Pediatr Radiol. (1994) 24:504–5. doi: 10.1007/BF02015013
13. Resl M, Navrátil P, Krajina A. Retroperitoneal bronchogenic cyst in a young adult. Respiration. (1996) 63:387–9. doi: 10.1159/000196583
14. Doggett RS, Carty SE, Clarke MR. Retroperitoneal bronchogenic cyst masquerading clinically and radiologically as a phaeochromocytoma. Virchows Arch. (1997) 431:73–6. doi: 10.1007/s004280050071
15. Buckley JA, Siegelman ES, Birnbaum BA, Rosato EF. Bronchogenic cyst appearing as a retroperitoneal mass. AJR Am J Roentgenol. (1998) 171:527–8. doi: 10.2214/ajr.171.2.9694496
16. Itoh H, Shitamura T, Kataoka H, Ide H, Akiyama Y, Hamasuna R, et al. Retroperitoneal bronchogenic cyst: report of a case and literature review. Pathol Int. (1999) 49:152–5. doi: 10.1046/j.1440-1827.1999.00837.x
17. Sullivan SM, Okada S, Kudo M, Ebihara Y. A retroperitoneal bronchogenic cyst with malignant change. Pathol Int. (1999) 49:338–41. doi: 10.1046/j.1440-1827.1999.00869.x
18. Yang SW, Linton JA, Ryu SJ, Shin DH, Park CS. Retroperitoneal multilocular bronchogenic cyst adjacent to adrenal gland. Yonsei Med J. (1999) 40:523–6. doi: 10.3349/ymj.1999.40.5.523
19. Bagolan P, Bilancioni E, Nahom A, Trucchi A, Inserra A, Neri M, et al. Prenatal diagnosis of a bronchogenic cyst in an unusual site. Ultrasound Obstet Gynecol. (2000) 15:66–8. doi: 10.1046/j.1469-0705.2000.00022.x
20. Reichelt O, Grieser T, Wunderlich H, Möller A, Schubert J. Bronchogenic cyst. A rare differential diagnosis of retroperitoneal tumors. Urol Int. (2000) 64:216–9. doi: 10.1159/000030534
21. Murakami R, Machida M, Kobayashi Y, Ogura J, Ichikawa T, Kumazaki T. Retroperitoneal bronchogenic cyst: CT and MR imaging. Abdom Imaging. (2000) 25:444–7. doi: 10.1007/s002610000019
22. Haddadin WJ, Reid R, Jindal RM. A retroperitoneal bronchogenic cyst: a rare cause of a mass in the adrenal region. J Clin Pathol. (2001) 54:801–2. doi: 10.1136/jcp.54.10.801
23. Ingu A, Watanabe A, Ichimiya Y, Saito T, Abe T. Retroperitoneal bronchogenic cyst: a case report. Chest. (2002) 121:1357–9. doi: 10.1378/chest.121.4.1357
24. Martín R, Sanz E, de Vicente E, Ortega P, Labrador E, Paumard A, et al. Differential diagnosis of asymptomatic retroperitoneal cystic lesion: a new case of retroperitoneal bronchogenic cyst. Eur Radiol. (2002) 12:949–50. doi: 10.1007/s003300101119
25. McCrystal DJ, Borzi PA. Retroperitoneoscopic resection of retroperitoneal bronchogenic cysts. Pediatr Surg Int. (2002) 18:375–7. doi: 10.1007/s00383-002-0829-9
26. Andersson R, Lindell G, Cwikiel W, Dawiskiba S. Retroperitoneal bronchogenic cyst as a differential diagnosis of pancreatic mucinous cystic tumor. Dig Surg. (2003) 20:55–7. doi: 10.1159/000068851
27. Hisatomi E, Miyajima K, Yasumori K, Okamura H, Nonaka M, Watanabe J, et al. Retroperitoneal bronchogenic cyst: a rare case showing the characteristic imaging feature of milk of calcium. Abdom Imaging. (2003) 28:716–20. doi: 10.1007/s00261-003-0003-4
28. Ishizuka O, Misawa K, Nakazawa M, Nishizawa O. A retroperitoneal bronchogenic cyst: laparoscopic treatment. Urol Int. (2004) 72:269–70. doi: 10.1159/000077129
29. Kim KH, Kim JI, Ahn CH, Kim JS, Ku YM, Shin OR, et al. The first case of intraperitoneal bronchogenic cyst in Korea mimicking a gallbladder tumor. J Korean Med Sci. (2004) 19:470–3. doi: 10.3346/jkms.2004.19.3.470
30. Goh BK, Chan HS, Wong WK. A rare case of “giant” right-sided retroperitoneal bronchogenic cyst. Dig Dis Sci. (2004) 49:1491–2. doi: 10.1023/B:DDAS.0000042253.60289.76
31. Song SY, Noh JH, Lee SJ, Son HJ. Bronchogenic cyst of the stomach masquerading as benign stromal tumor. Pathol Int. (2005) 55:87–91. doi: 10.1111/j.1440-1827.2005.01788.x
32. Paik SS, Jang KS, Han HX, Oh YH, Lee KG, Choi D. Retroperitoneal bronchogenic cyst mimicking pancreatic pseudocyst in a patient with colorectal cancer. J Gastroenterol Hepatol. (2005) 20:802–3. doi: 10.1111/j.1440-1746.2005.03763.x
33. Jo WM, Shin JS, Lee IS. Supradiaphragmatic bronchogenic cyst extending into the retroperitoneum. Ann Thorac Surg. (2006) 81:369–70. doi: 10.1016/j.athoracsur.2004.08.033
34. Sauvat F, Fusaro F, Jaubert F, Galifer B, Revillon Y. Paraesophageal bronchogenic cyst: first case reports in pediatric. Pediatr Surg Int. (2006) 22:849–51. doi: 10.1007/s00383-006-1738-0
35. Wang SE, Tsai YF, Su CH, Shyr YM, Lee RC, Tsai WC, et al. Retroperitoneal bronchogenic cyst mimicking pancreatic cystic lesion. J Chin Med Assoc. (2006) 69:538–42. doi: 10.1016/S1726-4901(09)70325-9
36. Kim EY, Lee WJ, Jang KT. Retroperitoneal bronchogenic cyst mimicking a pancreatic cystic tumour. Clin Radiol. (2007) 62:491–4. doi: 10.1016/j.crad.2006.10.012
37. Minei S, Igarashi T, Hirano D. A case of retroperitoneal bronchogenic cyst treated by laparoscopic surgery. Hinyokika Kiyo. (2007) 53:171–4.
38. Chu PY, Hwang TI, Teng TH, Lee CC. A retroperitoneal bronchogenic cyst successfully treated by laparoscopic surgery. Ann Saudi Med. (2007) 27:199–200. doi: 10.5144/0256-4947.2007.199
39. Chung JM, Jung MJ, Lee W, Choi S. Retroperitoneal bronchogenic cyst presenting as adrenal tumor in adult successfully treated with retroperitoneal laparoscopic surgery. Urology. (2009) 73:442.e13–5. doi: 10.1016/j.urology.2008.02.056
40. Elemen L, Tugay M, Tugay S, Gürcan NI, Erkus B, Gurbuz Y. Bronchogenic cyst of the right hemidiaphragm mimicking a hydatid cyst of the liver: report of the first pediatric case. Pediatr Surg Int. (2008) 24:957–9. doi: 10.1007/s00383-008-2187-8
41. Zügel NP, Kox M, Lang RA, Hüttl TP. Laparoscopic resection of an intradiaphragmatic bronchogenic cyst. JSLS. (2008) 12:318–20.
42. Obando J, Merkle E, Bean SM. A retroperitoneal bronchogenic cyst. Clin Gastroenterol Hepatol. (2009) 7:A24–21. doi: 10.1016/j.cgh.2008.11.011
43. Onol FF, Baytekin F, Dikbas O, Ergönenç T, Tanidir Y. A retroperitoneal bronchogenic cyst mimicking adrenal tumour in an adult: is differential diagnosis truly possible? J Clin Pathol. (2009) 62:187–9. doi: 10.1136/jcp.2008.061077
44. Shibahara H, Arai T, Yokoi S, Hayakawa S. Bronchogenic cyst of the stomach involved with gastric adenocarcinoma. Clin J Gastroenterol. (2009) 2:80–4. doi: 10.1007/s12328-008-0042-z
45. Díaz Nieto R, Naranjo Torres A, Gómez Alvarez M, Ruiz Rabelo JF, Pérez Manrique MC, Ciria Bru R, et al. Intraabdominal bronchogenic cyst. J Gastrointest Surg. (2010) 14:756–8. doi: 10.1007/s11605-009-0932-5
46. El Youssef R, Fleseriu M, Sheppard BC. Adrenal and pancreatic presentation of subdiaphragmatic retroperitoneal bronchogenic cysts. Arch Surg. (2010) 145:302–4. doi: 10.1001/archsurg.2010.12
47. Petrina A, Boselli C, Cirocchi R, Covarelli P, Eugeni E, Badolato M, et al. Bronchogenic cyst of the ileal mesentery: a case report and a review of literature. J Med Case Rep. (2010) 4:313. doi: 10.1186/1752-1947-4-313
48. Ubukata H, Satani T, Motohashi G, Konishi S, Goto Y, Watanabe Y, et al. Intra-abdominal bronchogenic cyst with gastric attachment: report of a case. Surg Today. (2011) 41:1095–100. doi: 10.1007/s00595-010-4398-6
49. Kim JB, Park CK, Kum DY, Lee DH, Jung HR. Bronchogenic cyst of the right hemidiaphragm presenting with pleural effusion. Korean J Thorac Cardiovasc Surg. (2011) 44:86–8. doi: 10.5090/kjtcs.2011.44.1.86
50. O’Neal PB, Moore FD, Gawande A, Cho NL, King EE, Moalem J, et al. Bronchogenic cyst masquerading as an adrenal tumor: a case of mistaken identity. Endocr Pract. (2012) 18:e102–5. doi: 10.4158/EP11186.CR
51. Govaerts K, Van Eyken P, Verswijvel G, Van der Speeten K. A bronchogenic cyst, presenting as a retroperitoneal cystic mass. Rare Tumors. (2012) 4:e13. doi: 10.4081/rt.2012.e13
52. Choi KK, Sung JY, Kim JS, Kim MJ, Park H, Choi DW, et al. Intra-abdominal bronchogenic cyst: report of five cases. Korean J Hepatobiliary Pancreat Surg. (2012) 16:75–9. doi: 10.14701/kjhbps.2012.16.2.75
53. Parray FQ, Sherwani AY, Dangroo SA, Bisati RA, Malik NS. Retroperitoneal bronchogenic cyst mimicking hydatid liver: a case report. Case Rep Surg. (2012) 2012:312147. doi: 10.1155/2012/312147
54. Fernández JL, Bauza G, McAneny DB. Minimally invasive management of lesser sac bronchogenic cyst. JSLS. (2011) 15:571–4. doi: 10.4293/108680811X13176785204715
55. Cai Y, Guo Z, Cai Q, Dai S, Gao W, Niu Y, et al. Bronchogenic cysts in retroperitoneal region. Abdom Imaging. (2013) 38:211–4.
56. Brient C, Muller C, Cassagneau P, Taieb D, Sebag F, Henry JF. A retroperitoneal bronchogenic cyst. J Visc Surg. (2012) 149:e361–3. doi: 10.1016/j.jviscsurg.2012.05.002
57. Kluger MD, Tayar C, Belli A, Salceda JA, van Nhieu JT, Luciani A, et al. A foregut cystic neoplasm with diagnostic and therapeutic similarities to mucinous cystic neoplasms of the pancreas. JOP. (2013) 14:446–9.
58. Ballehaninna UK, Shaw JP, Brichkov I. Subdiaphragmatic bronchogenic cyst at the gastroesophageal junction presenting with Dysphagia: a case report. Surg Laparosc Endosc Percutan Tech. (2013) 23:e170–2. doi: 10.1097/SLE.0b013e31828e3e54
59. Kurokawa T, Yamamoto M, Ueda T, Enomoto T, Inoue K, Uchida A, et al. Gastric bronchogenic cyst histologically diagnosed after laparoscopic excision: report of a case. Int Surg. (2013) 98:455–60. doi: 10.9738/INTSURG-D-12-00038.1
60. Castro R, Oliveira MI, Fernandes T, Madureira AJ. Retroperitoneal bronchogenic cyst: MRI findings. Case Rep Radiol. (2013) 2013:853795. doi: 10.1155/2013/853795
61. Runge T, Blank A, Schäfer SC, Candinas D, Gloor B, Angst E. A retroperitoneal bronchogenic cyst mimicking a pancreatic or adrenal mass. Case Rep Gastroenterol. (2013) 7:428–32. doi: 10.1159/000355879
62. Jannasch O, Büschel P, Wodner C, Seidensticker M, Kuhn R, Lippert H, et al. Retroperitoneoscopic and laparoscopic removal of periadrenally located bronchogenic cysts – a systematic review. Pol Przegl Chir. (2013) 85:706–13. doi: 10.2478/pjs-2013-0108
63. Terasaka T, Otsuka F, Ogura-Ochi K, Miyoshi T, Inagaki K, Kobayashi Y, et al. Retroperitoneal bronchogenic cyst: a rare incidentaloma discovered in a juvenile hypertensive patient. Hypertens Res. (2014) 37:595–7. doi: 10.1038/hr.2014.38
64. Maly T, Mihal V, Michalkova K, Tichy T, Neoral C, Zonca P. Retroperitoneal bronchogenic cyst: prenatal diagnosis of cystoid formation, its progression and surgery. Bratisl Lek Listy. (2014) 115:98–100. doi: 10.4149/BLL_2014_021
65. Dong B, Zhou H, Zhang J, Wang Y, Fu Y. Diagnosis and treatment of retroperitoneal bronchogenic cysts: a case report. Oncol Lett. (2014) 7:2157–9. doi: 10.3892/ol.2014.1974
66. Cao DH, Zheng S, Lv X, Yin R, Liu LR, Yang L, et al. Multilocular bronchogenic cyst of the bilateral adrenal: report of a rare case and review of literature. Int J Clin Exp Pathol. (2014) 7:3418–22.
67. Mirsadeghi A, Farrokhi F, Fazli-Shahri A, Gholipour B. Retroperitoneal bronchogenic cyst: a case report. Med J Islam Repub Iran. (2014) 28:56.
68. Jiang X, Zeng H, Gong J, Huang R. Unusual uptake of radioiodine in a retroperitoneal bronchogenic cyst in a patient with thyroid carcinoma. Clin Nucl Med. (2015) 40:435–6. doi: 10.1097/RLU.0000000000000664
69. Altieri MS, Zheng R, Pryor AD, Heimann A, Ahn S, Telem DA. Esophageal bronchogenic cyst and review of the literature. Surg Endosc. (2015) 29:3010–5. doi: 10.1007/s00464-015-4082-4
70. Bulut G, Bulut MD, Bahadır I, Kotan C. Bronchogenic cyst mimicking an adrenal mass in the retroperitoneal region: report of a rare case. Indian J Pathol Microbiol. (2015) 58:96–8. doi: 10.4103/0377-4929.151200
71. Yoon YR, Choi J, Lee SM, Kim YJ, Cho HD, Lee JW, et al. Retroperitoneal bronchogenic cyst presenting paraadrenal tumor incidentally detected by (18)F-FDG PET/CT. Nucl Med Mol Imaging. (2015) 49:69–72. doi: 10.1007/s13139-014-0306-0
72. Tong HX, Liu WS, Jiang Y, Liu JU, Zhou JJ, Zhang Y, et al. Giant retroperitoneal bronchogenic cyst mimicking a cystic teratoma: a case report. Oncol Lett. (2015) 9:2701–5. doi: 10.3892/ol.2015.3076
73. Leepalao M, Wernberg J. Multiple bronchogenic and gastroenteric cysts arising from the stomach in a patient with abdominal pain. Case Rep Surg. (2015) 2015:601491. doi: 10.1155/2015/601491
74. Trehan M, Singla S, Singh J, Garg N, Mahajan A. A rare case of intra- abdominal bronchogenic cyst- a case report. J Clin Diagn Res. (2015) 9:d03–4. doi: 10.7860/JCDR/2015/12949.6761
75. Zhang D, Zhang Y, Liu X, Zhu J, Feng C, Yang C, et al. Challenge in preoperative diagnosis of retroperitoneal mucinous cyst in a pediatric patient. Int J Clin Exp Med. (2015) 8:19540–7.
76. Gou Y, Wang Y, Fang H, Xu X, Yu W, Zhang K, et al. Bronchogenic cyst in the hepatogastric ligament masquerading as an esophageal mesenchymal tumor: a case report. Int J Clin Exp Pathol. (2015) 8:15307–11.
77. Herek D, Erbiş H, Kocyigit A, Yagci AB. Retroperitoneal bronchogenic cyst originating from diaphragmatic crura. Indian J Surg. (2015) 77(Suppl. 3):1397–8. doi: 10.1007/s12262-014-1045-2
78. Pasquer A, Djeudji F, Hervieu V, Rabeyrin M, Barth X. A rare retrorectal presentation of a bronchogenic cyst: a case report. Int J Surg Case Rep. (2016) 24:112–4. doi: 10.1016/j.ijscr.2016.05.028
79. Chhaidar A, Ammar H, Abdessayed N, Azzaza M, Gupta R, Abdennaceur N, et al. Large bronchogenic cyst of stomach: a case report. Int J Surg Case Rep. (2017) 34:126–9. doi: 10.1016/j.ijscr.2017.03.021
80. Gao X, Zhai M, Zhang H, Wang Y, Zhou J. A giant intradiaphragmatic bronchogenic cyst: case report and literature review. Tumori. (2017) 103(Suppl. 1):e25–7. doi: 10.5301/TJ.5000669
81. Wang M, He X, Qiu X, Tian C, Li J, Lv M. Retroperitoneal bronchogenic cyst resembling an adrenal tumor with high levels of serum carbohydrate antigen 19-9: a case report. Medicine (Baltimore). (2017) 96:e7678. doi: 10.1097/MD.0000000000007678
82. Byers JT, Gertz HE, French SW, Wang L. Case report: retroperitoneal bronchogenic cyst as a diagnostic dilemma after colon cancer diagnosis. Exp Mol Pathol. (2018) 104:158–60. doi: 10.1016/j.yexmp.2018.02.002
83. Liu Q, Gao Y, Zhao Z, Zhao G, Liu R, Lau WY. Robotic resection of benign nonadrenal retroperitoneal tumors: a consecutive case series. Int J Surg. (2018) 55:188–92. doi: 10.1016/j.ijsu.2018.04.013
84. Başoğlu M, Karabulut K, Özbalcı GS, Aykun N, Çamlıdağ I, Güngör BB, et al. Laparoscopic resection of retroperitoneal bronchogenic cyst clinically presenting as adrenal cyst. Turk J Surg. (2018):1–3. [Epub ahead of print]. doi: 10.5152/turkjsurg.2018.4033
85. Chen HY, Fu LY, Wang ZJ. Ileal bronchogenic cyst: a case report and review of literature. World J Clin Cases. (2018) 6:807–10. doi: 10.12998/wjcc.v6.i14.807
86. Ye L, Yang D, Qin X, Hu B. An abdominal bronchogenic cyst. Gastroenterol Hepatol. (2020) 43:140–1. doi: 10.1016/j.gastrohep.2019.07.011
87. Venkatesh K, Pillarisetty K. Bronchogenic cyst presenting as content of omphalocele – A case report. Indian J Pathol Microbiol. (2020) 63:116–8. doi: 10.4103/IJPM.IJPM_841_18
88. Wen Y, Chen W, Chen J, He X. Retroperitoneal bronchogenic cyst resembling an adrenal tumor: two case reports and literature review. J Int Med Res. (2020) 48:300060520925673. doi: 10.1177/0300060520925673
89. Bakshi N, Dhawan S, Sengar P, Dhir U. Neuroendocrine tumor in a lesser sac bronchogenic cyst – A hitherto unreported entity made rarer by concomitant hepatic hydatid cyst. Indian J Cancer. (2020) 57:205–8.
90. Subramanian JB, Suprajha KS, Selvarangam S. A case report- retroperitoneal bronchogenic cyst in relation to the hindgut. Int J Surg Case Rep. (2020) 75:140–2. doi: 10.1016/j.ijscr.2020.09.038
91. Qingyu J, Xiaolong L, Ruohan Z, Licong M, Zhichao T, Qingwei C, et al. Computed tomography helps pre-operative evaluation before laparoscopic resection of retroperitoneal bronchogenic cyst: a case report. J Minim Access Surg. (2021) 17:95–7. doi: 10.4103/jmas.JMAS_72_20
92. Sinha V, Nandi P, Shankar M, Sardana N. Retroperitoneal bronchogenic cyst: a rare case study. Cureus. (2020) 12:e10421. doi: 10.7759/cureus.10421
93. Cowan S, Gunawardene A, Davenport E. Retroperitoneal bronchogenic cyst mistaken as an adrenal adenoma. ANZ J Surg. (2021) 91:E526–7. doi: 10.1111/ans.16515
94. Cassiani J, Crinò SF, Manfrin E, Rivelli M, Gabbrielli A, Guglielmi A, et al. Endoscopic ultrasound through-the-needle biopsy for the diagnosis of an abdominal bronchogenic cyst. Clin Endosc. (2021) 54:767–70. doi: 10.5946/ce.2020.195
95. Hu L, Fan J, Wang T, Tang G. Ectopic bronchogenic cyst in abdomen- a rare special type. Am J Med Sci. (2021) 361:e59–60. doi: 10.1016/j.amjms.2020.11.014
96. Tadokoro T, Misumi T, Itamoto T, Nakahara H, Matsugu Y, Ikeda S, et al. Retroperitoneal bronchogenic cyst resected by single-incision laparoscopic surgery in an adolescent female: a case report. Asian J Endosc Surg. (2022) 15:206–10. doi: 10.1111/ases.12973
97. Wu LD, Wen K, Cheng ZR, Alwalid O, Han P. Retroperitoneal bronchogenic cyst in suprarenal region treated by laparoscopic resection: a case report. World J Clin Cases. (2021) 9:7245–50. doi: 10.12998/wjcc.v9.i24.7245
98. Kamimura G, Ueda K, Suzuki S, Aoki M, Nagata T, Sato M. A case of intradiaphragmatic bronchogenic cyst with an abnormally high serum level of CA19-9. Respirol Case Rep. (2021) 9:e0838. doi: 10.1002/rcr2.838
99. Clementino-Filho J, Surjan RCT, Taglieri E, Ardengh JC. Retroperitoneal bronchogenic cyst mimicking a pancreatic cystic lesion with extremely high level of intralesional fluid CA-19.9 antigen: benign in disguise. Indian J Surg. (2021) 84:1–6. doi: 10.1007/s12262-021-03137-x
100. Hu BY, Yu H, Shen J. A retroperitoneal bronchogenic cyst clinically mimicking an adrenal mass: three case reports and a literature review. J Int Med Res. (2022) 50:3000605211072664. doi: 10.1177/03000605211072664
101. Toi N, Kurajoh M, Noda S, Emoto M. Retroperitoneal bronchogenic cyst adjacent to adrenocortical adenoma. Intern Med. (2022) 61:2821–2.
Keywords: bronchogenic cyst, subdiaphragmatic, subdiaphragmatic bronchogenic cyst, case series, literature review
Citation: Xiao J, Zhang X, Zhou H, Hong T, Li B, He X and Liu W (2022) Subdiaphragmatic bronchogenic cysts: Case series and literature review. Front. Med. 9:993091. doi: 10.3389/fmed.2022.993091
Received: 13 July 2022; Accepted: 12 September 2022;
Published: 05 October 2022.
Edited by:
Luigi Tornillo, University of Basel, SwitzerlandReviewed by:
Janek Salatzki, Heidelberg University, GermanyCopyright © 2022 Xiao, Zhang, Zhou, Hong, Li, He and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Liu, d2VpbGl1amJ3a0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.