- 1Department of Respiratory and Critical Care Medicine, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Mathematics, Shanghai Normal University, Shanghai, China
Background: Many patients with cough variant asthma (CVA) are underdiagnosed and undertreated due to the atypical symptoms, low diagnostic sensitivity of bronchodilator response (BDR), and limited application of bronchial challenge test.
Objective: To investigate whether airway reversibility in BDR can predict CVA diagnosis in patients with chronic cough and negative BDR.
Methods: This open-label, prospective cohort study included patients with chronic cough, nearly normal chest CT scan, and negative BDR results. Inhaled corticosteroids and long-acting β2 agonists were given for 4 weeks. The confirmed diagnosis of CVA was defined as improved symptoms and an increase of forced expiratory volume in 1 s (FEV1) by >12% and >200 mL after 4 weeks of treatment.
Results: Of 155 patients recruited, 140 completed the study. Patients in the CVA positive diagnosis group had greater absolute (Δ) and percent (Δ%) improvements in FEV1 and forced expiratory flows (FEFs), and higher fractional exhaled nitric oxide (FENO) than in the CVA negative diagnosis group. The area under the receiver operating characteristic curves (AUCs) of ΔFEV1%, FEF25–75%pred (percentage of predicted forced expiratory flow at 25% to 75%) and FENO for CVA positive diagnosis was 0.825, 0.714, and 0.637, with cutoff values of 5.90%, 61.99% and 41.50 ppb, respectively. A joint model of ΔFEV1% combined with FEF25–75%pred or FENO increased the AUC to 0.848 and 0.847, respectively.
Conclusion: ΔFEV1% in BDR can predict a CVA diagnosis and response to anti-asthma treatment in patients with chronic cough and negative BDR.
Clinical trial registration: [http://www.chictr.org.cn/index.aspx], identifier [ChiCTR2000029065].
Introduction
Cough variant asthma (CVA), with a sole or main symptom of cough, is a special phenotype of asthma and one of the most common causes of chronic cough. In China, CVA accounts for more than one-third of the causes of chronic cough (1, 2). The hallmarks of CVA include bronchial hyperresponsiveness (BHR) and successful treatment with inhaled corticosteroids (ICS) and/or bronchodilators (3).
Despite atypical symptoms and high levels of heterogeneity among CVA patients, some patients develop classic asthma over time (4–6). On top of that, severe cough can be highly disruptive to individual life, leaving patients vulnerable to a variety of comorbidities such as incontinence, cough syncope, dysphonia, depression, and difficulties in relationships (7). Early detection and treatment with ICS prove crucial to those patients as it aids symptom control and may prevent the progression of CVA to classic asthma (4). However, given the atypical symptoms and near-normal forced expiratory volume in 1 s (FEV1) in spirometry, the precise diagnosis of CVA remains very challenging, especially for primary care physicians. The European and American guidelines recommend that the CVA diagnosis should be determined according to the documentation of variability in lung function and the therapeutic response (3, 8).
Objective indicators of CVA diagnosis require evidence of confirmed variable airway limitation defined by either a positive bronchodilator response (BDR), or BHR defined by a positive bronchial challenge test (BCT). Generally, the BCT is recommended for diagnosing CVA. However, in light of the costly, time-consuming process, requirements of professional technicians and equipment (9), as well as the potential risk of severe bronchospasm (10), it remains difficult to be appropriately carried out, especially in primary care settings. On the other hand, although BDR is safer, more convenient and widespread-used, and with a higher specificity, its sensitivity for CVA is low because a 12% improvement of FEV1 is difficult to achieve, especially among those patients with nearly normal baseline FEV1. Unfortunately, most CVA patients prefer primary care settings for their relatively mild and atypical symptoms, thus putting appropriate diagnosis and treatment of CVA in a dilemma due to the shortage of effective detection methods.
An increase in FEV1 by >12% and >200 ml from baseline after 4 weeks of anti-asthma treatment is also recommended as a diagnostic criterion of asthma in GINA (8), which can be considered a positive BDR after anti-asthma treatment (BAAT). However, the guidelines do not provide specific descriptions as to which group of potential CVA patients may benefit most from this diagnostic therapy. Additionally, primary care physicians tend to be reluctant to prescribe inhaled corticosteroids due to potential side effects, and instead, they prefer to prescribe antibiotics and antitussive drugs. Therefore, it would be very helpful if a more convenient and efficient method is developed, which aims to identify those CVA patients who can potentially benefit most from the diagnostic therapy at baseline.
Based on those facts, the purpose of our research was to investigate whether spirometric indices change alone or in their different combinations, could predict patients’ responses to anti-asthma treatment, and therefore help physicians prescribe ICS precisely and improve the diagnostic rate of CVA in patients with chronic cough and negative BDR.
Materials and methods
Study design and participants
This study protocol was approved by the Institutional Review Board at Shanghai General Hospital (no. [2020]30) and registered on http://www.chictr.org.cn/index.aspx (No. ChiCTR2000029065). From April 1, 2020, to January 30, 2021, participants were consecutively recruited via the Pulmonary Outpatient Clinic of Shanghai General Hospital (Shanghai, China). To be included in this study, participants must have been aged 18–65 years with a sole or predominant symptom of chronic cough, and the spirometry satisfied the criteria that FEV1/forced vital capacity (FVC) was more than 0.7 after administration of salbutamol, BDR was negative according to the GINA standards.
The exclusion criteria included a respiratory infection within 8 weeks before screening and cigarette smoking including current smoking, cessation within 2 months, and a smoking history of more than 10 pack-years. Patients with the symptom of gastroesophageal reflux disease and upper airway cough syndrome were also excluded. All patients underwent a visual analog scale score (VAS) of cough to assess the severity of symptoms, a fractional exhaled nitric oxide (FENO) and a spirometry to assess the airway inflammation and constriction, and a blood cell counts, an echocardiography, and a high-resolution computed tomography scan to exclude concomitant systemic respiratory and cardiac disease. The usage of drugs that may affect the spirometry results and lead to chronic cough was also an important exclusion criterion.
Enrolled patients were given 4 weeks of ICS and long-acting β2-agonist (LABA) treatment (Symbicort Turbuhaler, 160 mg budesonide and 4.5 mg formoterol per dose, 1 dose twice per day, AstraZeneca). Considering the circadian rhythms, after 4 weeks of ICS/LABA treatment, the follow-up spirometry and VAS were performed at the same time of the first visit (8 am–10 am) and allowed 2 days of adjustment before or after the scheduled date, according to the actual situation of the subjects. Symptom recovery time was assessed weekly by telephone or WeChat and defined as the time from the start of treatment to symptom improvement, and the symptom improvement was judged by a decrease in the VAS (ΔVAS = VAS1–VAS2 > 30 mm) (11).
Spirometry, bronchodilation test, and fractional exhaled nitric oxide measurements
Spirometry and BDR were performed by the same technologist with the same spirometer (Jaeger Co., Hochberg, Germany) following the specifications and performance criteria recommended in the American Thoracic Society/European Respiratory Society Standardization (12). Participants underwent spirometry before and 15 min after inhaling salbutamol (400ug, Ventolin, salbutamol sulfate inhaled aerosol, Registration ID: JX20080307, 400 mg, GlaxoSmithKline).
The response to the bronchodilator was expressed as the percentage change relative to the prebronchodilator value of FEV1 (ΔFEV1%), FVC (ΔFVC%), and forced expiratory flows (FEFs; ΔFEFs%) and as the absolute change of ΔFEV1, ΔFVC, and ΔFEFs. The response to the 4 weeks of anti-inflammation was also expressed as the percentage improvement relative to the baseline of improvement-FEV1%, improvement-FVC%, and improvement-FEFs% and as the absolute change of improvement-FEV1, improvement-FVC, and improvement-FEFs.
Fractional exhaled nitric oxide (FENO) (NIOX MINO, Aerocrine AB, Solna, Sweden) was performed before spirometry since the involved breathing maneuvers could distort FENO results.
Group definition
After 4 weeks of therapy, patients were divided into the CVA positive diagnosis group and the CVA negative diagnosis group according to their improvement of FEV1 and symptoms: a positive diagnosis of CVA was defined as an increase in FEV1 by more than 12% and 200 ml, and ΔVAS greater than 30mm from baseline to after 4 weeks of therapy. In CVA negative diagnosis group, according to their improvement in FEV1 and symptoms: patients with an increase in FEV1 by less than 12% and more than 200 ml, and ΔVAS greater than 30mm from baseline to after 4 weeks of therapy were divided into a suspected group. Patients with an increase in FEV1 by less than 12% and 200 ml, regardless of the symptom changes, were divided into a negative group.
Statistical analysis
Data analysis was performed with SPSS software version 23.0 (SPSS Inc, Chicago, Ill) and R studio version 4.0.2 (Window desktop, R packages “proc”). The normality of the data distribution was checked with the Kolmogorov-Smirnov test. Normally distributed data are presented as the mean ± SD. The independent t-test or Mann-Whitney test and Fisher exact test or chi-square test were performed for the analysis of intergroup differences for continuous variables and categorical variables, respectively. The differences among the three groups were analyzed with one-way analysis of variance (ANOVA) if normally distributed or by Kruskal-Wallis if not, and the differences between the two groups were analyzed with Student-Newman-Keuls.
The prediction performance of each variable was measured as the area under the curve (AUC) of the receiver-operating characteristic derived from the logistic regression models. The resultant AUC of multiple logistic models of the 2 variables was used as a measure of the joint prediction performance. The Delong test was used to determine whether the multiple logistic models would significantly improve the prediction performance (13). The threshold for statistical significance for all analyses was set at P less than 0.05.
Results
Demographic and clinical characteristics data
A total of 155 patients with chronic cough was enrolled, and 140 patients completed the 4 weeks of treatment and scheduled spirometry at the second visit; 9 patients were excluded because they did not attend the second visit on time, and 6 patients were excluded for taking medicine insufficient.
There were 98 patients in the CVA negative diagnosis group (70%), and 42 patients in the CVA positive diagnosis group (30%). All spirometric indices increased after bronchodilation for the two groups, and they increased further after the 4 weeks of treatment. FEV1%pred in most patients was more than 80%, only 16 (11%) patients were in 70%–80%, among whom 5 patients were in CVA negative diagnosis group and 11 patients were in CVA positive diagnosis group.
Most demographic data and eosinophils did not differ in the two groups at baseline (Table 1). However, all baseline spirometric indices including large airway indices (FVC%pred, FEV1%pred) and small airway indices (FEF50%pred, FEF75%pred, FEF25–75%pred) in the CVA positive diagnosis group were significantly lower than the CVA negative diagnosis group (p < 0.05). The improvement of spirometric in BDR including the percentage change (ΔFEV1%, ΔFVC%, ΔFEF75%, ΔFEF25–75%) and absolute change (ΔFEV1, ΔFVC, ΔFEF75, ΔFEF25–75) in the CVA positive diagnosis group was significantly higher than the CVA negative diagnosis group (p < 0.05). And the CVA positive diagnosis group had a higher baseline FENO (p = 0.010) and VAS score (p = 0.001). The symptom recovery time was longer in the CVA negative diagnosis group than in the CVA positive diagnosis group (p = 0.001) (Table 1).
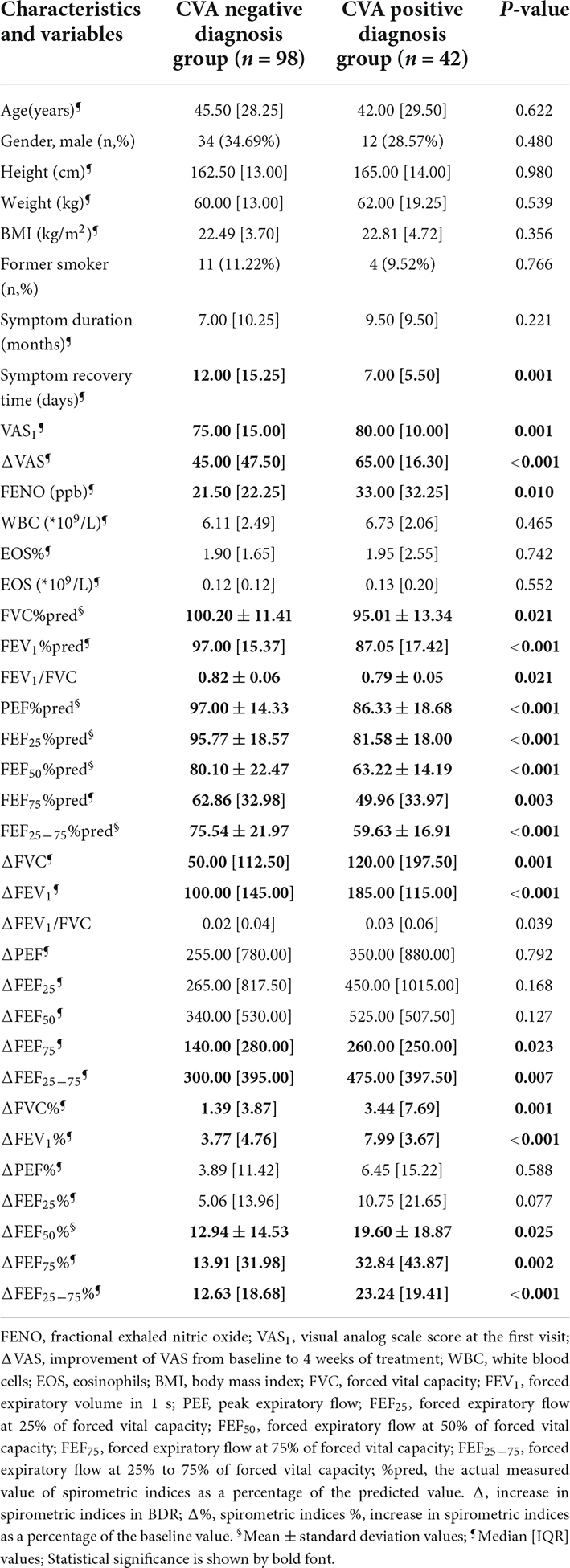
Table 1. Demographic data and clinical features of participants in the CVA negative diagnosis group and CVA positive diagnosis group.
Predictive values of a single measurement
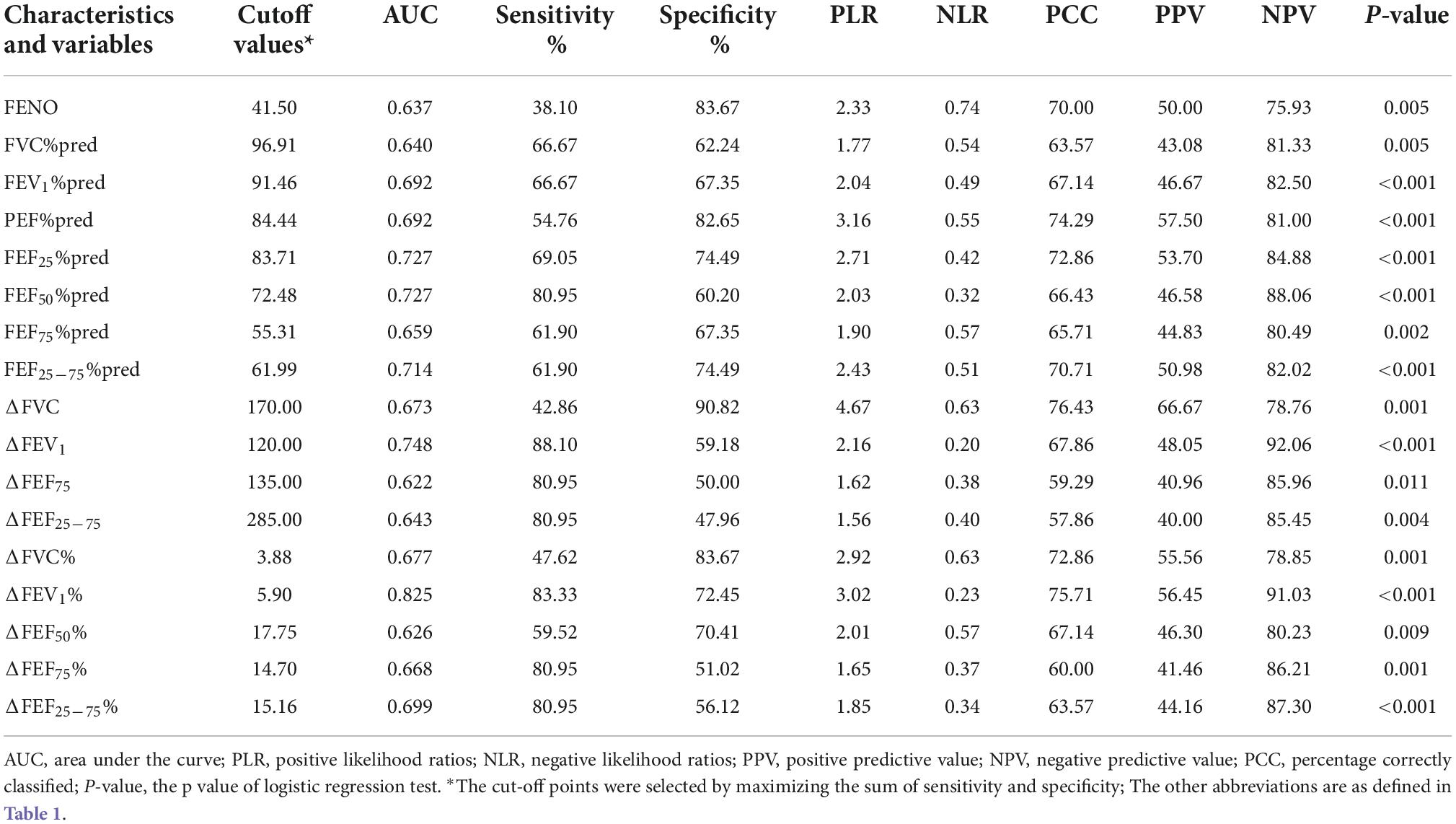
The prognostic value of these variables for CVA diagnosis was calculated by AUC (Table 2). The largest AUC was ΔFEV1% (0.825, 95% CI 0.752 to 0.897) in BDR, with cutoff values of 5.90%. The AUCs of the small airway, such as FEF50%pred, FEF75%pred, and FEF25–75%pred for CVA diagnosis were 0.727, 0.659, and 0.714 with cutoff values of 72.48%, 55.31%, and 61.99%, respectively. The AUC of FENO for CVA diagnosis was 0.637 with a cutoff value of 41.50 ppb.
Predictive value of joint models: ΔFEV1% and fractional exhaled nitric oxide, ΔFEF25–75%, or FEF25–75%pred
In the evaluated joint models, the two highest AUCs were the combination of ΔFEV1% + FEF25–75%pred (0.848, 95% CI 0.779 to 0.916) (Figure 1A), and the combination of ΔFEV1% + FENO (0.847, 95% CI 0.778 to 0.916) (Figure 1B). But the AUCs of the two joint models were not significantly higher than the AUC of ΔFEV1% alone (p = 0.069 and 0.076, respectively) (Table 3). The combination of FENO + FEF25–75%pred can increase AUC to 0.762 (Figure 1C). The results also demonstrated that the AUC of ΔFEV1% + FENO was higher than FEF25–75%pred + FENO (p = 0.051) (Figure 1D).
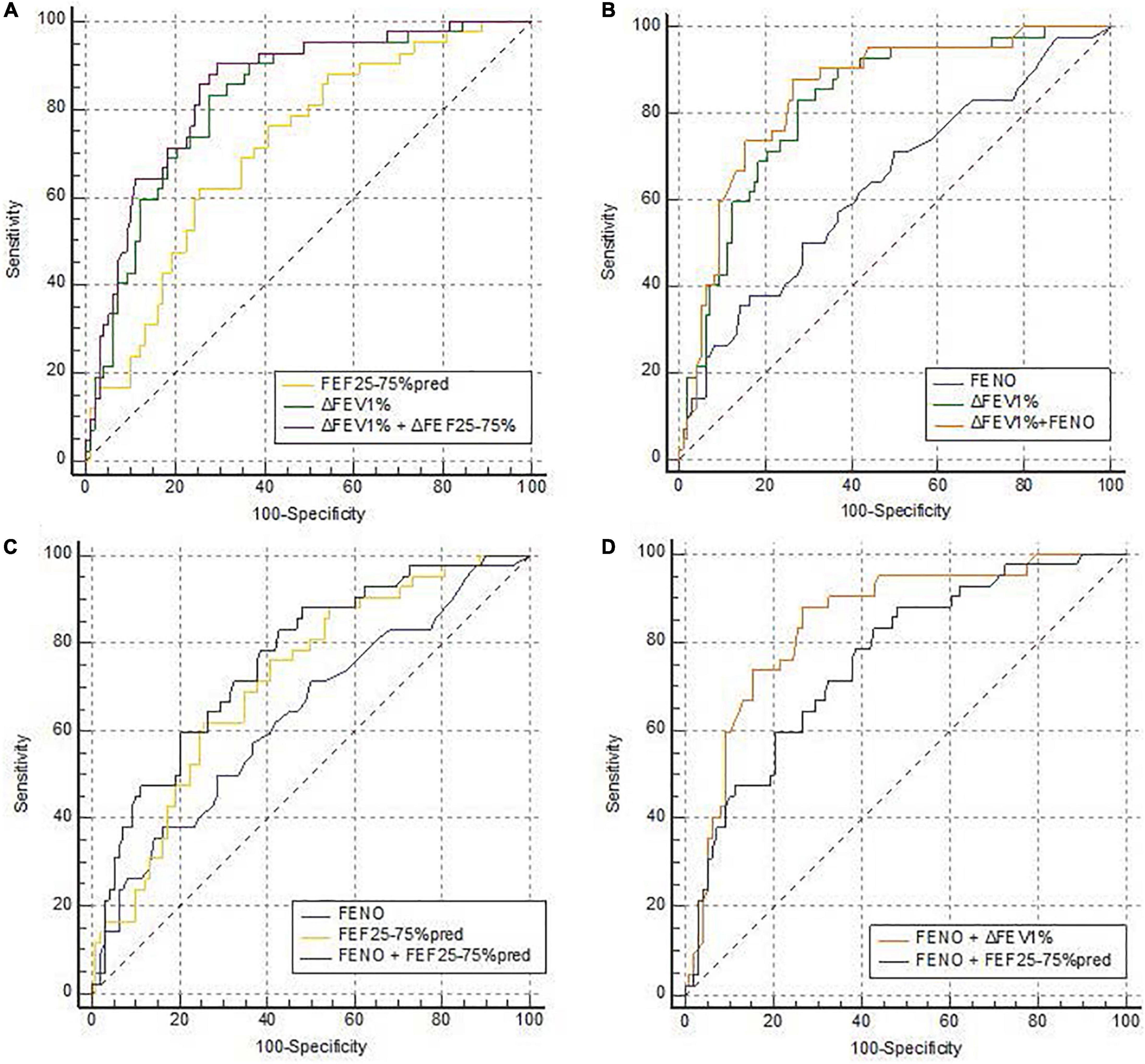
Figure 1. ROC curves of the joint models predicting CVA diagnosis. ROC curves for the joint models of (A) ΔFEV1% and FENO, (C) FENO and FEF25–75%pred, (B) ΔFEV1% and FEF25–75%pred, and (D) compare the AUC of FENO + ΔFEV1% and FENO + FEF25–75%pred in predicting CVA. (A) n = 140; AUCΔFEV1% + FENO = 0.847 (95% CI, 0.778 to 0.916); AUCFENO = 0.637 (95% CI, 0.535 to 0.739); AUCΔFEV1% = 0.825 (95% CI, 0.752 to 0.897). (C) n = 140; AUCFENO+FEF25–75%pred = 0.762 (95% CI, 0.677 to 0.847); AUCFENO = 0.703 (95% CI, 0.601 to 0.805); AUCFEF25–75%pred = 0.714 (95% CI, 0.624 to 0.804). (B) n = 140; AUC ΔFEV1%+FEF25–75%pred = 0.848 (95% CI, 0.779 to 0.916); AUCΔFEV1% = 0.825 (95% CI, 0.752 to 0.897); AUCFEF25–75%pred = 0.714 (95% CI, 0.624 to 0.804). CVA, cough variant asthma; ΔFEV1%, the increase in forced expiratory in 1 s as a percentage of baseline value in a bronchodilation test; FEF25–75%pred, percentage of predicted forced expiratory flow at 25% to 75%; FENO, fractional exhaled nitric oxide; ROC, receiver operating characteristic; AUC, area under the curve.
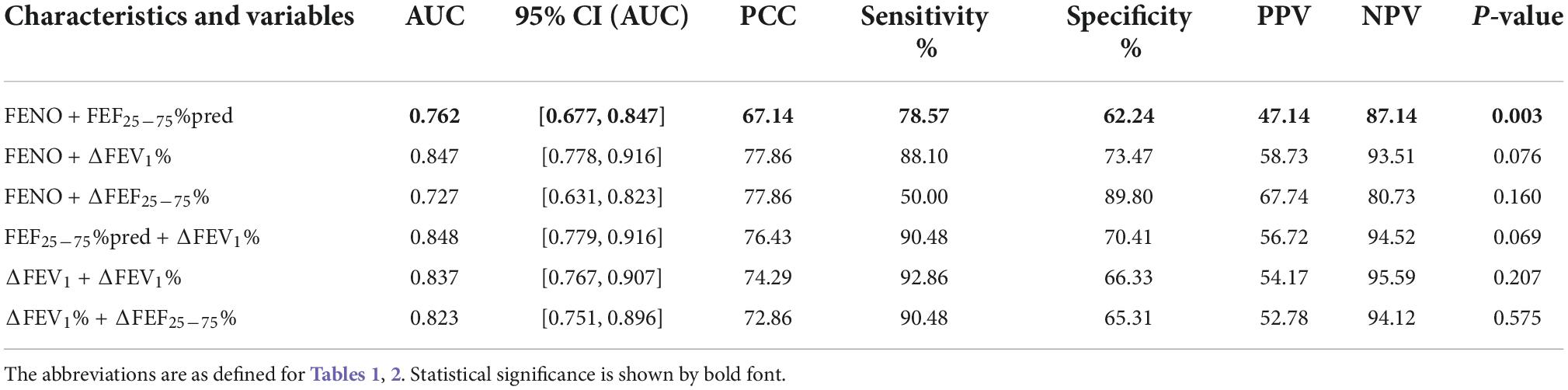
Table 3. Predictive values of the different joint models for the prediction of CVA positive diagnosis.
Venn diagram was showing the overlaps of CVA positive diagnosis patients with FENO ≥ 41.50ppb, ΔFEV1% ≥ 5.90%, and FEF25–75%pred ≤ 61.99%. There were 32 (76.19%) patients in the CVA positive diagnosis group with ΔFEV1% ≥ 5.90%, 16 (38.1%) patients with FENO ≥ 41.50ppb, and 23 (54.76%) patients with FEF25–75%pred ≤ 61.99% (Figure 2).
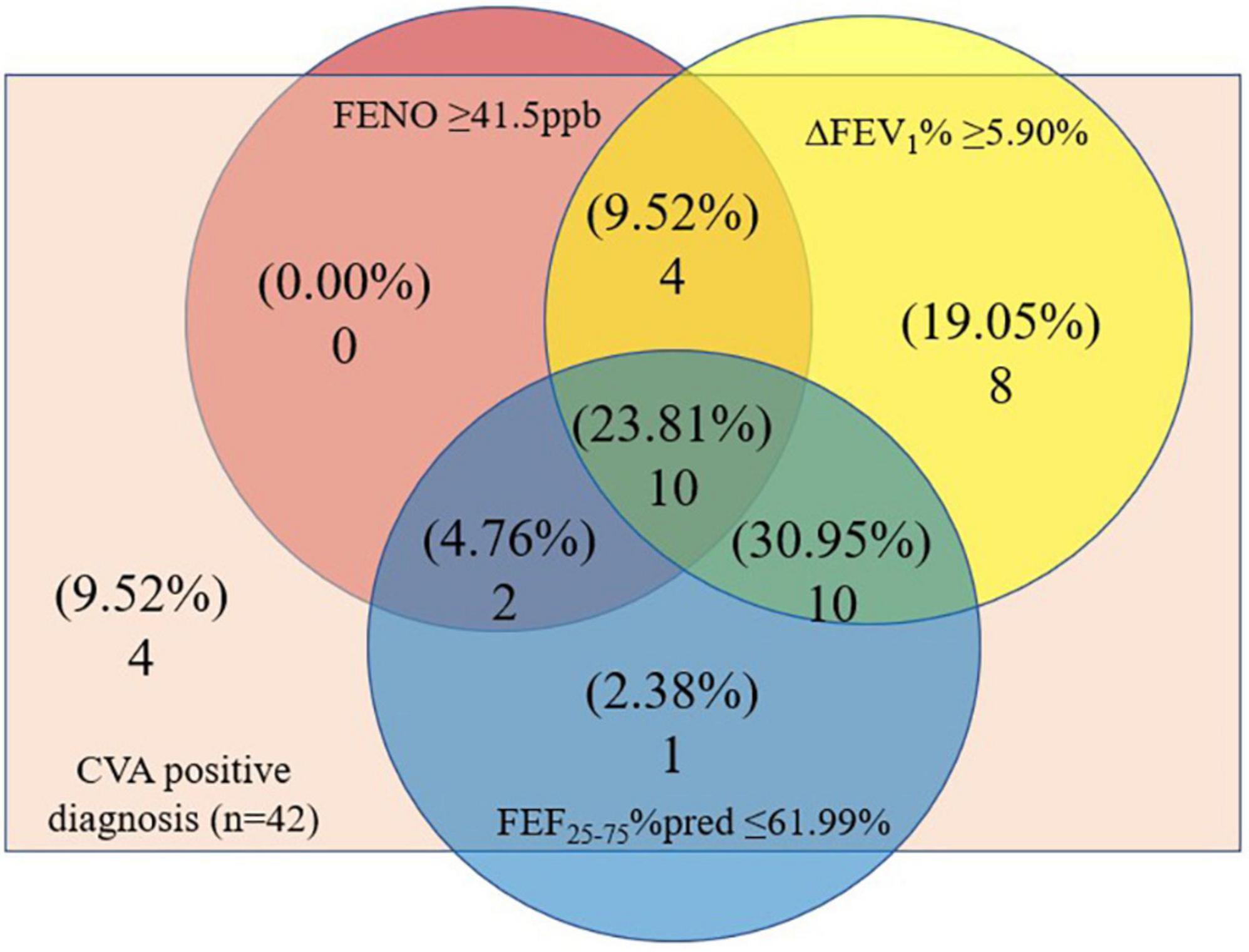
Figure 2. Venn diagram showing the overlaps of CVA positive diagnosis patients with ΔFEV1% ≥ 5.90%, FENO ≥ 41.5ppb, or FEF25–75%pred ≤ 61.99%. CVA, cough variant asthma; ΔFEV1%, the increase in forced expiratory in 1 s as a percentage of baseline value in a bronchodilation test; FEF25–75%pred, percentage of predicted forced expiratory flow at 25% to 75%; FENO, fractional exhaled nitric oxide.
Clinical characteristics of the suspected group
In the CVA negative diagnosis group, according to the improvement of FEV1 and VAS, 34 patients were up to the standard of the suspected group. Baseline spirometric indices including FEV1%pred, PEF%pred, and FEFs%pred, in the suspected group were significantly higher than those in the CVA positive diagnosis group (p < 0.05). The increases in absolute including improvement-FEV1, FVC, FEF25, FEF50, FEF75, and FEF25–75, and percentage including improvement-FEV1%, FEF25%, FEF50%, and FEF25–75%, from baseline to the posttreatment in the suspected group were significantly higher than the other 64 patients in the negative group whose FEV1 increased by less than 200 ml and less than 12% (p < 0.05). Improvements in each spirometric index from baseline to after bronchodilation and treatment were shown in Table 4.
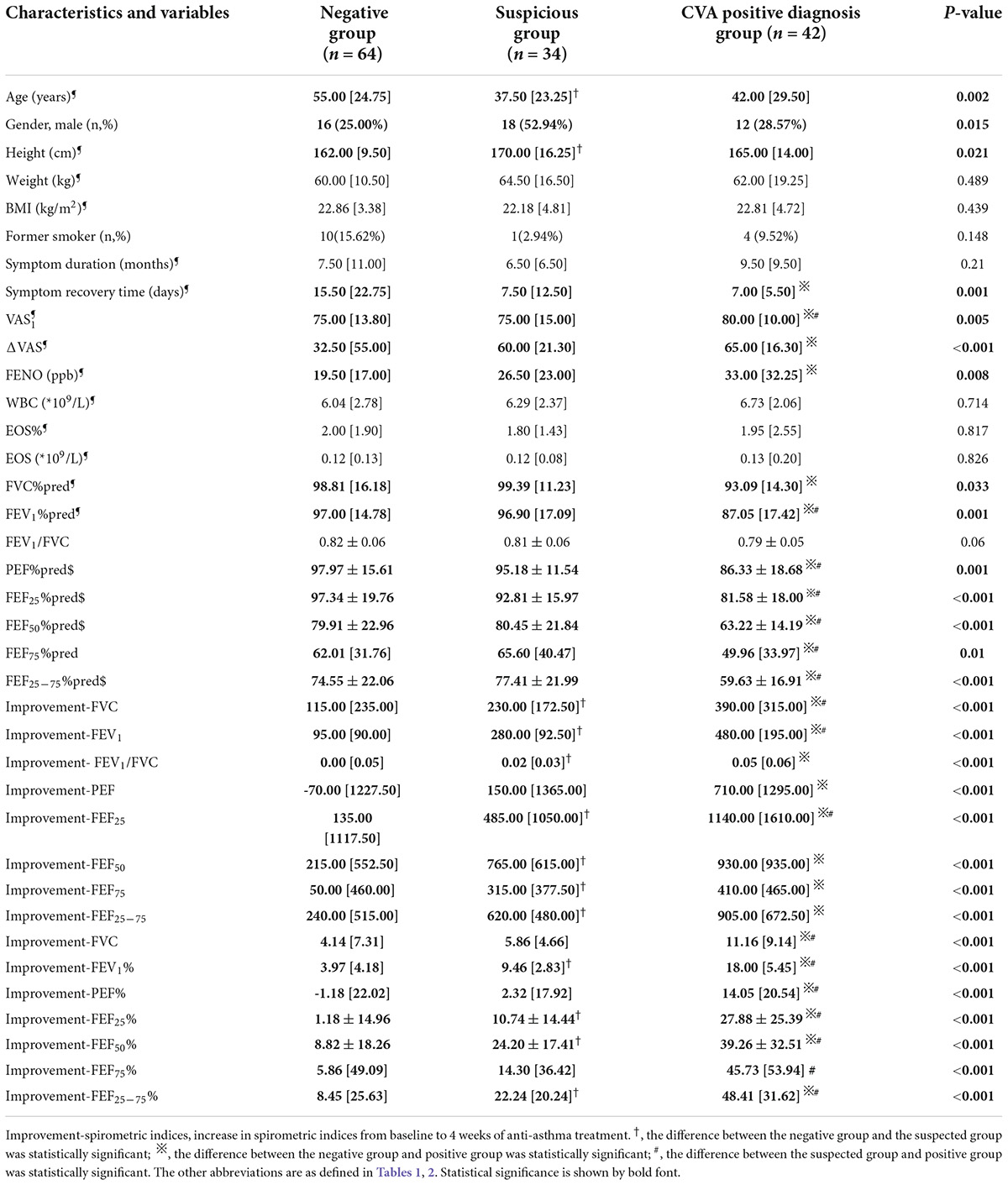
Table 4. Demographic data and clinical features of study participants in the negative group, suspected group, and CVA positive diagnosis group.
Clinical characteristics of patients with symptom recover time ≤7 days and symptom recover time >7 days
There were 67 patients with symptom recovery time ≤7 days (47.86%), and 73 patients with symptom recovery time >7 days (52.14%). Patients with symptom recovery time ≤7 days in the positive group, the suspected group, and the negative group accounted for 69.05%, 50.00%, and 32.81%, respectively (Supplementary Figure 1). Patients with symptom recovery time ≤7 days had lower baseline spirometry, and higher spirometric improvement in BDR including the percentage change (ΔFEV1%) and absolute change (ΔFEV1). The improvement of spirometric after 4 weeks of treatment includes the percentage change (improvemet-FEV1%, FEF25%, FEF25–75%) and absolute change (improvement-FEV1, FEF25, FEF75, FEF25–75) in patients with symptom recover time ≤7 days was significantly higher than patients with symptom recover time >7 days. And patients with symptom recovery time ≤7 days had a higher baseline FENO (p = 0.011) and VAS score (p = 0.002) (Supplementary Table 1). We also collected the cough characteristic of these patients with symptom recovery time ≤7 days, most of them had fixed inducing factors and nocturnal or daytime cough, and the cough was commonly stimulating dry cough with average sputum less than 10 ml/day.
Stratification analysis
Stratification analysis of baseline FEV1%pred divided the patients into 4 layers, and the percentage of ΔFEV1% > 12% was significantly different in the different subgroups. The percentage of the improvement of ΔFEV1% > 12% declined with increasing baseline FEV1%pred (p = 0.001) (Supplementary Figure 2A). However, the percentage of the improvement of ΔFEV1 > 200 ml had no significant correlation between baseline FEV1 stratification (p = 0.063) (Supplementary Figure 2B).
Discussion
To our knowledge, our research pioneered in analyzing the spirometric changes in BDR to predict the diagnosis of CVA in patients with chronic cough and negative BDR. ΔFEV1% ≥ 5.90% for predicting the diagnosis of CVA and response to anti-asthma treatment may provide a simple, economical, and accessible method for primary care physicians without access to BCT. FEF25–75%pred alone or combined with FENO also had a predictive value for CVA diagnosis. In addition, patients with higher baseline FEV1%pred had a smaller percentage of FEV1 improvement after anti-asthma treatment, so clinical physicians might need to take baseline FEV1%pred into consideration when evaluating the result of BAAT.
Although most primary hospitals own spirometers, BCT is, to a large extent, hard to be performed. On top of that, many CVA patients have essentially normal baseline FEV1, leading to a low rate of positive BDR results. In our study, the baseline FEV1%pred of most patients was above 80%, but there were still some patients with the baseline FEV1%pred between 70 and 80%, whose BDR results were also negative. All these factors contribute to the seriously low diagnostic rate of CVA (14). Therefore, it is important to provide a simple and effective method to identify CVA patients timely, and predict the treatment response to diagnostic therapy.
Diagnostic therapy is helpful for early detection and effective treatment in CVA patients, which may prevent the development to classic asthma (4, 5). ICS is a fundamental medication for CVA treatment, and recent investigations suggest that combination therapy of ICS and bronchodilator provides more rapid and effective relief of cough symptoms than ICS or bronchodilator therapy alone (15, 16). Therefore, ICS combined with formoterol was used under medical supervision.
Through the diagnostic therapy, a total of 42 patients who met the BAAT-positive criteria were diagnosed with CVA. All spirometric indices including large and small airways in the CVA positive diagnosis group were lower than those in the CVA negative diagnosis group. Notably, despite the near-normal large airway function in CVA patients, small airway dysfunction was remarkable, which was consistent with our previous study (17, 18). We also found a lower FEV1/FVC in the CVA positive diagnosis group, which indicated an expiatory airflow limitation according to GINA.
Some studies have shown that FENO has a certain value in predicting the diagnosis of CVA, but its independent diagnostic value remains controversial (19–21). In our study, when FENO was used alone for predicting CVA, the AUC was significantly lower than ΔFEV1%, indicating that FENO alone is insufficient for predicting CVA in patients with chronic cough. When combined with ΔFEV1%, its predictive accuracy improved but was not significantly higher than ΔFEV1% alone. Compared with FENO, we recommend routine BDR in primary care settings to help diagnose CVA, and the FENO test might be further performed if conditions permit.
Small-airway indices such as FEF25–75%pred alone or combined with FENO are effective in predicting CVA according to our previous research (22, 23). FEF25–75%pred also had a good predictive value for the diagnosis of CVA in this study, but compared with ΔFEV1%, it was lower. The different results of predicted models were likely due to the different evaluation criteria of the CVA diagnosis, the former was BCT and now was the response to anti-asthma treatment. In terms of predicting the effect of anti-asthma treatment, the predictive value of ΔFEV1% in BDR was higher than that of FEF25–75%pred. However, the Venn diagram showed that some patients with CVA were identified by FEF25–75%pred dysfunction rather than ΔFEV1% in BDR, indicating small airway dysfunction was also important in predicting the diagnosis of CVA and therapeutic response. Therefore, we recommended that small-airway indices should be conducted at the same time, because it may help to furtherly improve the diagnostic rate of CVA.
Patients with symptom recovery time ≤7 days may suffer from corticosteroid-responsive cough (CRC), which includes cough variant asthma, eosinophilic bronchitis, and atopic cough (24). Patients with the shorter symptom recovery time had a higher FENO and VAS at the baseline, and also had higher spirometric improvement in BDR after 4 weeks of treatment, which suggested a higher airway inflammation and well response to ICS + LABA. Furthermore, symptom recovery time ≤7 days may be a signal that the anti-asthma treatment will be effective. Therefore, patients in primary care with a stimulating dry cough in nocturnal or daytime, high FENO, and even no significant improvement in BDR should equally be considered a treatment with ICS and evaluated the response within 7 days.
The absolute change of FEV1 from baseline to 4 weeks of treatment in the suspected group was significantly greater than that in the negative group (ΔFEV1 ≤ 200 ml and ΔFEV1% ≤ 12%). But its baseline FEV1 was significantly higher than that in the CVA positive diagnosis group. It is worth mentioning that the percentage change of FEV1 is negatively correlated with baseline FEV1, which may cause difficulty for patients in the suspected group to meet the positive criteria of BAAT even if the absolute change is much more than 200 ml. Nevertheless, our results warrant further investigations to determine whether this group of patients has a limited percentage of improvement due to high baseline FEV1 or other diseases such as eosinophilic bronchitis that ICS was effective but the improvement of spirometry was limited.
Identically, an influence of baseline FEV1 on BAAT outcome was observed. By stratifying baseline FEV1, we found that the higher the baseline FEV1, the fewer patients achieved an improvement of FEV1% > 12% after anti-asthma treatment. Therefore, CVA may not be ruled out for those patients with high baseline FEV1 even if they didn’t meet the BAAT-positive criteria. In these scenarios, BCT should be performed to further confirm the diagnosis. If without access to BCT, it could be inferred by the response time of the treatment, because the symptom recovery time of the CVA positive diagnosis group and suspected group was both about 7 days. Meanwhile, there was no significant correlation between baseline FEV1 stratification and patients with an absolute change of FEV1 > 200 ml after anti-asthma treatment. Whether an improvement in FEV1 > 200 ml can be considered alone as a positive criterion for BAAT diagnosis of CVA with high baseline FEV1 requires further clinical research to verify.
It is important to recognize some limitations of our study. First, the study is single-centered with a relatively small sample size, therefore multicenter, large-scale, prospective studies may be needed in the future to further validate the results. Second, patients in the suspected group will need further observations to make a definitive diagnosis. Third, due to the limited availability of the cough monitoring system, we used patient-reported outcomes rather than objective cough measures. Fourth, due to lack of dynamic laryngoscopy, vocal cord dysfunction was rule out by spirometry with a low and straight inspiratory phase curve, which may result a mixed in this study.
In conclusion, our study proposed that ΔFEV1% ≥5.90% in BDR can be used to predict the diagnosis of CVA and the response of anti-asthma treatment in patients suffering from a chronic cough with negative BDR. In primary care settings without access to BCT, BDR is a simple and economical method to help physicians improve the diagnosis accuracy of CVA and help distinguish which patients should receive diagnostic trials of anti-asthma treatment. For chronic cough patients with high baseline FEV1%pred and significant improvement of the absolute value of FEV1 after anti-asthma treatment, the diagnosis of CVA may not be completely ruled out even if the positive criteria of BAAT are not met. While it is still premature to make any assumptions, physicians need to consider this new perspective when making personalized diagnoses and treatment plans for patients with chronic cough.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board at Shanghai General Hospital (no. [2020]30). The patients/participants provided their written informed consent to participate in this study.
Author contributions
MZ and HH conceived of and designed the entire study. YZ, WB, YX, and JL contributed to the data collection. CL performed spirometry, BDT, and FENO. YZ and MZ were involved in interpreting the clinical data. HH and ZeX performed statistical analyses. HH, ZiX, and YP wrote the manuscript and supervised by MZ. All authors critically reviewed and approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by the Appropriate Technique Application Program of Shanghai Municipal Health System (Grant No. 2019SY042); Scientific and Technological Innovation Program Funded by Science and Technology Commission of Shanghai Municipality (Grant No. 20Y11902400); and National Natural Science Foundation of China (Grant No. 81900036). All the grants were awarded to MZ, except No. 81900036, which was awarded to YZ.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.987887/full#supplementary-material
Supplementary Figure 1 | Symptom recovery time in the positive group, suspected group, and negative group.
Supplementary Figure 2 | The improvement of FEV1 from baseline to 4 weeks of anti-asthma treatment in different layers of FEV1%pred. FEV1%pred, percentage of predicted forced expiratory in 1 s.
References
1. Lai K, Chen R, Lin J, Huang K, Shen H, Kong L, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest. (2013) 143:613–20.
2. Irwin R, Corrao W, Pratter M. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. (1981) 123:413–7.
3. Morice A, Millqvist E, Bieksiene K, Birring S, Dicpinigaitis P, Domingo Ribas C, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. (2020) 55:1901136.
4. Matsumoto H, Niimi A, Takemura M, Ueda T, Tabuena R, Yamaguchi M, et al. Prognosis of cough variant asthma: a retrospective analysis. J Asthma. (2006) 43:131–5.
5. Nakajima T, Nishimura Y, Nishiuma T, Kotani Y, Funada Y, Nakata H, et al. Characteristics of patients with chronic cough who developed classic asthma during the course of cough variant asthma: a longitudinal study. Respiration. (2005) 72:606–11. doi: 10.1159/000087459
6. Fujimura M, Nishizawa Y, Nishitsuji M, Nomura S, Abo M, Ogawa H. Predictors for typical asthma onset from cough variant asthma. J Asthma. (2005) 42:107–11.
7. Chamberlain S, Garrod R, Douiri A, Masefield S, Powell P, Bücher C, et al. The impact of chronic cough: a cross-sectional European survey. Lung. (2015) 193:401–8.
8. Global Initiative for Asthma. Global strategy for asthma management and prevention. Fontana, WI: Global Initiative for Asthma (2021).
9. Coates A, Wanger J, Cockcroft D, Culver B, Bronchoprovocation Testing Task Force: Kai-Håkon Carlsen, Diamant Z, et al. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J. (2017) 49:1601526.
10. Bins J, Metting E, Muilwijk-Kroes J, Kocks J, T Veen J. The use of a direct bronchial challenge test in primary care to diagnose asthma. NPJ Prim Care Respir Med. (2020) 30:45.
11. Martin Nguyen A, Bacci E, Vernon M, Birring S, Rosa C, Muccino D, et al. Validation of a visual analog scale for assessing cough severity in patients with chronic cough. Ther Adv Respir Dis. (2021) 15:17534666211049743.
12. Graham B, Steenbruggen I, Miller M, Barjaktarevic I, Cooper B, Hall G, et al. Standardization of spirometry 2019 update. an official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. (2019) 200:e70–88. doi: 10.1164/rccm.201908-1590ST
13. DeLong E, DeLong D, Clarke-Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45.
14. Huang H, Hua W, Chen R, Hu Y, Ying S, Chi C, et al. Perspectives and management of atypical asthma in Chinese specialists and primary care practitioners-a nationwide questionnaire survey. Front Med. (2021) 8:727381. doi: 10.3389/fmed.2021.727381
15. Tagaya E, Kondo M, Kirishi S, Kawagoe M, Kubota N, Tamaoki J. Effects of regular treatment with combination of salmeterol/fluticasone propionate and salmeterol alone in cough variant asthma. J Asthma. (2015) 52:512–8. doi: 10.3109/02770903.2014.975358
16. Bao W, Chen Q, Lin Y, Liu H, Zhao G, Chen Z, et al. Efficacy of procaterol combined with inhaled budesonide for treatment of cough-variant asthma. Respirology. (2013) 18(Suppl. 3):53–61. doi: 10.1111/resp.12169
17. Hao H, Bao W, Xue Y, Zhou Y, Huang Z, Yin D, et al. Spirometric changes in bronchodilation tests as predictors of asthma diagnosis and treatment response in patients with FEV1 >/= 80% predicted. J Allergy Clin Immunol Pract. (2021) 9:3098–108.e4. doi: 10.1016/j.jaip.2021.03.015
18. Bao W, Tian X, Hao H, Jin Y, Xie X, Yin D, et al. Is small airway dysfunction an abnormal phenomenon for patients with normal forced expiratory volume in 1 second and the ratio of forced expiratory volume in 1 second to forced vital capacity? Ann Allergy Asthma Immunol. (2022) 128:68–77e61. doi: 10.1016/j.anai.2021.09.011
19. Chen L, Zeng G, Wu L, Zi M, Fang Z, Fan H, et al. Diagnostic value of FeNO and MMEF for predicting cough variant asthma in chronic cough patients with or without allergic rhinitis. J Asthma. (2021) 58:326–33.
20. Zhou J, Zhao X, Zhang X, Yu X, Wang Y, Jiang W, et al. Values of fractional exhaled nitric oxide for cough-variant asthma in children with chronic cough. J Thorac Dis. (2018) 10:6616–23.
21. Song W, Kim H, Shim J, Won H, Kang S, Sohn K, et al. Diagnostic accuracy of fractional exhaled nitric oxide measurement in predicting cough-variant asthma and eosinophilic bronchitis in adults with chronic cough: a systematic review and meta-analysis. J Allergy Clin Immunol. (2017) 140:701–9. doi: 10.1016/j.jaci.2016.11.037
22. Bao W, Zhang X, Lv C, Bao L, Yin J, Huang Z, et al. The value of fractional exhaled nitric oxide and forced mid-expiratory flow as predictive markers of bronchial hyperresponsiveness in adults with chronic cough. J Allergy Clin Immunol Pract. (2018) 6:1313–20. doi: 10.1016/j.jaip.2017.09.026
23. Hou L, Hao H, Huang G, Liu J, Yu L, Zhu L, et al. The value of small airway function parameters and fractional exhaled nitric oxide for predicting positive methacholine challenge test in asthmatics of different ages with FEV1 >/= 80% predicted. Clin Transl Allergy. (2021) 11:e12007. doi: 10.1002/clt2.12007
Keywords: cough variant asthma (CVA), bronchodilation test, forced expiratory volume in 1 s (FEV1), fractional exhaled nitric oxide (FENO), diagnosis
Citation: Hao H, Pan Y, Xu Z, Xu Z, Bao W, Xue Y, Lv C, Lin J, Zhang Y and Zhang M (2022) Prediction of bronchodilation test in adults with chronic cough suspected of cough variant asthma. Front. Med. 9:987887. doi: 10.3389/fmed.2022.987887
Received: 06 July 2022; Accepted: 23 November 2022;
Published: 09 December 2022.
Edited by:
Päivi Liisa Piirilä, Helsinki University Central Hospital, FinlandReviewed by:
Raffaele Campisi, Azienda Ospedaliero Universitaria Policlinico “G. Rodolico-San Marco”, ItalyDragan Mijakoski, Institute of Occupational Health of RNM, North Macedonia
Copyright © 2022 Hao, Pan, Xu, Xu, Bao, Xue, Lv, Lin, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Zhang, bWFnZ2llX3poYW5nbWluQDE2My5jb20=; Yingying Zhang, YWxwaGFmbHlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Huijuan Hao
Huijuan Hao Yilin Pan
Yilin Pan Zichong Xu
Zichong Xu Zengchao Xu
Zengchao Xu Wuping Bao
Wuping Bao Yishu Xue
Yishu Xue Chengjian Lv1
Chengjian Lv1 Yingying Zhang
Yingying Zhang Min Zhang
Min Zhang