- Institute of Management, Scuola Superiore Sant’Anna, Pisa, Italy
Background: As chronic conditions, rare and complex connective tissue and musculoskeletal diseases (rCTDs) significantly affect the quality of life generating an impact on the physical, psychological, social, and economic dimensions of the patients’ lives, having implications on the family, changing the lifestyle and interpersonal relationships. Traditionally, generic and disease-specific measures for Quality of Life (QoL) provide valuable information to clinicians since QoL affects healthcare services utilization, predicts morbidities and mortalities, workability, etc. Moreover, the assessment of unmet clinical needs, satisfaction, the experience with the treatment and the care, the psychological dimensions, and the effects of the diseases, such as fatigue, could represent valuable dimensions to be considered in the QoL impact assessment. It is also necessary to measure the impact of rCTDs by considering the perspectives of family members/informal caregivers, for instance considering values, beliefs, experiences, life circumstances, psychological aspects, family relationships, economic issues, changes in social activities, etc.
Objective: The aim of this scoping review is to better understand the status of QoL metrics used in clinical and economic research for the assessment of the individual’s perspective on living with rCTDs.
Research question: What are the main challenges in QoL measures (and/or) measurement/assessment in rCTDs?
Materials and methods: Scoping review of the literature referring to QoL measures in rCTDs. Database: PUBMED, ISI-Web of Science; last date: 21/09/2021.
Results: Anxiety and depression, body image satisfaction, daily activity, fatigue, illness perception, pain, personality, QoL, resilience, satisfaction with the relationship, self-management, sexual QoL, sleep quality, social support, stress, uncertainty, and work productivity are the observed dimensions covered by the included studies. However, “more shadows than lights” can summarize the review’s outcome in terms of Patient Reported Outcome Measures (PROMs) domains covered for each of the rCTDs. Also, for those diseases characterized by a relatively high prevalence and incidence, such as Systemic Lupus Erythematosus, Sjögren’s Syndrome, and Systemic Sclerosis, the analysis of patients’ resilience, satisfaction with the quality of the relationship, personality, and stress are still missing dimensions. It has been observed how reducing items, increasing the number of domains, and disease-specific questionnaires characterize the “technological trajectory,” such as the evolution of questionnaires’ characteristics for assessing QoL and QoL-related dimensions and the burden of rCTDs.
Conclusion: The scoping review presents an overview of studies focused on questionnaires used to evaluate the different dimensions of quality of life in terms of general instruments and disease-specific questionnaires. Future research should include the co-design with patients, caregivers, and patient representatives to create questionnaires focused on the unmet needs of people living with rCTDs.
Introduction
Rare and complex connective tissue and musculoskeletal diseases (rCTDs) are a heterogeneous group of immune-mediated inflammatory diseases. With heterogeneous symptoms, these diseases may affect various organs of the body and, at the latest stage, come to multiple organ systems impairment. rCTDs include diseases such as Systemic Lupus Erythematosus (SLE), Systemic Sclerosis (SSc), Mixed Connective Tissue Diseases (MCTD), Inflammatory Idiopathic Myopathies (IIM), Undifferentiated Connective Tissue Diseases (UCTD), Antiphospholipid Syndrome (APS), IgG4, Sjögren’s Syndrome (SS), Ehlers Danlos (EDS), and Relapsing Polychondritis (RP) (1). The accurate incidence and prevalence of each disease (2) are still undetermined; however, most rCTDs affect no more than 1 person per 2,000 (3), on average (meeting criteria defined by the European Union Regulation on Orphan Medicinal Products 1999), with few exceptions. Despite these diseases being characterized by a variegate spectrum of clinical manifestations (3) with often unpredictable courses that reduce health and wellbeing (4), their clinical and non-clinical burden is scarcely investigated. There are, of course, some exceptions, especially on SLE. It is the case of a comprehensive and recent survey that shows how SLE affected several spheres of patients’ life, in particular, education (50.7%), career (57.9%), and emotional/sexual life (38.2%) (5). Also, Shi et al. (6) showed that disease activities and organ damage are negatively correlated with HRQoL. Moreover, it has been shown that the cognitive impairments were negatively associated with social role and QoL (7) by Mendelsohn et al. Patients with SS are affected by physical and psychological disorders, such as digital ulcers, skeletal muscle weakness, fatigue, and depression, which impair working and social activities (8). Less investigated are family members’ and informal caregivers’ stress. Finally, poor and not detailed evidence on the economic implications and impacts of rCTDs on the sustainability of healthcare systems is available.
With the aim of reducing patients’ symptoms and disability and improving the Health-Related Quality of Life (HRQoL), clinicians are assessing the impact of selected specific symptoms on patients’ functional disability and QoL, and the factors associated with it (9, 10). On this, Patient Reported Outcome Measures (PROMs) are offering no available generic-and disease-specific instruments as in the case of the 36-item Short-Form Health Survey (SF-36) and the Lupus Quality of Life LupusQoL. HRQoL is the most explored dimension among PROMs (10). However, literature on PROMs in rCTDs is still scarce and instruments are characterized by great heterogeneity and applied to small sample sizes (11).
However, to the best of our knowledge, previous systematic reviews investigating the HRQoL of patients with Systemic Lupus Erythematosus (6–9, 12–17), Systemic Sclerosis (18–28), Ehlers-Danlos syndromes (29, 30), Sjögren’s Syndrome (31), and Idiopathic Inflammatory Myopathy (32) focused on the effect of new or existing therapies, and/or the burden of specific diseases on specific categories of patients. Studies included in the reviews adopted PROMs and related questionnaires as tools. It is also the case of those reviews related to pediatrics and adolescents (29), women (33), specific geographic areas and country (34), and also those papers that review the existing economic burden and health resource consumption (35).
Compared to the previous studies, this scoping review gave an overview of the tools (PROMs and QoL) adopted for investigating whether and how the scientific literature has measured the different dimensions of PROMs in rCTDs. To enhance our understanding of the global burden of rCTDs, in fact, there is an urgent need of reviewing all the instruments adopted, e.g., PROMs, and for each of the involved rCTDs, identifying which areas are still unexplored. This scoping review offers to clinicians and researchers a taxonomy of where we are in terms of existing tools for assessing dimensions of QoL, then it indicates the not investigated directions we should follow for a better understanding of the complexity of the non-clinical impact of different rCTDs.
Materials and methods
Study design and search strategy
To achieve the scope of the review, we adopted the PICO (Patient/Population Intervention Comparison Outcomes) framework to guide us in part for selecting the minimum quality and available information for including papers and to determine the boundaries of the scoping review; in part, for identifying some of the variables adopted for classifying the included paper (36).
Although in scopes, perspectives, outcomes, and suggestions, this paper is a scoping review, we adopted, as a searching and review method, the more rigorous PRISMA approach (37).
One author performed the literature research and three reviewers, working independently, screened all the studies, and extracted data using a template developed specifically for this study.
Research queries
For each database (PubMed and ISI-Web of Science), the research query consists of the intersection of two main fields: words related to QoL and other PROMs (125,143 records from PubMed and 19,497 from ISI-Web of Science); words related to rCTDs diseases (50,316 records from PubMed and 54,759 from ISI-Web of Science) for a total of 1,047 records from PubMed and 85 from ISI-Web of Science. The review process started with these records (see Figure 1). Research queries for the two databases are reported in the Supplementary material.
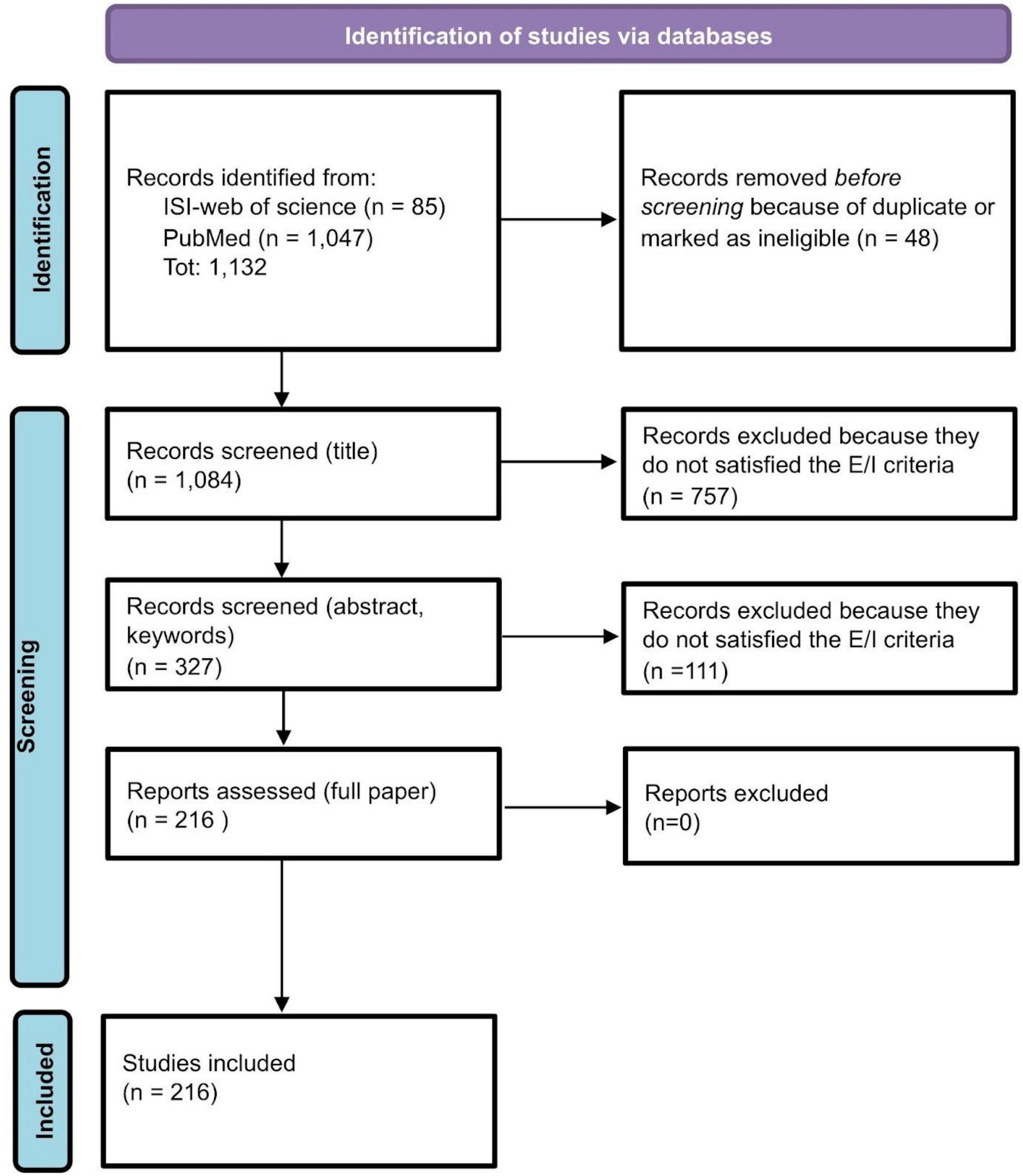
Figure 1. Review process: PRISMA flow-chart (37).
Including/excluding criteria
We included peer-review papers published between 2011 and 2021 in English. Within this time interval, non-English written papers, reports, reviews, theoretical and/or position papers without empirical analysis, and/or presentation of new questionnaires without empirical validation (see classification criteria), as well as papers with physical or clinical endpoints only and outputs of clinical studies have been excluded.
Statistics and test
Kruskal-Wallis test p-value and Chi-square test p-value tested median differences among groups of continuous variables and frequency tables of discrete variables, respectively.
Classification criteria for the included papers
Papers have been classified according to the type of studies, authors, year, title, journal, country, sample sizes, disease, questionnaire adopted, number of citations (from Google Scholar updated on May 20, 2022), and average number of citations per year. Types of studies have been classified as follows:
A. Studies that assess and compare the impact of new treatments, drugs, interventions, etc., in which QoL and psychological changes are adopted as criteria for assessing the efficacy of the proposed solutions.
B. Methodological studies that are devoted to:
B1: Validate existing metrics, questionnaires, and tools in specific populations and/or countries and/or compare existing questionnaires (e.g., mapping disease-specific vs. generic QoL questionnaires);
B2: Validate new metrics, questionnaires, and tools for assessing QoL and the psychological burden of rare diseases.
C. Studies that identify correlates of QoL and psychological burden with respect to the disease characteristics as ancillary analysis within specific studies. These studies can be analyses performed starting from A, through RCT, observational and interventional, and cohort studies.
D. Epidemiological studies including the socio-economic and psychological dimensions of the diseases, analyses of correlates of QoL, and psychological burden with respect to the disease characteristics in a specific population are included here.
Questionnaires classification
Questionnaires included in the paper have been classified according to the scope, number of dimensions, items, and disease specificity.
Outcomes
After the description of the number of studies selected and included in the scoping review through a PRISMA scheme (see subsection titled “Review process” and Figure 1), outcomes have been divided into three main topics.
Bibliometric analysis
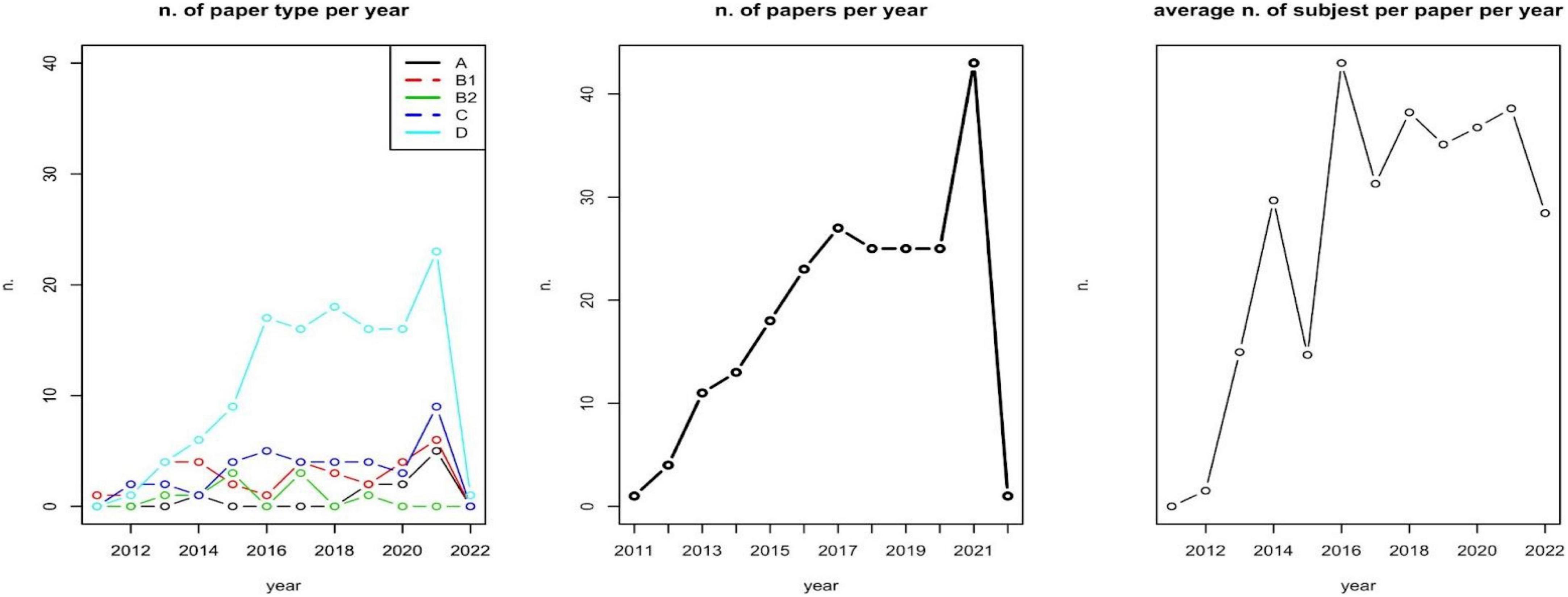
We first assessed the relationship between the number of subjects included and the type of papers; the number of involved subjects and the selected diseases; type of disease and type of paper involved. A first analysis describes the number of papers per type per year, the number of papers per year, and the average number of subjects involved (sample size) per year (Figure 2). These analyses aim at identifying an over year positive trend in sample size, and a focalization on methods and approaches for assessing patients’ PROMs.
A second descriptive analysis reports the number and distribution of citations per year (Figure 3), as a proxy of the field and specific paper’s impacts on the scientific community. A third analysis reports the per year most cited paper per included disease and PROMs’ domain (Table 1).
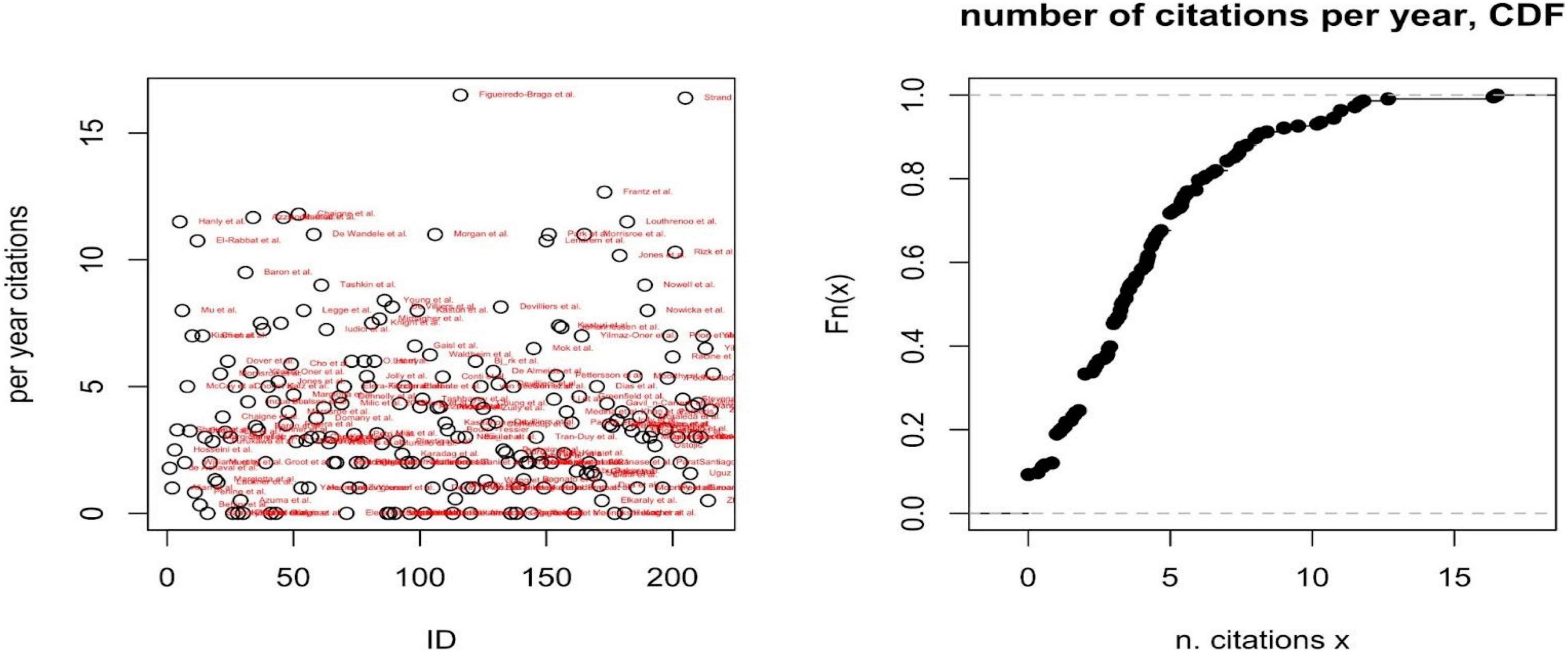
Figure 3. Per year number of citations (left) and cumulative distribution function (CDF) of the number of citations per year (right).
Analysis of questionnaires
It consists of three main analyses. First, we reported the number of total questionnaires administered per country, then, we reported the list of questionnaires adopted with indications of their domains and total items (Table 2). Finally, focusing on the most frequently covered dimensions of PROMs (i.e., anxiety and depression, fatigue, pain, and QoL), we analyzed the trend over the year of the related number of items, the number of domains, and the disease-specificity of questionnaires, to highlight a “technological” trajectory of the tools and metrics adopted for assessing patient’s PROMs (Figure 4).
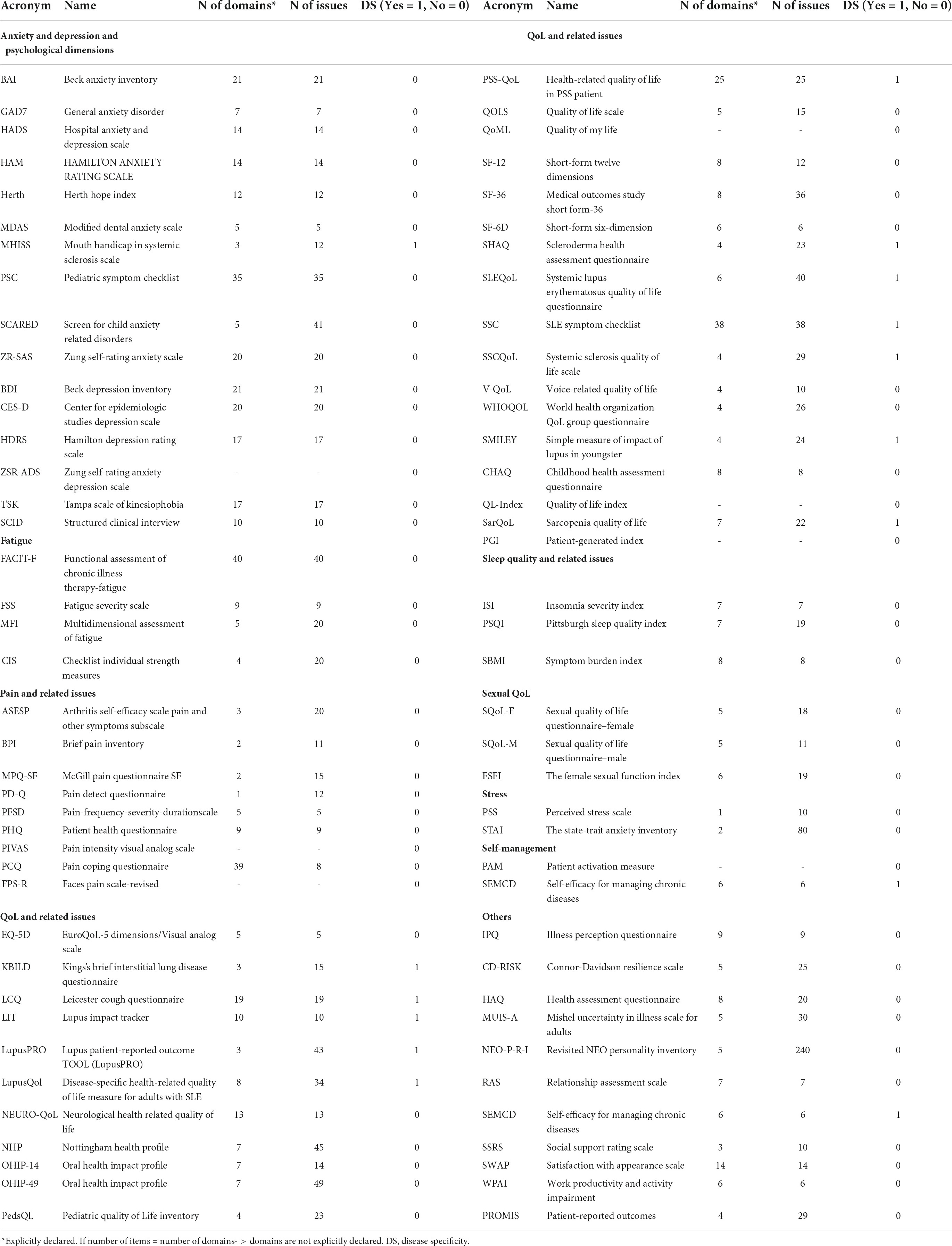
Table 2. Classification of questionnaires adopted per scope, no. of domains, no. of issues, and disease specificity.
Dimensions on patient reported outcome measures covered per disease
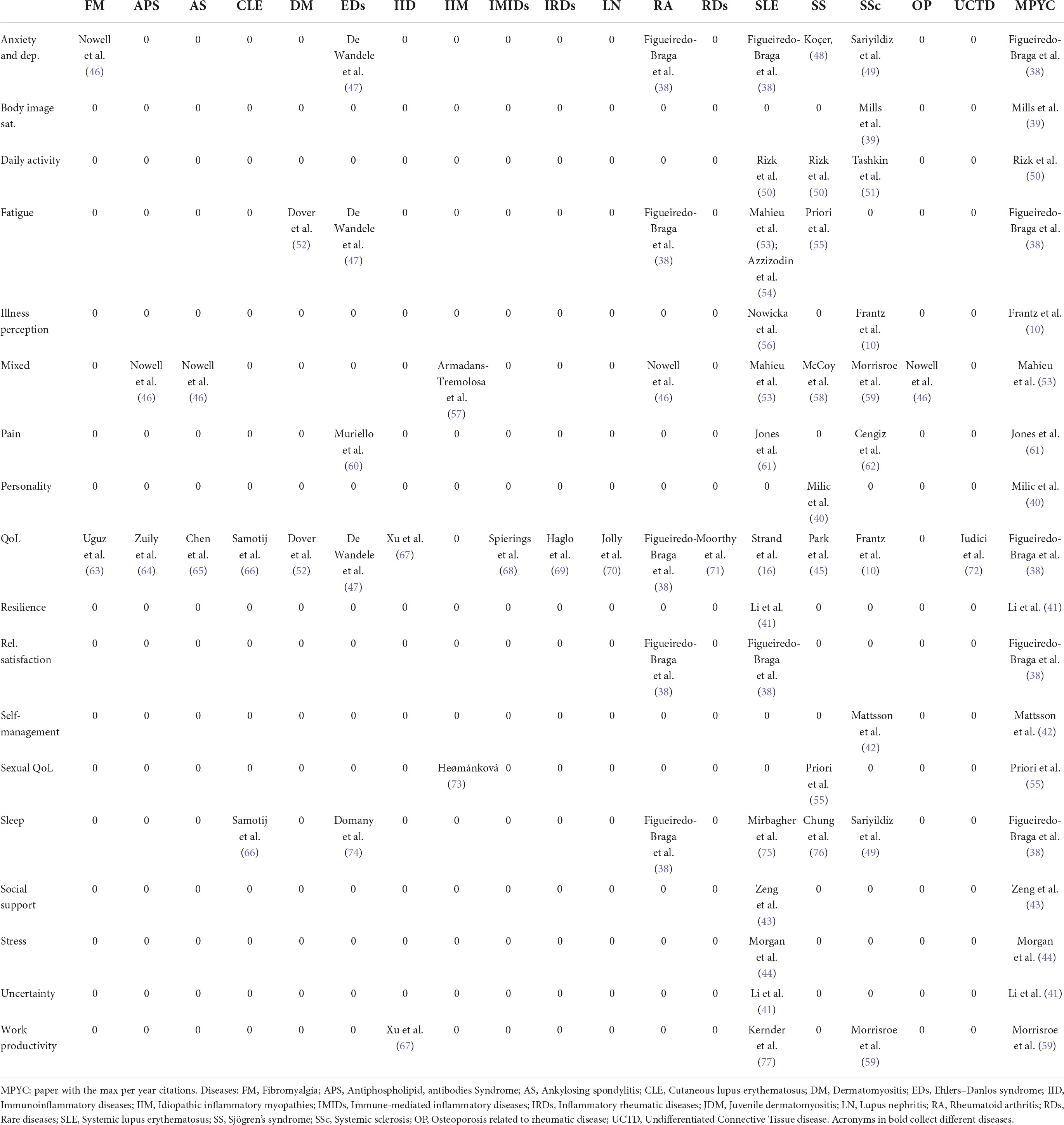
It is the core and the main outcome of the present scoping review. Each row of Table 3 refers to a specific PROM dimension over the different diseases. Each column of the table represents, for a specific disease, the dimensions of PROMs covered. Therefore, Table 1 shows the state of the art in our knowledge/ignorance of dimensions that describe how the selected diseases impact patients.
Results
Review process
After removing duplicates or ineligible papers because of the lack of abstracts and essential information, 1,084 papers’ abstracts from ISI-Web of Science and PubMed have been screened. A total of 327 papers survived the process. After excluding 111 articles because out of the objective of our review, we fully assessed 216 papers. All 216 papers have been included and classified in the present review (see Figure 1).
Bibliometric analysis
The majority of included papers are of type D (A: 4.63%; B1: 14.81%; B2: 4.17%; C: 17.59%; D: 58.80%). There is a non-significative positive relationship between the number of subjects included and A–D classifications (Kruskal-Wallis test p-value = 0.1699). The number of involved subjects also depends on the selected diseases (Kruskal-Wallis test p-value = 0.027). No effect of disease on the type of paper has been observed (Chi-square test p-value = 0.498).
As reported in Figure 2, during the years 2019–2020, a zero-growth rate can be observed in the number of scientific papers published as well as the number of subjects involved per paper (on average). A deeper and specific analysis could be carried out for identifying whether this is caused by the COVID-19 pandemic.
Diseases involved and questionnaires
A total of 7.8% of the included papers analyze/compare two diseases, 4.2% analyze/compare three diseases, 3.2% analyze/compare four diseases, and 0.46% analyze/compare five, six, and seven diseases. A total of 48.61% of the included papers’ outcomes are based on two validated questionnaires, 22.22% on three, 8.79% on four, and 5.09% on five validated questionnaires for assessing QoL and/or QoL-related dimensions and PROMs.
Impact of studies on the scientific community
The number of citations per year has been adopted as a proxy of the paper’s impact on the scientific community. The cumulative distribution function of the number of per year citations shows how the impact of each paper is relatively low, on average (i.e., the probability that a paper is cited at least five times per year is only 0.3); only two papers are cited more than 15 times per year, on average (Figueiredo-Braga et al. and Strand et al.), both focusing on SLE. For each disease and dimensions of PROMs, Supplementary material report the most cited or the only paper included.
Most cited papers per patient reported outcome measures’ domain and disease
Table 1 reports the most cited or the only available article per PROM’s domain and disease. The reported papers can be adopted as benchmarks in the covered field. Also, on the list of the most cited papers, there are some contributions that cover more than one PROM dimension and more than one disease, i.e., they recur on the table cells both horizontally (by PROMs dimension) and vertically (by disease). There are papers with high impact (measured in per year number of citations) with respect to PROMs and diseases covered. It is the case of Figeiredo-Braga et al. (38), in SLE and Rheumatoid arthritis (RA), that assesses anxiety and depression, QoL, satisfaction with the relation, and sleep quality. The last column of Table 1 collects the per-year most cited papers per PROM dimension, on the most cited papers per PROM and disease. With respect to dimensions that are not often covered, Mills et al. (39) investigate body image satisfaction for SSc patients; Milic et al. (40) analyze the personality of SS patients; Li et al. (41) investigate resilience in SLE; Mattsson et al. (42) analyzed self-management and sexual QoL (SS patients); Zeng et al. (43), Morgan et al. (44), and Park et al. (45) analyzed social support, stress, uncertainty in SLE patients, respectively.
Analysis of questionnaires
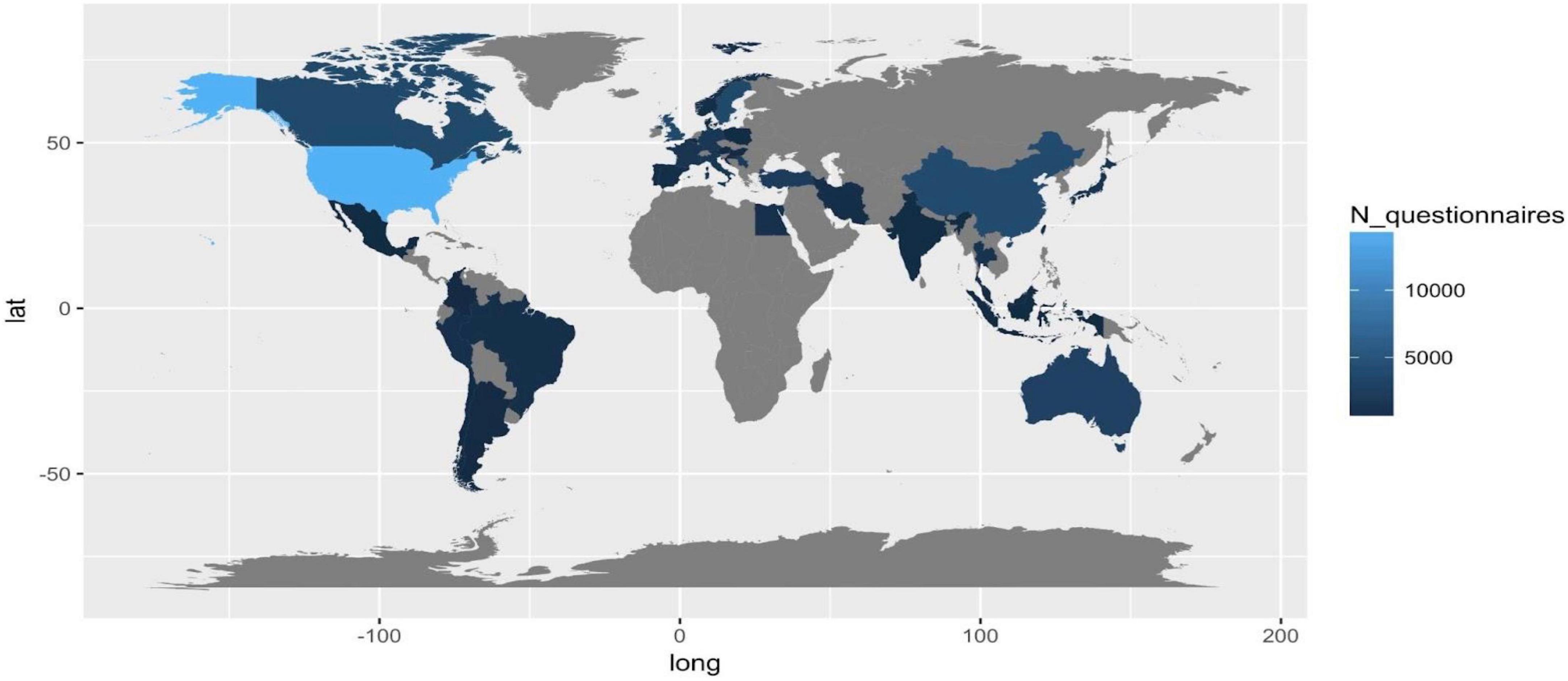
Administered questionnaires/subjects involved per countries
Considering the number of total questionnaires administered per county as a proxy of interest in PROMs related to the included disease, we observe the lack of information in Russia, South-Russia countries, Central America, Africa, and Arabia. The quality and efficiency of healthcare systems and data (e.g., USA, Canada), and the incidence and prevalence of country-specific diseases (e.g., Turkey and Iran) can explain the high frequency of studies in specific regions.
Technological trajectories of questionnaires
Treating questionnaires as “technologies” for assessing PROMs, three drivers guide their design: number of domains (D), number of issues (I), and disease specificity (DS).
Disease specificity
We observed a moderate tendency of increasing disease specificity in QoL and Pain questionnaires (Figure 5C).
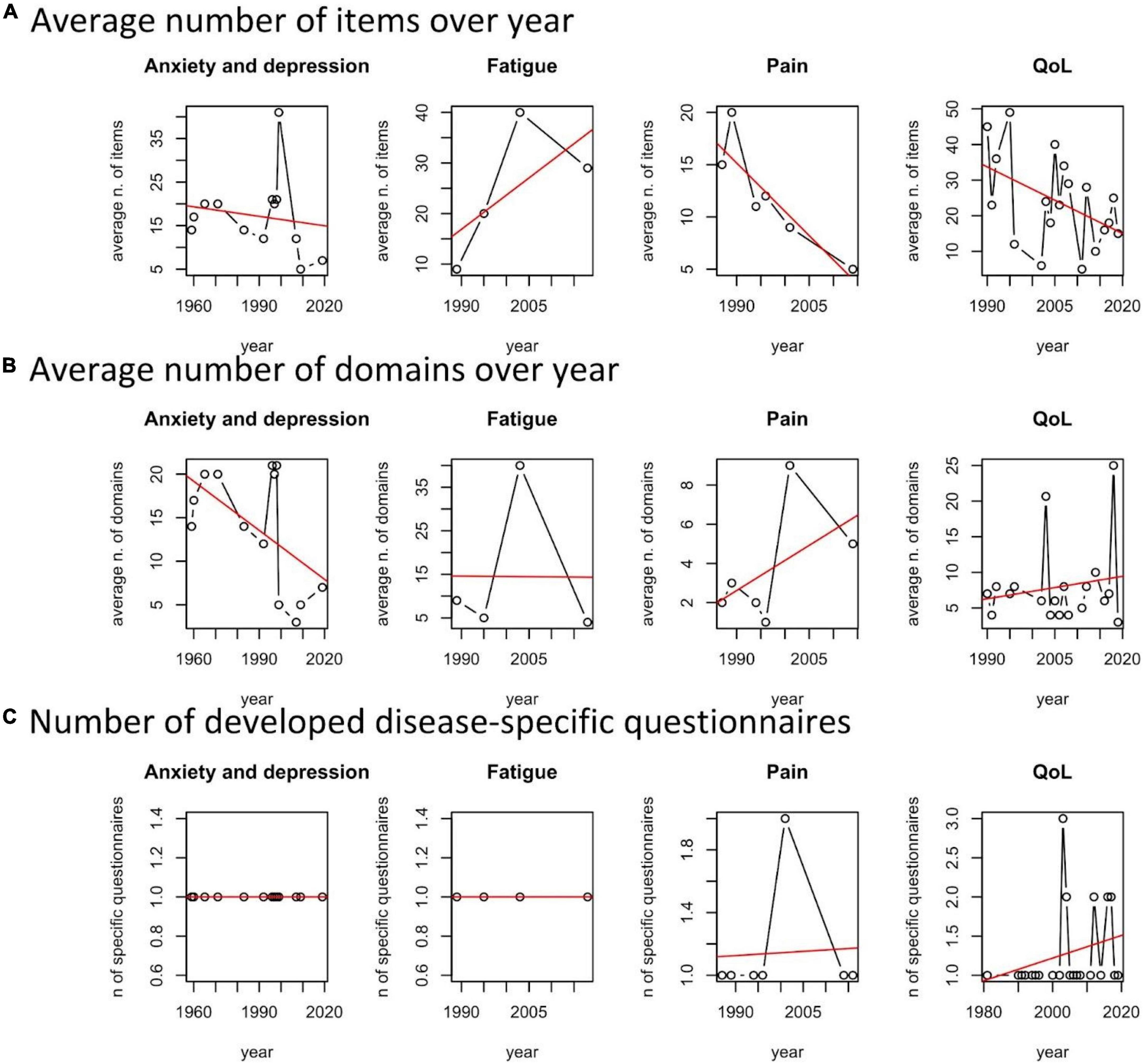
Figure 5. Average number of issues, domains, and number of developed disease-specific questionnaires per year and patient reported outcome measures (PROMs) area.
Number of domains and issues
With respect to the average number of issues of the developed questionnaires per year and scope, we noted a general tendency of improving the survey’s efficiency by cutting the number of questions. This is observed for validated questionnaires on anxiety and depression, pain and related issues, and QoL (Figure 5A). There is a tendency of increasing the number of domains for Pain and QoL (Figure 5B). The exception is the domain “fatigue,” probably because of increasing awareness of scholars on the different dimensions involved in describing it.
To summarize, reduction of items, increasing number of domains, and disease-specific questionnaires characterize the “technological trajectory” of questionnaires for assessing QoL and QoL-related dimensions and the burden of rCTDs.
Diseases and dimensions of patient reported outcome measures covered
Table 3 reports the number of papers that adopted validated questionnaires for assessing the dimensions of the disease. Black cells indicate that, for a particular disease, a specific dimension is not covered.
As one can observe, only questionnaires on QoL are frequently adopted for many of the involved diseases. pSS, RA, SLE, SS, and SSc are diseases for which we have more information on anxiety and depression, illness perception, pain, stress, and social support (especially for SLE). Also, considering the most studied diseases because of a relatively higher number of available patients to be enrolled, body image satisfaction and self-management are not assessed for SS, RA, SLE, and SS. It is also the case for illness perception in SS and RA (in general). Social support is covered only for SLE. The less covered dimensions, in general, and among the different diseases are body image satisfaction, resilience, and the assessment of personality. These are no less important dimensions, influencing medical-patient interactions and communication, adherence, behavioral changes, and the effect of social marketing initiatives, as well as the efficacy of therapies and treatments.
Discussion
The state of the art on QoL and other PROMs related to rCTDs suggests five areas of challenges and area of future research.
First, there are diseases that need to be investigated beyond the generic questionnaires on QoL (e.g., APS, Antiphospholipid, antibodies syndrome; AS, Ankylosing spondylitis; JDM, Juvenile dermatomyositis; LN, Lupus nephritis).
Second, there are dimensions that are not covered for some relatively well-investigated diseases, e.g., stress, satisfaction with the relation, and resilience. We wonder if, over the years, Table 3 of our review will reduce the number of black cells, also for those diseases that are not in the current spotlight as it happens for SLE and SSc.
Third, Table 3 collects dimensions that are covered at least one time over the included papers and disease. Therefore, there are dimensions still not covered by the current literature, such as prescription and therapy compliance, family organization’ changes induced by the disease, etc., and other dimensions that could emerge only through a bottom-up approach and by adopting complementary qualitative methods, such as Narrative Medicine, focus groups, etc.
A fourth emerged theme (see Figure 5) is related to the available and adopted questionnaires for each dimension of PROMs. Although the average number of questions per questionnaire is falling in a specific domain like QoL, SF-36 is the standard, the heterogeneity of the adopted questionnaires and the lack of specific mapping among the related questionnaires make it very difficult to offer a valid suggestion for selecting the most appropriate questionnaire, neither to summarize too heterogeneous scores into a general synthesis. This mainly depends on the lack of a general consensus on the meaning of quality of life among scholars. Mapping scores of different questionnaires could fill the current complexity and uncertainty, supporting researchers in selecting the most appropriate questionnaires covering different dimensions of PROMs.
Finally, although it is a fundamental topic in improving the sustainability, quality, and equity of healthcare systems, no evidence emerged on the relationship between QoL and the other dimensions of PROMs, on one side, and Patient Reported Experience Measures (PREMs) on the quality of existing and new healthcare service delivery, both from the perspective of patients and healthcare providers, on the other side. Mapping validated questionnaires related to PROMs and PREMs could improve our understanding of patients’ needs and reaction to the offered healthcare services.
Also, specific QoL assessment techniques in rCTDs may fill the gap with respect to well-known diseases. For instance, patients can report their quality of life and experiences in dedicated digital diaries, and/or through specific texts. The techniques adopted in narrative medicine are promising and new dimensions related to the quality of life may rise in the future to come.
Moreover, Big Data could be adopted for detecting and analyzing rCTD patients’ behaviors, quality of life, and decision-making without dedicated questionnaires. However, the role of Big Data is limited by sample sizes and ethical and legal/privacy constraints.
Finally, note that all dimensions reported in the scoping review and those indicated for future investigations refer to subjective and conscious answers coming from the patient. New advanced techniques can also detect positive or negative experiences with care and treatment by means of objective measures of unconscious feelings. Neuroscience and consumer neuroscience techniques and new measures, such as frontal alpha-wave asymmetry (78), detected through electroencephalography (EEG) (79) are promising solutions. However, if they solve biases of the current questionnaires through which conscious answers on ex post rationalized experiences are collected, these interesting and new techniques are not able to overcome and usually increase the problem of insufficient sample sizes.
Strength and limitations
The strength of the current scoping review is in the number of included and classified papers (216), on the capability to check, for disease and PROMs domain, and the availability or lack of studies and instruments. Since the scoping review covers the field of rCTDs, it overcomes the existing methodological reviews that are usually disease specific (9, 78). Also, to our knowledge, this is the first work that quantitatively analyzes “technological” trajectories of questionnaires on the basis of the number of items, number of domains, and disease specificity.
However, this scoping review has some limitations and weaknesses. First, although it mentions the most cited papers, it does not report their outcome. As clarified in the introduction, the review can answer whether and how some dimensions have been covered but not what and the relation with the clinical outcomes. Since it was not the aim of our scoping review, scholars and/or professionals that are interested in dimensions not covered in the present work can refer to disease-specific reviews. Second, our analysis is limited to the specific questionnaires adopted and used and it does not refer to all the available questionnaires and PROMs. Third, specific analyses on questionnaire subdomains have not been conducted. As a consequence, we did not take into account other questionnaires’ subdomains that refer to the selected PROMs. Fourth, we focus our attention on disease-specific vs. generic questionnaires. Therefore, although Table 2 reports population-specific questionnaires (e.g., questionnaires for pediatric patients), we did not conduct analysis on this topic. Fifth, with respect to the number of questionnaires administered per country, we did not detail data for a specific disease but we referred to the general field of rCTDs. Finally, the time-interval selection is not justified by some discoveries or facts that justify the adoption of a 10-year period for including/excluding papers. Fixing a “related to the field” factor for selecting time-interval would have shifted the objective of the review, from assessing how QoL and the related dimensions are covered, to assess the impact of the specific factor (e.g., a new drug and a new specific questionnaire) on the assessment of QoL and the other dimensions for rCTDs patients.
Conclusion
This scoping review presents an overview of studies focused on questionnaires used to evaluate the different dimensions of quality of life in terms of general instruments and disease-specific questionnaires. Future research should include the co-design with patients, caregivers, and patient representatives to create questionnaires focused on the unmet needs of people living with rare and complex connective tissue and musculoskeletal diseases.
Author contributions
SC was responsible for the manuscript’s abstract and keywords. IT and LT were responsible for the manuscript introduction. SC and LT edited methods. LT, IP, and IT reviewed and classified the included manuscripts. LT quantitatively analyzed the classified manuscript and edited results, tables, and figures in results. All authors contributed equally to the discussion and conclusion, and also revised the final version of the manuscript and the Supplementary material.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.986218/full#supplementary-material
References
1. Talarico R, Aguilera S, Alexander T, Amoura Z, Andersen J, Arnaud L, et al. The added value of a European reference network on rare and complex connective tissue and musculoskeletal diseases: Insights after the first 5 years of the ERN ReCONNET. Clin Exp Rheumatol. (2022) 40:3–11. doi: 10.55563/clinexprheumatol/d2qz38
2. Carter EE, Barr SG, Clarke AE. The global burden of SLE: Prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. (2016) 12:605–20. doi: 10.1038/nrrheum.2016.137
3. Cannizzo S, Lorenzoni V, Palla I, Pirri S, Trieste L, Triulzi I, et al. Rare diseases under different levels of economic analysis: Current activities, challenges and perspectives. RMD Open. (2018) 4:e000794. doi: 10.1136/rmdopen-2018-000794
4. Tsokos GC. Systemic lupus erythematosus. N Engl J Med. (2011) 365:2110–21. doi: 10.1056/NEJMra1100359
5. Cornet A, Andersen J, Myllys K, Edwards A, Arnaud L. Living with systemic lupus erythematosus in 2020: A European patient survey. Lupus Sci Med. (2021) 8:e000469. doi: 10.1136/lupus-2020-000469
6. Shi Y, Li M, Liu L, Wang Z, Wang Y, Zhao J, et al. Relationship between disease activity, organ damage and health-related quality of life in patients with systemic lupus erythematosus: A systemic review and meta-analysis. Autoimmun Rev. (2021) 20:102691. doi: 10.1016/j.autrev.2020.102691
7. Mendelsohn S, Khoja L, Alfred S, He J, Anderson M, DuBois D, et al. Cognitive impairment in systemic lupus erythematosus is negatively related to social role participation and quality of life: A systematic review. Lupus. (2021) 30:1617–30. doi: 10.1177/09612033211031008
8. Hudson M, Thombs BD, Steele R, Panopalis P, Newton E, Baron M. Health-related quality of life in systemic sclerosis: A systematic review. Arthritis Rheum. (2009) 61:1112–20. doi: 10.1002/art.24676
9. Nguyen MH, Huang FF, O’Neill SG. Patient-reported outcomes for quality of life in SLE: Essential in clinical trials and ready for routine care. J Clin Med. (2021) 10:3754. doi: 10.3390/jcm10163754
10. Frantz C, Avouac J, Distler O, Amrouche F, Godard D, Kennedy AT, et al. Impaired quality of life in systemic sclerosis and patient perception of the disease: A large international survey. Semin Arthritis Rheum. (2016) 46:115–23. doi: 10.1016/j.semarthrit.2016.02.005
11. Miedany Y. Patient reported outcome measures in rheumatic diseases. Berlin: Springer (2016). doi: 10.1007/978-3-319-32851-5
12. Castelino M, Abbott J, McElhone K, Teh L-S. Comparison of the psychometric properties of health-related quality of life measures used in adults with systemic lupus erythematosus: A review of the literature. Rheumatology. (2013) 52:684–96. doi: 10.1093/rheumatology/kes370
13. Elera-Fitzcarrald C, Fuentes A, González LA, Burgos PI, Alarcón GS, Ugarte-Gil MF. Factors affecting quality of life in patients with systemic lupus erythematosus: Important considerations and potential interventions. Expert Rev Clin Immunol. (2018) 14:915–31. doi: 10.1080/1744666X.2018.1529566
14. Barber MRW, Clarke AE. Socioeconomic consequences of systemic lupus erythematosus. Curr Opin Rheumatol. (2017) 29:480–5. doi: 10.1097/BOR.0000000000000416
15. Puyade M, Maltez N, Lansiaux P, Pugnet G, Roblot P, Colmegna I, et al. Health-related quality of life in systemic sclerosis before and after autologous haematopoietic stem cell transplant-a systematic review. Rheumatology. (2020) 59:779–89. doi: 10.1093/rheumatology/kez300
16. Strand V, Simon LS, Meara AS, Touma Z. Measurement properties of selected patient-reported outcome measures for use in randomised controlled trials in patients with systemic lupus erythematosus: A systematic review. Lupus Sci Med. (2020) 7:e000373. doi: 10.1136/lupus-2019-000373
17. Rodríguez Huerta MD, Trujillo-Martín MM, Rúa-Figueroa Í, Cuellar-Pompa L, Quirós-López R, Serrano-Aguilar P. Healthy lifestyle habits for patients with systemic lupus erythematosus: A systematic review. Semin Arthritis Rheum. (2016) 45:463–70. doi: 10.1016/j.semarthrit.2015.09.003
18. Almeida C, Almeida I, Vasconcelos C. Quality of life in systemic sclerosis. Autoimmun Rev. (2015) 14:1087–96. doi: 10.1016/j.autrev.2015.07.012
19. Maddali-Bongi S, Del Rosso A. Systemic sclerosis: Rehabilitation as a tool to cope with disability. Clin Exp Rheumatol. (2016) 34:162–9.
20. Nakayama A, Tunnicliffe DJ, Thakkar V, Singh-Grewal D, O’Neill S, Craig JC, et al. Patients’ perspectives and experiences living with systemic sclerosis: A systematic review and thematic synthesis of qualitative studies. J Rheumatol. (2016) 43:1363–75. doi: 10.3899/jrheum.151309
21. Li L, Cui Y, Chen S, Zhao Q, Fu T, Ji J, et al. The impact of systemic sclerosis on health-related quality of life assessed by SF-36: A systematic review and meta-analysis. Int J Rheum Dis. (2018) 21:1884–93. doi: 10.1111/1756-185X.13438
22. Smirani R, Truchetet M-E, Poursac N, Naveau A, Schaeverbeke T, Devillard R. Impact of systemic sclerosis oral manifestations on patients’ health-related quality of life: A systematic review. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. (2018) 47:808–15. doi: 10.1111/jop.12739
23. Moradzadeh M, Aghaei M, Mehrbakhsh Z, Arab-Bafrani Z, Abdollahi N. Efficacy and safety of rituximab therapy in patients with systemic sclerosis disease (SSc): Systematic review and meta-analysis. Clin Rheumatol. (2021) 40:3897–918. doi: 10.1007/s10067-021-05698-4
24. Fischer A, Zimovetz E, Ling C, Esser D, Schoof N. Humanistic and cost burden of systemic sclerosis: A review of the literature. Autoimmun Rev. (2017) 16:1147–54. doi: 10.1016/j.autrev.2017.09.010
25. DeQuattro K, Yelin E. Socioeconomic status, health care, and outcomes in systemic lupus erythematosus. Rheum Dis Clin North Am. (2020) 46:639–49. doi: 10.1016/j.rdc.2020.07.004
26. Bangert E, Wakani L, Merchant M, Strand V, Touma Z. Impact of belimumab on patient-reported outcomes in systemic lupus erythematosus: Review of clinical studies. Patient Relat Outcome Meas. (2019) 10:1–7. doi: 10.2147/PROM.S134326
27. Wang Y, Zhao R, Gu C, Gu Z, Li L, Li Z, et al. The impact of systemic lupus erythematosus on health-related quality of life assessed using the SF-36: A systematic review and meta-analysis. Psychol Health Med. (2019) 24:978–91. doi: 10.1080/13548506.2019.1587479
28. Basta F, Afeltra A, Margiotta DPE. Fatigue in systemic sclerosis: A systematic review. Clin Exp Rheumatol. (2018) 36:S150–60.
29. Feldman ECH, Hivick DP, Slepian PM, Tran ST, Chopra P, Greenley RN. Pain symptomatology and management in pediatric ehlers-danlos syndrome: A review. Child. (2020) 7:146. doi: 10.3390/children7090146
30. Malfait F, Colman M, Vroman R, De Wandele I, Rombaut L, Miller RE, et al. Pain in the ehlers-danlos syndromes: Mechanisms, models, and challenges. Am J Med Genet C Semin Med Genet. (2021) 187:429–45. doi: 10.1002/ajmg.c.31950
31. Wu G-L, Li T-Y. Focus on effects of chinese medicine on improving anxiety-depression and quality of life of patients with primary sjögren’s syndrome. Chin J Integr Med. (2020) 26:486–9. doi: 10.1007/s11655-020-3473-0
32. Leclair V, Regardt M, Wojcik S, Hudson M. Health-related quality of life (HRQoL) in idiopathic inflammatory myopathy: A systematic review. PLoS One. (2016) 11:e0160753. doi: 10.1371/journal.pone.0160753
33. Oliveira FAP, Santos FMMD, Dias AFMP, Neiva CLS, Telles RW, Lanna CCD. Cosmetic camouflage improves health-related quality of life in women with systemic lupus erythematosus and permanent skin damage: A controlled intervention study. Lupus. (2020) 29:1438–48. doi: 10.1177/0961203320947802
34. Phuti A, Schneider M, Tikly M, Hodkinson B. Living with systemic lupus erythematosus in the developing world. Rheumatol Int. (2018) 38:1601–13. doi: 10.1007/s00296-018-4017-1
35. Martin Calderon L, Chaudhury M, Pope JE. Healthcare utilization and economic burden in systemic sclerosis: A systematic review. Rheumatology. (2021) 61:3123–31. doi: 10.1093/rheumatology/keab847
36. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. (2007) 7:16. doi: 10.1186/1472-6947-7-16
37. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
38. Figueiredo-Braga M, Cornaby C, Cortez A, Bernardes M, Terroso G, Figueiredo M, et al. Depression and anxiety in systemic lupus erythematosus: The crosstalk between immunological, clinical, and psychosocial factors. Medicine. (2018) 97:e11376. doi: 10.1097/MD.0000000000011376
39. Mills SD, Fox RS, Merz EL, Clements PJ, Kafaja S, Malcarne VL, et al. Evaluation of the satisfaction with appearance scale and its short form in systemic sclerosis: Analysis from the UCLA scleroderma quality of life study. J Rheumatol. (2015) 42:1624–30. doi: 10.3899/jrheum.141482
40. Milic V, Grujic M, Barisic J, Marinkovic-Eric J, Duisin D, Cirkovic A, et al. Personality, depression and anxiety in primary Sjogren’s syndrome - Association with sociodemographic factors and comorbidity. PLoS One. (2019) 14:e0210466. doi: 10.1371/journal.pone.0210466
41. Li T, Cui C, Li Y, Wang L. The impacts of resilience on the association between illness uncertainty and sleep quality among Chinese women with systemic lupus erythematosus. Clin Rheumatol. (2020) 39:1609–16. doi: 10.1007/s10067-019-04898-3
42. Mattsson M, Sandqvist G, Hesselstrand R, Olsson D, Kwakkenbos L, Nordin A, et al. Validity and reliability of the Swedish version of the self-efficacy for managing chronic disease scale for individuals with systemic sclerosis. Scand J Rheumatol. (2022) 51:110–9. doi: 10.1080/03009742.2021.1917142
43. Zeng F, Xu Q, Liu D, Luo H, Zhou Y-O, Ning W, et al. Relatives’ quality of life and psychological disturbance: A new concern of SLE management. Clin Rheumatol. (2018) 37:67–73. doi: 10.1007/s10067-017-3743-1
44. Morgan C, Bland AR, Maker C, Dunnage J, Bruce IN. Individuals living with lupus: Findings from the LUPUS UK members survey 2014. Lupus. (2018) 27:681–7. doi: 10.1177/0961203317749746
45. Park J-B. Using big data to understand rare diseases. Int J Hear Fail. (2021) 3:194–6. doi: 10.36628/ijhf.2021.0026
46. Nowell WB, Gavigan K, Kannowski CL, Cai Z, Hunter T, Venkatachalam S, et al. Which patient-reported outcomes do rheumatology patients find important to track digitally? A real-world longitudinal study in arthritis power. Arthritis Res Ther. (2021) 23:53. doi: 10.1186/s13075-021-02430-0
47. De Wandele I, Calders P, Peersman W, Rimbaut S, De Backer T, Malfait F, et al. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin Arthritis Rheum. (2014) 44:353–61. doi: 10.1016/j.semarthrit.2014.05.013
48. Koçer B, Tezcan ME, Batur HZ, Haznedaroğlu Ş, Göker B, İrkeç C, et al. Cognition, depression, fatigue, and quality of life in primary Sjögren’s syndrome: correlations. Brain Behav. (2016) 6:e00586. doi: 10.1002/brb3.586
49. Sariyildiz MA, Batmaz I, Budulgan M, Bozkurt M, Yazmalar L, Inanir A, et al. Sleep quality in patients with systemic sclerosis: relationship between the clinical variables, depressive symptoms, functional status, and the quality of life. Rheumatol Int. (2013) 33:1973–9. doi: 10.1007/s00296-013-2680-9
50. Rizk A, Gheita TA, Nassef S, Abdallah A. The impact of obesity in systemic lupus erythematosus on disease parameters, quality of life, functional capacity and the risk of atherosclerosis. Int J Rheum Dis. (2012) 15:261–7. doi: 10.1111/j.1756-185X.2011.01698.x
51. Tashkin DP, Volkmann ER, Tseng C-H, Roth MD, Khanna D, Furst DE, et al. Improved cough and cough-specific quality of life in patients treated for scleroderma-related interstitial lung disease: results of scleroderma lung study II. Chest. (2017) 151:813–20. doi: 10.1016/j.chest.2016.11.052
52. Dover S, Stephens S, Schneiderman JE, Pullenayegum E, Wells GD, Levy DM, et al. The effect of creatine supplementation on muscle function in childhood myositis: a randomized, double-blind, placebo-controlled feasibility study. J Rheumatol. (2021) 48:434–41. doi: 10.3899/jrheum.191375
53. Mahieu M, Yount S, Ramsey-Goldman R. Patient-reported outcomes in systemic lupus erythematosus. Rheum Dis Clin North Am. (2016) 42:253–63. doi: 10.1016/j.rdc.2016.01.001
54. Azizoddin DR, Gandhi N, Weinberg S, Sengupta M, Nicassio PM, Jolly M. Fatigue in systemic lupus: the role of disease activity and its correlates. Lupus. (2019) 28:163–73. doi: 10.1177/0961203318817826
55. Priori R, Minniti A, Derme M, Antonazzo B, Brancatisano F, Ghirini S, et al. Quality of sexual life in women with primary sjögren syndrome. J Rheumatol. (2015) 42:1427–31. doi: 10.3899/jrheum.141475
56. Nowicka-Sauer K, Hajduk A, Kujawska-Danecka H, Banaszkiewicz D, Smoleńska Ż, Czuszyńska Z, et al. Illness perception is significantly determined by depression and anxiety in systemic lupus erythematosus. Lupus. (2018) 27:454–60. doi: 10.1177/0961203317751858
57. Armadans-Tremolosa I, Guilera G, Las Heras M, Castrechini A, Selva-O’Callaghan A. Functioning in adult patients with idiopathic inflammatory myopathy: Exploring the role of environmental factors using focus groups. PLoS One. (2021) 16:e0244959. doi: 10.1371/journal.pone.0244959
58. McCoy SS, Bartels CM, Saldanha IJ, Bunya VY, Akpek EK, Makara MA, et al. National sjögren’s foundation survey: burden of oral and systemic involvement on quality of life. J Rheumatol. (2021) 48:1029–36. doi: 10.3899/jrheum.200733
59. Morrisroe K, Hudson M, Baron M, De Vries-Bouwstra J, Carreira PE, Wuttge DM, et al. Determinants of health-related quality of life in a multinational systemic sclerosis inception cohort. Clin Exp Rheumatol. (2018) 36:S53–60.
60. Muriello M, Clemens JL, Mu W, Tran PT, Rowe PC, Smith CH, et al. Pain and sleep quality in children with non-vascular ehlers-danlos syndromes. Am J Med Genet A. (2018) 176:1858–64. doi: 10.1002/ajmg.a.40371
61. Jones JT, Cunningham N, Kashikar-Zuck S, Brunner HI. Pain, fatigue, and psychological impact on health-related quality of life in childhood-onset lupus. Arthritis Care Res (Hoboken). (2016) 68:73–80. doi: 10.1002/acr.22650
62. Cengiz G, Erol K, Gok K, Ozgocmen S. Comparison of pain characteristics in patients with rheumatoid arthritis and systemic sclerosis with particular reference to the neuropathic pain component: cross-sectional study. Med Princ Pract Int J Kuwait Univ Heal Sci Cent. (2018) 27:537–42. doi: 10.1159/000493480
63. Uguz F, Kucuk A, Cicek E, Kayhan F, Salli A, Guncu H, et al. Quality of life in rheumatological patients: the impact of personality disorders. Int J Psychiatry Med. (2015) 49:199–207. doi: 10.1177/0091217415582183
64. Zuily S, Rat A-C, Regnault V, Kaminsky P, Mismetti P, Ninet J, et al. Impairment of quality of life in patients with antiphospholipid syndrome. Lupus. (2015) 24:1161–8. doi: 10.1177/0961203315580871
65. Chen H-H, Chen D-Y, Chen Y-M, Lai K-L. Health-related quality of life and utility: comparison of ankylosing spondylitis, rheumatoid arthritis, and systemic lupus erythematosus patients in Taiwan. Clin Rheumatol. (2017) 36:133–42. doi: 10.1007/s10067-016-3471-y
66. Samotij D, Szczêch J, Antiga E, Bonciani D, Caproni M, Chasset F, et al. Clinical characteristics of itch in cutaneous lupus erythematosus: a prospective, multicenter, multinational, cross-sectional study. Lupus. (2021) 30:1385–93. doi: 10.1177/09612033211016098
67. Xu A, Sun C, Metcalf R, Limaye V. Health-related quality of life and work impairment in idiopathic inflammatory myopathies in South Australia. Int J Rheum Dis. (2021) 24:809–14. doi: 10.1111/1756-185X.14120
68. Spierings J, Sloeserwij A, Vianen ME, de Boer JH, Sigurdsson V, van de Wijgert JHHM, et al. Health-related quality of life in patients with immune mediated inflammatory diseases: a cross-sectional, multidisciplinary study. Clin Immunol. (2020) 214:108392. doi: 10.1016/j.clim.2020.108392
69. Haglo H, Wang E, Berg OK, Hoff J, Helgerud J. Smartphone-assisted high-intensity interval training in inflammatory rheumatic disease patients: randomized controlled trial. JMIR Mhealth Uhealth. (2021) 9:e28124. doi: 10.2196/28124
70. Jolly M, Toloza S, Goker B, Clarke AE, Navarra SV, Wallace D, et al. Disease-specific quality of life in patients with lupus nephritis. Lupus. (2018) 27:257–64. doi: 10.1177/0961203317717082
71. Moorthy LN, Baldino ME, Kurra V, Puwar D, Llanos A, Peterson MGE, et al. Relationship between health-related quality of life, disease activity and disease damage in a prospective international multicenter cohort of childhood onset systemic lupus erythematosus patients. Lupus. (2017) 26:255–65. doi: 10.1177/0961203316659546
72. Iudici M, Pantano I, Fasano S, Pierro L, Charlier B, Pingeon M, et al. Health status and concomitant prescription of immunosuppressants are risk factors for hydroxychloroquine non-adherence in systemic lupus patients with prolonged inactive disease. Lupus. (2018) 27:265–72. doi: 10.1177/0961203317717631
73. Heømánková B, Špiritoviæ M, Oreská S, Štorkánová H, Komarc M, Klein M, et al. Sexual function in patients with idiopathic inflammatory myopathies: a cross-sectional study. Rheumatology (Oxford). (2021) 60:5060–72. doi: 10.1093/rheumatology/keab397
74. Domany KA, Hantragool S, Smith DF, Xu Y, Hossain M, Simakajornboon N. Sleep disorders and their management in children with ehlers-danlos syndrome referred to sleep clinics. J Clin sleep Med. (2018) 14:623–9. doi: 10.5664/jcsm.7058
75. Mirbagher L, Gholamrezaei A, Hosseini N, Sayed Bonakdar Z. Sleep quality in women with systemic lupus erythematosus: contributing factors and effects on health-related quality of life. Int J Rheum Dis. (2016) 19:305–11. doi: 10.1111/1756-185X.12418
76. Chung SW, Hur J, Ha Y-J, Kang EH, Hyon JY, Lee H-J, et al. Impact of sleep quality on clinical features of primary Sjögren’s syndrome. Korean J Intern Med. (2019) 34:1154–64. doi: 10.3904/kjim.2017.158
77. Kernder A, Richter JG, Fischer-Betz R, Winkler-Rohlfing B, Brinks R, Aringer M, et al. Delayed diagnosis adversely affects outcome in systemic lupus erythematosus: cross sectional analysis of the LuLa cohort. Lupus. (2021) 30:431–8. doi: 10.1177/0961203320983445
78. Zhao G, Zhang Y, Ge Y. Frontal. EEG asymmetry and middle line power difference in discrete emotions. Front Behav Neurosci. (2018) 12:1–14. doi: 10.3389/fnbeh.2018.00225
Keywords: daily activity, fatigue, pain, sleep quality, quality of life, rheumatic diseases, PROMS, musculoskeletal diseases
Citation: Trieste L, Cannizzo S, Palla I, Triulzi I and Turchetti G (2022) State of the art and future directions in assessing the quality of life in rare and complex connective tissue and musculoskeletal diseases. Front. Med. 9:986218. doi: 10.3389/fmed.2022.986218
Received: 04 July 2022; Accepted: 25 August 2022;
Published: 23 September 2022.
Edited by:
Rosaria Talarico, University of Pisa, ItalyReviewed by:
Massimo Radin, University of Turin, ItalyLucía Silva-Fernández, Hospital Universitario Son Espases, Spain
Copyright © 2022 Trieste, Cannizzo, Palla, Triulzi and Turchetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Turchetti, Z2l1c2VwcGUudHVyY2hldHRpQHNhbnRhbm5hcGlzYS5pdA==
 Leopoldo Trieste
Leopoldo Trieste Sara Cannizzo
Sara Cannizzo Ilaria Palla
Ilaria Palla Isotta Triulzi
Isotta Triulzi Giuseppe Turchetti
Giuseppe Turchetti