
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 27 September 2022
Sec. Intensive Care Medicine and Anesthesiology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.985955
This article is part of the Research Topic Endothelial Activation and Microcirculatory Disorders in Sepsis and Critical Illness View all 10 articles
 Keiko Suzuki1,2†
Keiko Suzuki1,2† Hideshi Okada3*†
Hideshi Okada3*† Kazuyuki Sumi2
Kazuyuki Sumi2 Hiroyuki Tomita4
Hiroyuki Tomita4 Ryo Kobayashi2,5
Ryo Kobayashi2,5 Takuma Ishihara6
Takuma Ishihara6 Yosuke Mizuno3
Yosuke Mizuno3 Fuminori Yamaji3
Fuminori Yamaji3 Ryo Kamidani3
Ryo Kamidani3 Tomotaka Miura1,3
Tomotaka Miura1,3 Ryu Yasuda3
Ryu Yasuda3 Yuichiro Kitagawa3
Yuichiro Kitagawa3 Tetsuya Fukuta3
Tetsuya Fukuta3 Kodai Suzuki3
Kodai Suzuki3 Takahito Miyake3
Takahito Miyake3 Norihide Kanda3
Norihide Kanda3 Tomoaki Doi3
Tomoaki Doi3 Takahiro Yoshida3
Takahiro Yoshida3 Shozo Yoshida3,7
Shozo Yoshida3,7 Nobuyuki Tetsuka1
Nobuyuki Tetsuka1 Shinji Ogura3
Shinji Ogura3 Akio Suzuki2,5*
Akio Suzuki2,5*Tissue injury and hemorrhage induced by trauma lead to degradation of the endothelial glycocalyx, causing syndecan-1 (SDC-1) to be shed into the blood. In this study, we investigated whether serum SDC-1 is useful for evaluating trauma severity in patients. A single-center, retrospective, observational study was conducted at Gifu University Hospital. Patients transported to the emergency room for trauma and subsequently admitted to the intensive care unit from January 2019 to December 2021 were enrolled. A linear regression model was constructed to evaluate the association of serum SDC-1 with injury severity score (ISS) and probability of survival (Ps). A total of 76 trauma patients (54 men and 22 women) were analyzed. ISS was significantly associated with serum SDC-1 level in trauma patients. Among the six body regions defined in the AIS used to calculate the ISS score, “chest” and “abdominal or pelvic contents” were significantly associated with serum SDC-1 level, and “extremities or pelvic girdle” also tended to show an association with serum SDC-1 level. Moreover, increasing serum SDC-1 level was significantly correlated with decreasing Ps. Serum SDC-1 may be a useful biomarker for monitoring the severity of trauma in patients. Further large-scale studies are warranted to verify these results.
Trauma is a global phenomenon and one of the main causes of death worldwide (1). The major causes of trauma-related death within the first 24 h are hemorrhage and brain injury, and respiratory distress, organ failure, and infection in the period thereafter (2). Because trauma has a wide range of presentations, both in injury type and the location and effect of individual wounds, the ability to measure injury severity is particularly important for comparison of disparate types of trauma. The Injury Severity Score (ISS) is an anatomical scoring system that provides an overall score for patients with multiple injuries, in which each injury is assigned an Abbreviated Injury Scale (AIS) score (3, 4). While the ISS is regarded as the gold standard for grading trauma severity (5, 6), it is important to note that trauma severity measured using the ISS is anatomically based, and reveals no information on disease pathophysiology. However, the systemic response induced by tissue injury and hemorrhage, which includes the release of damage-associated molecular patterns (DAMPs), hypoperfusion and reperfusion, inflammatory responses, and activation of endocrine and neurological systems, leads to endothelial cell activation, resulting in cellular and organ dysfunction (6). Thus, although the pathophysiology of trauma is characterized by endothelial injury, this is not considered by the ISS.
The endothelial glycocalyx, a gel-like layer of glycoproteins that covers the luminal surface of the capillary endothelium, is thought to maintain organ and vascular homeostasis (7). Syndecan-1 (SDC-1), the core protein in heparan sulfate proteoglycan, is found in the endothelial glycocalyx and shed into the blood in various systemic inflammatory conditions, including trauma (7, 8), sepsis (9), acute respiratory distress syndrome (10), acute kidney injury (11) and cardiovascular disease (12, 13). Additionally, several studies have demonstrated an association between serum SDC-1 level and mortality in trauma patients (14, 15). A prospective cohort study of 75 trauma patients reported that serum SDC-1 level ≥ 63 ng/ml was an independent factor for 30-day mortality after adjusting for age and ISS (15). Similarly, a prospective observational study of 410 trauma patients revealed that serum SDC-1 level ≥ 40 ng/ml was a significant factor for 30-day in-hospital mortality using multivariable logistic analysis adjusted for age, ISS, arrival systolic blood pressure, and base excess (14). However, it is unclear whether serum SDC-1 is a useful marker of severity in patients with trauma.
The aim of this study was to investigate the relationship between ISS and serum SDC-1 levels on admission to the emergency room in trauma patients.
This single-center retrospective observational study was conducted at Gifu University Hospital, which is affiliated with Gifu University (Gifu, Japan). Patients transported to the emergency room (ER) for trauma and admitted to the intensive care unit at Gifu University from January 2019 to December 2021 were included. Patients were excluded from the present analysis if they were transported to hospital 24 h or more after the onset of injury, had an unclear onset time of injury, were diagnosed with an AIS of < 3, received cardiopulmonary resuscitation or fluid resuscitation during transport, were undergoing maintenance dialysis, or were aged under 20 years.
Upon transport to the ER, blood was sampled from eligible patients. Data derived from these blood samples were used in the present analysis. All laboratory data, except serum SDC-1, and patient demographics were extracted from the hospital's electronic medical records.
AIS was scored by physicians using AIS2008 (16). To calculate the ISS score, we used the six body regions defined in the AIS: 1. Head and neck, 2. Face, 3. Chest, 4. Abdomen, 5. Extremities pelvis, 6. Surface. The ISS score was calculated from the three most severely injured of the six body regions using the equation: ISS = (AIS1)2 ± (AIS2)2 ± (AIS3)2.
The probability of survival (Ps) is a commonly used parameter in the field of trauma care. It can be calculated from the AIS, ISS and Trauma and Injury Severity Score (TRISS) as follows:
where RTS is the revised trauma score and b0, b1, b2, b3 are coefficients. The coefficients b0–b3 are derived from multiple-regression analysis of the Major Trauma Outcome Study database (17). For patients under 55 years old, the age index is 0, while for patients > 55 years old, the age index is 1. These coefficients change depending on whether subjects are pediatric patients or have blunt trauma. RTS can be calculated from the following three physiological parameters:
where GCS is the Glasgow Coma Scale, SBP is systolic blood pressure (mmHg), and RR is respiratory rate (/min). Serum SDC-1 concentrations were measured using an enzyme-linked immunosorbent assay (950.640.192; Diaclone, Besancon, Cedex, France). These data were retrospectively analyzed.
Patients' baseline characteristics are presented as median and interquartile range (IQR) for continuous variables, and frequency and proportion for categorical variables. To evaluate the association between ISS and serum SDC-1, we performed multivariable regression analysis adjusted for covariates we defined a priori based on factors previously shown to be strongly associated with serum SDC-1 and ISS. For serum SDC-1, these were age (18, 19), gender (20, 21), previous treatment with transfusion and/or angiography at another hospital before being transported to our hospital (22). For ISS, these were: medication history of antiplatelet use and/or anticoagulant use (23, 24). Because the distribution of serum SDC-1 was skewed, natural log transformation was used in the regression model and the results are presented with back-transformation.
To evaluate which of the six body regions defined in the AIS used to calculate the ISS score was associated with serum SDC-1, a similar analysis was performed by replacing the ISS with the body region-specific score. The association between Ps and serum SDC-1 was also evaluated using a multivariable linear regression model. A two-sided P-value < 0.05 was considered significant. All analyses were performed using R 4.1.1 (The R Project for Statistical Computing).
The investigation conformed with the principles outlined in the Declaration of Helsinki. Ethics approval was obtained from the medical ethics committee of Gifu University Graduate School of Medicine, Gifu, Japan (Institutional review board approval No. 2021-B097). In view of the retrospective nature of the study, subject informed consent was not required.
The disposition of the enrolled patients is shown in Figure 1. A total of 447 patients with trauma were transferred to our ER during the study period. Of these, 228 patients were excluded for the following reasons: AIS < 3 (n = 137), unclear onset time of injury (n = 54), transported to hospital 24 h or more after onset of injury (n = 21), under 20 years old (n = 13), and received cardiopulmonary resuscitation or fluid resuscitation during transport (n = 3). A total of 219 patients met the eligibility criteria. However, 143 of these patients had missing blood samples, which meant we could not determine their serum SDC-1 levels. Thus, the remaining 76 patients (54 men and 22 women) were analyzed in this study.
Patient demographics are shown in Table 1. Median age was 66.0 years (IQR, 47.0–78.0) and median duration of hospital stay was 29.0 days (IQR, 20.0–48.8). The most common cause of trauma was falling from a height (n = 35, 46.0%), followed by motor vehicle accident (n = 33, 43.4%) and others (n = 8, 10.5%). A total of 3 (3.9%) and 5 (6.6%) patients had received transfusion and angiography at another hospital before being transported to our hospital, respectively. Further, 6 (7.9%) and 3 (3.9%) patients were taking antiplatelet agents and anticoagulants, respectively.
The median time from onset of trauma to blood collection in the ER was 62.5 min (IQR, 39.75–150.25). The median serum SDC-1 level was 34.6 ng/mL (IQR, 27.1–67.8), and the median ISS score was 20 (IQR 13.8–29.0). The results of the multivariable linear regression model adjusted for age, gender, treatment history (transfusion and angiography) and medication history (antiplatelet agents and anticoagulants) are shown in Table 2 and Figure 2. ISS was significantly associated with serum SDC-1 level in trauma patients with AIS ≥3 [Exp (β) = 1.046, 95% confidence interval (CI) = 1.020–1.072, P = 0.001, R2 = 0.23].
We subsequently used a multivariable linear regression model adjusted for age, gender, treatment history (transfusion and angiography) and medication history (antiplatelet agents and anticoagulants) to determine whether the ISS score for each of the six body regions defined in the AIS was associated with serum SDC-1 level. As shown in Table 3 and Figure 3, the body region-specific score for “chest” and “abdominal or pelvic contents” were significantly associated with serum SDC-1 level in trauma patients (chest: Exp (β) = 1.273, 95%CI = 1.076–1.506, P = 0.006, R2 = 0.18; abdominal or pelvic contents: Exp (β) = 1.326, 95%CI = 1.090–1.614, P = 0.005, R2 = 0.18), and the score for “extremities or pelvic girdle” also tended to show an association with serum SDC-1 level (Exp (β) = 1.178, 95%CI = 0.982–1.412, P = 0.077, R2 = 0.12).
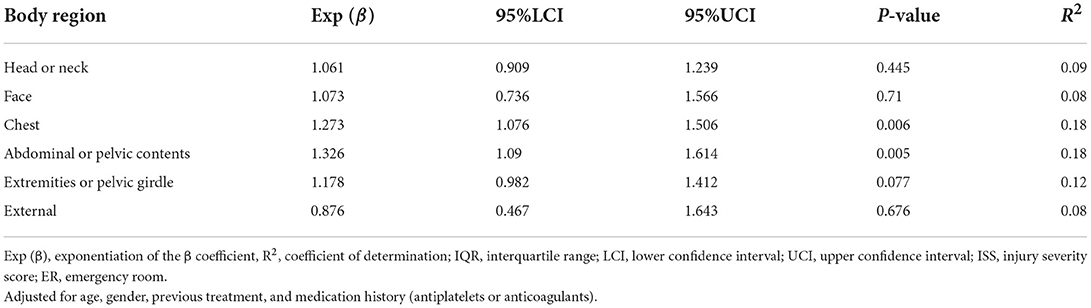
Table 3. Linear regression between serum SDC-1 and six body regions defined in the AIS used to calculate the ISS score in trauma patients on arrival at the ER.
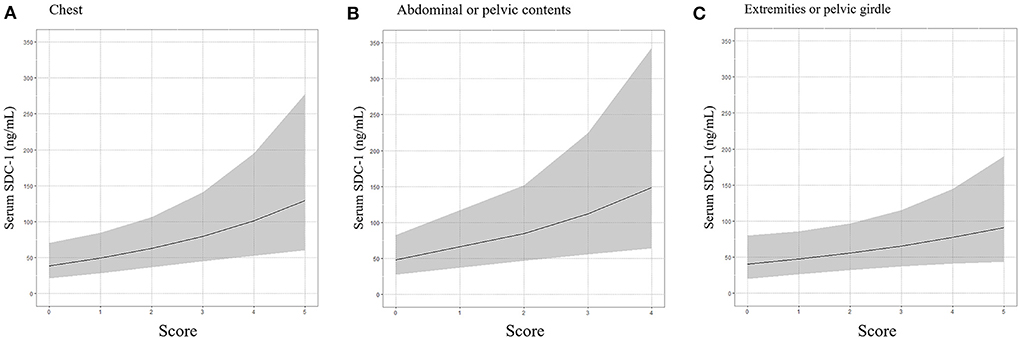
Figure 3. Association between serum SDC-1 and chest (A), abdominal or pelvic contents (B) and extremities or pelvic girdle (C), body regions defined by the AIS used to calculate ISS score.
The median Ps was 0.9225 (IQR 0.8134–0.9655). The multivariable linear regression model showed that increasing serum SDC-1 level was significantly correlated with decreasing Ps after adjustment for age, gender, treatment history (transfusion and angiography) and medication history (antiplatelet agents and anticoagulants) (Exp (β) = 0.973, 95%CI = 0.96–0.986, P < 0.001, R2 = 0.26, Table 4; Figure 4).
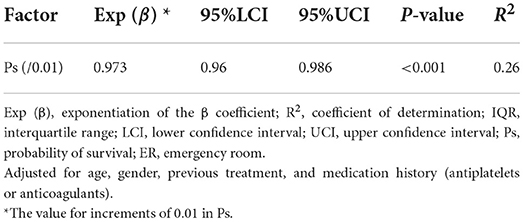
Table 4. Linear regression between serum SDC-1 and probability of survival in trauma patients at arrival at the ER.
We demonstrated that serum SDC-1 level measured on admission to the emergency room in patients with trauma was significantly associated with ISS. Among the six body regions defined in the AIS used to calculate the ISS score, “chest” and “abdominal or pelvic contents” were significantly associated with serum SDC-1 level in trauma patients, and “extremities or pelvic girdle” tended to show an association with serum SDC-1 level. Moreover, increasing serum SDC-1 level was significantly correlated with decreasing Ps.
The composition and function of the endothelial glycocalyx are related to the pathophysiology of trauma (8), with the shedding of SDC-1 from the endothelial glycocalyx into serum having been shown to be associated with several inflammatory cytokines and chemical mediators related to the pathophysiology of trauma (14, 15, 25). The ISS is correlated with adrenaline, interleukin (IL)-6, histone-complexed DNA fragments, high-mobility group box 1 (HMGB1), soluble thrombomodulin (sTM) and protein C in trauma patients with high serum SDC-1 levels (≥ 63 ng/ml) but not in those with low SDC-1 levels (< 63 ng/ml) (15). Patients with serum SDC-1 ≥ 40 ng/ml suffering from blunt or penetrating trauma have nearly 1.5-fold higher sTM levels in the initial 24 h after the trauma than patients with serum SDC-1 < 40 ng/ml (14). Serum SDC-1 levels in severely injured patients experiencing hemorrhagic shock are positively correlated with interleukin-10 and inversely correlated with interferon (IF)-γ, fractalkine and IL-1β (25). These findings suggest that serum SDC-1 may reflect the pathophysiology of trauma.
Two clinical studies in trauma patients have also reported relationships between serum SDC-1 and ISS. In an observational study of 80 trauma patients, Johansson et al. showed that those with acute coagulopathy of trauma shock (ACoTS), defined as activated partial thromboplastin time or international normalized ratio above the normal reference level, had significantly higher median ISS values and serum SDC-1 levels than non-ACoTS patients [ISS: 34 (IQR 30–43) vs. 17 (IQR 10–25), P < 0.001; SDC-1: 62 (IQR 34–107) vs 31 (IQR 18–48), P = 0.013] (26). The same group also reported in a prospective cohort study of 75 trauma patients that the ISS did not significantly differ between a high serum SDC-1 group and low serum SDC-1 group (15). However, it is important to note that the median ISS was higher in the high serum SDC-1 group than in the low serum SDC-1 group [23 (IQR 14–37) vs. 17 (IQR 14–28)]. Additionally, neither of the two studies described above used continuous values to analyze the relationship between SDC-1 level and ISS, nor did they discuss the validity of grouping by the median value. In fact, the median serum SDC-1 level and ISS score in the present study was different to those of the prior studies. In the present study, multivariable regression analysis adjusted for age, gender, previous treatment, and medication history demonstrated that serum SDC-1 level measured on admission to the emergency room in trauma patients was significantly associated with ISS. This result indicates that serum SDC-1 may be a useful biomarker of severity in patients with trauma.
Moreover, we showed that among the six body regions defined in the AIS used to calculate the ISS score, “chest” and “abdominal or pelvic contents” were significantly associated with serum SDC-1 level, and that “extremities or pelvic girdle” tended to be associated with serum SDC-1 level. In contrast, “head or neck,” “face” and “external” regions were not associated with serum SDC-1 level. The reason for the difference in association between serum SDC-1 and the ISS score for each body region defined by the AIS is unclear. However, we speculate that the total surface area of the endothelium in each region may differentially influence the amount of serum SDC-1. For example, the “head and neck,” “face” and “external” regions, mainly categorized as ectodermic tissue, have a small surface area of endothelium, whereas “chest” and “abdominal or pelvic contents” and “extremities or pelvic girdle,” mainly categorized as endodermic or mesodermic tissue, have a greater surface area of endothelium. Reports from surgeries of the abdominal, cardiac and thoracic regions have indicated elevated levels of serum SDC-1, which is consistent with the present results (27–29). Additionally, the structure and components of the endothelial glycocalyx differ among organs, as does its susceptibility to injury (7, 30). The endothelial glycocalyx in the capillaries of the brain, for example, unlike that in the capillaries of the heart and lungs, is extremely thick. As a result, lipopolysaccharide-induced vascular injury completely eliminates the endothelial glycocalyx in cardiac and pulmonary capillaries but leaves much of it in the cerebral capillaries intact (30). These factors may explain the difference in correlation between SDC-1 and the ISS score for some body regions defined by the AIS but not others.
In the present study, we used probability of survival to evaluate the association between serum SDC-1 level and mortality in trauma patients because mortality was only 3.947% (3/76). In the multivariable linear regression model, increasing serum SDC-1 level was significantly correlated with decreasing Ps, a result that is consistent with those of previous reports (15, 25).
This study has several limitations. First, we were unable to evaluate the extent of endothelium injury and serum inflammatory cytokine and chemical mediator levels (e.g., IL-6, histone-complexed DNA fragments, HMGB1, sTM, protein C) related to the pathophysiology of trauma. Second, in addition to the endothelial glycocalyx, SDC-1 is also expressed in other organs. However, we did not evaluate SDC-1 expression in different organs in this study. Third, we evaluated the Ps but not mortality in this study. Fourth, due to the retrospective nature of this study, a large number of patients (n = 143) who met the eligibility criteria were excluded because of missing blood samples, as this prevented us from determining their serum SDC-1 levels. Moreover, potentially relevant confounding factors may have been missed. Finally, the sample size was small and data were obtained from a single institution.
In conclusion, serum SDC-1 may be a useful biomarker for evaluating severity in trauma patients, especially trauma in the “chest,” “abdominal or pelvic contents” and “extremities or pelvic girdle” regions. Additionally, elevated serum SDC-1 levels may be an important risk factor for mortality in patients with trauma. Further large-scale studies are warranted to verify the usefulness of serum SDC-1 as a marker of severity in trauma patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by ethics approval was obtained from the Medical Ethics Committee of the Gifu University Graduate School of Medicine, Gifu, Japan (record no: 2021-B097). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
KeS and AS wrote the manuscript. KeS, KaS, YM, FY, RKo, TMiu, RY, YK, TF, KoS, TMiy, and NK collected the blood samples. KeS, KaS, and RKo measured the syndecan-1 concentration using ELISA. KeS and RY created the database. TI performed the statistical analysis. YM, FY, RKa, TMiu, RY, YK, TF, KoS, TMiy, NK, TD, TY, and SY treated the patients. HT, SY, NT, and SO supervised the study. HO and AS revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
We would like to thank DMC Corp. (www.dmed.co.jp) for providing English language editing of this paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sakran JV, Greer SE, Werlin E, McCunn M. Care of the injured worldwide: trauma still the neglected disease of modern society. Scand J Trauma Resusc Emerg Med. (2012) 20:64. doi: 10.1186/1757-7241-20-64
2. Holcomb JB. del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. (2013) 148:127–36. doi: 10.1001/2013.jamasurg.387
3. Baker SP, O'Neill B, Haddon W Jr. Long WB The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. (1974) 14:187–96. doi: 10.1097/00005373-197403000-00001
4. Baker SP, O'Neill B. The injury severity score: an update. J Trauma. (1976) 16:882–5. doi: 10.1097/00005373-197611000-00006
5. Lavoie A, Moore L, LeSage N, Liberman M, Sampalis JS. The injury severity score or the new injury severity score for predicting intensive care unit admission and hospital length of stay? Injury. (2005) 36:477–83. doi: 10.1016/j.injury.2004.09.039
6. Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. (2014) 384:1455–65. doi: 10.1016/S0140-6736(14)60687-5
7. Suzuki A, Tomita H, Okada H. Form follows function: the endothelial glycocalyx. Transl Res. (2022). doi: 10.1016/j.trsl.2022.03.014
8. Chignalia AZ, Yetimakman F, Christiaans SC, Unal S, Bayrakci B, Wagener BM, et al. The glycocalyx and trauma: a review. Shock. (2016) 45:338–48. doi: 10.1097/SHK.0000000000000513
9. Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. (2019) 23:16. doi: 10.1186/s13054-018-2292-6
10. Schmidt EP, Overdier KH, Sun X, Lin L, Liu X, Yang Y, et al. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med. (2016) 194:439–49. doi: 10.1164/rccm.201511-2281OC
11. Libório AB, Braz MB, Seguro AC, Meneses GC, Neves FM, Pedrosa DC, et al. Endothelial glycocalyx damage is associated with leptospirosis acute kidney injury. Am J Trop Med Hyg. (2015) 92:611–6. doi: 10.4269/ajtmh.14-0232
12. Padberg JS, Wiesinger A, di Marco GS, Reuter S, Grabner A, Kentrup D, et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. (2014) 234:335–43. doi: 10.1016/j.atherosclerosis.2014.03.016
13. Kitagawa Y, Kawamura I, Suzuki K, Okada H, Ishihara T, Tomita H, et al. Serum syndecan-1 concentration in hospitalized patients with heart failure may predict readmission-free survival. PLoS ONE. (2021) 16:e0260350. doi: 10.1371/journal.pone.0260350
14. Gonzalez Rodriguez E, Ostrowski SR, Cardenas JC, Baer LA, Tomasek JS, Henriksen HH, et al. Syndecan-1: a quantitative marker for the endotheliopathy of trauma. J Am Coll Surg. (2017) 225:419–27. doi: 10.1016/j.jamcollsurg.2017.05.012
15. Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR A. high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. (2011) 254:194–200. doi: 10.1097/SLA.0b013e318226113d
16. Gennarelli TAWEAftAoAM. Abbreviated Injury Scale 2005: Update 2008. Barrington, Ill: Association for the Advancement of Automative Medicine (2008).
17. Boyd CR, Tolson MA, Copes WS. Evaluating trauma care: the TRISS method. Trauma score and the injury severity score. J Trauma. (1987) 27:370–8. doi: 10.1097/00005373-198704000-00005
18. Oda K, Okada H, Suzuki A, Tomita H, Kobayashi R, Sumi K, et al. Factors Enhancing Serum Syndecan-1 Concentrations: A Large-Scale Comprehensive Medical Examination. J Clin Med. 2019;8(9). doi: 10.3390/jcm8091320
19. Bruijns SR, Guly HR, Bouamra O, Lecky F, Lee WA. The value of traditional vital signs, shock index, and age-based markers in predicting trauma mortality. J Trauma Acute Care Surg. (2013) 74:1432–7. doi: 10.1097/TA.0b013e31829246c7
20. van Teeffelen J, Jansen C, Spaan J, Vink H. Gender difference in systemic glycocalyx volume in mice. FASEB J. (2007) 21:A491. doi: 10.1096/fasebj.21.5.A491-a
21. Magnotti LJ, Fischer PE, Zarzaur BL, Fabian TC, Croce MA. Impact of gender on outcomes after blunt injury: a definitive analysis of more than 36,000 trauma patients. J Am Coll Surg. (2008) 206:984–91. doi: 10.1016/j.jamcollsurg.2007.12.038
22. Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M, et al. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. (2014) 18:538. doi: 10.1186/s13054-014-0538-5
23. Francesco V, Roberto B, Giulia C, Piero CS, Michele A, Andrea S, et al. All elderly are fragile, but some are more fragile than others: an epidemiological study from one of the busiest trauma centers in Italy. Updates Surg. (2022). doi: 10.1007/s13304-022-01337-y
24. Kerschbaum M, Lang S, Henssler L, Ernstberger A, Alt V, Pfeifer C, et al. Influence of oral anticoagulation and antiplatelet drugs on outcome of elderly severely injured patients. J Clin Med. (2021) 10:1649 doi: 10.3390/jcm10081649
25. Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. (2011) 6:e23530. doi: 10.1371/journal.pone.0023530
26. Johansson PI, Sørensen AM, Perner A, Welling KL, Wanscher M, Larsen CF, et al. Disseminated intravascular coagulation or acute coagulopathy of trauma shock early after trauma? An observational study. Crit Care. (2011) 15:R272. doi: 10.1186/cc10553
27. Pesonen E, Passov A, Andersson S, Suojaranta R, Niemi T, Raivio P, et al. Glycocalyx degradation and inflammation in cardiac surgery. J Cardiothorac Vasc Anesth. (2019) 33:341–5. doi: 10.1053/j.jvca.2018.04.007
28. Pustetto M, Goldsztejn N, Touihri K, Engelman E, Ickx B, Van Obbergh L. Intravenous lidocaine to prevent endothelial dysfunction after major abdominal surgery: a randomized controlled pilot trial. BMC Anesthesiol. (2020) 20:155. doi: 10.1186/s12871-020-01075-x
29. Arthur A, McCall PJ, Jolly L, Kinsella J, Kirk A, Shelley BG. Endothelial glycocalyx layer shedding following lung resection. Biomark Med. (2016) 10:1033–8. doi: 10.2217/bmm-2016-0163
Keywords: trauma, syndecan-1, injury severity score, glycocalyx, probability of survival
Citation: Suzuki K, Okada H, Sumi K, Tomita H, Kobayashi R, Ishihara T, Mizuno Y, Yamaji F, Kamidani R, Miura T, Yasuda R, Kitagawa Y, Fukuta T, Suzuki K, Miyake T, Kanda N, Doi T, Yoshida T, Yoshida S, Tetsuka N, Ogura S and Suzuki A (2022) Syndecan-1 as a severity biomarker for patients with trauma. Front. Med. 9:985955. doi: 10.3389/fmed.2022.985955
Received: 04 July 2022; Accepted: 29 August 2022;
Published: 27 September 2022.
Edited by:
W. Conrad Liles, University of Washington, United StatesReviewed by:
Tobias Piegeler, University Hospital Leipzig, GermanyCopyright © 2022 Suzuki, Okada, Sumi, Tomita, Kobayashi, Ishihara, Mizuno, Yamaji, Kamidani, Miura, Yasuda, Kitagawa, Fukuta, Suzuki, Miyake, Kanda, Doi, Yoshida, Yoshida, Tetsuka, Ogura and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akio Suzuki, YWtpb0BnaWZ1LXUuYWMuanA=; Hideshi Okada, aGlkZXNoaUBnaWZ1LXUuYWMuanA=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.