
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 24 February 2023
Sec. Obstetrics and Gynecology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.985235
 Yun Yang1†
Yun Yang1† Mengru Zhao1†
Mengru Zhao1† Mengyue Chen1
Mengyue Chen1 Huimin Tang2
Huimin Tang2 Zhenyue Qin1
Zhenyue Qin1 Junling Liu2
Junling Liu2 Huihui Wang1
Huihui Wang1 Mingyue Bao1
Mingyue Bao1 Jiming Chen2*
Jiming Chen2* Bairong Xia3*
Bairong Xia3*Teratomas often occur in the gonads, while Extragonadal mature cystic teratomas are reported occasionally, with the most common site being the omentum. Teratoma in the Douglas sac is extremely rare. we report a rare case of mature cystic Teratoma in the Douglas sac in a 71-year-old woman who underwent laparoscopic surgery. A cyst with a diameter of approximately 6 cm from Douglas was found during surgery, and the mass was separated from both ovaries. Microscopically, the cyst was a mature cystic teratoma that did not originate from the ovary.
Teratomas are germ cell-derived tumors with the potential to differentiate into somatic cells. Most teratomas contain at least two types of embryonic tissue components. According to differentiation degrees of the tissues contained, teratomas can be divided into mature ones and immature ones. Mature cystic ovarian teratoma, also known as dermoid cyst, is a benign tumor, accounting for 85–97% of germ cell tumors and more than 95% of teratomas. It is primarily composed of ectodermal, mesodermal, and endodermal mature tissues, and usually dominated by ectoderm, mostly cystic, which can be monocystic or polycystic. The content is oil-like, often mixed with hair, and the cyst wall is often lined with multi-layered squamous epithelium and appendage hair follicles, sebaceous glands, sweat glands, etc. Immature teratoma is a malignant germ cell tumor that reproduces the differentiation characteristics of embryos and fetal tissues. Its tissues contain different amounts of immature tissues, largely primitive and embryonic neuroectodermal tissues. Teratomas often occur in the gonads, while extragonadal mature cystic teratomas are occasionally reported, with the most common site being the omentum majus (1). However, teratoma in Douglas’ pouch is extremely rare and its exact etiology is unknown. Here we have a rare case of mature cystic teratoma in Douglas’ pouch in a 71-year-old woman who underwent laparoscopic surgery. A cyst with a diameter of approximately 6 cm in Douglas’ pouch was found during surgery, and the mass was separated from both ovaries. Microscopically, the cyst was a mature cystic teratoma.
A 71-year-old women was admitted to the hospital because of “a prolapse of vaginal mass for more than 3 years and a pelvic mass found 1 month ago.” When the patient stood upright, a broad-bean-sized mass prolapsed out of the vagina, and the symptom disappeared by itself when the patient rested. She has been post-menopausal for 25 years, with 4 pregnancies and 3 children, and received cataract surgery 6 years ago. Physical examination showed no obvious mass palpated in the abdomen and no tenderness. Gynecological examination showed no abnormality in the vulva and vagina, atrophic and smooth cervix, uterus in the middle position and metratrophia, no tenderness, and a cystic mass of a diameter of 6 cm felt behind the uterus with clear boundary, smooth surface, and acceptable mobility without tenderness. No obvious mass was palpated in bilateral adnexal areas. Tumor markers are largely normal. Pelvic ultrasound showed a mixed echogenic mass on the left side of the pelvis. The mass size was about 7.4*5.4 cm.
A laparoscopic exploratory operation was performed under general anesthesia on 10 August 2017. During the operation, it could be observed that the uterine surface was smooth, and a mass with a diameter of about 6 cm was seen in Douglas’ pouch. The surface of the mass was smooth, without obvious connection with the uterus and bilateral appendages, but its back was tightly adhered to the posterior wall of the uterus, the right appendages, and the mesentery (Figure 1A). The bilateral ovarian atrophied, being grayish-white and with an intact surface. We used scissors to separate the adhesion between the mass and its surrounding tissues (Figure 1B) until the mass was completely isolated (Figure 1C). Then, the specimen was put into a specimen bag made of a sterile glove. Yellow liquid flew out after the mass was cut and contained hair, bone, and tooth-like tissues (Figure 2A). Both ovaries were regarded normal considering the patient’s age (Figure 2B).
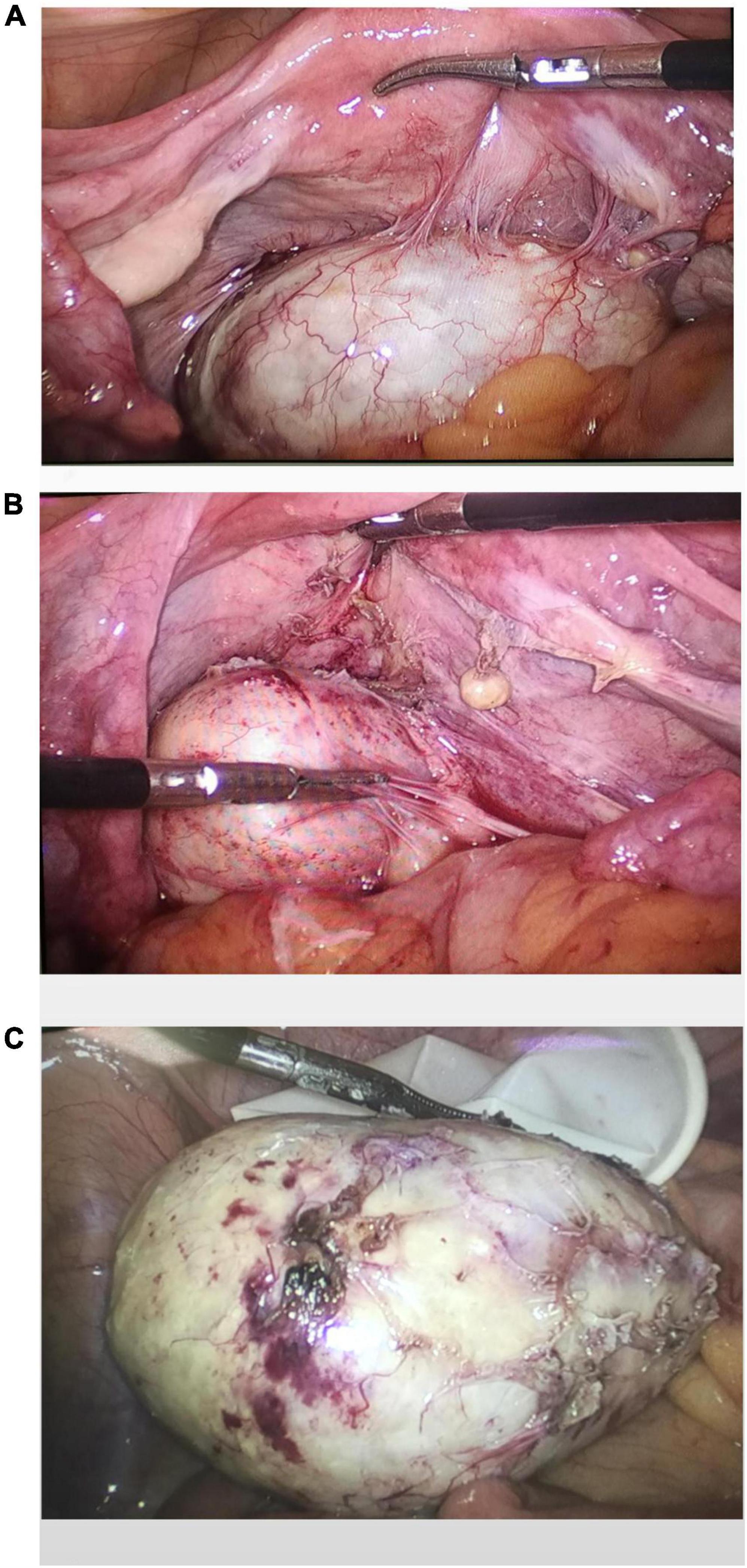
Figure 1. Intraoperative exploration revealed a teratoma in the utero-rectal fossa, adherent to the posterior uterine wall, right adnexa, and mesentery (A). The teratoma was disconnected from the posterior uterine wall, right adnexa, and mesentery (B). Gross observation of the specimen (C).
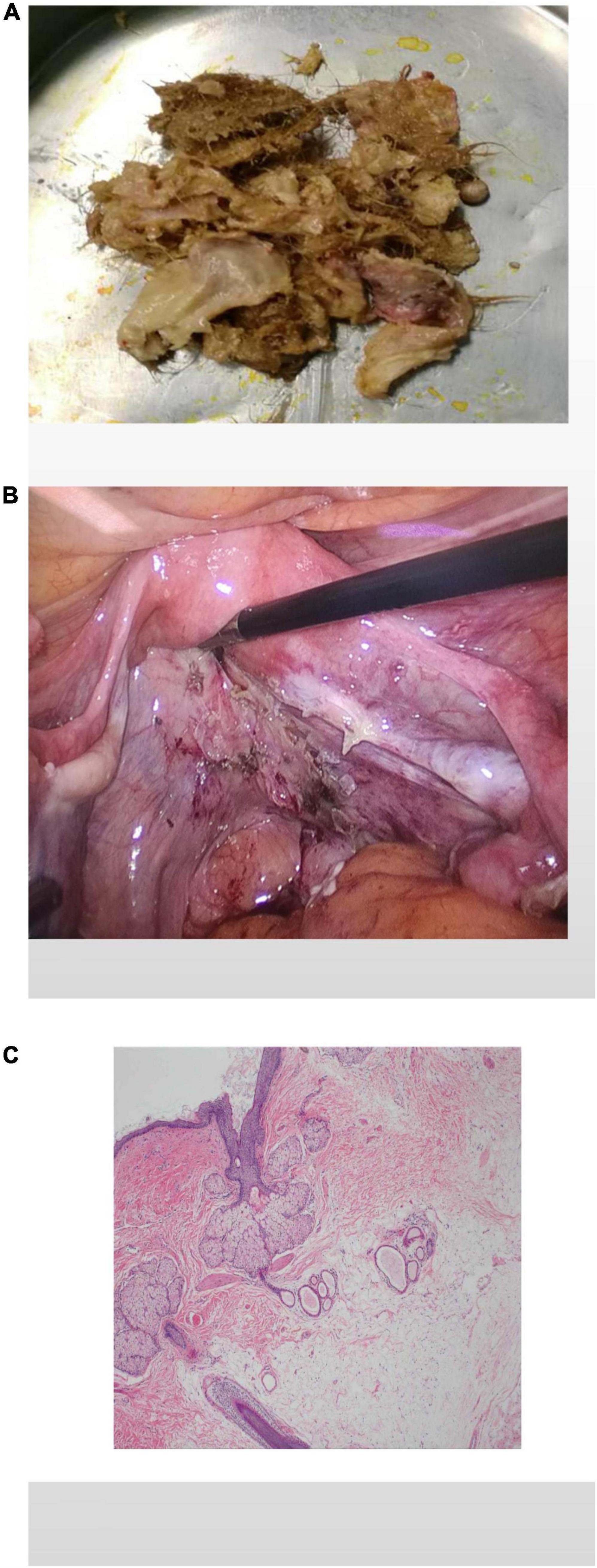
Figure 2. The specimen contains hair, bone, and tooth-like tissues (A). The pelvis and bilateral adnexal areas after complete resection of the teratoma showed no obvious abnormality (B). Histological pictures (C).
The patient had an uneventful postoperative course. Pathological examination after surgery showed that the tumor was mature cystic teratoma (Figure 2C).
Mature cystic teratoma is a congenital tumor that originates from pluripotent stem cells and contains tissues from at least two of the three germ cell layers. They usually have a parthenogenetic origin with a 46, XX karyotype and characteristic stagnation after Meiosis I. Meckel (2) first described extragonadal teratomas in 1815. The most common location was the omentum, followed by Douglas’ pouch, liver, and diaphragmatic inguinal canal. In addition, teratomas may also be found in the reproductive tract of the uterus, cervix, and oviduct.
There are three proposed theories about the causes of these extragonadal sites: (1) Primary teratoma may originate from displaced germ cells. (2) Teratoma may occur in the supernumerary ovary. (3) Teratoma may be caused by automatic amputation of ovarian dermoid cyst. The first mechanism is during early fetal development when germ cells migrate from the yolk sac along the hindgut to the reproductive crest. Germ cells become arrested between the yolk sac entoderm and the dorsal mesentery (3). The second mechanism is teratoma caused by multiple or accessory ovaries. It was first described by Wharton (4). Supernumerary ovaries are completely separated from normal ovaries and come from single primordial ones. Accessory ovaries refer to the multiple ovarian tissues found in near-normal ovaries, which may be connected to or developed from normal ovaries. In embryology, the migration of primitive cells from the yolk sac to a gonadal ridge and delayed germ cell migration may be the cause for the formation of polycystic ovaries. The third mechanism was first described by Thornton. In subacute or chronic torsion, teratomas may adhere to adjacent structures and form new collateral circulation. In rare cases, the tumors may be completely detached from their pedicle, leading to parasitic dermoid cysts (5). Auto amputation is considered the most common cause of omentum EGT and Douglas’ pouch and sacral ligament EGT, and 70% of upper abdominal EGT may be caused by the displacement of primordial germ cells (6). It seemed to be more frequently seen on the right side, which is likely due to the colon sigmoideum preventing left-sided torsion (7). In our case, the possibility of ovarian auto amputation could be ruled out for the time being, because the bilateral ovarian morphology was intact and the mechanism needed further exploration.
The cases in many reports are parasitic dermoid cysts in the omentum majus, while the cases of teratomas located in the Douglas’ pouch are rare. We focused on the parasitic cysts located in the Douglas’ pouch to investigate the disease characteristics as reference for clinical diagnosis and treatment. We performed a PubMed search using keywords “extragonadal” or “parasitic,” “teratoma,” “Douglas,” or “cul-de-sac,” and found 24 articles. The clinical data of the 24 cases of parasitic dermoid cysts in Douglas’ pouch is given in Table 1 (7–29). The patient age ranged from 23 to 83, and 11 patients had abdominal pain as the primary symptom and the other patients experienced no abdominal pain. One of the patients had no obvious clinical symptoms, and Douglas’ pouch teratoma was found during cesarean section at 39 weeks of gestation. Another patient found the teratoma by physical examination at 39 days of gestation. Three of 24 patients had teratoma in the uterosacral ligament. The maximum size of the tumor was 15 cm × 15 cm × 10 cm. Nine of 24 patients had normal levels of tumor markers (CA-125, CA-19-9, alpha fetoprotein, and carcinoembryonic antigen), four had elevated levels of tumor markers, with CA 19-9 up to 631.23 U/mL, and CA125 up to 110.5 U/mL. A total of 12 of 24 cases were considered to be a result of auto amputation, 3 were considered a result of displaced primordial germ cells, and the causes of the remaining cases were unknown.
When extragonadal teratomas develop in the uterus, cervix, and oviduct, symptoms such as abnormal uterine bleeding and vaginal masses may occur. Patients with Douglas’ pouch teratomas may suffer from hypo gastralgia (30). Some patients may have no clinical symptoms until their teratomas were found during physical examinations. In this case, the abdominal pain was not obvious, and the patient was in menopause, with prolapse of vaginal mass and less vaginal secretion as the main manifestations. Preoperative diagnosis of this disease is highly difficult, and transvaginal ultrasound is the preferred method of examination. MRI or CT scan of the pelvis can help with preoperative diagnosis. CT images often show the presence of fat and calcification. The current data shows that tumor markers have little effect on the diagnosis of the disease. Intraoperative and postoperative pathological analysis is the main evidence to establish the diagnosis. Although not all cases have accurate histopathological diagnosis, the mass is usually composed of necrotic and calcified tissues, and residual ovarian components can be found in a small number of cases (15). The preferred method for the treatment of this disease is surgery. For benign teratomas, laparoscopic cystectomy can be the preferred surgical method, and the tumor-free principle should be strictly followed during surgery (31). For immature teratomas, a standard bleomycin, etoposide, and platinum (BEP) chemotherapy regimen should be established postoperatively. For malignant teratoma, surgery should be the main treatment, supplemented by chemotherapy and radiotherapy. In this case, the tumor was completely resected, and the postoperative pathology was cystic and mature teratoma, which was a benign tumor.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YY and MZ: acquisition of data and writing—original draft. MC, HT, and ZQ: analysis and interpretation of data and visualization. JL, HW, and MB: perform the analysis with constructive discussions. JC: conceptualization, funding acquisition, resources, supervision, and writing. BX: conceptualization, supervision, and writing. All authors contributed to the article and approved the submitted version.
This research was supported by grants from the Maternal and Child Health Research Project of Jiangsu Province (F202138), the Scientific Research Support Program for Postdoctoral of Jiangsu Province (2019K064), and the Scientific Research Support Program for “333 Project” of Jiangsu Province (BRA2019161).
We are grateful to JC for his photography of Douglas’ pouch teratoma during the surgery.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hegde P. Extragonadal omental teratoma: a case report. J Obstet Gynaecol Res. (2014) 40:618–21. doi: 10.1111/jog.12198
3. Oosterhuis JW, Stoop H, Honecker F, Looijenga LH. Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. Int J Androl. (2007) 30:256–63;discussion263–4. doi: 10.1111/j.1365-2605.2007.00793.x
4. Wharton LR. Two cases of supernumerary ovary and one of accessory ovary, with an analysis of previously reported cases. Am J Obstet Gynecol. (1959) 78:1101–19. doi: 10.1016/s0002-9378(16)36660-1
5. Ushakov FB, Meirow D, Prus D, Libson E, BenShushan A, Rojansky N. Parasitic ovarian dermoid tumor of the omentum–a review of the literature and report of two new cases. Eur J Obstet Gynecol Reprod Biol. (1998) 81:77–82. doi: 10.1016/s0301-2115(98)00144-4
6. Kanneganti A, Bhadiraju P, Tong PSY. Extragonadal teratomas in women and adolescent girls: a systematic review. Eur J Obstet Gynecol Reprod Biol. (2021) 262:134–41. doi: 10.1016/j.ejogrb.2021.05.005
7. Kusaka M, Mikuni M. Ectopic ovary: a case of autoamputated ovary with mature cystic teratoma into the cul-de-sac. J Obstet Gynaecol Res. (2007) 33:368–70. doi: 10.1111/j.1447-0756.2007.00538.x
8. Lefkowitch JH, Fenoglio CM, Richart RM. Benign cystic teratoma of the retrouterine pouch of douglas. Am J Obstet Gynecol. (1978) 131:818–20. doi: 10.1016/0002-9378(78)90258-2
9. Turhan NO, Dilmen G, Ustun H. Benign cystic teratoma of the douglas. Eur J Obstet Gynecol Reprod Biol. (2000) 93:213–4.
10. Chen H-J, Hsieh B-C, Pan H-S, Seow K-M, Tsai Y-L, Hwang J-L. Dermoid cyst of the retrouterine pouch of douglas. Taiwan J Obstet Gynecol. (2004) 43:226–8. doi: 10.1016/0002-9378(78)90258-2
11. Takeda A, Manabe S, Hosono S, Nakamura H. A case of a mature cystic teratoma of the uterosacral ligament successfully treated by laparoscopic surgery. J Minim Invasive Gynecol. (2005) 12:34–6. doi: 10.1016/j.jmig.2004.12.015
12. Kobayashi Y, Kiguchi K, Ishizuka B. Mature cystic teratoma of the pouch of douglas containing multiple mobile spherules. Int J Gynaecol Obstet. (2006) 92:81–2. doi: 10.1016/j.ijgo.2005.07.018
13. Khoo CK, Chua I, Siow A, Chern B. Parasitic dermoid cyst of the pouch of douglas: a case report. J Minim Invasive Gynecol. (2008) 15:761–3.
14. Moawad NS, Starks D, Ashby K. Ectopic ovarian teratoma of the uterosacral ligament associated with a large ovarian dermoid. J Minim Invasiv Gynecol. (2008) 15:523–4. doi: 10.1016/j.jmig.2008.04.017
15. Bartlett CE, Khan A, Pisal N. Parasitic dermoid cyst managed laparoscopically in a 29-year-old woman: a case report. J Med Case Rep. (2009) 3:63. doi: 10.1186/1752-1947-3-63
16. Peitsidou A, Peitsidis P, Goumalatsos N, Papaspyrou R, Mitropoulou G, Georgoulias N. Diagnosis of an autoamputated ovary with dermoid cyst during a cesarean section. Fertil Steril. (2009) 91:.e9–12. doi: 10.1016/j.fertnstert.2008.12.029
17. Sinha R, Sundaram M, Lakhotia S. Multiple intraabdominal parasitic cystic teratomas. J Minim Invasive Gynecol. (2009) 16:789–91.
18. Matsushita H, Kurabayashi T, Yanase T, Hashidate H. Autoamputation of an ovarian cyst: a case report. J Reprod Med. (2009) 54:709–11.
20. Tokunaga M, Seta M, Yamada M, Nishio M, Yamamoto K, Koyasu Y. Coexistent dermoid cysts of the pouch of the douglas and ovary resected by laparoscopy. Asian J Endosc Surg. (2012) 5:31–3. doi: 10.1111/j.1758-5910.2011.00107.x
21. Eda M, Kaidoh T, Takanashi Y, Inoue T. A stone-like ovarian dermoid cyst in the douglas’ pouch of an elderly woman. Pathol Int. (2012) 62:771–3. doi: 10.1111/pin.12002
22. Koo YJ, Im KS, Jung HJ, Kwon YS. Mature cystic teratoma of the uterosacral ligament successfully treated with laparoendoscopic single-site surgery. Taiwan J Obstet Gynecol. (2012) 51:86–8. doi: 10.1016/j.tjog.2012.01.017
23. Makni A, Ben Rhouma S, Farah J, Jouini M, Kacem M, Ben Safta Z. A case of mature teratoma of the douglas. Tunis Med. (2013) 91:473–4.
24. Ohshima K, Umeda A, Hosoi A, Yamamoto T, Munakata S. Mature cystic teratoma in douglas’. Pouch. Case Rep Pathol. (2015) 2015:202853. doi: 10.1155/2015/202853
25. Kakuda M, Matsuzaki S, Kobayashi E, Yoshino K, Morii E, Kimura TA. Case of extragonadal teratoma in the pouch of douglas and literature review. J Minim Invasive Gynecol. (2015) 22:1311–7. doi: 10.1016/j.jmig.2015.07.008
26. John BM. Ectopic ovary with dermoid cyst as a result of possible asymptomatic autoamputation. J Hum Reprod Sci. (2017) 10:226–30. doi: 10.4103/jhrs.JHRS_67_17
27. Kim HG, Song YJ, Na YJ, Yang J, Choi OHA. Rare case of an autoamputated ovary with mature cystic teratoma. J Menopausal Med. (2017) 23:74–6. doi: 10.6118/jmm.2017.23.1.74
28. Sethi P, Purkait S. Mature cystic teratoma of douglas’ pouch: a rare entity. Cureus. (2019) 11:e5515. doi: 10.7759/cureus.5515
29. Daccache A, Feghali E, Assi R, Sleiman Z. Unplanned adnexectomy for ovarian cystadenoma with undiagnosed autoamputation of the contralateral ovary, lessons learned from medical mistakes. Facts Views Vis Obgyn. (2021) 13:187–90. doi: 10.52054/FVVO.13.2.017
30. Litos MG, Furara S, Chin K. Supernumerary ovary: a case report and literature review. J Obstet Gynaecol. (2003) 23:325–7. doi: 10.1080/01443610310000106055
Keywords: mature cystic teratoma, Douglas’ pouch, case report, old women, literature review
Citation: Yang Y, Zhao M, Chen M, Tang H, Qin Z, Liu J, Wang H, Bao M, Chen J and Xia B (2023) Mature cystic extragonadal teratoma in Douglas’ pouch: Case report and literature review. Front. Med. 9:985235. doi: 10.3389/fmed.2022.985235
Received: 03 July 2022; Accepted: 27 October 2022;
Published: 24 February 2023.
Edited by:
Ambrogio P. Londero, University of Genoa, ItalyReviewed by:
Lovenish Bains, University of Delhi, IndiaCopyright © 2023 Yang, Zhao, Chen, Tang, Qin, Liu, Wang, Bao, Chen and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiming Chen, Y2ptaW5nQDEyNi5jb20=; Bairong Xia, eGlhYmFpcm9uZ0B1c3RjLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.