- 1Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy
- 2University Hospital “Azienda Ospedaliero-Universitaria” of Cagliari, Cagliari, Italy
Background: The assessment process of elderly people considers all aspects of an individual’s life, including physical, mental, and social aspects. Frailty refers to a decline in physiological functions or strengths leading to increased vulnerability to stressors and decreased ability to cope with them. Comprehensive Geriatric Assessment (CGA) is a validated and useful tool in this context to holistically study elderly people. The primary aim of this study was to determine the prevalence of impaired health status in a large geriatric population turning to outpatient service, based on the components of the CGA, and thus to describe its usefulness in real-life clinical practice. The secondary aim of this study was the evaluation of the association between nutritional status, assessed with Mini Nutritional Assessment (MNA)—within the CGA—and cognitive-affective and functional capacities, and multimorbidity.
Materials and methods: This real-life, retrospective cross-sectional study included subjects consecutively evaluated from January 2009 to December 2020 at the Geriatric Outpatient Service, University Hospital of Monserrato, Cagliari, Italy. A sum of 3,260 patients were subjected to CGA.
Results: Only a small proportion of the sample (2.24%) showed an absence of impairment in cognitive-affective, functional, and nutritional domains. Moderate correlations were found between MNA and several other CGA variables, namely, Geriatric Depression Scale (GDS; ϱ = −0.41, p < 0.0001), Barthel Index of Independence in Activities of Daily Living (ADL) (ϱ = 0.51, p < 0.0001), Instrumental Activities of Daily Living (IADL) (ϱ = 0.43, p < 0.0001), and Performance-Oriented Mobility Assessment (ϱ = 0.44, p < 0.0001). A multiple regression also highlighted these variables as significant regressors of MNA. Finally, malnutrition showed a significant association with depression (odds ratio [OR]: 4.97), dependence on ADL (OR: 19.8) and IADL (OR: 7.04), and falling risk (OR: 5.16).
Conclusion: This study has figured out the complex situation in which geriatric care finds itself the complexity and severe impairment of elderly people. The possibilities of intervention are often limited, but the literature confirms the benefits of good nutritional status on the general health status. The data that emerged from our study fit into this assumption, highlighting the close association between the nutritional domain and the other CGA domains.
Introduction
With a higher life expectancy, and general health awareness on the rise, the world is witnessing an increase in geriatric patients (1). Moreover, it is known that Italy, and especially Sardinia, is one of the countries with a larger prevalence of elderly people (2). Primary care providers and geriatricians have tailored their diagnostic and therapeutic practices toward this section of the population. More articles in scientific literature deepen the specifics of assessment markers and how predictive tools can assist in improving these patients’ quality of life (1, 3, 4). In this regard, Comprehensive Geriatric Assessment (CGA) represents a specialistic tool that takes a deep look into several areas, i.e., cognitive, mood, functional status, nutrition, social and interpersonal relationship, and caregivers’ status in order to holistically frame, and thus help, the patient, where possible (3–6). The years have yielded evidence that make CGA essential in clinical practice, as long as it is exploited when there is still room for maneuver; otherwise, it may turn into a useful tool to take note of a critical clinical condition and, where appropriate, the receipt of indemnity, but mournfully not to significantly improve the patient’s health and quality of life (7). At the current state of the art, namely, an advanced cognitive impairment on organic or vascular basis, affecting a high percentage of subjects from 5 to 7% in most countries (8), does not find treatments to reverse the history of the disease (9) though some recent studies encourage research in this direction (10–12). Similarly, a severe dependence on the execution of daily activities can hardly be restored—this condition also affects a considerable proportion of the geriatric population (13). That is, the real-life use of CGA as a diagnostic tool should be preceded by a standardized selection of patients who can benefit from it (7). Nevertheless, evidence has been produced that adequate interventions in single CGA domains can also improve the performances in other domains (14). This applies, in particular, to the nutritional status, an important actor of the health and wellbeing of elderly people, and also considers one of the most important factors involved in the complex etiology of sarcopenia and frailty (14). For its part, due to many factors, such as frailty, multimorbidity, polypharmacotherapy, and inappropriate use of drugs (15), nutritional intake is often compromised. The prevalence of malnutrition is generally < 10% in the elderly people who live in their house and increases up to two-thirds of the elderly admitted to the acute ward or rehabilitation facilities (16). The risk of malnutrition is similar in different settings, i.e., generally ≥ 40% (16). A recent global consensus approach [Global Leadership Initiative on Malnutrition (GLIM)] defines malnutrition as the combination of at least one phenotype criterion, i.e., non-volitional weight loss, low body mass index (BMI), and reduced muscle mass, and one etiologic criterion, i.e., reduced food intake/malabsorption or severe disease with inflammation (17). Furthermore, a close relationship between malnutrition and negative outcomes is well documented in the elderly, e.g., the frequency of infections or pressure ulcers and the length of hospitalization and convalescence following an acute disease (14). In particular, in patients with chronic diseases, malnutrition represents an independent risk factor for increased mortality (18).
Moreover, recent studies suggest that malnutrition is associated with cognitive decline and the degree of impairment in daily functioning in patients with dementia (19). The above epidemiological, clinical, and pathophysiological aspects underline the importance of periodical screening. Given this necessity, new studies are needed to understand the real-life state of the geriatric population and to study and deepen the correlations between the domains of geriatric assessment. This study will try to answer these questions with a cross-sectional approach. It fits into a thriving field of study, contributing to explain the growing burden of multimorbidity and demand for care.
The primary aim of this study was to determine the prevalence of impaired health status in a large geriatric population turning to outpatient service, based on the components of the CGA, and thus to describe its usefulness in real-life clinical practice.
The secondary aim of this study was the evaluation of the association between nutritional status, assessed with MNA—within the CGA—and cognitive-affective and functional capacities, and multimorbidity.
This study fits into a thriving field of study, contributing to explain the growing burden of multimorbidity and demand for care.
Materials and methods
Design of the study
This real-life, retrospective cross-sectional study included subjects consecutively evaluated from January 2009 to December 2020 at the Geriatric Outpatient Service, University Hospital of Monserrato, Cagliari, Italy.
Inclusion criteria: It includes those with the age of ≥65 years and has been subjected to CGA from January 2009 to December 2020.
Exclusion criteria: It includes those with the age of <65 years and has not been subjected to CGA.
A total of 3,260 subjects met the inclusion criteria.
Assessment
The enrolled subjects were evaluated with a validated battery of tools, including
Mini-Mental State Examination (MMSE), which is a screening test to detect severity and changes in time of cognitive impairment. The total score, given by the sum of the exact answers in each item, corrected for age and education, with scores from 30 (absence of cognitive impairment) to 0 (maximum cognitive impairment). A score of <24 is suggestive of cognitive impairment (20, 21).
Geriatric Depression Scale (GDS): It is a screening tool designed to evaluate the presence of depression in elderly subjects with MMSE > 14. This test is made up of 15 Yes/No questions, each of which is translated into a relative score of “0” or “1” as a result of the absence/presence of the investigated depressive symptom. The score ranges from 0 (absence of depression) to 15. A score of >5 is suggestive of depression (22).
Barthel Index of Independence in Activities of Daily Living (ADL): It is used to assess the ability to perform tasks such as taking a bath, using the toilet, walking, maintaining urinary and fecal continence, dressing up, and feeding. The total score ranges from 100 (independence) to 0 (complete dependence) (23). In its modified version, the various grades of dependence are distinguished as follows: 0–24 (complete dependence), 25–49 (severe dependence), 50–74 (moderate dependence), and 75–90 (mild dependence) (24).
Instrumental Activities of Daily Living (IADL): It is used to assess the ability to perform tasks such as using a telephone, doing laundry, and handling finances. The score ranges from 8 (independence) to 0 (complete dependence). A score of <6 is suggesting of dependence (25).
Mini Nutritional Assessment (MNA): It is a tool to assess malnutrition or its risk. The total score is obtained from the sum of the scores assigned to the answers of the 18 questions, which can be divided into four sections (anthropometric, global, dietetic, and subjective). The total score classifies the subject as follows: malnourished (<17), risk of malnutrition (17–23.5), and well-nourished (≥24) (26).
Performance-Oriented Mobility Assessment (POMA): It is a tool for the evaluation of balance and gait in the elderly without cognitive impairment or affected by mild-to-moderate dementia. It identifies subjects at falling risk. The total score (0–28) is obtained by adding the resulting partial scores of two sections, namely, Balance and Gait. The total score classifies the subject in the following way: not able to walk (<2); high fall risk (2–19); moderate fall risk (20–23); and not increased fall risk (≥24) (27).
Cumulative Illness Rating Scale (CIRS): It is a tool to measure the elderly state of health. It evaluates 14 categories of pathologies concerning some organs and systems (e.g., heart vascular system, lungs, liver, and kidneys), hypertension, and psychiatric and behavioral aspects. Every item is evaluated on an ordinal scale with increasing severity levels, from 1 (absence of pathology) to 5 (severe pathology). Using this tool, we can evaluate the total score (CIRS Tot.), the Severity Index (CIRS ISC), which is obtained from the average of the 13 categories scores—excluding psychiatric and behavioral problems—, and the Comorbidity Index Score (CIRS ICC), which corresponds to the number of categories with a score of ≥3 (28).
Statistical analysis
Quantitative variables were expressed as median and interquartile ranges (IQRs). The Mann–Whitney test for continuous variables was used to study gender differences in CGA variables. Spearman’s rank correlation coefficient (ϱ) and, since this is a retrospective study, odds ratios (ORs) were used to study the relationship between the variables. The Kruskal–Wallis test was used to compare the three groups deriving from MNA scores. The Conover test was performed for post-hoc analysis. Multivariate analysis was performed with a stepwise multiple regression (p-values > 0.1 were excluded by the model): MNA scores were considered as “dependent variable”; the remaining CGA scores (MMSE, GDS, ADL, IADL, POMA, CIRS Tot., CIRS ICC, and CIRS ISC), age, and gender were considered “independent variables”.
The results are reported that indicate p-values in reference to 95% confidence intervals.
The MedCalc software (version 19.5; Ostend, Belgium) was used for the statistical analysis.
Results
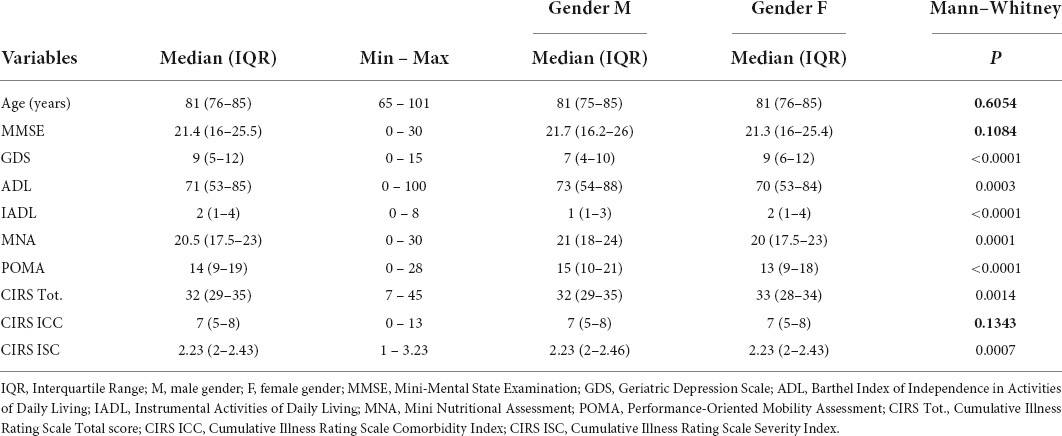
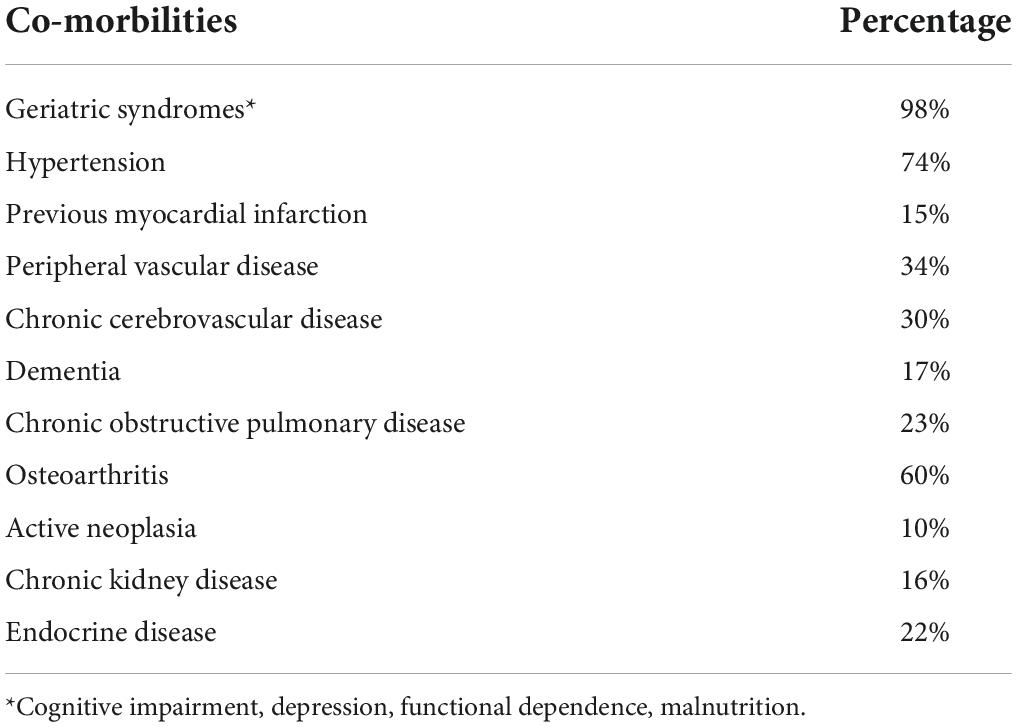
This retrospective cross-sectional study included 3,260 participants, of whom 2,350 (72.1%) were women. The characteristics of the enrolled subjects are shown in Table 1. Comorbidities are summarized in Table 2.
Table 1 also shows gender differences in performing CGA: women seemed to be more vulnerable than men in GDS, ADL, CIRS Tot., MNA, and POMA (p < 0.0001), while no gender-related differences were found in age, MMSE, and CIRS ICC.
Figure 1 shows the percentage of patients who were found to be compromised on CGA variables: cognitive impairment was suspected in 64.6%, and depression was suspected in 74.6%. Regarding nutritional assessment, it was poor in 80.5% of the subjects (19.9% showed malnourishment, and 60.6% was considered at risk of malnutrition). Total dependence on ADL was found in 3.9% of patients, severe dependence in 17.1%, and moderate dependence in 35.2%; regarding IADLs, 89.1% of patients needed assistance in completing them. Lastly, POMA revealed a high risk of falling in 72.8% of participants (3.4% was not able to walk).
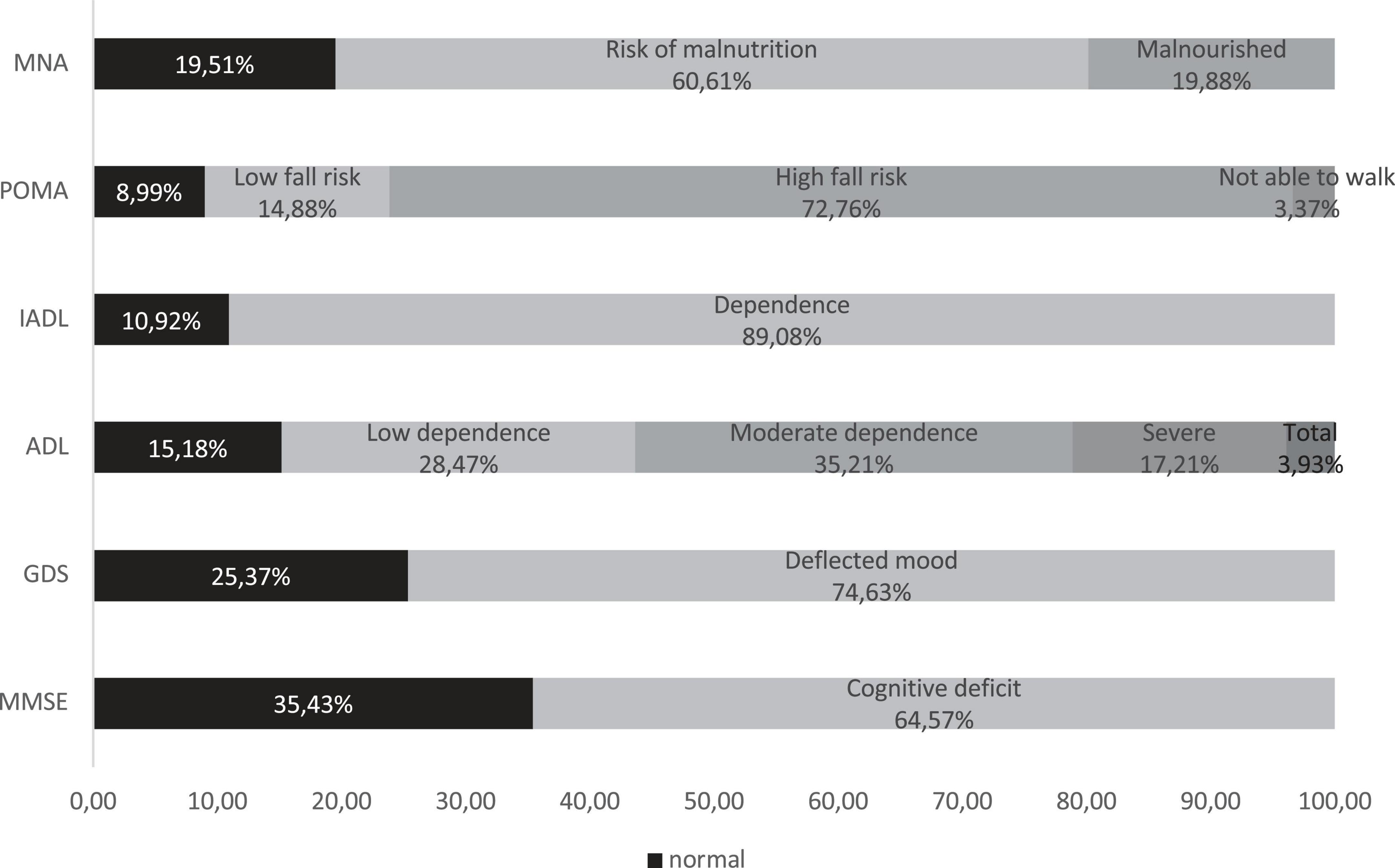
Figure 1. Comprehensive Geriatric Assessment. MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; ADL, Barthel Index of Independence in Activities of Daily Living; IADL, Instrumental Activities of Daily Living; POMA, Performance-Oriented Mobility Assessment; MNA, Mini Nutritional Assessment.
Only 73 (2.24%) subjects have proved good performances in MMSE, GDS, ADL, IADL, and MNA.
A sum of 1,819 (55.8%) people were revealed dependent on ADL (e.g., moderate, severe, and total dependence) and IADL, but only 495 (27.2% of them) could be considered cognitively intact.
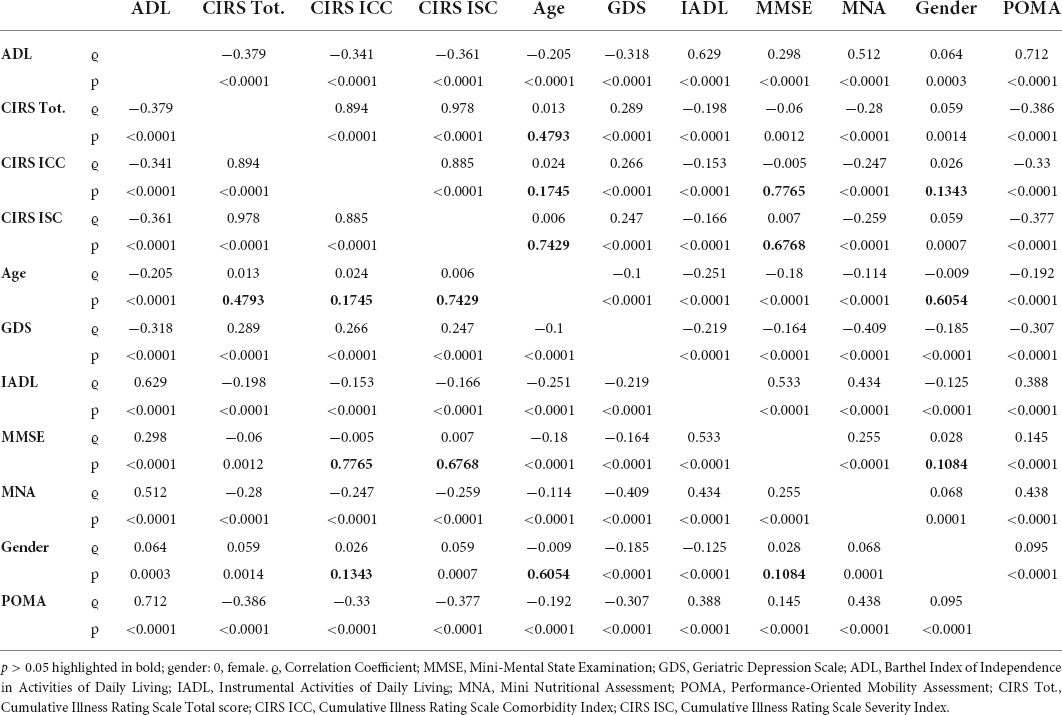
The correlation between the variables has been studied using Spearman’s rank correlation coefficient (ϱ), and the results are shown in Table 3. Very strong correlation (0.8 < ϱ < 0.99) was found between CIRS Tot., CIRS ICC, and CIRS ISC; strong correlation (0.6 < ϱ < 0.79) was found between ADL and IADL (ϱ = 0.63, p < 0.0001), ADL and POMA (ϱ = 0.71, p < 0.0001), and MMSE and IADL (ϱ = 0.53, p < 0.0001); moderate correlation (0.4 < ϱ < 0.59) was found between MNA and ADL (ϱ = 0.51, p < 0.0001), MNA and IADL (ϱ = 0.43, p < 0.0001), MNA and POMA (ϱ = 0.44, p < 0.0001), and MNA and GDS (ϱ = −0.41; p < 0.0001). The other correlations were weak or very weak (ϱ < 0.4) or not statistically significant.
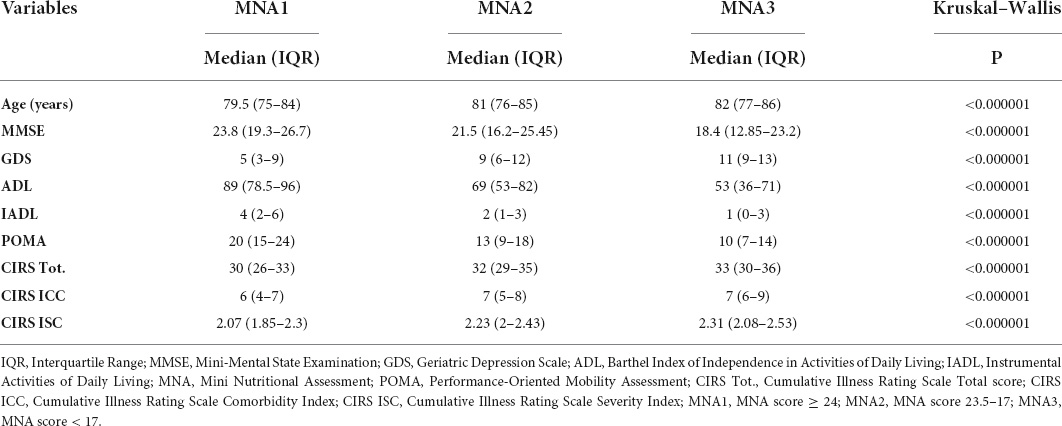
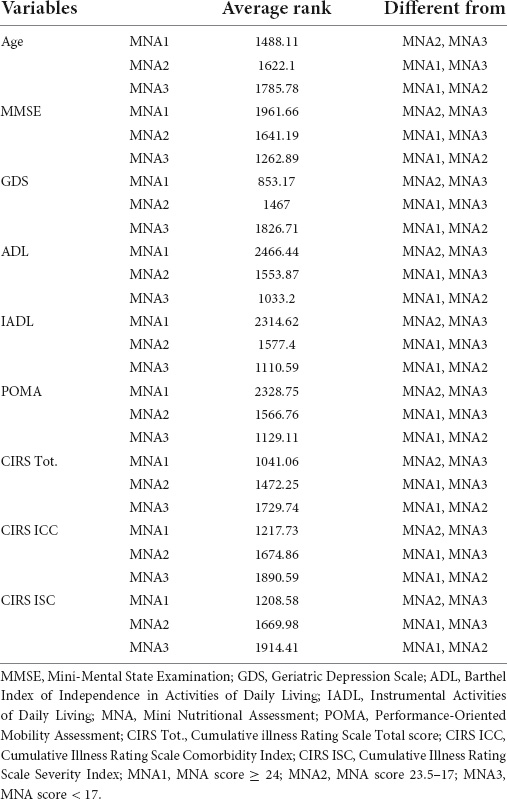
The study population was divided into three groups according to MNA scores, namely, MNA1 (score ≥ 24), MNA2 (24–17), and MNA3 (<17). The Kruskal–Wallis test was conducted to examine the differences in CGA scores according to the nutritional status. Significant differences (p < 0.000001) were found among the three groups (Table 4). Post-hoc analysis, conducted with the Conover test, showed a decrease in average ranks for MMSE, ADL, IADL, and POMA, and an increase in average ranks for age, GDS, CIRS Tot., CIRS ICC, and CIRS ISC, in MNA3 vs. MNA2 and MNA1, and in MNA2 vs. MNA1 (Table 5).
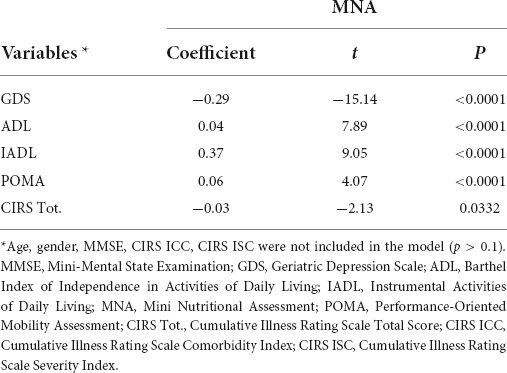
A stepwise multiple regression, conducted with the least squares method, considered GDS, ADL, IADL, POMA (p < 0.0001), and CIRS Tot. (p = 0.0332) as regressors of MNA; the other variables were not included in the model (Table 6).
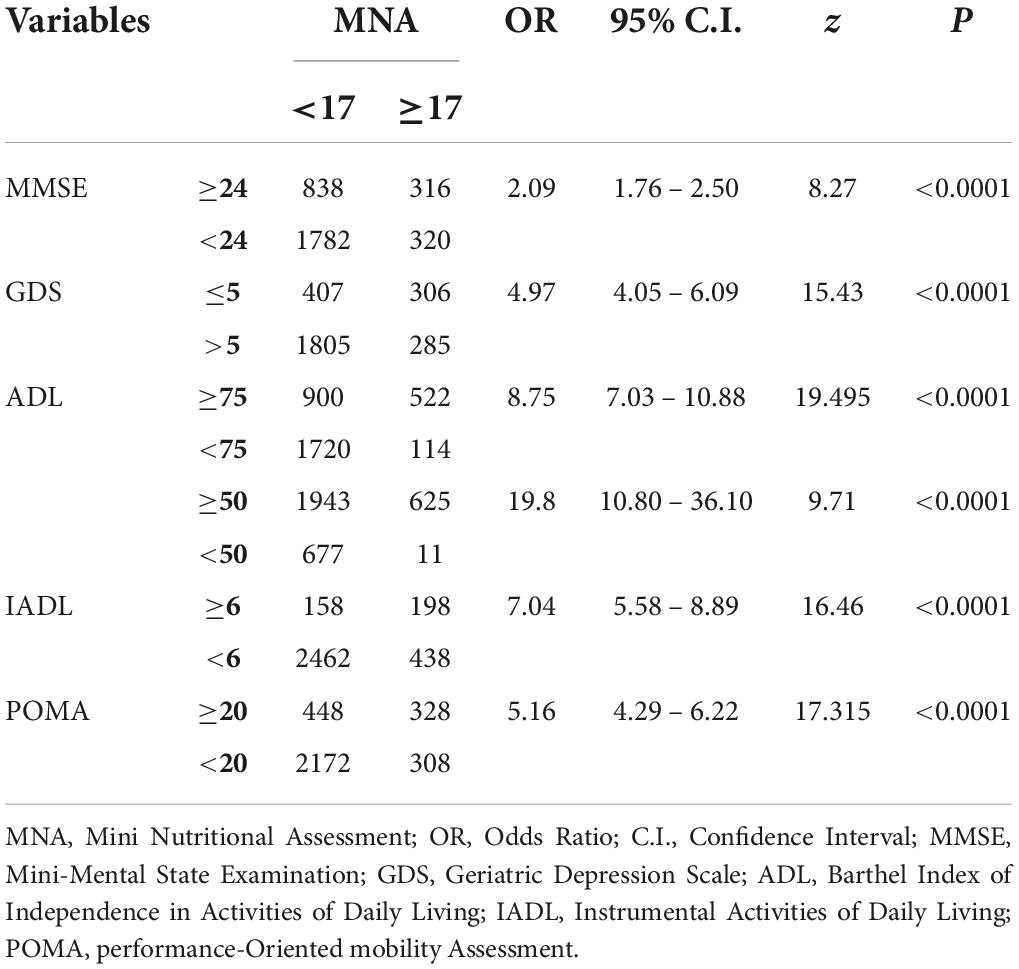
For the purpose of further examining the role of the nutritional status in influencing the other CGA domains, Table 7 shows that malnutrition revealed a significant association (p < 0.0001) with cognitive impairment (OR: 2.09, 95%CI 1.76–2.5), depression (OR: 4.97, 95%CI 4.05–6.09), dependence on ADL (OR: 19.8, 95%CI 10.8–36.1) and IADL (OR: 7.04, 95%CI 5.58–8.89), and risk of fall (OR: 5.16, 95%CI 4.29–6.22).
Discussion
We collected and examined data from a sample of 3,260 subjects referred from January 2009 to December 2020. The primary aim of this study was to examine the performance status presented by geriatric patients who turn to outpatient service, based on the components of the CGA. Understanding this aspect is fundamental to be able to build appropriate assistance and care plans. In our population, only 15.5% of the patients showed intact cognitive function, and 25.4% showed adequate mood; the percentages of the subjects that considered independent in performing basic and IADL were 15.2 and 10.9%, respectively. Low or absent increased risk of fall was found in 23.9% of the patients, and adequate nutritional status was found in 19.5%. These data denounce that too often, the people come to the attention of the specialist clinician when their status is compromised, leaving less room for maneuver to interventions, whether pharmacological or not. To confirm this thesis, it can be seen that only 2.24% of the study population could be considered “fit” in one of the CGA areas, such as cognitive, affective, functional, and nutritional areas; moreover, unfortunately, only 27.2% of functional-dependent people showed adequate cognitive performances.
About the gender-related differences in CGA scores, statistically significant in GDS, ADL, CIRS Tot., MNA, and POMA, are actually not clinically significant, except for GDS. By way of illustration, the median of ADL scores was 73 in men and 70 in women: this difference, although showing p = 0.0003, does not evidence any variation from the point of view of real dependence in performing daily living activities. On the other way, as mentioned earlier, GDS scores were higher, highlighting a more deflected mood, in women (median: 9 vs. 7), and this difference cannot fail to be considered relevant also from the clinical point of view, as well as pharmacological. Nevertheless, this data are consistent with the literature (29–31).
Regarding the correlations between the variables, as expected (28), CIRS Tot., CIRS ICC, and CIRS ISC were mutually very strongly correlated with each other—since a single index is insufficient (32), we also summarized the principal comorbidities—, and also the strong relationship between ADL, IADL, and POMA is consistent with the literature (14, 33). It is interesting to underline that MNA is the most correlated variable with ϱ > 0.4: it is correlated with GDS, ADL, IADL, and POMA.
Given this correlation, but also the compromised state of the population, and the limitation of the areas of intervention, the secondary aim of this study was to observe the association between nutritional status and the other CGA areas: we have chosen to look more deeply at this domain—assessed with MNA—due to its offering possibilities for intervention, according to the literature (14, 34, 35). Another domain with different possibilities of intervention could be the functional one, but these are limited, in our study population, by the cognitive impairment showed by the patients, as mentioned earlier (7, 36).
We performed an analysis to study the difference in mean ranks of the various CGA tests in three groups, namely, MNA1 (well-nourished people), MNA2 (people at risk of malnutrition), and MNA3 (malnourished people). Every variable showed the same pattern that is a worsening of the performed results to the worsening of the nutritional status. To better explain this trend, the multiple regression highlighted GDS, ADL, IADL, POMA, and CIRS Tot. as significant regressors of MNA. The strength of the association between the malnutrition and the various outcomes suggested by CGA was measured through ORs. The most powerful associations emerged with ADL, IADL, and POMA: it is intuitive, as well as confirmed in clinical studies (37–39), that malnutrition, linked to physical weakness and sarcopenia, affects functional ability, which in turn increases fall risk. It is interesting to also highlight a clear association between malnutrition and depressed mood, consistent with other previous studies (39–41). The lowest OR (2.09, p < 0.0001) emerged between malnutrition and cognitive impairment: although it is confirmed that a poor nutritional status impacts cognitive function, MMSE was excluded by stepwise multiple regression (variable removed by the model if p > 0.1) and also showed poor correlation with MNA (ϱ = 0.255, p < 0.0001), our data have not been able to clearly comply with this evidence (19).
This real-life, cross-sectional study analyzed the performances of geriatric people evaluated in an outpatient setting. We have sadly become aware of the delay for patients to undergo a holistic geriatric assessment for the first time: this delay can strongly limit every area of intervention.
One of the fields more correlated to the others, and in which it is easier to intervene, is the nutritional one, which is also strongly associated with the other CGA domains: our data seem to suggest that maintaining an adequate nutritional status is strongly associated with a maintenance of affective and functional status, and also of the general health of the elderly people.
The strengths of the study are represented by the size of the sample, as well as its design: the real-life approach allows to have an effective idea of the conditions of multimorbidity and demand for care of the elderly population. The main limitation of the study is represented by the fact it is single-center and did not prospectively examine the patients: in this sense, by longitudinally monitoring the patients, it would also be possible to establish causal associations between the variables.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the University of Cagliari (certification NP/2022/1382). The patients/participants provided their written informed consent to participate in this study.
Author contributions
FS, SL, and AM were principal investigators and contributed to the study design, data analyses, interpretation of the findings, and wrote the manuscript. FZ and LS contributed to the data collection. MP carried out the data analyses. All authors have read and approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CGA, Comprehensive Geriatric Assessment; BMI, Body Mass Index: MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; ADL, Barthel Index of Independence in Activities of Daily Living; IADL, Instrumental Activities of Daily Living; MNA, Mini Nutritional Assessment; MNA1, MNA score ≥ 24; MNA2, MNA score 23.5–17; MNA3, MNA score < 17; POMA, Performance-Oriented Mobility Assessment; CIRS Tot., Cumulative Illness Rating Scale Total score; CIRS ICC, Cumulative Illness Rating Scale Comorbidity Index; CIRS ISC, Cumulative Illness Rating Scale Severity Index; IQR, Interquartile Range; OR, Odds Ratio.
References
1. Roser M, Ritchie H, Ortiz-Ospina E. World Population Growth. OurWorldInData.org. 2013. [Online Resource]. Oxford: Our WorldInData. (2013).
2. Istat. (2022). Available online at: http://dati.istat.it/Index.aspx?DataSetCode=DCIS_INDDEMOG1 (accessed June 2022).
3. Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. (1993) 342:1032–6. doi: 10.1016/0140-6736(93)92884-v
4. Boreskie KF, Hay JL, Boreskie PE, Arora RC, Duhamel TA. Frailty-aware care: giving value to frailty assessment across different healthcare settings. BMC Geriatr. (2022) 22:13. doi: 10.1186/s12877-021-02722-9
5. Onder G, Vetrano DL, Palmer K, Trevisan C, Amato L, Berti F, et al. Italian guidelines on management of persons with multimorbidity and polypharmacy. Aging Clin Exp Res. (2022) 34:989–96. doi: 10.1007/s40520-022-02094-z
6. Mahmoud AM, Biello F, Maggiora PM, Bruna R, Burrafato G, Cappelli M, et al. A randomized clinical study on the impact of Comprehensive Geriatric Assessment (CGA) based interventions on the quality of life of elderly, frail, onco-hematologic patients candidate to anticancer therapy: protocol of the ONCO-Aging study. BMC Geriatr. (2021) 21:320. doi: 10.1186/s12877-021-02237-3
7. Trentini M, Semeraro S, Motta M. Italian study group for geriatric assessment and management. effectiveness of geriatric evaluation and care. one-year results of a multicenter randomized clinical trial. Aging. (2001) 13:395–405. doi: 10.1007/BF03351509
8. Lopez OL, Kuller LH. Epidemiology of aging and associated cognitive disorders: prevalence and incidence of Alzheimer’s disease and other dementias. Handb Clin Neurol. (2019) 167:139–48. doi: 10.1016/B978-0-12-804766-8.00009-1
9. Hlavka JP, Mattke S, Liu JL. Assessing the preparedness of the health care system infrastructure in six european countries for an Alzheimer’s treatment. Rand Health Q. (2019) 8:2.
10. Mo JJ, Li JY, Yang Z, Liu Z, Feng JS. Efficacy and safety of anti-amyloid-β immunotherapy for Alzheimer’s disease: a systematic review and network meta-analysis. Ann Clin Transl Neurol. (2017) 4:931–42. doi: 10.1002/acn3.469
11. Artusi CA, Rinaldi D, Balestrino R, Lopiano L. Deep brain stimulation for atypical parkinsonism: a systematic review on efficacy and safety. Parkinsonism Relat Disord. (2022) 96:109–18. doi: 10.1016/j.parkreldis.2022.03.002
12. Braz de Oliveira MP, Moreira Padovez RFC, Serrão PRMDS, de Noronha MA, Cezar NOC, Andrade LP. Effectiveness of physical exercise at improving functional capacity in older adults living with Alzheimer’s disease: a systematic review of randomized controlled trials. Disabil Rehabil. (2022) 1–12. doi: 10.1080/09638288.2022.2037744 [Epub ahead of print].
13. Zamudio-Rodríguez A, Avila-Funes JA, Tabue-Teguo M, Dartigues JF, Amieva H, Pérès K. Towards an approach of disability along a continuum from robustness, pre-frailty, frailty to disability. Age Ageing. (2022) 51:afac025. doi: 10.1093/ageing/afac025
14. Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Hooper L, Kiesswetter E, et al. ESPEN practical guideline: clinical nutrition and hydration in geriatrics. Clin Nutr. (2022) 41:958–89. doi: 10.1016/j.clnu.2022.01.024
15. Loddo S, Salis F, Rundeddu S, Serchisu L, Peralta MM, Mandas A. Nutritional status and potentially inappropriate medications in elderly. J Clin Med. (2022) 11:3465. doi: 10.3390/jcm11123465
16. Cereda E, Pedrolli C, Klersy C, Bonardi C, Quarleri L, Cappello S, et al. Nutritional status in older persons according to healthcare setting: a systematic review and meta-analysis of prevalence data using MNA§. Clin Nutr. (2016) 35:1282–90. doi: 10.1016/j.clnu.2016.03.008
17. Jensen GL, Cederholm T, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. J Parenter Enteral Nutr. (2019) 43:32–40. doi: 10.1002/jpen.1440
18. Aquilani R, Dossena M, Verri M, Buongiorno AI, Bosch F. Diagnosi di malnutrizione e terapia nutrizionale nel paziente ambulatoriale/domiciliare con patologia cronica: un approccio pratico e rapido. [Diagnosis of malnutrition and nutritional therapy in the outpatient/home patient with chronic pathology: a practical and rapid approach]. Riv Soc Ital di Med Gen. (2015) 2:17–21.
19. Yu W, Yu W, Liu X, Wan T, Chen C, Xiong L, et al. Associations between malnutrition and cognitive impairment in an elderly Chinese population: an analysis based on a 7-year database. Psychogeriatrics. (2021) 21:80–8. doi: 10.1111/psyg.12631
20. Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
21. Measso G, Cavarzeran F, Zappalà G, Lebowitz BD, Crook TH, Francis J, et al. The mini-mental state examination: normative study of a random sample of italian population. Dev Neuropsychol. (1993) 9:77–85.
22. Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. (1986) 5:165–73. doi: 10.3109/09638288.2010.503835
23. Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. (1965) 14:61–5.
24. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel index for stroke rehabilitation. J Clin Epidemiol. (1989) 42:703–9. doi: 10.1016/0895-4356(89)90065-6
25. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. (1969) 9:179–86.
26. Guigoz Y, Vellas B, Garry PJ. Mini nutritional assessment: a practical assessment tool for grading the nutritional state of elderly patients. Facts Res Gerontol. (1994) 15:116–22.
27. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. (1986) 34:119–26. doi: 10.1111/j.1532-5415.1986.tb05480.x
28. Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. (1995) 43:130–7. doi: 10.1111/j.1532-5415.1995.tb06377.x
29. Smith L, Shin JI, Butler L, Barnett Y, Oh H, Jacob L, et al. Physical multimorbidity and depression: a mediation analysis of influential factors among 34,129 adults aged = 50?years from low- and middle-income countries. Depress Anxiety. (2022) 39:376–86. doi: 10.1002/da.23250
30. Korf JM, Ganesh BP, McCullough LD. Gut dysbiosis and age-related neurological diseases in females. Neurobiol Dis. (2022) 168:105695. doi: 10.1016/j.nbd.2022.105695
31. Coin A, Devita M, Bizzotto M, Bubola A, Manzato E, Sergi G, et al. The association between cognitive reserve and depressive mood in older inpatients: gender and age differences. Exp Aging Res (2022) 1–10. doi: 10.1080/0361073X.2022.2041324 [Epub ahead of print].
32. Canaslan K, Ates Bulut E, Kocyigit SE, Aydin AE, Isik AT. Predictivity of the comorbidity indices for geriatric syndromes. BMC Geriatr. (2022) 22:440. doi: 10.1186/s12877-022-03066-8
33. Nagarkar A, Kulkarni S. Association between daily activities and fall in older adults: an analysis of longitudinal ageing study in India (2017-18). BMC Geriatr. (2022) 22:203. doi: 10.1186/s12877-022-02879-x
34. Meyer AM, Podolski N, Pickert L, Polidori MC. Präventive Geriatrie: kognitiven Abbau verhindern [Strategies to prevent age-related cognitive decline]. Dtsch Med Wochenschr. (2020) 145:146–50. doi: 10.1055/a-0955-9587
35. Hammond D, Vanderlee L, White CM, Acton RB, White M, Roberto CA, et al. The conceptual framework for the international food policy study: evaluating the population-level impact of food policy. J Nutr. (2022) 152(Suppl, 1):1S–12. doi: 10.1093/jn/nxac042
36. Roswiyani R, Kwakkenbos L, Spijker J, Witteman CLM. The effectiveness of combining visual art activities and physical exercise for older adults on well-being or quality of life and mood: a scoping review. J Appl Gerontol. (2019) 38:1784–804. doi: 10.1177/0733464817743332
37. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. (2012) 31:652–8. doi: 10.1016/j.clnu.2012.02.007
38. Nagai T, Uei H, Nakanishi K. Association among geriatric nutritional risk index and functional prognosis in elderly patients with osteoporotic vertebral compression fractures. Indian J Orthop. (2021) 56:338–44. doi: 10.1007/s43465-021-00478-3
39. Klimova B, Novotny M, Valis M. The impact of nutrition and intestinal microbiome on elderly depression-a systematic review. Nutrients. (2020) 12:710. doi: 10.3390/nu12030710
40. Lang UE, Beglinger C, Schweinfurth N, Walter M, Borgwardt S. Nutritional aspects of depression. Cell Physiol Biochem. (2015) 37:1029–43. doi: 10.1159/000430229
Keywords: elderly, real-life, Comprehensive Geriatric Assessment (CGA), nutritional status, Mini Nutritional Assessment (MNA)
Citation: Salis F, Loddo S, Zanda F, Peralta MM, Serchisu L and Mandas A (2022) Comprehensive Geriatric Assessment: Application and correlations in a real-life cross-sectional study. Front. Med. 9:984046. doi: 10.3389/fmed.2022.984046
Received: 01 July 2022; Accepted: 15 August 2022;
Published: 13 September 2022.
Edited by:
Mario Ulises Pérez-Zepeda, Instituto Nacional de Geriatría, MexicoReviewed by:
Elena Vladimirovna Frolova, North Western State Medical University, RussiaAhmet Turan Isik, Dokuz Eylül University, Turkey
Copyright © 2022 Salis, Loddo, Zanda, Peralta, Serchisu and Mandas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Salis, ZnJhbmNlc2NvLXNhbGlzQHRpc2NhbGkuaXQ=
†These authors share first authorship
 Francesco Salis
Francesco Salis Simona Loddo1†
Simona Loddo1† Luca Serchisu
Luca Serchisu