- 1Department of Medical Parasitology and Mycology, Arak University of Medical Sciences, Arak, Iran
- 2Department of Microbiology, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
- 3Department of Microbiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 4Molecular and Medicine Research Center, Khomein University of Medical Sciences, Khomein, Iran
Background: Renal mucormycosis (RM) is a rare presentation of invasive mucormycosis with a high mortality rate. There is no single systematic review of the literature that indicates the different clinical aspects of RM.
Methods: A systematic search of PubMed/Medline was performed to collect individual case reports of RM in patients of all ages published between 2010 and April 2022.
Results: Seventy-one individual cases were detected through PubMed bibliographic database searches, with a final assessment performed on 60 patients with RM. India and Asia had the largest number of reported cases, with 30 (50%) and 42 (70%) reports, respectively. Also, 74 and 26% of the patients with a mean age of 33 years were male and female, respectively. RM showed 44% mortality rate in the analyzed cases. Immunosuppressive agent therapy followed by tissue transplantation (kidney and liver) and diabetes were the most remarkable risk factors in patients. Nevertheless, 22% of the patients were immunocompetent with no apparent underlying condition. COVID-19 positivity was detected in eight adult patients with an 87% mortality rate. The most common signs of infection were fever, flank pain, and oliguria; additionally, isolated RM was reported in 57% of the cases. In 55% of the patients, histopathologic examination alone was sufficient to diagnose RM, whereas molecular methods and culture were used in only 18 and 35% of patients, respectively. Surgery alone, surgery plus anti-infection therapy, and anti-infection therapy alone were used in 12, 60, and 13% of patients, respectively. Furthermore, 15% of the patients died before any treatment.
Conclusion: The early diagnosis of RM is necessary. In this regard, the use of molecular-based diagnostic assays can help identify the fungus at the genus and species levels and use an appropriate treatment in the shortest possible amount of time. Because of the increase in antibiotic resistance in recent years, determining microbial susceptibility tests can lead to the better infection management. Additionally, withdrawal of immunosuppressant, appropriate surgical intervention, and antifungal therapy are the main factors associated with a successful outcome in RM.
Introduction
Mucormycosis, previously called Zygomycosis, comprises a wide range of invasive infections caused by different Mucorales species such as Rhizopus, Apophysomyces, Lichtheimia Mucor, Rhizomucor, Cunninghamella, and Saksenaea (1, 2). Mucormycosis has been considered as a rare fungal infection but has a high mortality rate (3, 4). Patients with underlying conditions such as tissue transplantation, diabetes, and immunosuppressive agent's therapy are more at risk of developing mucormycosis (5). The common mucormycosis clinical presentations are rhino-orbito-cerebral (ROCM), pulmonary, cutaneous, gastrointestinal (GI), and disseminated forms.
Kidney involvement, renal mucormycosis (RM), has been reported in up to 20–22% of the cases with disseminated forms (5, 6). The exact mechanism of RM is not clear yet; however, retrograde spread from lower urinary tract infection and blood dissemination to the kidneys have been suggested (7). Thus, in high-risk patients, early demonstration of the infection in the urine and cystoscopy assessment of the bladder could be helpful to avert dissemination as bladder can also be a portal of entry for Mucorales (8).
Mucorales have angioinvasive ability and invade the blood vessels, thus leading to vascular thrombosis and related ischemic necrosis of the kidney. Furthermore, these fungi could also invade the tubules, the glomeruli, and the parenchyma, in addition to the renal vessels. Collectively, medullary and cortical necrosis leads to renal failure and irreversible kidney damage (8, 9).
Note that the psoas and quadratus lumborum muscles posteriorly and with the peritoneal contents anteriorly provide good protection for kidneys. However, the kidneys could sustain lacerations and contusions from lower chest or blunt abdominal trauma, as occurs not infrequently in cases of high-impact motor vehicle crashes (10). Another study also reported that following intravenous inoculation in mice, the predominant sites of focal infection were the kidney and brain (11). Accordingly, different surgical interventions and traumas might have led to the entrance of the Mucorales into the patients' kidneys. Further, an intravascular catheter should be considered as a common portal of Mucorales' entrance to the body which could lead to the nosocomial disseminated mucormycosis (1, 12, 13).
Because of the lack of a comprehensive review of the literature indicating the different clinical perspectives of RM, this review paper was performed to compile available data about this infection and its patients in order to help in their diagnosis as well as management procedure.
Methods
Literature search
The Medline (via PubMed) was searched from January 1, 2010, to April 29, 2022, where Boolean Operators were used to extracting search keywords from the National Library of Medicine's Medical Subject Heading (MeSH) terms, abstracts, or titles (and, or): “Mucor” or “Mucorales” “Rhizopus” or “Lichtheimia” or “Rhizomucor” or “Absidia” or “Cunninghamella” or “Apophysomyces” or “Mucormycosis” or “Zygomycosis” and “Renal” or “Kidney.” Further, we also evaluated the references of the found articles and cross-checked for any other cases that may have been overlooked or missed during the initial investigation. Non-English written papers were excluded. Hickey et al. study, as well as our most recent publications, were used as the protocol for this study (14–16).
Inclusion criteria
In the current review paper, RM individual case reports in patients of all ages published in English between 2010 and April 2022 and available online in Medline (via PubMed) were included. Analysis of the included studies was performed carefully by both authors (MD and AS).
Exclusion criteria
Guidelines, review articles (systematic or meta-analysis), renal infections resulting from other pathogens, animal models, and studies with insufficient reported data were excluded from the final analysis (Figure 1).
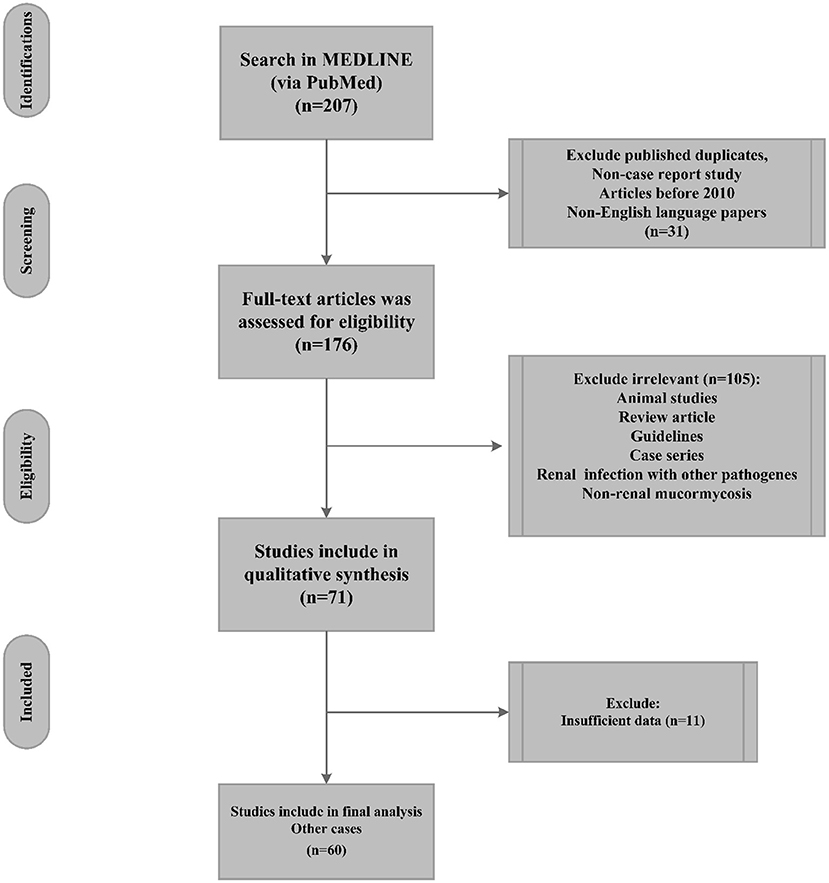
Figure 1. Flow chart of renal mucormycosis publication selection (PubMed reported cases 2010 until April 2022) and their inclusion in the systematic review.
Study selection and data extraction
MD and AS evaluated the articles, and in case of a discrepancy, both authors were obligated to inspect the paper for eligibility secured for review. Individual case reports were assessed to elicit data about the epidemiological features, clinical signs, diagnostic methods, and infection management of RM in patients of all ages. In the end, Excel software (Microsoft, Redmond, WA, USA) was performed to collect and evaluate the following information extracted from articles: publication year, country, age/gender, underlying conditions, causative Mucorales, clinical signs, surgical intervention, antifungal treatment, and outcomes.
Quality assessment
The Joanna Briggs Institute's (JBI) critical appraisal checklist was used for the quality assessment of studies (17).
Results
Epidemiology
Seventy-one cases, as individual case reports, were detected via searches of the PubMed bibliographic database; afterward, 11 cases were eliminated from the final analysis because of insufficient data or non-RM (Figure 1). Accordingly, sixty cases were included in the present study and were used for final analysis. The geographical distribution of the detected cases was as follows: India (30 cases), USA (nine cases), China (four cases), and Japan (three cases). Other countries, including Saudi Arabia, France, Germany, Korea, Netherland, Qatar, Romania, and South Africa, reported only one case. Thus, Asia had the largest number of cases (70%, 42 cases), followed by the Americas (15%, 9 cases) and Europe (10%, six reports). In this regard, only two and one reports were detected from Australia and Africa, respectively. In addition, 74 and 26% of the patients with a mean age of 33 years (range: 0.25–76, SD: 19.22 years) were male and female, respectively. The analyzed cases had a 44% mortality rate.
According to our analysis, 11 patients (11/60, 18%) had blood disorders, including acute myelogenous leukemia (AML), acute lymphocytic leukemia (ALL) (five cases), non-Hodgkin's lymphoma and Idiopathic CD4 lymphocytopenia, as well as anemia (three cases). Notably, one of these patients had a history of Hematopoietic Stem Cell Transplantation (HSCT) and two deaths occurred due to RM. Additionally, immunosuppressive agent therapy, tissue transplantation (kidney and liver), diabetes, and chemotherapy were reported in 27, 20, 18, and 14% of the patients, respectively.
In addition to the mentioned underlying conditions, different disorders such as Behçet's disease, injury and alcoholism, intravenous drug abuse, prolonged hospitalization with broad-spectrum antibiotic usage, and HIV were considered predisposing factors for RM in patients. Further, eight of the patients (13%) had a COVID-19 positive history, while six of these patients were treated with corticosteroids. Note that 87% (7/8) of the COVID-19 positive patients died because of the RM. On the other hand, 13 (22%) patients did not have any major risk factors; accordingly, these patients were considered immunocompetent patients. The mortality rate among these patients was 38% (5/13).
Only 22 studies (37%, 22/60) identified species at the species level, where Rhizopus spp. was the most frequently isolated pathogen from patients, with nine reports. In this regard, Rhizopus oryzae and Rhizopus microsporus caused RM in six patients. Further, other Mucorales, including Apophysomyces elegans (five cases), Rhizomucor spp. (two cases), Lichtheimia corymbifera (two cases), Absidia corymbifera, Cunninghamella spp, Mycocladus corymbifer, and Lichtheimia ramosa were detected in the patients with RM (Table 1).
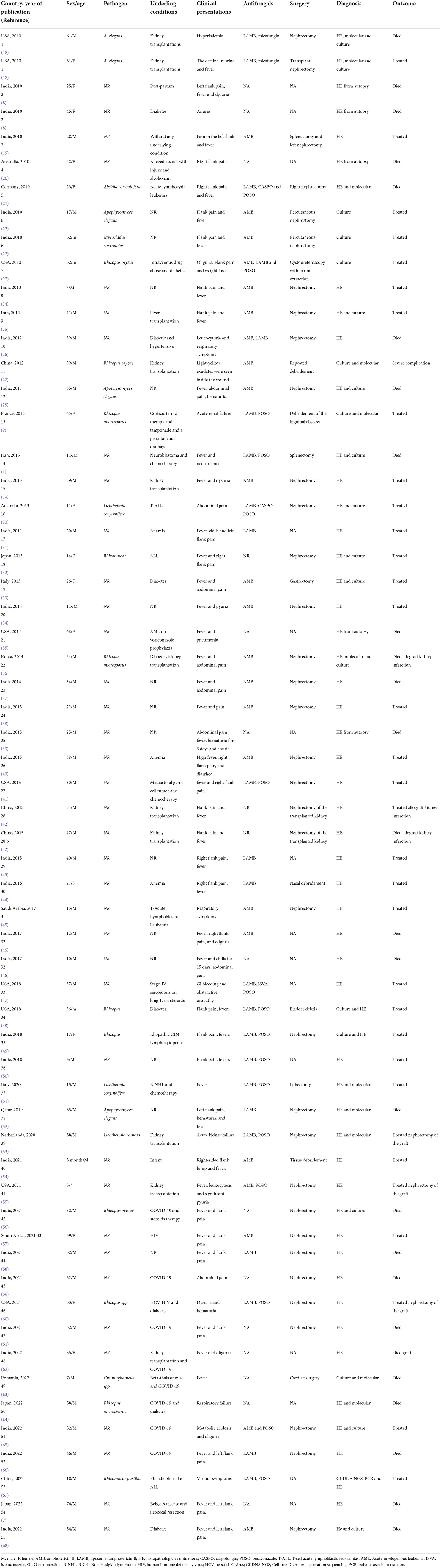
Table 1. Different clinical aspects of patients with renal mucormycosis (PubMed reported cases 2010 until April 2022).
Clinical manifestations
Fever (73%, 44/60), flank pain (may be unilateral or bilateral depending upon the extent of disease) (57%, 34/60), oliguria 17% (10/60), hematuria (12%, 7/60), pyuria 10% (6/60), anuria 8% (5/60), and dysuria 5% (3/60) were the most prevalent RM clinical signs reported in the patients. Note that the isolated RM was reported in 57% (34/60) of the cases. Additionally, in five patients, bacterial co-infection was detected: vancomycin-resistant Enterococcus faecium, bacterial intraabdominal abscesses, carbapenems resistant Enterobacter cloacae, Mycobacterium tuberculosis, and Pseudomonas aeruginosa.
Diagnosis
With respect to laboratory analysis, in 55% (33/60) of the patients, histopathologic examination (HE) alone was sufficient to diagnose RM. Ribbon-like and aseptate hyphae of Mucor, with surrounding tissue necrosis, were the most common reported characteristic after HE. Tissue specimens were collected from surgery and biopsy; additionally, in 10 (17%) patients, tissue specimens were collected from an autopsy. Notably, in eight (13%) patients with disseminated mucormycosis, the specimens were collected from other organs apart from kidneys, which included liver, lung, spleen, nasal, gastric, mitral plus tricuspid vegetations, as well as left cervical soft tissue. Furthermore, findings from the combined use of HE + molecular evaluation and HE + culture led to the detection of infection in six (10%) and 12 (20%) patients, respectively. In three (5%) other patients, the combined use of all diagnostic methods (HE + culture + molecular evaluations) resulted in a definite diagnosis of RM.
On the other hand, HE was not used in six (10%) patients. In three of these patients, culture + molecular evaluation led to the diagnosis, while in three other patients, microscopic assessment and culture led to the definite diagnosis. Collectively, positive culture and polymerase chain reaction (PCR) plus sequencing were used to detect infection in 21 (35%) and 11 (18%) patients, respectively. Notably, different specimens, including tissue (15 patients), drainage (three patients), urine (two patients), and pus (one patient), resulted in a positive culture in patients with RM. Nevertheless, blood culture did not identify the Mucorales in any of the patients.
Finally, the data about medical imaging modalities, including Computed Tomography (CT) and Ultrasonography, were reported in 67% (40/60) and 47% (28/60) of patients, respectively. The findings of imaging modalities did not yield a conclusive diagnosis for any of the patients. In this regard, in one patient, CT findings led to the suspicion of cancer (38). Nevertheless, among the patients with imaging modalities, enlarged kidney (32%, 19/60), hydronephrosis, and pyelonephritis-like signs (12%, 7/60) were the most common signs reported.
Treatment
RM has been managed using various approaches. Combined use of surgery and antifungals was the main treatment approach employed in 60% (36/60) of the patients. In addition, surgery alone and antifungal alone were used in 12% (7/60) and 13% (8/60) of the patients, respectively. On the other hand, nine patients (15%) died before any treatment. Notably, among the patients managed without surgery, RM was controlled in three of them with antifungals + drainage without major surgery (22, 43). Another patient was treated with deoxycholate amphotericin B (AMB), but he deteriorated further and he succumbed before surgery (46). In two other patients, surgery was considered by physicians for the management of RM; however, due to the patient's frail state as well as multiple comorbidities and parental opposition, the surgery was not applied to the patients (47, 50).
The most common antifungal agents used in patients with RM were AMB (23 cases, 52%, 23/44), liposomal amphotericin B (LAMB) (22 cases, 50%, 22/44), and posaconazole (17 cases, 39%, 17/44). Note that isavuconazole and echinocandins were used only for two patients (Table 1). The LAMB, posaconazole, and isavuconazole were considered by physicians for the treatment of RM in other patients; however, these antifungals were not available in the clinical setting (26, 57).
Fifty-seven percent (13/23) of patients that had AMB in their treatment regime revealed desired therapeutic results; meanwhile, this drug was ineffective in the rest of the patients, with the LAMB treatment rate being 64% (14/22). Notably, deranged renal function was identified in two patients as AMB adverse events; additionally, renal failure was detected in one patient after LAMB therapy (26, 47, 57).
In two cases, AMB was administered, and both patients recovered completely without surgery (22). On the other hand, in two other patients AMB only caused partial resolution of the mucormycosis mass, and surgical intervention was needed to control the infection (24, 25). LAMB also showed good therapeutic effects in two patients. Both of these patients survived on antifungal therapy alone with additional drainage (31, 43).
Another antifungal agent that was used to treat RM was posaconazole. Sixty-one percent (12/17) of patients treated with this antibiotic showed favorable outcomes. In one patient, the combined used of LAMB and posaconazole (step-down therapy) resolved infection in a 3-year-old boy (50). LAMB (3 mg/kg/day) was used for the treatment of disseminated mucormycosis in another patient with ALL. This antifungal inhibited the infection, but CT indicated no significant decrease in the focus of the liver, spleen, and kidneys, but a new lesion appeared in his brain. Posaconazole combined with LAMB and CT (at the 4 and 8th week of the combination antifungal treatment) indicated that all lesions in the mentioned organs significantly decreased. Collectively, the patient had a desirable outcome through treatment with LAMB sequential posaconazole (67).
Nevertheless, the use of posaconazole for the treatment of three patients with RM failed due to some issues. For example, in one patient, the physicians were not able to demonstrate acceptable systemic posaconazole blood concentrations despite aggressive attempts to maximize absorption (18). In another patient, posaconazole was discontinued after 3 months because of the failure to achieve satisfactory therapeutic levels (30, 31). Finally, this antifungal was avoided initially because the patient was on cyclosporine, while posaconazole is a CYP enzyme inhibitor, which could lead to the drug interaction with cyclosporine (31).
Isavuconazole, one of the triazole class antifungal agents, was used to treat RM in only two patients. Both of these patients recovered from the infection. In one of these patients, a surgical approach was considered; however, given the patient's multiple comorbidities and frail state, such an approach was deferred. Additionally, LAMB was switched to oral posaconazole due to the deterioration of renal function. Nevertheless, posaconazole did not control the infection well; accordingly, treatment was switched to intravenous isavuconazole at a dose of 372 mg every 8 h. The patient completed 6 months of isavuconazole therapy, whose kidney function improved and remained stable (47).
Surgery was another approach that was used for the treatment of patients with RM. Different types of surgery were performed on 72% (43/60) of patients, and in 61% of them, a surgical procedure was necessary to inhibit the infection. Nephrectomy was the most surgical intervention that was used on the patient. In one case, AMB was discontinued due to renal failure, and nephrectomy managed the infection (57). In two other patients, treatment with AMB only led to the partial resolution of the mass, and nephrectomy controlled the infection (24, 25).
For better understanding, all mentioned findings are presented in Figure 2.
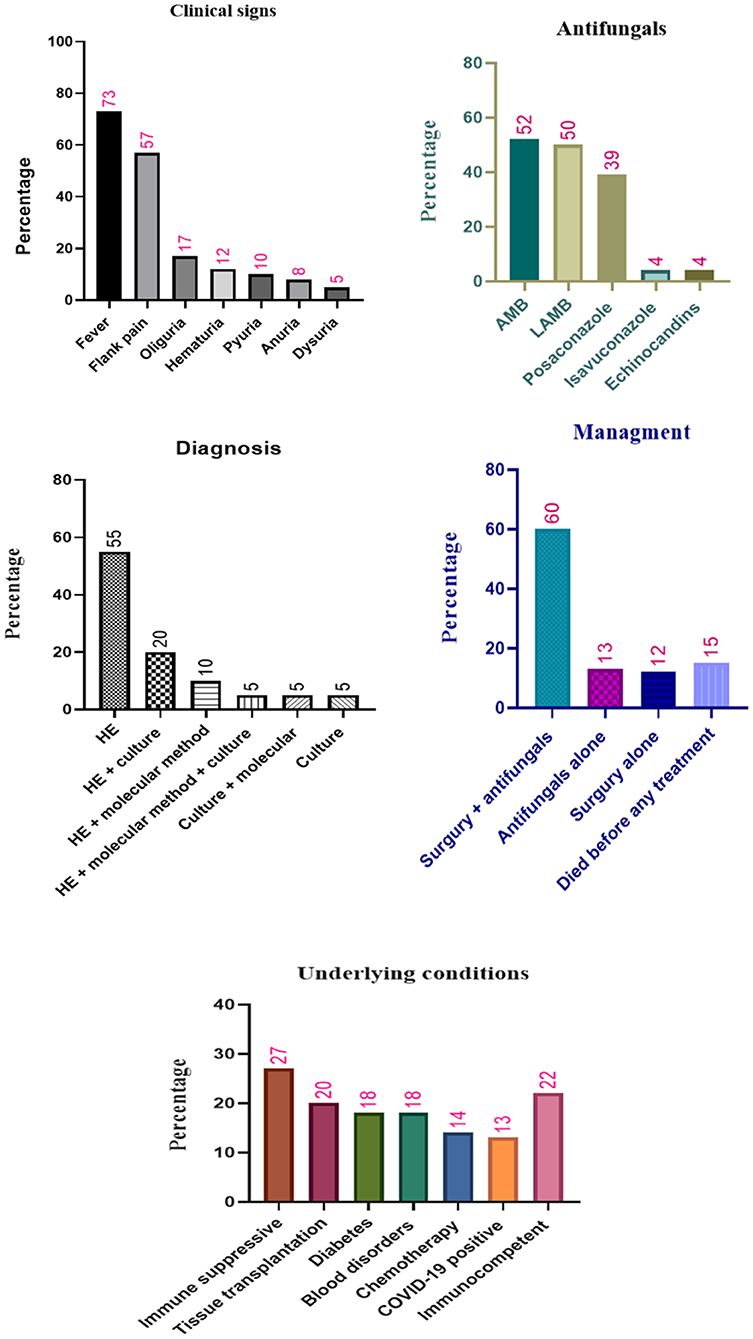
Figure 2. Different characteristics and antifungals treatment of patients with renal mucormycosis. HE, histopathologic examination; AMB, amphotericin B; LAMB, liposomal amphotericin B.
Discussion
Kidney involvement is one of the rare forms of mucormycosis with a high rate of mortality. Our analysis showed a 44% mortality rate in patients with RM (PubMed reported cases 2010–2022). Our recent investigations also showed 46.3 and 44% mortality rates in patients with ROCM and GI mucormycosis (2, 15). As with GI mucormycosis and ROCM, India had the highest incidence of RM. The high prevalence of diabetes in India is one of the most important reasons for such a high prevalence of mucormycosis in this country. Additionally, some other factors such as socio-economic situation, scarce hygienic conditions, climate, malnutrition, and lack of early diagnosis of mucormycosis can elevate this infection mortality rate in Indian patients. Nevertheless, deficiencies of the health care facilities have caused a broad range of the population of Indian diabetic patients to remain undiagnosed and uncontrolled; it seems that screening and controlling diabetes could be a useful tool to control the spread of mucormycosis in this country (69–71). Furthermore, the lack of effective drugs against mucormycosis such as LAMB, Posaconazole, and isavuconazole in low-income countries could increase the mortality rate of mucormycosis (26). Thus, better management of diabetes and the use of the most effective antifungal against mucormycosis could control this infection in patients from low-income countries.
Our investigation indicated that tissue transplantations, especially kidney transplantation, are among the most common related risk factors in patients with RM. The occurrence of mucormycosis in renal transplant is associated with various factors such as increased usage of immunosuppression in the initial post-transplant period with induction protocols and antirejection therapy such as anti-lymphocyte antibodies, pulse steroids, and interleukin (IL)-2 receptor antagonists, as well as intraoperative or postoperative surgical complications. Further, infected organ transmissions and the existence of immunomodulating viruses such as hepatitis C and cytomegalovirus should also be considered (29, 72). In this regard, a recently published study suggested that metabolic disorders and the nutritional status of the patients requiring tissue transplantation should be improved. Recipients and donors should also be strictly selected to reduce the risk of graft rejection, and antifungal drugs could be added to the renal perfusion fluid before surgery. Additionally, operation room condition is also very important since it could lead to tissue contamination during kidney collection, kidney perfusion, and transplant operation (42).
As with transparent patients, the use of corticosteroids in COVID-19-positive patients also increased the prevalence of mucormycosis in three recent years. The results of the present study also showed an 87% mortality rate for RM in COVID-19-positive patients. Coronavirus causes ciliary dysfunction, cytokine storm, microvascular hypercoagulability, and consequently innate immune dysregulation. Anti-IL-6-directed therapies and corticosteroid treatment that are used for the treatment of COVID-19 patients lead to immune suppression. Additionally, other risk factors such as mechanical ventilation, invasive procedures, industrial-grade oxygen administration, as well as prolonged hospital stays provide the perfect setting for invasive mucormycosis (56, 64, 73). Collectively, during COVID-19, systemic steroid treatments and uncontrolled diabetes are the main risk factors for RM. Thus, physicians should always be cautious of this infection in patients suffering from severe COVID-19 who are treated with extensive immunosuppressive therapy (64). Notably, Mucorales have an ability for blood invasion; thus, these fungi are associated with infarction and embolism. However, thrombosis is diagnosed frequently in COVID-19 patients in the critical care unit. Thus, it remains difficult to clinically distinguish SARS-CoV-2-associated thrombosis from mucormycosis-associated thrombosis where the possible presence of mucormycosis should be considered by the physicians in the patients presenting with sequential multiple thromboses (59, 64).
The results of the present investigation indicated that 22% of the patients with RM did not have any mucormycosis-associated risk factors. One study reported that the use of multiple antibiotics prior to admission for the treatment of urinary infection could be related to the development of RM (35). Additionally, hospital-acquired mucormycosis should be considered because of the contaminated air filters and wound dressings, administration of intravenous fluid contaminated with the fungus, and heavy air fungal loads of construction, as well as even tongue depressors (39, 44). Thus, RM also should be considered in immunocompetent patients who have been hospitalized for a long time.
Fever, flank pain, and oliguria were reported as the most commonly RM symptoms in the literature. Thus, this infection, in most cases, shows non-specific clinical manifestations which are comparable to those of other diseases such as other fungal infections, tuberculosis, and renal cell carcinoma (38). However, timely diagnosis is essential for early therapeutic intervention to limit dissemination and progressive angioinvasion, reduce the extent of surgical resection, as well as improve survival and outcome (30).
Imaging modalities have a contributory role in detecting the RM, but the diagnosis cannot be established merely on imaging. Nevertheless, perinephric stranding and enlarged non-hydronephrotic kidneys with hypodensities, as well as areas of low attenuation and perinephric fluid collection, are common findings in contrast-enhanced CT in patients with RM. Additionally, the combined use of Doppler ultrasonography and Magnetic resonance imaging (MRI)/CT could enhance the efficacy of RM diagnosis (1, 28, 39). Thus, physicians should be aware and fungal etiology must be considered after observing the mentioned signs.
In most cases, HE, the gold standard for ruling out and confirming the mucormycosis diagnosis, leads to the definitive diagnosis of RM. Mucorales are recognized by their broad aseptate hyphae branching at right angles at irregular intervals. Slender, dichotomously branching and septate could indicate Fusarium, Scedosporium, or Aspergillus species. Yeast with pseudohyphae may be associated with infections caused by Candida species (74–76).
However, unlike other pathogenic fungi which strongly react with histochemical stains, Mucorales are often difficult to detect. In this regard, pathologists should maintain a high index of suspicion for invasive mucormycosis, particularly in recipients with clinical symptoms compatible with an infectious process (18, 19). As mentioned, RM must be differentiated from cancer, tuberculosis, and other fungal infections. Atypical malignant cells could differentiate RM from renal cell carcinoma in HE. Nevertheless, fungal infections and tuberculosis both have multinucleated giant cells, neutrophil and macrophage infiltration, as well as granulomas. In this regard, the exact diagnosis of tuberculosis and fungal infections could be achieved from positive acid-fast bacilli and the presence of fungal hyphae in periodic acid-Schiff stain or Gomori silver methenamine, respectively (38).
Furthermore, our evaluations indicated that culture helped detect RM in 30% of cases and the chance of isolating Mucorales when biopsies or surgery specimens were used was higher for this method. Notably, culture could not differentiate between invasive infection and harmless contamination without repeated isolation of the fungus from sterile body sites (77). Further, several days were needed to identify the fungus species even after detecting a mold on the culture media (78). Accordingly, HE remains the gold standard for the detection of invasive mucormycosis.
However, in different patients, such as hematological patients, the use of surgery or biopsy to collect tissue specimens is almost unfeasible, and due to severe conditions, empirical antifungal therapy is started before biopsy, which would lower the chance of distinguishing the different fungal pathogens (67, 79).
To this end, the use of molecular methods has been considered for the diagnosis of RM in recent years. The present study showed that only 11 cases were studied using molecular methods based on PCR amplification and sequencing of the large nuclear subunit (28S) along with small nuclear subunit (18S) rRNA genes, as well as the internal transcribed spacer (ITS) region of rRNA. Molecular methods could facilitate an early diagnosis of RM and detect infrequently isolated organisms. Further, quantitative PCR (qPCR) could be used within 7 days after the initial treatment to monitor the response to antifungals. A negative qPCR indicates the response to the treatment (80, 81). However, molecular methods have a high cost, are not used in many laboratories, the positive threshold is not strictly defined and still, large-scale data are required in order to verify their specificity (27, 67). Collectively, combining molecular methods and HE can lead to an accurate and timely diagnosis of RM where culture is needed to ascertain microbial susceptibility to various antifungal agents.
After a timely and accurate diagnosis, the use of appropriate antifungals is essential in the treatment of RM. AMB and LAMB, based on the availability of antifungal drugs, are suggested for mucormycosis treatment as a first-line treatment regime (82). Our assessment also indicated that the mentioned antifungals had been widely used in patients with RM. LAMB showed better efficiency and fewer adverse event in comparison to the AMB. However, the use of LAMB is mainly contingent on the availability of antifungal agents in the clinical setting (83).
Furthermore, delayed-release tablets or intravenous posaconazole and intravenous isavuconazole are recommended for the treatment of mucormycosis (82). Our results indicated acceptable efficacy for posaconazole in the treatment of RM. However, this antifungal was used in only two patients, and further studies are required for better evaluation of isavuconazole efficacy in RM treatment. Posaconazole was also used in 17 patients and demonstrated good performance in managing Polymerase chain reaction (PCR) in some patients (23, 50, 67). However, this antifungal, as with LAMB and isavuconazole, is not available in low-income countries (26). On the other hand, these antifungals did not show an acceptable therapeutic effect in five patients (1, 18, 21, 30, 47). In this regard, the concentration of posaconazole should be monitored in patients at the beginning of the treatment, where a low concentration of this antifungal could lead to initial therapeutic failure. The resultant bioavailability and absorption of posaconazole are best achieved when taken with a high-fat meal (9, 30).
In 72% of patients, surgery was another therapeutic approach. Mucorales have an angioinvasive ability; thus, mucormycosis leads to the thrombosis of small and large arteries and tissue necrosis. In this regard, mucormycosis would reduce the penetration of antifungals and leukocytes to the site of interest; accordingly, the removal of infected and devitalized tissue by surgical intervention could be effective (84).
Finally, it should be noted that some patients died despite the appropriate use of surgery and antifungals (1, 21). Thus, management of a patient's underlying condition is essential in the treatment of RM. For instance, a patient died due to the RM despite intensive treatment (surgery + AMB and posaconazole). This patient had a history of allogeneic stem cell transplantation and suffered from graft-vs. -host disease; accordingly, if immune reconstitution cannot be achieved, successful eradication of invasive mucormycosis is rarely possible (21). Immunosuppressant impair the interferon gamma-driven response to fungal pathogens, decrease neutrophils and macrophage phagocytic function, as well as impair their migration. Hence, if it is possible, the immunosuppressive regimen should be withdrawn.
Limitations
The present study included only PubMed/MEDLINE studies available in the English language, and that contained an abstract, which may have reduced the number of relevant publications. Only individual case reports were included in the present review, and the observational studies were excluded because of their insufficient or absence of information. In addition, many studies have not reported information on the type of microorganism that causes the infection. Details of treatment such as reasons for choosing the drug, dose, duration, adverse events, and drug interactions for treatment of this subset of patients were not reported in some studies. It's noteworthy to mention that case reports and case series are more likely to be biased than other studies; additionally, these studies are descriptive and describe the patient's signs and symptoms. The prevalence and percentage of co-infection in them have not been studied. Finally, it was not possible to discuss the bias, risks, or individual limitations of the studies since these were not reported.
Conclusion
Renal involvement is a rare form of invasive mucormycosis with a high mortality rate. Our results showed that Rhizopus spp. was the most frequently isolated pathogen from patients. COVID-19 has increased the prevalence of mucormycosis in recent years, and management of corticosteroid therapy is critical in COVID-19-positive patients. The early diagnosis of RM is necessary; accordingly, the combined use of imaging, HE, and molecular methods could enhance the efficacy of diagnosis. In addition to timely diagnosis, withdrawal of immunosuppressant, appropriate surgical intervention and antifungal therapy plus immunotherapy with interferon-gamma, as well as bacterial superinfection management are the main factors related to the successful outcome in RM. Noteworthy, some antifungal such as LAMB, isavuconazole, and posaconazole are mostly used by rich countries, and perhaps providing conditions for wider use of these treatments can be effective in controlling RM in scarce hygienic conditions. Additionally, a multidisciplinary team consisting of an expert physician, radiologist, histopathologist, surgeons, and microbiologist is frequently needed for the successful management of RM.
Data availability statement
The authors confirm that the data supporting the findings of this study is available within the article and its supplementary material.
Author contributions
AS and MD conceived and designed the study, as well as analyzed the cases. AS, AK, and AM contributed to comprehensive research. AS, MD, and ZC wrote the paper. Notably, all authors have read and approved the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ebadi M, Alavi S, Ghojevand N, Aghdam MK, Yazdi MK, Zahiri A. Infantile splenorenopancreatic mucormycosis complicating neuroblastoma. Pediatr Int. (2013) 55:e152–5. doi: 10.1111/ped.12182
2. Didehdar M, Chegini Z, Moradabadi A, Anoushirvani AA, Tabaeian SP, Yousefimashouf M, et al. Gastrointestinal mucormycosis: a periodic systematic review of case reports from 2015 to 2021. Microbial Pathogenesis. (2022) 163:105388. doi: 10.1016/j.micpath.2022.105388
3. Dolatabadi S, Ahmadi B, Rezaei-Matehkolaei A, Zarrinfar H, Skiada A, Mirhendi H, et al. Mucormycosis in iran: a six-year retrospective experience. J Mycol Med. (2018) 28:269–73. doi: 10.1016/j.mycmed.2018.02.014
4. Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux JP, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. (2022) 3:e543–52. doi: 10.1016/S2666-5247(21)00237-8
5. Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. (2012) 1(Suppl. 54):S23–34. doi: 10.1093/cid/cir866
6. Tathe SP, Dani AA, Chawhan SM, Meshram SA, Randale AA, Raut WK. Gastric mucormycosis: diagnosis by imprint cytology. Diagn Cytopathol. (2016) 44:820–2. doi: 10.1002/dc.23518
7. Shimoyama K, Niwa T, Furukawa S, Morishita N, Nagakura Y, Yonezawa H, et al. Behçet's disease with bilateral renal infarction due to mucormycosis. Intern Med. (2022) 61:1077–83. doi: 10.2169/internalmedicine.7462-21
8. Gupta K, Nada R, Joshi K, Rohilla M, Walia R. Can ascending infection from bladder serve as the portal of entry for primary renal zygomycosis? Mycopathologia. (2010) 170:357–60. doi: 10.1007/s11046-010-9329-y
9. Charles P, Kahn JE, Ackermann F, Honderlick P, Lortholary O. Renal mucormycosis complicating extracorporeal membrane oxygenation. Med Mycol. (2013) 51:193–5. doi: 10.3109/13693786.2012.686069
10. Lee YJ, Oh SN, Rha SE, Byun JY. Renal trauma. Radiol Clin North Am. (2007) 45:581–92. doi: 10.1016/j.rcl.2007.04.004
11. Smith JM, Jones RH. Localization and fate of Absidia ramosa spores after intravenous inoculation of mice. J Comp Pathol. (1973) 83:49–55. doi: 10.1016/0021-9975(73)90026-1
12. Cherney CL, Chutuape A, Fikrig MK. Fatal invasive gastric mucormycosis occurring with emphysematous gastritis: case report and literature review. Am J Gastroenterol. (1999) 94:252–6. doi: 10.1111/j.1572-0241.1999.00809.x
13. Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol. (2007) 45:321–46. doi: 10.1080/13693780701218689
14. Hickey AJ, Gounder L, Moosa M-YS, Drain PK. A systematic review of hepatic tuberculosis with considerations in human immunodeficiency virus co-infection. BMC Infect Dis. (2015) 15:209. doi: 10.1186/s12879-015-0944-6
15. Chegini Z, Didehdar M, Khoshbayan A, Rajaeih S, Salehi M, Shariati A. Epidemiology, clinical features, diagnosis and treatment of cerebral mucormycosis in diabetic patients: a systematic review of case reports and case series. Mycoses. (2020) 63:1264–82. doi: 10.1111/myc.13187
16. Chegini Z, Didehdar M, Tabaeian SP, Khoshbayan A, Shariati A. A systematic review of case reports of hepatic actinomycosis. Orphanet J Rare Dis. (2021) 16:1–13. doi: 10.1186/s13023-021-01821-5
17. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: systematic reviews of etiology and risk. Joanna Brig Inst Rev Manual. (2017). doi: 10.46658/JBIRM-17-06
18. Alexander BD, Schell WA, Siston AM, Rao CY, Bower WA, Balajee SA, et al. Fatal apophysomyces elegans infection transmitted by deceased donor renal allografts. Am J Transplant. (2010) 10:2161–7. doi: 10.1111/j.1600-6143.2010.03216.x
19. Gupta V, Singh SK, Kakkar N, Jain S, Kalra N, Sakia UN. Splenic and renal mucormycosis in a healthy host: successful management by aggressive treatment. Trop Gastroenterol. (2010) 31:57–8.
20. Ingham A, Gilbert JD, Byard RW. Disseminated fungal infection with renal infarction simulating homicide. Am J Forensic Med Pathol. (2010) 31:390–2. doi: 10.1097/PAF.0b013e3181f69cb6
21. Leithauser M, Kahl C, Aepinus C, Prall F, Maruschke M, Riemer H, et al. Invasive zygomycosis in patients with graft-versus-host disease after allogeneic stem cell transplantation. Transpl Infect Dis. (2010) 12:251–7. doi: 10.1111/j.1399-3062.2009.00480.x
22. Marak RS, Misra R, Ansari MS, Dixit A, Poornima P., rasad KN, et al. Successful medical management of renal zygomycosis: a summary of two cases and a review of the Indian literature. Med Mycol. (2010) 48:1088–95. doi: 10.3109/13693781003753477
23. Palacio-Bedoya F, Cadena JA, Thompson GR, Sutton DA, Owens AD, Patterson TF. A noninvasive renal fungus ball caused by rhizopus–a previously unreported manifestation of zygomycosis. Med Mycol. (2010) 48:866–9. doi: 10.3109/13693781003694796
24. Dhua AK, Sinha S, Sarin YK, Khurana N. Isolated mucormycosis in a post-pyeloplasty kidney in an immuno-competent child. J Ind Assoc Pediatr Surg. (2012) 17:132–4. doi: 10.4103/0971-9261.98136
25. Geramizadeh B, Kazemi K, Shamsaifar AR, Bahraini A, Nikeghbalian S, Malekhosseini SA. Isolated renal mucormycosis after liver transplantation:an unusual case report. Iran Red Crescent Med J. (2012) 14:447–50. doi: 10.5812/ircmj.1134
26. Khandelwal D, Gadodia A, Sood R, Vikram NK, Singh P, Kumar R. Disseminated zygomycosis with renal involvement simulating malignancy in a diabetic patient. Indian J Urol. (2012) 28:347–9. doi: 10.4103/0970-1591.102726
27. Zhao L, Wang CX, Zhang L, Tu XA, Wang W, Chen Y, et al. Mucormycosis extending from the surgical wound to the transplanted kidney: case report and literature review. Exp Clin Transplant. (2012) 10:403–5. doi: 10.6002/ect.2011.0107
28. Ranjan P, Lal H, Chipde SS, Singh R, Kapoor R. Novel magnetic resonance imaging findings in renal zygomycosis. Urology. (2012) 79:e55–6. doi: 10.1016/j.urology.2011.07.1400
29. Gupta KL, Joshi K, Bhat A, Kohli HS, Jha V, Sakhuja V. Mucormycosis of the transplanted kidney with renal papillary necrosis. Exp Clin Transplant. (2013) 11:554–7. doi: 10.6002/ect.2012.0238
30. Guymer C, Khurana S, Suppiah R, Hennessey I, Cooper C. Successful treatment of disseminated mucormycosis in a neutropenic patient with T-cell acute lymphoblastic leukaemia. BMJ Case Rep. (2013) 2013:bcr2013009577. doi: 10.1136/bcr-2013-009577
31. Khandelwal A, Gupta P, Gupta A, Virmani V. Renal mucormycosis in aplastic anemia: a novel presentation. Int Urol Nephrol. (2013) 45:285–8. doi: 10.1007/s11255-011-0078-8
32. Sunagawa K, Ishige T, Kusumi Y, Asano M, Nisihikawa E, Kato M, et al. Renal abscess involving mucormycosis by immunohistochemical detection in a patient with acute lymphocytic leukemia: a case report and literature review. Jpn J Infect Dis. (2013) 66:345–7. doi: 10.7883/yoken.66.345
33. Bini R, Addeo A, Maganuco L, Fontana D, Viora T, Leli R. The role of surgery in a case of diffuse mucormycosis with haematemesis and gastric necrosis. Ann R Coll Surg Engl. (2014) 96:e31–3. doi: 10.1308/003588414X13946184901687
34. Nayagam LS, Vijayanand B, Balasubramanian S. Isolated renal mucormycosis in an immunocompetent child. Indian J Nephrol. (2014) 24:321–3. doi: 10.4103/0971-4065.133015
35. Non L, Sta Cruz JP, Tuazon S. Sudden death in a patient with bone marrow transplant by a fungus among us. BMJ Case Rep. (2014) 2014:bcr2014207403. doi: 10.1136/bcr-2014-207403
36. Park W, Jang M, Hwang E, Han S, Park S, Kim H, et al. Allograft mucormycosis due to rhizopus microsporus in a kidney transplant recipient. Transplant Proc. (2014) 46:623–5. doi: 10.1016/j.transproceed.2013.12.017
37. Ranjan P, Chipde SS, Vashistha S, Kumari N, Kapoor R. Reno-invasive fungal infection presenting as acute renal failure: importance of renal biopsy for early diagnosis. Saudi J Kidney Dis Transpl. (2014) 25:1282–4. doi: 10.4103/1319-2442.144268
38. Singh AK, Goel MM, Gupta C, Kumar S. Isolated renal zygomycosis in an immunocompetent patient. BMJ Case Rep. (2014) 2014:bcr2013200060. doi: 10.1136/bcr-2013-200060
39. Annigeri RA, Parameswaran A. Zygomycosis presenting as acute bilateral renal artery thrombosis in a healthy young male. J Assoc Physicians India. (2015) 63:77–9.
40. Dhaliwal HS, Singh A, Sinha SK, Nampoothiri RV, Goyal A, Chatterjee D, et al. Diagnosed only if considered: isolated renal mucormycosis. Lancet. (2015) 385:2322. doi: 10.1016/S0140-6736(15)60730-9
41. Hamdi A, Mulanovich VE, Matin SF, Landon G, Sircar K, Tu SM, et al. Isolated renal mucormycosis in a transplantation recipient. J Clin Oncol. (2015) 33:e50–1. doi: 10.1200/JCO.2013.49.1969
42. Zhu X, Liu H, Wang W, Song S, Jin M, Hu X, et al. Two cases of transplant renal artery thrombosis and spontaneous rupture caused by mucormycosis. Transpl Infect Dis. (2015) 17:442–8. doi: 10.1111/tid.12387
43. Devana SK, Bora GS, Mavuduru RS, Panwar P, Kakkar N, Mandal AK. Successful management of renal mucormycosis with antifungal therapy and drainage. Indian J Urol. (2016) 32:154–5. doi: 10.4103/0970-1591.179192
44. Shukla A, Shrivastava N, Singh CA, Nayak B. Percutaneous management of systemic fungal infection presenting as bilateral renal fungal ball. J Endourol Case Rep. (2016) 2:152–4. doi: 10.1089/cren.2016.0085
45. Munshi S, Moazin M, Abu-Daff S, Urrahman MA, Alamoudi OJ, Almehrej AH. Renal mucormycosis in immunocompromised patient, treated with robotic nephrectomy: case report and review of articles. Urol Case Rep. (2017) 15:53–5. doi: 10.1016/j.eucr.2017.08.007
46. Saran S, Naranje K, Gurjar M, Bhadauria D, Kaul A, Poddar B. Isolated renal mucormycosis in immunocompetent children: a report of two cases. Indian J Crit Care Med. (2017) 21:457–9. doi: 10.4103/ijccm.IJCCM_184_17
47. Izaguirre-Anariba DE, Chee F, Thet Z, Lanza J. An interesting case of a 57-year-old male with an upper gastrointestinal bleeding and obstructive uropathy with bilateral hydronephrosis diagnosed with systemic mucormycosis. Case Rep Infect Dis. (2018) 2018:6283701. doi: 10.1155/2018/6283701
48. Marsh BM, Rajasingham R, Tawfic SH, Borofsky MS. Successful conservative management of bilateral renal mucormycosis. Urology. (2018) 120:2–5. doi: 10.1016/j.urology.2018.03.010
49. Sethi J, Ramachandran R, Kohli HS, Gupta KL. Isolated renal mucormycosis in a patient with idiopathic CD4 lymphocytopenia. BMJ Case Rep. (2018) 2018:bcr2018225234. doi: 10.1136/bcr-2018-225234
50. Mathew G, Arumugam V, Murugesan S, Duhli N, Agarwal I. Renal mucormycosis: a rare cause of urinary tract infection leading to end-stage renal disease (ESRD). J Trop Pediatr. (2019) 65:405–8. doi: 10.1093/tropej/fmy059
51. Mauro M, Lo Cascio G, Balter R, Zaccaron A, Bonetti E, Vitale V, et al. The diagnostic pitfalls of mucormycosis. Mediterr J Hematol Infect Dis. (2020) 12:e2020079. doi: 10.4084/mjhid.2020.079
52. Rashid S, Ben Abid F, Babu S, Christner M, Alobaidly A, Al Ansari A, et al. Fatal renal mucormycosis with apophysomyces elegans in an apparently healthy male. Aging Male. (2020) 23:746–9. doi: 10.1080/13685538.2019.1586871
53. Spithoven EM, Bruns AHW, Petri BJ, Haas PJ, Nguyen TQ, Hagen F, et al. Renal transplant patient survives a donor-derived abdominal invasive mucormycosis (Lichtheimia ramos a). Med Mycol Case Rep. (2020) 30:39–42. doi: 10.1016/j.mmcr.2020.10.002
54. Anand S, Kainth D, Dhua AK, Sehgal M, Bajpai M, Das P, et al. Isolated renal mucormycosis in children: a case report and review of the literature. J Indian Assoc Pediatr Surg. (2021) 26:338–41. doi: 10.4103/jiaps.JIAPS_142_20
55. Baldwin XL, Serrano Rodriguez P, Nickeleit V, Toledo A. Graft nephrectomy as rescue therapy for posttransplant rhizopus pyelonephritis in a pediatric patient. Exp Clin Transplant. (2021) 19:489–92. doi: 10.6002/ect.2020.0356
56. Choudhary GR, Aggarwal A, Jain V, Jena R. COVID-19 and fatal renal mucormycosis: contributory or coincidental? Indian J Urol. (2021) 37:270–3. doi: 10.4103/iju.iju_197_21
57. Khaba MC, Nevondo LM, Moroatshehla SM, Makhado NA. Disseminated mucormycosis presenting as a renal mass in an human immunodeficiency virus-infected patient: a case report. S Afr J Infect Dis. (2021) 36:202. doi: 10.4102/sajid.v36i1.202
58. Parmar KM, Akif S, Kumar S, Kaundal P. Isolated unilateral renal mucormycosis in a young immunocompetent male. BMJ Case Rep. (2021) 14:e245309. doi: 10.1136/bcr-2021-245309
59. Singh T, Chaudhari R, Gupta A. Renal artery thrombosis and mucormycosis in a COVID-19 patient. Indian J Urol. (2021) 37:267–9. doi: 10.4103/iju.IJU_76_21
60. Thatipelli S, Santoiemma P, Echenique IA, Green R, Ison MG, Ladner D, et al. Donor-derived renal allograft mucormycosis in a combined liver and kidney transplantation: case report and review of the literature. Transpl Infect Dis. (2021) 23:e13534. doi: 10.1111/tid.13534
61. Varshney VK, Swami A, Thirunavukkarasu B, Agarwal A, Baid G. Synchronous small bowel gangrene with pyelonephritis secondary to mucormycosis: a disastrous complication of COVID-19 pandemic. Cureus. (2021) 13:e15911. doi: 10.7759/cureus.15911
62. Chandra A, Rao SN, Malhotra KP. Fatal allograft mucormycosis complicating severe COVID-19 infection and bacterial pyelonephritis. Transpl Infect Dis. (2022) 24:e13793. doi: 10.1111/tid.13793
63. Cinteza E, Nicolescu A, Ciomartan T, Gavriliu LC, Voicu C, Carabas A, et al. Disseminated cunninghamella spp. Endocarditis in a beta-thalassemia patient after asymptomatic COVID-19 infection. Diagnostics. (2022) 12:657. doi: 10.3390/diagnostics12030657
64. Horiguchi T, Tsukamoto T, Toyama Y, Sasaki T, Nakamura T, Sakurai A, et al. Fatal disseminated mucormycosis associated with COVID-19. Respirol Case Rep. (2022) 10:e0912. doi: 10.1002/rcr2.912
65. Kurien AA, Srinivasaprasad ND, Valavan KT. Renal infarction due to COVID-19-associated renal mucormycosis. Kidney Int. (2022) 101:655. doi: 10.1016/j.kint.2021.07.027
66. Sethia RK, Charag AH. A case of isolated unilateral renal mucormycosis post COVID-19 pneumonia with fatal outcome. Urol Case Rep. (2022) 43:102061. doi: 10.1016/j.eucr.2022.102061
67. Shi L, Zhao X, Yan X, Liu Y, Liu Y, Cao H, et al. Aggressive disseminated rhizomucor pusillus infection in a Ph-like acute lymphoblastic leukemia patient: early detection by cell-free DNA next-generation sequencing. J Infect Chemother. (2022) 28:459–64. doi: 10.1016/j.jiac.2021.12.007
68. Samuel SV, George TK, Gopinathan VR, Abraham OC. Community-acquired fungal pyelonephritis with renal infarction and gangrene of the colon: an uncommon diagnosis. BMJ Case Rep. (2022) 15:e241685. doi: 10.1136/bcr-2021-241685
69. Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, et al. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. (2004) 80:670–4. doi: 10.1136/pgmj.2003.016030
70. Chakrabarti A, Chatterjee S, Das A, Panda N, Shivaprakash M, Kaur A, et al. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. (2009) 85:573–81. doi: 10.1136/pgmj.2008.076463
71. Mignogna MD, Fortuna G, Leuci S, Adamo D, Ruoppo E, Siano M, et al. Mucormycosis in immunocompetent patients: a case-series of patients with maxillary sinus involvement and a critical review of the literature. Int J Infect Dis. (2011) 15:e533–40. doi: 10.1016/j.ijid.2011.02.005
72. Llorente A, Perez-Valero I, García E, Heras I, Fraile V, García P, et al. Mortality risk factors in patients with zygomycosis: a retrospective and multicentre study of 25 cases. Enferm Infecc Microbiol Clin. (2011) 29:263–8. doi: 10.1016/j.eimc.2010.09.008
73. Bretagne S, Sitbon K, Botterel F, Dellière S, Letscher-Bru V, Chouaki T, et al. COVID-19-associated pulmonary aspergillosis, fungemia, and pneumocystosis in the intensive care unit: a retrospective multicenter observational cohort during the first french pandemic wave. Microbiol Spectr. (2021) 9:e0113821. doi: 10.1128/Spectrum.01138-21
74. Bakhshizadeh M, Hashemian HR, Najafzadeh MJ, Dolatabadi S, Zarrinfar H. First report of rhinosinusitis caused by neoscytalidium dimidiatum in Iran. J Med Microbiol. (2014) 63:1017–9. doi: 10.1099/jmm.0.065292-0
75. Nourizadeh N, Adabizadeh A, Zarrinfar H, Majidi M, Jafarian AH, Najafzadeh MJ. Fungal biofilms in sinonasal polyposis: the role of fungal agents is notable? J Oral Maxillofacial Surg Med Pathol. (2019) 31:295–8. doi: 10.1016/j.ajoms.2019.01.007
76. Hosseinikargar N, Basiri R, Asadzadeh M, Najafzadeh MJ, Zarrinfar H. First report of invasive aspergillus rhinosinusitis in a critically ill COVID-19 patient affected by acute myeloid leukemia, northeastern Iran. Clin Case Rep. (2021) 9:e04889. doi: 10.1002/ccr3.4889
77. Rammaert B, Lanternier F, Zahar J-R, Dannaoui E, Bougnoux M-E, Lecuit M, et al. Healthcare-associated mucormycosis. Clin Infect Dis. (2012) 54(suppl_1):S44–54. doi: 10.1093/cid/cir867
78. Modi DA, Farrell JJ, Sampath R, Bhatia NS, Massire C, Ranken R, et al. Rapid identification of aspergillus terreus from bronchoalveolar lavage fluid by PCR and electrospray ionization with mass spectrometry. J Clin Microbiol. (2012) 50:2529–30. doi: 10.1128/JCM.00325-12
79. Gomes MZ, Lewis RE, Kontoyiannis DP. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, -Lichtheimia species. Clin Microbiol Rev. (2011) 24:411–45. doi: 10.1128/CMR.00056-10
80. Kasai M, Harrington SM, Francesconi A, Petraitis V, Petraitiene R, Beveridge MG, et al. Detection of a molecular biomarker for zygomycetes by quantitative PCR assays of plasma, bronchoalveolar lavage, and lung tissue in a rabbit model of experimental pulmonary zygomycosis. J Clin Microbiol. (2008) 46:3690–702. doi: 10.1128/JCM.00917-08
81. Millon L, Caillot D, Berceanu A, Bretagne S, Lanternier F, Morio F, et al. Evaluation of serum mucorales PCR for the diagnosis of mucormycoses: the MODIMUCOR prospective trial. Clin Infect Dis. (2022) 5:ciab1066. doi: 10.1093/cid/ciab1066
82. Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SC, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. (2019) 19:e405–21. doi: 10.1016/S1473-3099(19)30312-3
83. Chaudhary RJ, Choudhary NS, Saraf N, Gautam D, Piplani T, Thiagrajan S, et al. Delayed graft dysfunction due to invasive hepatic mucormycosis after living donor liver transplantation. J Clin Exp Hepatol. (2020) 10:629–32. doi: 10.1016/j.jceh.2020.04.008
Keywords: renal, kidneys, mucormycosis, COVID-19, invasive fungal
Citation: Didehdar M, Chegini Z, Khoshbayan A, Moradabadi A and Shariati A (2022) Clinical presentations, diagnosis, management, and outcomes of renal mucormycosis: An overview of case reports. Front. Med. 9:983612. doi: 10.3389/fmed.2022.983612
Received: 01 July 2022; Accepted: 08 August 2022;
Published: 24 August 2022.
Edited by:
Sunil Dhiman, Defence Research and Development Establishment (DRDE), IndiaReviewed by:
Georgie Mathew, Christian Medical College and Hospital, IndiaHossein Zarrinfar, Mashhad University of Medical Sciences, Iran
Copyright © 2022 Didehdar, Chegini, Khoshbayan, Moradabadi and Shariati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aref Shariati, YXJlZnNoYXJpYXRpMDExMUBzYm11LmFjLmly; Zahra Chegini, cGFyaXNhLmNoZWdpbmk3MkBnbWFpbC5jb20=
 Mojtaba Didehdar1
Mojtaba Didehdar1 Zahra Chegini
Zahra Chegini Aref Shariati
Aref Shariati