- 1Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom
- 2Department of Obstetrics and Gynecology, Newham University Hospital NHS Trust, London, United Kingdom
- 3Department of Health Sciences, School of Sciences, European University Cyprus, Nicosia, Cyprus
Anovulation is very common and has several different clinical manifestations, including amenorrhea, oligomenorrhea and abnormal uterine bleeding. Various mechanisms can cause anovulation. The clinical consequences and commonest chronic anovulatory disorder, polycystic ovary syndrome (PCOS), has a prevalence that ranges between 6 to 10% of the global population. While multiple causes can eventually result in PCOS, various methods have been described in the literature for its management, often without ascertaining the underlying cause. Ovulation Induction (OI) is a group of techniques that is used in women with PCOS who are looking to conceive and are unbale to do so with natural means. This narrative review presents a summary of the current evidence and available techniques for OI in women with PCOS, highlighting their performance and applicability.
Introduction
Among the classification criteria for stratifying anovulatory women, normogonadotropic normoestrogenic patients, also known as World Health Organization (WHO) group II, account for around 80% of all anovulatory patients (1). Polycystic Ovary Syndrome (PCOS) is the most common disorder affecting this subgroup of patients, with prevalence ranging from 6 to 10% of the global population depending on the classification criteria used (2). It is recognized that PCOS is not a cause of anovulation but rather a symptom of chronic anovulation caused by a range of both endocrinologic and functional abnormalities (3). As opposed to normal cycling hormone concentrations in ovulating women, women with PCOS are often described as being in a “hormonally steady state,” with both gonadotropin and sex steroid concentrations similar to those seen in early follicular phase (4). Increased Luteinizing Hormone (LH) levels are a result of abnormal LH secretory dynamic due to increased LH pulse frequency and pulse amplitude (5–8), where decreased FSH levels results from the increase in Gonadotropin Releasing Hormone (GnRH) pulse frequency, the negative feedback effects of chronically elevated estrone concentrations and increased inhibin B levels (9, 10). These changes often result in an increased LH/FSH ratio (11–13).
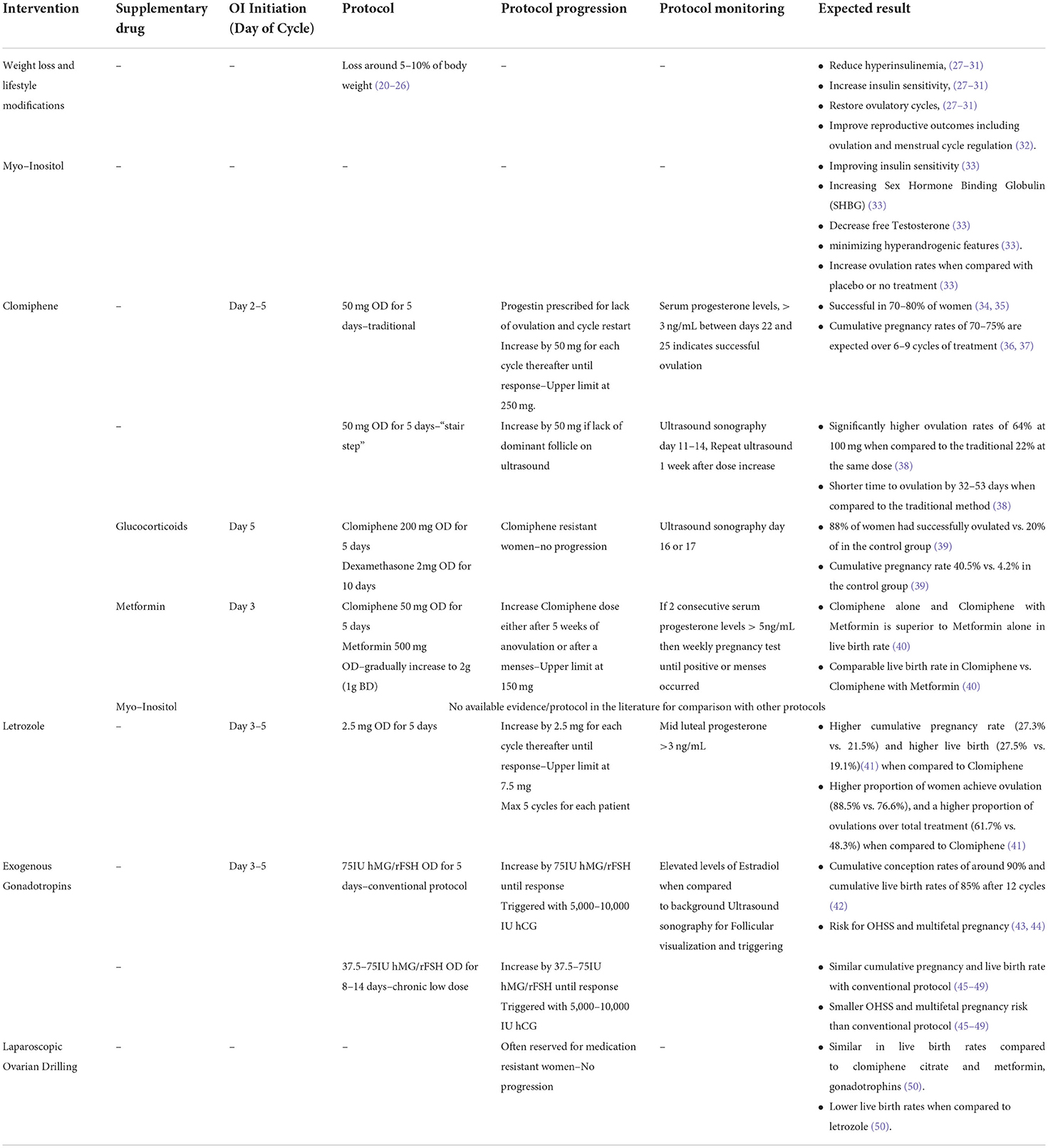
While menstrual irregularities, and by association infertility, are the most common complaints in women with PCOS, they are seldom isolated findings and are accompanied by an increase in both androgen and estrogenic hormone production. Elevated levels of serum testosterone, androstenedione, and 17α-hydroxyprogesterone have been identified to be LH dependent, derived mainly from the ovaries, while levels of dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and estrone are mainly derived from the adrenals (14–16). High concentrations of androstenedione are converted into estrone by peripheral tissues, while FSH and oestradiol levels are relatively stable and comparable with levels of a normal follicular phase cycle (4). A positive feedback of increased ovarian androgens contributes to insulin resistance which in turn inhibits hepatic sex hormone binding globulin production, further increasing androgen levels (17–19). These changes are responsible for the pathophysiological symptoms of PCOS, such as insulin resistance, which predisposes to type 2 diabetes, obesity, hirsutism, and anovulation (3). A stepwise approach in treating and managing patients diagnosed with PCOS can range from lifestyle interventions to invasive operations such as Laparoscopic Ovarian Drilling (LOD), which is often reserved for medication resistant cases. The present narrative review summarizes the available evidence and current techniques commonly used in clinical practice for Ovulation Induction (OI) in women with PCOS. Table 1 summarizes the approaches listed in this review.
Weight loss and lifestyle modifications
The prevalence of obesity in PCOS patients varies in different populations, ranging from 42 to 65% (51, 52). While the diagnosis of PCOS does not always indicate an anovulatory state, obese patients are more likely to suffer from it, hindering their ability conceive (53). Modest weight loss around 5–10% of body weight, often restores ovulatory cycles in obese anovulatory women with PCOS (20–26). In a population of PCOS patients, 70–80% of obese patients were found to be insulin resistant while only 20–30% of lean patients were insulin resistant (54). The primary aim in any patient with PCOS would be to promote weight loss through caloric deficit and physical activity, which would reduce hyperinsulinemia, increase insulin sensitivity, and often restore ovulatory cycles (27–31). According to a recent analysis of PCOS weight loss trials, moderate weight loss is associated with the occurrence of sporadic ovulation in a considerable proportion of patients, and that decreases in hyperandrogenism and insulin resistance likely precede any changes in reproductive outcomes (55). The reduction in hyperandrogenism and insulin resistance with or without the reflective reduction in weight loss has also been demonstrated to improve reproductive outcomes including ovulation and menstrual cycle regulation (32). Obesity is a risk factor for poor obstetric outcomes and maternal complications (e.g., spontaneous abortions and thromboembolisms), thus patients should be counseled on maintaining a normal weight throughout pregnancy (53).
Clomiphine citrate
Acting as a Selective Estrogen Receptor Modulator (SERM), clomiphene has been available for clinical use since 1967 and has been identified to act both as an agonist and an estrogenic antagonist (56–58). As an estrogenic antagonist at the hypothalamic level, it depletes nuclear receptors, therefore indirectly preventing accurate interpretation of circulating estrogen levels, without affecting existing levels. This causes a normal compensatory mechanism by the hypothalamus, altering GnRH secretion, stimulating gonadotropin release and driving ovarian follicular development (56, 59). While the optimal day to begin clomiphene for OI would be day 5 of a cycle, ovulation rates, conception rates and pregnancy outcomes are similar regardless of whether induction begins anytime between day 2 and day 5 (60). Clomiphene dose required for induction is positively correlated with both bodyweight and obesity (61). Despite higher doses required, both obese and lean women achieve similar pregnancy rates (62, 63). There is a reverse association between the likelihood of response to clomiphene and increasing body mass index (BMI) as well as the patient's age (34). While the association between clomiphene and bodyweight for OI has been previously described, to date, there is no laboratory parameter that can precisely predict the required dose for each individual patient (61, 63).
Using the traditional method for OI, treatment is initiated with daily 50 mg tablets for 5 days beginning on the 5th day of the cycle. Even though most women will respond to either 50 mg or 100 mg (64, 65) as per manufacturer recommendations (66), some clinicians have used doses as high as 250 mg (64, 65). As the success rate at doses over 150 mg per day is relatively low, it is often recommended to seek alternative induction methods before using these doses (64, 65). Cycle monitoring for ovulation using the traditional method can be achieved using serum progesterone levels, where levels over 3ng/mL between days 22 and 25 are considered sufficient evidence indicating successful ovulation (67, 68). Whenever ovulation does not occur, a progestin is prescribed to induce menses before the next cycle begins at a higher dose. Induction of ovulation will be successful in 70–80% of women (34, 35) and cumulative pregnancy rates of 70–75% are expected over 6–9 cycles of treatment (36, 37).
A novel “stair step” method uses ultrasound sonography for the identification of successful ovulation while avoiding the use of progestins to induce menses at the end of an unsuccessful cycle (38). Patients are placed on 5 days of clomiphene starting at 50 mg, where ultrasound sonography is used between days 11 and 14 to identify non responders who lacked a dominant follicle. Patients who did not successfully ovulate would be given 5 days of clomiphene at 100 mg, followed with an ultrasound 1 week later. In cases where no follicle was visualized on the 2nd ultrasound, the dose would be increased to 150 mg for 5 days and a 3rd ultrasound would be obtained 1 week thereafter (38). This protocol has demonstrated significantly higher ovulation rates of 64% at 100 mg when compared to the traditional 22% at the same dose (95% CI 45–81, p = 0.001) (38). An additional advantage with this method is the time to ovulation was significantly reduced by 32–53 days when compared to the traditional method described above (38).
While the side effects from clomiphene treatment are few and far between mainly due to the short treatment duration, vasomotor symptoms account for around 20%, followed by adnexal tenderness in 5%, nausea in 3%, headache in 1%, and very rarely blurred vision or scotomata (37). The main risk factor to consider would be multifetal pregnancy due to multi follicular development, which is increased to around 7–10% (37, 69–71). Clomiphene's anti estrogenic action on the marginally thinner endometrium is one of its drawbacks in these cycles, but the association between endometrial thickness (EMT) and pregnancy rates has not been elucidated in the literature (72).
Clomiphene and glucocorticoids
The use of prednisolone and dexamethasone has been described to increase the success rates of OI in clomiphene resistant women. These steroids have been shown to be effective in both continuous treatment, as well as in follicular phase regiments which are often given during days 5 to 14 (39, 73–77). It was initially demonstrated that this method is best suited for women with elevated DHEA-S levels, but further studies revealed that it is also effective in unselected patient groups (39, 73, 74, 78).
A large-scale randomized controlled trial (RCT) with 230 clomiphene resistant women, compared a combined treatment of clomiphene citrate 200 mg daily over days 5 to 9 and dexamethasone 2 mg daily over days 5 to 14 against clomiphene citrate 200 mg and placebo for the same amount of time. In the combined group, 88% of women had successfully ovulated whereas only 20% of women ovulated in the control group. The cumulative pregnancy rate in the combined treatment group was also significantly higher with 40.5 vs. 4.2% in the control group (p < 0.0001) (39).
The exact mechanism of which glucocorticoids act on clomiphene resistant women has not been elucidated, although suggested mechanisms include suppression of hyperandrogenism, synergistic actions with FSH's direct effects on oocyte development, and indirect effects of intrafollicular growth factors and cytokines (79). Coadministration of clomiphene and steroids has been argued to be justified for a few cycles and should not be used for extended periods of time to minimize the risks and side effects associated with long term steroid use.
Clomiphene and insulin sensitizing drugs
Several insulin sensitizing drugs have been investigated for their role in managing PCOS. Metformin acts by reducing hepatic gluconeogenesis, decreasing intestinal glucose absorption, increasing peripheral glucose uptake and utilization, and reducing fatty acid oxidation, resulting in the reduction of circulating insulin levels (80). While treatment with metformin might be considered as an adjuvant for weight management in PCOS patients (81–84) by facilitating weight loss via the suppression of appetite (85), the overall effect is modest and often inconsistent. Therefore, it should not be used as an alternative to regular physical exercise and caloric restriction (86, 87).
While metformin and other insulin sensitizing drugs such as myo-inositol has been shown to be effective in increasing ovulation rates in in some women with PCOS (33), a large triple-arm RCT (40) of 626 women with PCOS comparing fertility outcomes between metformin with placebo, clomiphene with placebo, and combined treatment of clomiphene with metformin, concluded that clomiphene is superior to metformin in achieving live birth in infertile women with PCOS (40). They also found no evidence in support for extended release metformin, either alone or in combination with clomiphene citrate, to improve live birth rates in women with PCOS (40). Of interest, adverse event rates in the clomiphene group were comparable to the ones described by other studies mentioned above (37, 69–71).
In a large recent meta-analysis (88), despite the fact that it was limited due to the small number of primary studies with low to medium quality of evidence, there was no observable difference in live birth rates between combined treatment of clomiphene and metformin vs. clomiphene alone, (OR 1.21, 95% CI: 0.92–1.59). However, the authors found gastrointestinal side effects more common in combination therapy (OR 3.97, 95% CI: 2.59–6.08) (88). While the combination therapy group had higher clinical pregnancy rates (OR 1.59, 95% CI: 1.27–1.99) and ovulation rates, (OR 1.57, 95% CI: 1.28–1.92) it suffered from an increased miscarriage rate per woman which was statistically significant (OR 1.59, 95% CI: 1.03–2.46) (88).
Myo-inositol alone or in combination with D-chiro-inositol has been shown to be beneficial in the management of both the endocrine and metabolic profile of women with PCOS, by improving insulin sensitivity and increasing Sex Hormone Binding Globulin (SHBG), leading to binding of Testosterone and therefore minimizing hyperandrogenic features (33). In this extent, it is able to significantly increase ovulation rates in PCOS women when compared with placebo or no treatment (OR 3.57, 95% CI: 1.72–7.45) (88), but its effect on pregnancy and live birth rate has not been elucidated in the literature, nor has it been adequately compared with or against clomiphene (89).
It is evident from the available data that stratification of results using BMI is an important factor which further stresses the importance of lifestyle modifications such as physical activity and diet. The available evidence also suggests that clomiphene use is preferable to metformin for OI in obese women with PCOS due to the improvement of clinical pregnancy and ovulation rates (40), while clomiphene use in combination with metformin is avoided due to a higher risk profile with increased miscarriage rate, against marginal improvements in clinical pregnancy and ovulation rates.
Aromatase inhibitors
Aromatase is the rate limiting step in the estrogen production in both the periphery and the brain (90, 91). By inhibiting the rate limiting step, thereby decreasing the levels and effect of peripheral estrogen, aromatase inhibitors cause a compensatory increase in the pituitary gonadotropin secretion leading to the development of ovarian follicles (92–94). While this process seems suspiciously similar to that of clomiphene, they vary in that clomiphene blocks central estrogen receptors without changing circulating estrogen levels, whereas aromatase inhibitors restrict estrogen synthesis by blocking its rate-limiting enzyme. Initially it was only indicated for postmenopausal women with breast cancer, but a proof-of-concept study successfully demonstrated letrozole as an effective method for OI in clomiphene resistant women, mainly targeting patients with PCOS and ovulatory infertility (95).
A large double blind, multi-center trial (41), recruited 750 women who were diagnosed with PCOS according to the modified Rotterdam criteria (96), compared the efficacy of clomiphene against letrozole. All patients were randomly assigned on a treatment arm initiated between day 3 and 5 of their cycle after spontaneous menses or withdrawal bleeding induced by medroxyprogesterone acetate, 5 mg per day, for 10 days. Patients were either started on letrozole 2.5 mg for 5 days, or on clomiphene 50 mg for 5 days. In each subsequent cycle, the patients were given up to a maximum of 7.5 mg of letrozole, increasing in steps of 2.5 mg at each cycle, or a maximum of 150 mg of clomiphene increasing in steps of 50 mg at each cycle. Non responders were identified using mid luteal progesterone <3 ng/mL. Patients underwent a maximum of 5 cycles and no additional ovulation trigger was used in either group. The letrozole group had a significantly higher cumulative pregnancy rate (27.3 vs. 21.5%) and significantly higher live birth (27.5 vs. 19.1%, RR 1.44; 95% CI: 1.10–1.87, p = 0.007) (41). In addition, the letrozole group had a significantly higher proportion of women achieve ovulation (88.5 vs. 76.6%, RR 1.16; 95% CI: 1.08–1.24, p < 0.001), and a significantly higher proportion of ovulations over total treatment (61.7 vs. 48.3%, RR 1.28; 95% CI: 1.19–1.37, p < 0.001) (41).
Despite producing a similar estrogenic effect through different mechanisms, one major difference between letrozole and clomiphene citrate is their effect on EMT (97). In a recent meta-analysis (97) targeting WHO group II anovulatory patients and comparing clomiphene with several other OI interventions, concluded that midcycle EMT (WMD −1.39; 95% CI: −2.27 to −0.51) (97) and pregnancy rates (RR 0.78; 95% CI: 0.63–0.95) (97) were both lower in clomiphene groups compared to letrozole groups, even though both groups had comparable ovulation rates (RR 0.97; 95% CI, 0.90–1.04) (97). Results from the same meta-analysis showed no statistical difference in EMT (WMD −0.12; 95% CI: −2.17 to 1.94) and no statistical difference in pregnancy rates (RR 2.44; 95% CI: 0.90–6.66) between the clomiphene group and the 1 mg anastrozole group (97), and no difference in EMT (WMD −1.34; 95% CI: −2.70 to 0.01) and a comparable pregnancy rate (RR 1.36; 95% CI: 0.86–2.15) (97) between clomiphene and tamoxifen groups. Although all drugs compared to clomiphene belong to the same drug class, the current results, while heterogenous, suggest superior pregnancy outcomes when comparing letrozole with clomiphene, whereas no discernible differences were found when using other aromatase inhibitors such as tamoxifen and anastrozole.
Exogenous Gonadotropins
Initially, from crude urinary extracts used as early as the 1960s (98, 99) containing equal amounts of FSH and LH (100), gonadotropin preparations have evolved over the years into highly purified recombinant preparations that are widely used today (101, 102). They have been shown as effective inducers in both hypogonadotropic hypogonadism (WHO Group I) (103, 104) and PCOS patients (WHO Group II) (105). While it could be argued that human menopausal gonadotropin (hMG/menotropins) should be superior for OI in clomiphene resistant PCOS women when compared with recombinant FSH preparations (rFSH), due to their erratic background FSH and LH secretion, they can be used interchangeably since a meta-analysis of trials comparing their effects found no difference in ovulation rate, pregnancy rate, multiple pregnancy rate and incidence of ovarian hyperstimulation syndrome (OHSS) (105). OI with Gonadotropins is seldom first line due to their less-than-ideal risk profile, which include high rates of multifetal pregnancy, significant risk for OHSS due to the required hCG trigger for ovum release and the higher spontaneous miscarriage rate of 20–25% when compared to background of 15% (43, 44). Despite the fact that there is a statistical linear correlation between patient weight and gonadotropins dose required for response, dosage should be assessed clinically, since no laboratory parameter can precisely predict response threshold for each patient (106).
The conventional protocol consists of daily intramuscular injections (IM) of 75IU of gonadotropins for 5 to 6 days between day 3 and 5 of the cycle, until ovarian response. In the event of non-responders, the dose increases in steps of 75IU. Response is identified by elevated serum Estradiol levels compared to background levels and verified by visualizing follicular development on ultrasound sonography. Targeted ovum size is between 16 and 18 mm, trigged with 5,000 to 10,000 IU of human Chorionic Gonadotropin (hCG) IM.
A “chronic low dose” protocol was developed specifically for PCOS patients who are known to be more sensitive to gonadotropin stimulation, often starting from a lower dose between 37.5 and 75IU. Each cycle would last for 7 to 14 days and subsequent cycles would often increase the dose by 37.5 to 75IU (45–49).
OI with gonadotropins have demonstrated cumulative pregnancy rates of 90% and cumulative live birth rates of 85% after 12 cycles (42). Both protocols have similar live birth rates although “chronic low dose” protocol is preferable due to its main advantages in the reduced rate of multifetal pregnancy and reduced rates of OHSS at the price of longer duration of treatment (45–49).
Laparoscopic ovariann drilling
The bilateral ovarian wedge resection by Stein and Leventhal in 1935 (96), was the first procedure which brought forth the associations between amenorrhea with the presence of bilateral polycystic ovaries and the underlying hormonal influences which were not the result of inflammatory changes (96). While the same paper describing the wedge resection successfully restored physiologic function of the ovaries with menstruation and allowed two of the seven patients to become pregnant, the procedure fell out of favor with the introduction of clomiphene citrate and the inherent risks associated with invasive operations, such as adnexal adhesions (96).
As newer laparoscopic techniques have been developed, the traditional ovarian wedge technique has transformed to ovarian “drilling,” which aims to use electrocautery or laser vaporization to cause focal destruction of the ovarian stroma in an effort to reduce intraovarian and systemic androgen concentrations (50, 107–111). Despite the fact that LOD may reduce the risk of postoperative adnexal adhesion formation (112–115), a meta-analysis found no statistically significant differences in live birth rates following LOD when compared to clomiphene citrate and metformin (OR 0.59; 95% CI: 0.32–1.09 p = 0.09), nor gonadotrophins (OR 0.87; 95% CI: 0.56–1.36; p = 0.55) (50). The same study found statistically significant lower live birth rates following LOD only when compared to letrozole (OR 0.55; 95% CI: 0.32–0.92; p = 0.02) (50).
Concluding remarks
Menstrual irregularities, chronic anovulation and infertility are some of the most common complaints of women with PCOS. One of the first and simplest steps in the management of PCOS would be lifestyle modifications such as exercise and caloric restriction, with the goal of lowering the patient's BMI and insulin resistance. This would then further decrease potential complications in the event of a successful pregnancy. Even in the absence of significant weight loss, regular exercise can result in spontaneous ovulation and an enhanced likelihood of a successful OI cycle. Clomiphene citrate is the best known and most widely used ovulation inducing agent, due to its good performance and broad use since its first introduction in 1967. Recent evidence in aromatase inhibitors and more specifically letrozole has shown that it can be more efficacious in OI when compared with clomiphene, with higher cumulative pregnancy rates and higher live birth rates. It could be argued that letrozole should instead be used as a first line treatment as it leads to higher midcycle EMT when compared to clomiphene, although the importance of such measurement remains controversial. Insulin sensitizing agents such as metformin are not suitable for use as OI agents but can be used as adjuvants in lifestyle modifications, aiming to reduce bodyweight, although their results are modest and often inconsistent. Myo-inositol has been shown to Increase ovulation rates in PCOS patients, but no studies have assessed its efficacy on pregnancy and live birth rates. Recombinant gonadotropins, while highly effective, are often reserved for doctors with specialists training and women who have failed all other OI protocols due to their risk involving OHSS, multifetal pregnancy and high spontaneous miscarriage rate. LOD has been reported to be effective in small studies in clomiphene resistant patients, however a meta-analysis of the available data suggests similar live birth rates when compared to clomiphene and metformin cycles and significantly lower live birth rates when compared to letrozole. Clinicians should be able to assess the likelihood of a successful pregnancy outcome in PCOS patients undergoing OI, taking into account age, bodyweight, different protocols used, and duration of infertility. If the foregoing treatments fail to produce a pregnancy, a referral to a specialist fertility clinic for in vitro fertilization would be an effective alternative. Future studies should aim to compare the pregnancy outcomes of different OI methodologies described above as well as stratify the effectiveness of LOD against existing medical management.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Laven JSE, Imani B, Eijkemans MJC, Fauser BCJM. New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstet Gynecol Surv. (2002) 57:755–67. doi: 10.1097/00006254-200211000-00022
2. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-Analysis. Hum Reprod. (2016) 31:2841–55. doi: 10.1093/humrep/dew218
3. Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: What's new? Adv Clin Exp Med. (2017) 26:359–67. doi: 10.17219/acem/59380
4. Venturoli S, Porcu E, Fabbri R, Magrini O, Gammi L, Paradisi R, et al. episodic pulsatile secretion of fsh, Lh, prolactin, oestradiol, oestrone, and Lh circadian variations in polycystic ovary syndrome. Clin Endocrinol (Oxf). (1988) 28:93–107. doi: 10.1111/j.1365-2265.1988.tb01208.x
5. Hayes FJ, Taylor AE, Martin KA, Hall JE. Use of a gonadotropin-releasing hormone antagonist as a physiologic probe in polycystic ovary syndrome: assessment of neuroendocrine and androgen dynamics 1. J Clin Endocrinol Metab. (1998) 83:2343–9. doi: 10.1210/jcem.83.7.4925
6. Imse V, Holzapfel G, Hinney B, Kuhn W, Wuttke W. Comparison of luteinizing hormone pulsatility in the serum of women suffering from polycystic ovarian disease using a bioassay and five different immunoassays. J Clin Endocrinol Metab. (1992) 74:1053–61. doi: 10.1210/JCEM.74.5.1569153
7. Kazer RR, Kessel B, Yen SSC. Circulating luteinizing hormone pulse frequency in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (1987) 65:233–6. doi: 10.1210/jcem-65-2-233
8. Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: Indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. (1988) 66:165–72. doi: 10.1210/jcem-66-1-165
9. Laven JSE, Imani B, Eijkemans MJC, De Jong FH, Fauser BCJM. Absent biologically relevant associations between serum inhibin B concentrations and characteristics of polycystic ovary syndrome in normogonadotrophic anovulatory infertility. Hum Reprod. (2001) 16:1359–64. doi: 10.1093/HUMREP/16.7.1359
10. Lockwood GM, Muttukrishna S, Groome NP, Matthews DR, Ledger WL. Mid-follicular phase pulses of inhibin B are absent in polycystic ovarian syndrome and are initiated by successful laparoscopic ovarian diathermy: a possible mechanism regulating emergence of the dominant follicle. J Clin Endocrinol Metab. (1998) 83:1730–5. doi: 10.1210/JCEM.83.5.4756
11. Balen AH. Hypersecretion of luteinizing hormone and the polycystic ovary syndrome. Hum Reprod. (1993) 8:123–8. doi: 10.1093/humrep/8.suppl_2.123
12. Rebar R, Judd HL, Yen SSC, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. (1976) 57:1320–9. doi: 10.1172/JCI108400
13. Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome1. J Clin Endocrinol Metab. (1997) 82:2248–56. doi: 10.1210/jcem.82.7.4105
14. Gonzalez F, Chang L, Horab T, Lobo RA. Evidence for heterogeneous etiologies of adrenal dysfunction in polycystic ovary syndrome. Fertil Steril. (1996) 66:354–61. doi: 10.1016/s0015-0282(16)58500-8
15. Gonzalez F, Hatala DA, Speroff L. Adrenal and ovarian steroid hormone responses to gonadotropin-releasing hormone agonist treatment in polycystic ovary syndrome. Am J Obstet Gynecol. (1991) 165:535–45. doi: 10.1016/0002-9378(91)90280-5
16. Dunaif A, Green G, Futterweit W, Dobrjansky A. Suppression of hyperandrogenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. (1990) 70:699–704. doi: 10.1210/jcem-70-3-699
17. Cohen JC, Hickman R. Insulin resistance and diminished glucose tolerance in powerlifters ingesting anabolic steroids. J Clin Endocrinol Metab. (1987) 64:960–3. doi: 10.1210/jcem-64-5-960
18. Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. (1988) 67:460–4. doi: 10.1210/jcem-67-3-460
19. Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, et al. direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. (1991) 72:83–9. doi: 10.1210/jcem-72-1-83
20. Hollmann M, Runnebaum B, Gerhard I. Effects of weight loss on the hormonal profile in obese, infertile women. Hum Reprod. (1996) 11:1884–91. doi: 10.1093/oxfordjournals.humrep.a019512
21. Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. (1995) 10:2705–12. doi: 10.1093/oxfordjournals.humrep.a135772
22. Jakubowicz DJ, Nestler JE. 17α-Hydroxyprogesterone responses to leuprolide and serum androgens in obese women with and without polycystic ovary syndrome after dietary weight loss 1. J Clin Endocrinol Metab. (1997) 82:556–60. doi: 10.1210/jcem.82.2.3753
23. Andersen P, Seljeflot I, Abdelnoor M, Arnesen H, Dale PO, Løvik A, et al. Increased insulin sensitivity and fibrinolytic capacity after dietary intervention in obese women with polycystic ovary syndrome. Metabolism. (1995) 44:611–6. doi: 10.1016/0026-0495(95)90118-3
24. Guzick DS, Wing R, Smith D, Berga SL, Winters SJ. Endocrine consequences of weight loss in obese, hyperandrogenic, anovulatory women. Fertil Steril. (1994) 61:598–604. doi: 10.1016/s0015-0282(16)56632-1
25. Kiddy DS, Hamilton-Fairley D, Seppälä M, Koistinen R, James VHT, Reed MJ, et al. Diet-Induced changes in sex hormone binding globulin and free testosterone in women with normal or polycystic ovaries: correlation with serum insulin and insulin-like growth factor-I. Clin Endocrinol (Oxf). (1989) 31:757–64. doi: 10.1111/j.1365-2265.1989.tb01297.x
26. Pasquali R, Antenucci D, Casimirri F, Venturoli S, Paradisi R, Fabbri R, et al. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab. (1989) 68:173–9. doi: 10.1210/jcem-68-1-173
27. Araújo-Vilar D, Osifo E, Kirk M, García-Estévez DA, Cabezas-Cerrato J, Hockaday TDR. Influence of moderate physical exercise on insulin-mediated and non- insulin-mediated glucose uptake in healthy subjects. Metabolism. (1997) 46:203–9. doi: 10.1016/S0026-0495(97)90303-6
28. Thyfault JP, Wright DC. Weighing the effects of exercise and intrinsic aerobic capacity: Are there beneficial effects independent of changes in weight? Appl Physiol Nutr Metab. (2016) 41:911–6. doi: 10.1139/apnm-2016-0122
29. Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. (1998) 13:1502–5. doi: 10.1093/humrep/13.6.1502
30. Bates GW, Whitworth NS. Effect of body weight reduction on plasma androgens in obese, infertile women. Fertil Steril. (1982) 38:406–9. doi: 10.1016/s0015-0282(16)46571-4
31. Tolino A, Gambardella V, Caccavale C, D'Ettore A, Giannotti F, D'Antò V, et al. Evaluation of ovarian functionality after a dietary treatment in obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. (2005) 119:87–93. doi: 10.1016/j.ejogrb.2004.06.043
32. Harrison CL, Lombard CB, Moran LJ, Teede HJ. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update. (2011) 17:171–83. doi: 10.1093/humupd/dmq045
33. Facchinetti F, Bizzarri M, Benvenga S, D'Anna R, Lanzone A, Soulage C, et al. Results from the international consensus conference on Myo-inositol and d-chiro-inositol in obstetrics and gynecology: the link between metabolic syndrome and PCOS. Eur J Obstet Gynecol Reprod Biol. (2015) 195:72–6. doi: 10.1016/j.ejogrb.2015.09.024
34. Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab. (1998) 83:2361–5. doi: 10.1210/jcem.83.7.4919
35. Homburg R. Clomiphene citrate - End of an era? a mini-review. Hum Reprod. (2005) 20:2043–51. doi: 10.1093/humrep/dei042
36. Imani B, Eijkemans MJC, Te Velde ER, Fauser BCJM. A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil Steril. (2002) 77:91–7. doi: 10.1016/S0015-0282(01)02929-6
37. Imani B, Eijkemans MJC. te Velde ER, Habbema JDF, Fauser BCJM. Predictors of Chances to Conceive in Ovulatory Patients during Clomiphene Citrate Induction of Ovulation in Normogonadotropic Oligoamenorrheic Infertility1. J Clin Endocrinol Metab. (1999) 84:1617–22. doi: 10.1210/jcem.84.5.5705
38. Hurst BS, Hickman JM, Matthews ML, Usadi RS, Marshburn PB. Novel clomiphene “stair-step” protocol reduces time to ovulation in women with polycystic ovarian syndrome. Am J Obstet Gynecol. (2009) 200:510.e1–510.e4. doi: 10.1016/j.ajog.2008.10.031
39. Parsanezhad ME, Alborzi S, Motazedian S, Omrani G. Use of dexamethasone and clomiphene citrate in the treatment of clomiphene citrate-resistant patients with polycystic ovary syndrome and normal dehydroepiandrosterone sulfate levels: A prospective, double-blind, placebo-controlled trial. Fertil Steril. (2002) 78:1001–4. doi: 10.1016/S0015-0282(02)04206-1
40. Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, Metformin, or Both for Infertility in the Polycystic Ovary Syndrome. N Engl J Med. (2007) 356:551–66. doi: 10.1056/nejmoa063971
41. Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. (2014) 371:119–29. doi: 10.1056/nejmoa1313517
42. Balen AH, Braat DD, West C, Patel A, Jacobs HS. Cumulative conception and live birth rates after the treatment of anovulatory infertility: safety and efficacy of ovulation induction in 200 patients. Hum Reprod. (1994) 9:1563–70.
43. Schwartz M, Jewelewicz R, Dyrenfurth I, Tropper P, Vande Wiele RL. The use of human menopausal and chorionic gonadotropins for induction of ovulation. sixteen years' experience at the Sloane Hospital for Women. Am J Obstet Gynecol. (1980) 138:801–7. doi: 10.1016/S0002-9378(16)32740-5
44. Fluker MR, Urman B, MacKinnon M, Barrow SR, Pride SM, Yuen BH. Exogenous gonadotropin therapy in world health organization groups I and II ovulatory disorders. Obstet Gynecol. (1994) 83:189–96.
45. Calaf Alsina J, Ruiz Balda JA, Romeu Sarrio A, Caballero Fernandez V, Cano Trigo I, Gomez Parga JL, et al. Ovulation induction with a starting dose of 50 IU of recombinant follicle stimulating hormone in WHO group II anovulatory women: the IO-50 study, a prospective, observational, multicentre, open trial. BJOG An Int J Obstet Gynaecol. (2003) 110:1072–7. doi: 10.1111/j.1471-0528.2003.02290.x
46. Homburg R, Howles CM. Low-dose FSH therapy for anovulatory infertility associated with polycystic ovary syndrome: rationale, results, reflections and refinements. Hum Reprod Update. (1999) 5:493–9. doi: 10.1093/HUMUPD/5.5.493
47. Hedon B, Hugues JN, Emperaire JC, Chabaud JJ, Barbereau D, Boujenah A, et al. comparative prospective study of a chronic low dose versus a conventional ovulation stimulation regimen using recombinant human follicle stimulating hormone in anovulatory infertile women. Hum Reprod. (1998) 13:2688–92. doi: 10.1093/HUMREP/13.10.2688
48. White DM, Polson DW, Kiddy D, Sagle P, Watson H, Gilling-Smith C, et al. Induction of ovulation with low-dose gonadotropins in polycystic ovary syndrome: an analysis of 109 pregnancies in 225 women. J Clin Endocrinol Metab. (1996) 81:3821–4. doi: 10.1210/JCEM.81.11.8923819
49. Homburg R, Levy T, Ben-Rafael Z. A comparative prospective study of conventional regimen with chronic low-dose administration of follicle-stimulating hormone for anovulation associated with polycystic ovary syndrome. Fertil Steril. (1995) 63:729–33. doi: 10.1016/S0015-0282(16)57473-1
50. Bordewijk EM, Ng KYB, Rakic L, Mol BWJ, Brown J, Crawford TJ, van Wely M. Laparoscopic ovarian drilling for ovulation induction in women with anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. (2020) 2:CD001122. doi: 10.1002/14651858.CD001122.pub5
51. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. (2004) 89:2745–9. doi: 10.1210/jc.2003-032046
52. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women 1. J Clin Endocrinol Metab. (1999) 84:165–9. doi: 10.1210/jcem.84.1.5393
53. Al-Azemi 3, 4 M 1, Omu FE 2, Omu 3 AE 1, Al-Azemi M, Omu FE 2, Omu AE. The effect of obesity on the outcome of infertility management in women with polycystic ovary syndrome. Arch Gynecol Obstet. (2004) 270:205–10. doi: 10.1007/s00404-003-0537-2
54. Taylor HS, Pal L, Sell E. Speroff's Clinical Gynecologic Endocrinology and Infertility. 9th ed. Wolters Kluwer (2019).
55. Jarrett BY, Lujan ME. Impact of hypocaloric dietary intervention on ovulation in obese women with PCOS. Reproduction. (2017) 153:R15–27. doi: 10.1530/REP-16-0385
56. Clark JH, Markaverich BM. The agonistic-antagonistic properties of clomiphene: a review. Pharmacol Ther. (1981) 15:467–519. doi: 10.1016/0163-7258(81)90055-3
57. Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41: preliminary report. JAMA J Am Med Assoc. (1961) 178:101–4. doi: 10.1001/jama.1961.03040410001001
58. Dickey RP, Holtkamp DE. Development, pharmacology and clinical experience with clomiphene citrate. Hum Reprod Update. (1996) 2:483–506. doi: 10.1093/humupd/2.6.483
59. Hsueh AJW, Erickson GF, Yen SSC. Sensitisation of pituitary cells to luteinising hormone releasing hormone by clomiphene citrate in vitro. Nature. (1978) 273:57–9. doi: 10.1038/273057a0
60. Wu CH, Winkel CA. The effect of therapy initiation day on clomiphene citrate therapy. Fertil Steril. (1989) 52:564–8. doi: 10.1016/S0015-0282(16)60964-0
61. Lobo RA, Gysler M, March CM, Goebelsmann U, Mishell DR. Clinical and laboratory predictors of clomiphene response. Fertil Steril. (1982) 37:168–74. doi: 10.1016/s0015-0282(16)46034-6
62. Hammond MG, Halme JK, Talbert LM. Factors affecting the pregnancy rate in clomiphene citrate induction of ovulation. Obstet Gynecol. (1983) 62:196–202.
63. Homburg R, Orvieto R, Bar-Hava I, Ben-Rafael Z. Serum levels of insulin-like growth factor-1, IGF binding protein-1 and insulin and the response to human menopausal gonadotrophins in women with polycystic ovary syndrome. Hum Reprod. (1996)11:716–19. doi: 10.1093/oxfordjournals.humrep.a019239
64. Gysler M, March CM, Mishell DR, Bailey EJ. A decade's experience with an individualized clomiphene treatment regimen including its effect on the postcoital test. Fertil Steril. (1982) 37:161–7. doi: 10.1016/S0015-0282(16)46033-4
65. Gorlitsky GA, Kase NG, Speroff L. Ovulation and pregnancy rates with clomiphene citrate. Obstet Gynecol. (1978) 51:265–9. doi: 10.1097/00006250-197803000-00002
66. Center for Drug Evaluation Research. Approval Package for: Clomid. Application Number NDA 016131/S-126. (2012). p. 1–49. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/016131Orig1s026.pdf
67. Wathen NC, Perry L, Lilford RJ, Chard T. Interpretation of single progesterone measurement in diagnosis of anovulation and defective luteal phase: Observations on analysis of the normal range. Br Med J. (1984) 288:7–9. doi: 10.1136/bmj.288.6410.7
68. Abraham GE, Maroulis GB, Marshall JR. Evaluation of ovulation and corpus luteum function using measurements of plasma progesterone. Obstet Gynecol. (1974) 44:522–25.
69. Schenker JG, Yarkoni S, Granat M. Multiple pregnancies following induction of ovulation. Fertil Steril. (1981) 35:105–23. doi: 10.1016/s0015-0282(16)45308-2
70. Ahlgren M, Källen B, Rannevik G. Outcome of Pregnancy After Clomiphene Therapy. Acta Obstet Gynecol Scand. (1976) 55:371–5. doi: 10.3109/00016347609158516
71. Correy JF, Marsden DE, Schokman FCM. The outcome of pregnancy resulting from clomiphene-induced ovulation. Aust New Zeal J Obstet Gynaecol. (1982) 22:18–21. doi: 10.1111/j.1479-828X.1982.tb01391.x
72. Weiss NS, Van Vliet MN, Limpens J, Hompes PGA, Lambalk CB, Mochtar MH, et al. Endometrial thickness in women undergoing IUI with ovarian stimulation. how thick is too thin? A systematic review and meta-analysis. Hum Reprod. (2017) 32:1009–18. doi: 10.1093/humrep/dex035
73. Vital Reyes VS, Tellez Velasco S, Hinojosa Cruz JC, Reyes Fuentes A. [Clomiphene acetate and prednisone: alternative approach for the management of patients with chronic anovulation and clomiphene treatment failure]. Ginecol Obstet Mex. (2000) 68:266–70.
74. Trott EA, Plouffe LJ, Hansen K, Hines R, Brann DW, Mahesh VB. Ovulation induction in clomiphene-resistant anovulatory women with normal dehydroepiandrosterone sulfate levels: Beneficial effects of the addition of dexamethasone during the follicular phase. Fertil Steril. (1996) 66:484–6. doi: 10.1016/s0015-0282(16)58525-2
75. Daly DC, Walters CA, Soto-Albors CE. A randomized study of dexamethasone in ovulation induction with clomiphene citrate. Fertil Steril. (1984) 41:844–8. doi: 10.1016/S0015-0282(16)47896-9
76. Lobo RA, Paul W, March CM, Granger L, Kletzky OA. Clomiphene and dexamethasone in women unresponsive to clomiphene alone. Obstet Gynecol. (1982) 60:497–501.
77. Isaacs JD, Lincoln SR, Cowan BD. Extended clomiphene citrate (CC) and prednisone for the treatment of chronic anovulation resistant to CC alone. Fertil Steril. (1997) 67:641–3. doi: 10.1016/S0015-0282(97)81359-3
78. Elnashar A, Abdelmageed E, Fayed M, Sharaf M. Clomiphene citrate and dexamethazone in treatment of clomiphene citrate-resistant polycystic ovary syndrome: a prospective placebo-controlled study. Hum Reprod. (2006) 21:1805–8. doi: 10.1093/humrep/del053
79. Keay SD, Jenkins JM, Parsanezhad ME, Alborzi S, Motazedian S, Omrani G. Adjunctive use of dexamethasome in Clomid resistant patients [5] (multiple letters). Fertil Steril. (2003) 80:230–1. doi: 10.1016/S0015-0282(03)00587-9
80. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. (2002) 137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009
81. Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. (2001) 107:e55. doi: 10.1542/peds.107.4.e55
82. Kay JP, Alemzadeh R, Langley G, D'Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. (2001) 50:1457–61. doi: 10.1053/meta.2001.28078
83. Gokcel A, Gumurdulu Y, Karakose H, Melek Ertorer E, Tanaci N, Bascil Tutuncu N, et al. Evaluation of the safety and efficacy of sibutramine, orlistat and metformin in the treatment of obesity. Diabetes, Obes Metab. (2002) 4:49–55. doi: 10.1046/j.1463-1326.2002.00181.x
84. Pasquali R, Gambineri A, Biscotti D, Vicennati V, Gagliardi L, Colitta D, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab. (2000) 85:2767–74. doi: 10.1210/jcem.85.8.6738
85. Dokras A. Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders. Fertil Steril. (2008) 89:1848. doi: 10.1016/j.fertnstert.2007.12.018
86. Lord JM, Flight IHK, Norman RJ. Metformin in polycystic ovary syndrome: Systematic review and meta-analysis. Br Med J. (2003) 327:951–5. doi: 10.1136/bmj.327.7421.951
87. Metformin Therapy for the Management of Infertility in Women with Polycystic Ovary Syndrome: Scientific Impact Paper No. 13. BJOG An Int J Obstet Gynaecol. (2017) 124:e306–313. doi: 10.1111/1471-0528.14764
88. Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. (2017) 11:CD003053. doi: 10.1002/14651858.CD003053.pub6
89. Pundir J, Psaroudakis D, Savnur P, Bhide P, Sabatini L, Teede H, et al. Inositol treatment of anovulation in women with polycystic ovary syndrome: a meta-analysis of randomised trials. BJOG An Int J Obstet Gynaecol. (2018) 125:299–308. doi: 10.1111/1471-0528.14754
90. Buzdar A, Howell A. Advances in aromatase inhibition:clinical efficacy and tolerability in the treatment of breast cancer. Clin Cancer Res. (2001) 7:2620–2635.
91. Winer EP, Hudis C, Burstein HJ, Chlebowski RT, Ingle JN, Edge SB, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: Status report 2002. J Clin Oncol. (2002) 20:3317–27. doi: 10.1200/JCO.2002.06.020
92. Kamat A, Hinshelwood MM, Murry BA, Mendelson CR. Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol Metab. (2002) 13:122–8. doi: 10.1016/S1043-2760(02)00567-2
93. Naftolin F, Maclusky NJ, Leranth CZ, Sakamoto HS, Garcia-Segura LM. The cellular effects of estrogens on neuroendocrine tissues. J Steroid Biochem. (1988) 30:195–207. doi: 10.1016/0022-4731(88)90093-3
95. Mitwally MFM, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. (2001) 75:305–9. doi: 10.1016/S0015-0282(00)01705-2
96. Fauser BCJM. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
97. Gadalla MA, Huang S, Wang R, Norman RJ, Abdullah SA, El Saman AM, et al. Effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2018) 51:64–76. doi: 10.1002/uog.18933
98. Rosemberg E, Coleman J, Demany M, Garcia CR. Clinical effect of human urinary postmenopausal gonado-tropin. J Clin Endocrinol Metab. (1963) 23:181–90. doi: 10.1210/jcem-23-2-181
99. Lunenfeld B. Gonadotropin stimulation: past, present and future. Reprod Med Biol. (2012) 11:11. doi: 10.1007/S12522-011-0097-2
100. The The Practice Committee of the American Society for Reproductive Medicine, Birmingham A. Gonadotropin preparations: past, present, and future perspectives. Fertil Steril. (2008) 90:31. doi: 10.1016/j.fertnstert.2008.08.031
101. Casper RF. Are recombinant gonadotrophins safer, purer and more effective than urinary gonadotrophins? Reprod Biomed Online. (2005) 11:539–40. doi: 10.1016/S1472-6483(10)61155-8
102. Lunenfeld B. Historical perspectives in gonadotrophin therapy. Hum Reprod Update. (2004) 10:453–67. doi: 10.1093/humupd/dmh044
103. Group TERHLS. Recombinant human Luteinizing Hormone (LH) to support recombinant human Follicle-Stimulating Hormone (FSH)-Induced Follicular Development in LH- and FSH-Deficient anovulatory women: a dose-finding study1. J Clin Endocrinol Metab. (1998) 83:1507–14. doi: 10.1210/jcem.83.5.4770
104. Schoot DC, Harlin J, Shoham Z, Mannaerts BMJ, Lahlou N, Bouchard P, et al. Recombinant human follicle-stimulating hormone and ovarian response in gonadotrophin-deficient women. Hum Reprod. (1994) 9:1237–42. doi: 10.1093/OXFORDJOURNALS.HUMREP.A138685
105. Weiss NS, Nahuis M, Bayram N, Mol BWJ, Van der Veen F, van Wely M. Gonadotrophins for ovulation induction in women with polycystic ovarian syndrome. Cochrane Database Syst Rev. (2015) 1:CD010290. doi: 10.1002/14651858.CD010290.pub2
106. Chong AP, Rafael RW, Forte CC. Influence of weight in the induction of ovulation with human menopausal gonadotropin and human chorionic gonadotropin. Fertil Steril. (1986) 46:599–603. doi: 10.1016/s0015-0282(16)49634-2
107. Gjonnaess H. Polycystic ovarian syndrome treated by ovarian electrocautery through the laparoscope. Fertil Steril. (1984) 41:20–5. doi: 10.1016/S0015-0282(16)47534-5
108. El-Sayed MLM, Ahmed MA, Mansour MAA, Mansour SAA. Unilateral versus bilateral laparoscopic ovarian drilling using thermal dose adjusted according to ovarian volume in CC-resistant PCOS, a randomized study. J Obstet Gynecol India. (2017) 67:356–62. doi: 10.1007/s13224-017-1010-7
109. Sorouri ZZ, Sharami SH, Tahersima Z, Salamat F. Comparison between unilateral and bilateral ovarian drilling in clomiphene citrate resistance polycystic ovary syndrome patients: A randomized clinical trial of efficacy. Int J Fertil Steril. (2015) 9:9–16. doi: 10.22074/ijfs.2015.4202
110. Daniell JF, Miller W. Polycystic ovaries treated by laparoscopic laser vaporization. Fertil Steril. (1989) 51:232–6. doi: 10.1016/S0015-0282(16)60482-X
111. Donesky BW, Adashi EY. Surgically induced ovulation in the polycystic ovary syndrome: Wedge resection revisited in the age of laparoscopy. Fertil Steril. (1995) 63:439–63. doi: 10.1016/s0015-0282(16)57408-1
112. Greenblatt EM, Casper RF. Adhesion formation after laparoscopic ovarian cautery for polycystic ovarian syndrome: Lack of correlation with pregnancy rate. Fertil Steril. (1993) 60:766–70. doi: 10.1016/s0015-0282(16)56273-6
113. Gurgan T, Kisnisci H, Yarali H, Develioglu O, Zeyneloglu H, Aksu T. Evaluation of adhesion formation after laparoscopic treatment of polycystic ovarian disease. Fertil Steril. (1991) 56:1176–8. doi: 10.1016/S0015-0282(16)54737-2
114. Naether OGJ, Fischer R, Weise HC, Geiger-Kotzler L, Delfs T, Rudolf K. Laparoscopic electrocoagulation of the ovarian surface in infertile patients with polycystic ovarian disease. Fertil Steril. (1993) 60:88–94. doi: 10.1016/s0015-0282(16)56042-7
Keywords: polycystic ovary syndrome, Ovulation Induction, PCOS (polycystic ovarian syndrome), polycystic ovarian disease, infertility
Citation: Vyrides AA, El Mahdi E and Giannakou K (2022) Ovulation induction techniques in women with polycystic ovary syndrome. Front. Med. 9:982230. doi: 10.3389/fmed.2022.982230
Received: 30 June 2022; Accepted: 25 July 2022;
Published: 12 August 2022.
Edited by:
Andrea Tinelli, Xi'an Jiaotong University, ChinaReviewed by:
Harpal Singh Randeva, University Hospitals Coventry and Warwickshire NHS Trust, United KingdomCopyright © 2022 Vyrides, El Mahdi and Giannakou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantinos Giannakou, Sy5HaWFubmFrb3VAZXVjLmFjLmN5
 Andreas A. Vyrides
Andreas A. Vyrides Essam El Mahdi2
Essam El Mahdi2 Konstantinos Giannakou
Konstantinos Giannakou