
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 03 October 2022
Sec. Rheumatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.981659
This article is part of the Research Topic Ultrasound in Rheumatology - a Polyhedric Imaging Tool View all 13 articles
Ultrasound (US) is being increasingly used to diagnose Giant Cell Arteritis (GCA). The traditional diagnostic Gold Standard has been temporal artery biopsy (TAB), but this is expensive, invasive, has a false-negative rate as high as 60% and has little impact on clinical decision-making. A non-compressible halo with a thickened intima-media complex (IMC) is the sonographic hallmark of GCA. The superficial temporal arteries (STA) and axillary arteries (AA) are the most consistently inflamed arteries sonographically and imaging protocols for evaluating suspected GCA should include at least these two arterial territories. Studies evaluating temporal artery ultrasound (TAUS) have varied considerably in size and methodology with results showing wide discrepancies in sensitivity (9–100%), specificity (66–100%), positive predictive value (36–100%) and negative predictive value (33–100%). Bilateral halos increase sensitivity as does the incorporation of pre-test probability, while prior corticosteroid use decreases sensitivity. Quantifying sonographic vasculitis using Halo Counts and Halo Scores can predict disease extent/severity, risk of specific complications and likelihood of treatment response. Regression of the Halo sign has been observed from as little as 2 days to as late as 7 months after initiation of immunosuppressive treatment and occurs at different rates in STAs than AAs. US is more sensitive than TAB and has comparable sensitivity to MRI and PET/CT. It is time-efficient, cost-effective and allows for the implementation of fast-track GCA clinics which substantially mitigate the risk of irreversible blindness. Algorithms incorporating combinations of imaging modalities can achieve a 100% sensitivity and specificity for a diagnosis of GCA. US should be a standard first line investigation in routine clinical care of patients with suspected GCA with TAB reserved only for those having had a normal US in the context of a high pre-test probability.
Giant Cell Arteritis (GCA) is a vasculitis of large- and medium-sized vessels. It is the commonest idiopathic systemic vasculitis and incidence increases with age, predominantly affecting those aged > 70 years (1). Typical symptoms include headache, visual disturbance, jaw claudication and polymyalgia rheumatica (PMR). Prompt diagnosis and initiation of corticosteroids is key to prevent the most severe complications of stroke and/or irreversible blindness (2). The traditional gold standard for diagnosis involves performing a temporal artery biopsy (TAB) (3).
TAB has many shortcomings when assessing suspected cases of GCA. Not only is it costly and invasive, but it has repeatedly been shown to have a false negative rate as high as 60%, most likely due to inadequate sampling, skip lesions and pre-operative steroid use (4). Additionally, its impact on clinical decision-making is questionable. In recent years, the use of temporal artery ultrasound (TAUS) in assessing suspected GCA has increased considerably.
The definitions of what constitutes vasculitis on US are still evolving, as is our understanding of its true place not only in the diagnosis, but also in the long-term monitoring of GCA. Advances in technology have undoubtedly contributed hugely to this growing body of knowledge and we suggest where future innovations might lead to. We also compare TAUS to other imaging modalities in GCA and discuss how TAUS is currently utilized in routine clinical care with reference to current international guidelines. Lastly we describe our current understanding of the reliability and applicability of TAUS and suggest where US may ultimately be incorporated into a diagnostic algorithm for GCA.
TAB still has high value as a diagnostic test due to specificity of 100% for a diagnosis of GCA. However, given the high false-negative rate it is clear that many, if not the majority, of GCA patients are diagnosed based on clinical criteria despite the presence of a negative TAB result. A number of studies have examined the impact of TAB results on clinical decisions within this context. In one retrospective cohort of 290 patients in whom GCA was suspected with a subsequent negative diagnostic test (147 of whom had a negative bilateral TAUS and 143 of whom had a negative unilateral TAB), there was no between-group difference in the number of patients who had steroids discontinued, despite further stratification accounting for pre-test probability of having GCA. Additionally, there was no between-group differences noted in adverse outcomes (including blindness) or number of alternative diagnoses considered. These findings suggest that TAUS serves the same purpose as TAB but without the associated procedural risks while other large retrospective cohorts have shown that 41–87% of those with negative biopsies have corticosteroid therapy continued anyway (5–7). Thus, while most TABs that are performed are negative, in most cases negative TABs have no impact on clinical decision-making. Importantly, data suggests that incorporating TAUS into the workup for suspected GCA increases the positive yield of TABs from 8.5 to 24% with an associated 38% reduction in the number of TABs being performed overall and with a substantial cost-saving (8, 9).
In 1995 Schmidt first described, what still remains to this day, the cardinal sonographic hallmark of vasculitis- “The Halo Sign” (Figure 1) (10). It describes a sonographically hypoechoic ring of inflamed, oedematous vessel wall, surrounding the lumen of an artery. In a prospective study of 30 patients with clinically diagnosed GCA, confirmed by two independent rheumatologists, 22/30 had a Halo Sign identified in their superficial temporal arteries (STA), bilaterally in 17, and the rate of agreement between the two sonographers was 100%. No Halo Sign was identified in the 82 patients who had GCA excluded on clinical grounds (11).
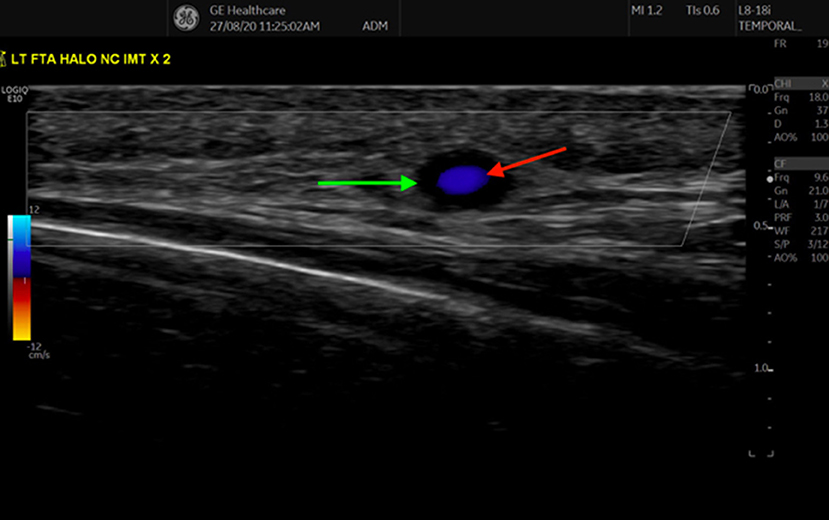
Figure 1. Transverse view of the frontal branch of the Superficial Temporal Artery, demonstrating a halo sign, as indicated by the anechoic region (green arrow) surrounding the inner Doppler (red arrow) signal.
In 2012, a new sonographic hallmark of vasculitis was reported: “The Compression Sign” (Figure 2). In a prospective study of 80 suspected cases of GCA (43 ultimately diagnosed as GCA based an ACR criteria), all participants had bilateral TAUS performed, examining for the presence of Halo Sign and/or Compression Sign. The Compression Sign was defined as persistent visibility of the STA despite transducer-imposed arterial compression (i.e., persistent contrasting echogenicity between vessel wall and surrounding tissue). Three physician-sonographers were involved in scanning and were blinded to the clinical details of the case. Interestingly, the Halo Sign and Compression Sign were both observed in 34/43 GCA patients and both signs were absent in all patients in the non-GCA group, showing a sensitivity and specificity of 79 and 100% respectively, for both signs in diagnosing GCA (12, 13). In 2018, the OMERACT LVV US working group defined the Halo and Compression Signs as the most significant sonographic abnormalities of GCA with inter-rater agreements of 91–99% and mean kappa values of 0.83–0.98 for both inter-rater and intra-rater reliabilities. The group defined the Halo Sign as “homogenous, hypoechoic wall thickening, well delineated toward the luminal side, visible both in longitudinal and transverse planes, most commonly concentric in transverse scans.” The Compression Sign was defined as being assessed “by applying pressure via the transducer until the lumen of the temporal artery occludes and no arterial pulsation remains visible” (14).
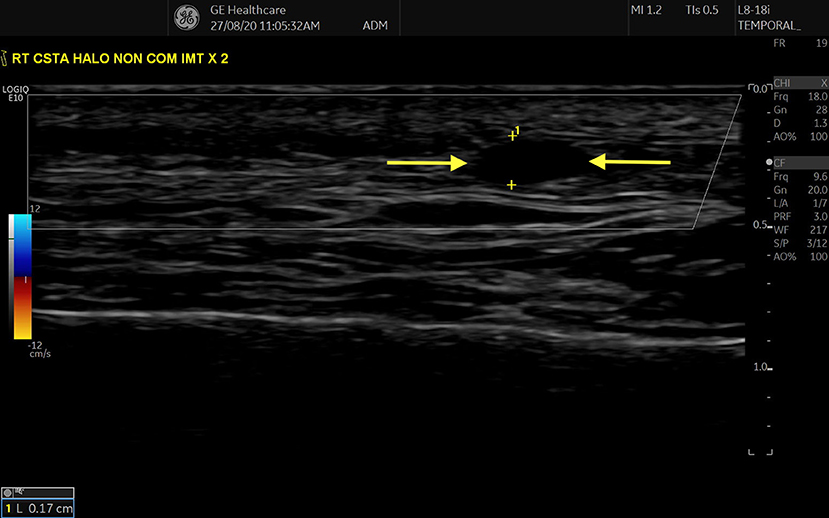
Figure 2. “Compression” sign in STA, transverse view. Hypoechoic/ anechoic region between two parallel hyperechoic lines (adventitia) represents an oedematous Intima-Media Complex (region between two yellow arrows).
More recently, the “Slope Sign” of axillary artery (AA) vasculitis has been described. This sign describes a long, thickened segment of inflamed arterial wall that slides down to a normal intima-media structure (double line) (Figure 3). In 214 patients referred to a fast-track GCA clinic, 81 were diagnosed with GCA, 23 of whom had axillary vasculitis. The slope sign was observed in all patients with AA vasculitis (15, 16).
In 2017, normal cut-off values for intima-media thickness (IMT) of arteries involved in GCA were first published. IMT measurements of the STAs, facial arteries and AAs in 40 new GCA cases and 40 controls were obtained, with the gold standard being a clinical diagnosis of GCA. The cut-off values with sensitivities and specificities of the various arterial segments for a diagnosis of GCA are outlined in Table 1 (17). A recent study looked at 101 patients aged > 50 years, without a diagnosis of GCA or PMR, but with varying degrees of perceived cardiovascular (CV) risk. US of STAs and AAs were performed on all and notably, in those deemed to have high/very high CV risk, mean IMT was greater than in those with moderate/low risk both in STAs and AAs. IMT was greater than standard normal cut-off values in at least one artery in 10.1% of patients, 80% of whom had very high/ high CV risk (18).
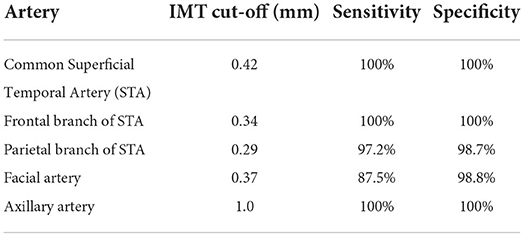
Table 1. Cut-off values for distinguishing vasculitic artery from normal artery in suspected cases of GCA with sensitivities and specificities for a clinical diagnosis of GCA (17).
Thus, while early sonographic definitions of vasculitis included the presence of vessel stenosis and occlusions, the current standard is to diagnose vasculitis based on the presence of a Halo Sign, a non-compressible artery (Compression Sign) and a thickened intima-media complex (IMC). The precision of these definitions remains a constant process of refinement and further research is needed in this field to further specify normal/abnormal IMT values. Additionally, false-positive ultrasounds can occur, as demonstrated in a cohort of 305 patients in whom TAUS confirmed the presence of a Halo Sign, but 14 of whom ultimately had a variety of diagnoses other than GCA (19).
Up to 2002, the frequency and location of peripheral arterial sonographic changes in GCA was unknown. In 10/33 GCA patients in one study, a Halo Sign could be demonstrated in peripheral arteries but importantly, sonographic vasculitis was consistently present in STAs and/or AAs if also present elsewhere (21). Additionally, it has been demonstrated that performing AAUS increases the diagnostic yield for large-vessel GCA with a detection-rate of 98 vs. 62% for TAUS alone, while asymptomatic abdominal aortic aneurysms may be detected in 33% of biopsy-proven GCA cases on US despite no clinical evidence of same (22–25). Other studies have consistently shown varying degrees of involvement of occipital, vertebral, carotid and femero-popliteal arteries (26–30). However, STA and AA are the most consistently inflamed arteries sonographically and imaging protocols for evaluating suspected GCA should include at least these two arterial territories.
Over the past 25 years, many studies have examined the diagnostic performance of TAUS against that of biopsy and clinical criteria (Table 2). In 2005, Karassa et al. undertook the first meta-analysis including all studies of >4 patients, which investigated the sensitivity and specificity of TAUS in GCA, using TAB or ACR classification criteria as the gold standard. Twenty-three studies of 2,036 patients demonstrated a weighted sensitivity and specificity of the Halo sign of 69 and 82%, respectively, compared with biopsy, and 55 and 94%, respectively, compared with ACR criteria. The studies included were mostly small with heterogenous methodology but they did show that in the presence of a low pre-test probability of GCA, a negative ultrasound can help out-rule the disease (31). A second meta-analysis was published in 2010 specifically examining the Halo sign and included eight studies of 575 patients (204 with GCA). Unilateral Halo sign achieved an overall sensitivity and specificity of 68 and 91%, respectively for GCA. The odds of having GCA in patients with a Halo Sign vs. in those without (pooled diagnostic odds ratio) was 34 (32). A systematic review by Ball looked at trials comparing TAUS and TAB and included 17 homogenous studies of 998 patients. When the sonographic halo was compared with TAB, the sensitivity was 75% and the specificity was 83%, leading the authors to conclude that TAUS was relatively accurate for diagnosing GCA and had promise as a first-line investigation, perhaps with TAB being reserved only for those with a normal US (33).
The seminal TABUL study was published in 2016 and showed that US was more sensitive and cost effective than TAB in investigating suspected GCA and importantly, the sensitivity of TAB was only 39% vs. previously published figures of >80%. In this prospective, multicentre study of 381 patients (257 with a reference standard clinical diagnosis of GCA, 124 without), all patients underwent US followed by TAB within 7 days of commencing treatment for GCA. 101 patients and 162 patients had positive TAB and US, respectively, with concordant results in 70% (kappa value 0.35). The sensitivities and specificities of biopsy and ultrasound were 39 and 100%, and 54 and 81%, respectively. Positive biopsy rate fell after 3 days of high-dose glucocorticoids whereas US abnormalities regressed within 4 days [a finding which concurred with those of a number of other studies highlighting the prompt regression of Halo Sign in those on corticosteroids (11, 34, 35)]. Of note, the authors demonstrated that a strategy of combining clinical assessment with US results was substantially cheaper (£485 per patient) than combining clinical assessment with biopsy (4).
Many studies therefore, have interrogated US as a diagnostic tool in GCA over the past two decades but have varied considerably in size and methodology with results showing wide discrepancies in sensitivity (9–100%), specificity (66–100%), positive predictive value (36–100%) and negative predictive value (33–100%) of US for a clinical or histological diagnosis of GCA. Most of the conflicting results are likely related to heterogenous methodologies, variances in sonographer technique and advances in ultrasound technology. The ever-expanding literature in this field has also shown us that the presence of bilateral halos increases sensitivity of US as does the incorporation of pre-test probability, while duration of prior corticosteroid use correlates inversely with likelihood of having a positive scan. A prospective study at Southend University Hospital assessing the validity of their pre-test probability score will be published in late 2022 (36).
Early studies evaluating the role of US in GCA disease-monitoring seemed to agree that Halo regression occurred within 3–4 weeks of initiating treatment (11, 21, 37–40). Later studies however, presumably due to improvements in US technology, identified halo persistence as late as 6 months after commencing immunosuppressive treatment for GCA (41–43). It is also notable that halo regression appears to occur more quickly in STA than AA, in those with relapsing vs. new-onset disease, in those who achieve clinical remission earlier, and in those with fewer STA branches affected at baseline (44–46). By contrast however, it has been demonstrated that there is no difference in relapse rate/steroid consumption between those with and without wall-thickening regression (47). In 2021, a prospective study evaluated the role of US in monitoring GCA in a cohort of 49 patients. The number of arterial segments with halo and the maximal IMT were measured at weeks 1, 3, 6, 12 and 24 and showed significant differences at all time points in STAs and after 6 weeks in AAs. Higher halo numbers/thickness correlated with inflammatory markers, cumulative steroid dose and lower likelihood of achieving remission with no such associations seen for AA halo. In cases of relapse, 16/17 cases had increased halo IMT compared to last measured value (48). However, no reliable conclusions can be drawn regarding the use of US in monitoring GCA based on available data.
A sub-study of the TABUL cohort demonstrated that an US score incorporating maximal IMT and bilaterality of STA/AA halos was useful for predicting likelihood for positive TAB but not for predicting clinical outcome at 6 months (49). More recently, Halo Counts (HC, number STA/AA branches with Halo) and Halo Scores (HS, composite of number and size of halos, Table 3) have been described. Both have shown a high degree of sensitivity for a clinical diagnosis of GCA (area under ROC curve 0.892 and 0.921) and strong associations with degrees of systemic inflammation and likelihood of ocular complications. In addition, the scores correlate positively with likelihood of having a subsequent positive TAB and they appear to be unaffected by cumulative steroid dose over the first week of treatment (50). These scores have so far been validated in one inception cohort for diagnosis but research is ongoing to assess their utility in monitoring disease activity long-term (20).
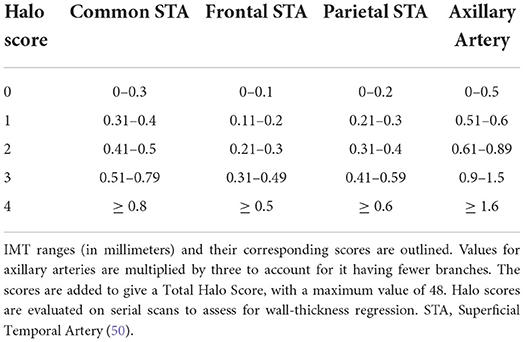
Table 3. Each branch is assigned a score based on the maximal intima-media thickness (IMT) identified in that branch.
In a number of studies, STA involvement without AA involvement is predictive of ocular disease with a stronger association being seen for those with bilateral halos (50–54). Involvement of both STAs and AAs infers a significantly higher risk of relapse and a more frequent requirement for steroid-sparing agents relative to those patients with either isolated cranial or isolated upper limb GCA with a similar association being seen for higher baseline HC and HS (48, 55).
MRI has the resolution to accurately depict vessel wall thickening and oedema using contrast agents. While primarily used for large vessels, recent protocols specifically for STAs have shown promise but sensitivities for a diagnosis of GCA vary widely (33.3–69% sensitivity, 87.5–91% specificity) and a combination of clinical examination and US have shown higher sensitivities (66.7 and 77.7%) and specificities (100%) relative to MRI (56, 57). Additionally, the sensitivity of baseline US and MRI of STAs for diagnosing GCA reduce rapidly with corticosteroid treatment. With TAB as the reference gold-standard, the respective sensitivities of MRI and US in a cohort of 59 suspected cases of GCA were as follows: 90 and 92% (when scanned within 1st day after steroid initiation), 77.8 and 80% (when scanned within 2–4 days after steroid initiation) and 80 and 50% (when scanned more than 4 days after steroid initiation) (58). Notably, other data have shown no statistical difference between US and MRI for detecting superficial cranial vessel vasculitis while US appears to detect vasculitic change more frequently than MRI both in those with new-onset disease and in those with chronic disease in the axillary, subclavian and carotid arteries (59). Multiple studies have also evaluated PET/CT relative to US in diagnosing GCA. PET/CT has shown greater sensitivity than US for identifying vertebral artery lesions but comparable sensitivity for diagnosing large-vessel disease, although abnormalities are often incongruous within single vascular regions (60–62).
While US is clearly a very useful clinical tool in rheumatology, as recently as 2014 only 1% of its use among rheumatologists was for the purpose of diagnosing vasculitis while 74–94% of rheumatologists prefer TAB over TAUS as a confirmatory test for GCA (63–65). However, since the publication of updated EULAR guidelines on imaging in LVV in 2018, its use has increased considerably, as reported by De Miguel et al., citing data from the Spanish ARTESER registry.
A number of European rheumatology centers have equipped themselves with the technology and expertise to operate Fast-track GCA clinics, which consist of same-day TAUS and initiation of treatment. The relative risk of permanent blindness in the GCA patients diagnosed through the Fast-track clinic is 88% lower compared with those diagnosed by the conventional route with a shorter mean duration of inpatient care by 3 days (66). The effectiveness of standardized training programmes for TAUS has shown excellent inter-reader reliability. In a study of 112 GCA patients who has vascular US (VUS) performed by five sonographers who underwent standardized training, an interobserver agreement of 95–96% with mean kappa values of 0.88–0.92 (95% CI 0.78 to 0.99) were achieved (67).
Recently, very-high resolution ultrasound (VHRU, 55 MHz) has been shown to non-invasively and reliably, define the thickness of the arterial intima layer. In 37 patients who had negative TAB, intimal thickening (IT >0.06 mm on histology) could be identified as a “four-line pattern” in VHRU with a sensitivity and specificity of 96.3 and 100% respectively and excellent agreement between histologic and VHRU IT measurement (68). Recently, in a proof-of-concept study in 24 GCA patients, contrast-enhanced US (CEUS) of large vessels had a sensitivity and specificity of 91.7 and 100% for detecting active LVV (69). It provides detailed images of lumen-to-vessel wall border and abnormalities correlate well with those seen on FDG-PET (70, 71). Most significantly however, Roncato et al. have described an automated image analysis tool for diagnosis of GCA using artificial intelligence (AI) algorithms. They reported on a retrospective cohort of 137 patients with suspected GCA who had VUS performed and labelled with VIA software. They obtained a sensitivity of 60% and specificity of 95% for their test set (72). Yet, while inter-rater agreements for US are high, it is an inherently subjective test with interpretation relying upon sonographer expertise/ experience. Incorporation of AI algorithms will provide more objectivity and standardization of US between individuals/centers and, we expect, eliminate the disparities between study results that we have observed to date.
In 2018, EULAR issued its first guidance document on the use of imaging in LVV, including ultrasound, with a new taskforce expected to update these recommendations in 2023 (73). In addition to some technical specifications, they state the following:
1. In patients with suspected GCA, an early imaging test is recommended to complement the clinical criteria for diagnosing GCA, assuming high expertise and prompt availability of the imaging technique.
2. In patients in whom there is a high clinical suspicion of GCA and a positive imaging test, the diagnosis of GCA may be made without an additional test (biopsy or further imaging). In patients with a low clinical probability and a negative imaging result, the diagnosis of GCA can be considered unlikely.
3. Ultrasound of temporal ± axillary arteries is recommended as the first imaging modality in patients with suspected predominantly cranial GCA. A non-compressible “Halo” sign is the ultrasound finding most suggestive of GCA.
The BSR has also issued recommendations for evaluating and managing GCA (74). They strongly recommended using a confirmatory diagnostic test, either TAUS, TAB, or both and they stress the importance of considering the pre-test probability prior to initiating investigations.
US should be a standard first line investigation in routine clinical care of patients with suspected GCA with TAB perhaps reserved for those only having had a normal US in the context of a high pre-test probability. It is more sensitive and cost-effective than TAB with an estimated saving of approximately €500 per patient and performs as well as MRI and PET/CT with the added benefit of easier access and lower relative cost when compared to those two investigations. Importantly however, it has been shown that algorithms incorporating combinations of imaging modalities can achieve a 100% sensitivity and specificity (62, 75). Moving forward, it is likely that such algorithms will become the Gold Standard in diagnosing GCA, rather than clinicians having to rely upon one specific test.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Duftner C, Dejaco C, Sepriano A, Falzon L, Schmidt WA, Ramiro S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD open. (2018) 4:e000612. doi: 10.1136/rmdopen-2017-000612
2. Salvarani C, Pipitone N, Versari A, Hunder GG. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol. (2012) 8:509–21. doi: 10.1038/nrrheum.2012.97
3. Hall S, Persellin S, Lie JT, O'Brien PC, Kurland LT, Hunder GG. The therapeutic impact of temporal artery biopsy. Lancet. (1983) 2:1217–20. doi: 10.1016/S0140-6736(83)91269-2
4. Luqmani R, Lee E, Singh S, Gillett M, Schmidt WA, Bradburn M, et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess. (2016) 20:1–238. doi: 10.3310/hta20900
5. Alberts MS, Mosen DM. Diagnosing temporal arteritis: duplex vs. biopsy. QJM: Monthly Journal of the Association of Physicians. (2007) 100:785–9. doi: 10.1093/qjmed/hcm103
6. Deyholos C, Sytek MC, Smith S, Cardella J, Orion KC. Impact of temporal artery biopsy on clinical management of suspected giant cell arteritis. Ann Vasc Surg. (2020) 69:254–60. doi: 10.1016/j.avsg.2020.06.012
7. Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: Does the end justify the means? Ann Med Surg. (2012). 20:1–5. doi: 10.1016/j.amsu.2017.06.020
8. Cristaudo AT, Mizumoto R, Hendahewa R. The impact of temporal artery biopsy on surgical practice. Ann Med Surg. (2012) 11:47–51. doi: 10.1016/j.amsu.2016.09.004
9. Alberts M. Temporal arteritis: improving patient evaluation with a new protocol. Perm J. (2013) 17:56–62. doi: 10.7812/TPP/12-067
10. Schmidt WA, Kraft HE, Völker L, Vorpahl K, Gromnica-Ihle EJ. Colour Doppler sonography to diagnose temporal arteritis. Lancet. (1995) 345:866. doi: 10.1016/S0140-6736(95)93005-1
11. Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. (1997) 337:1336–42. doi: 10.1056/NEJM199711063371902
12. Aschwanden M, Daikeler T, Kesten F, Baldi T, Benz D, Tyndall A, et al. Temporal artery compression sign–a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall in der Medizin. (2013) 34:47–50. doi: 10.1055/s-0032-1312821
13. Aschwanden M, Imfeld S, Staub D, Baldi T, Walker UA, Berger CT, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol. (2015) 33( 2 Suppl 89):S-113–5.
14. Chrysidis S, Duftner C, Dejaco C, Schäfer VS, Ramiro S, Carrara G, et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: a study from the OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open. (2018) 4:e000598. doi: 10.1136/rmdopen-2017-000598
15. Milchert M, Brzosko M, Bull Haaversen A, Diamantopoulos AP. Correspondence to ‘Slope sign': a feature of large vessel vasculitis? Ann Rheum Dis. (2019) 80:e198. doi: 10.1136/annrheumdis-2019-216601
16. Dasgupta B, Smith K, Khan AAS, Coath F, Wakefield RJ. ‘Slope sign': a feature of large vessel vasculitis? Ann Rheum Dis. (2019) 78:1738. doi: 10.1136/annrheumdis-2019-216213
17. Schäfer VS, Juche A, Ramiro S, Krause A, Schmidt WA. Ultrasound cut-off values for intima-media thickness of temporal, facial and axillary arteries in giant cell arteritis. Rheumatology. (2017) 56:1479–83. doi: 10.1093/rheumatology/kex143
18. Martire MV, Cipolletta E, Di Matteo A, Di Carlo M, Jesus D, Grassi W, et al. Is the intima-media thickness of temporal and axillary arteries influenced by cardiovascular risk? Rheumatology. (2021) 60:5362–8. doi: 10.1093/rheumatology/keab117
19. Fernández-Fernández E, Monjo-Henry I, Bonilla G, Plasencia C, Miranda-Carús ME, Balsa A, et al. False positives in the ultrasound diagnosis of giant cell arteritis: some diseases can also show the halo sign. Rheumatology. (2020) 59:2443–7. doi: 10.1136/annrheumdis-2019-eular.4008
20. Molina Collada J, Martínez-Barrio J, Serrano-Benavente B, Castrejón I, Caballero Motta LR, Trives Folguera L, et al. Diagnostic value of ultrasound halo count and Halo Score in giant cell arteritis: a retrospective study from routine care. Ann Rheum Dis. (2020) 81:e175. doi: 10.1136/annrheumdis-2020-218631
21. Schmidt WA, Natusch A, Möller DE, Vorpahl K, Gromnica-Ihle E. Involvement of peripheral arteries in giant cell arteritis: a color Doppler sonography study. Clin Exp Rheumatol. (2002) 20:309–18.
22. Schmidt WA, Seifert A, Gromnica-Ihle E, Krause A, Natusch A. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology. (2008) 47:96–101. doi: 10.1093/rheumatology/kem322
23. Agard C, Hamidou MA, Said L, Ponge T, Connault J, Chevalet P, et al. [Screening of abdominal aortic involvement using Doppler sonography in active giant cell (temporal) arteritis at the time of diagnosis. A prospective study of 30 patients]. La Revue de Medecine Interne. (2007) 28:363–70. doi: 10.1016/j.revmed.2006.12.018
24. Hop H, Mulder DJ, Sandovici M, Glaudemans A, van Roon AM, Slart R, et al. Diagnostic value of axillary artery ultrasound in patients with suspected giant cell arteritis. Rheumatology. (2020) 59:3676–84. doi: 10.1093/rheumatology/keaa102
25. Nielsen BD, Hansen IT, Keller KK, Therkildsen P, Gormsen LC, Hauge EM. Diagnostic accuracy of ultrasound for detecting large-vessel giant cell arteritis using FDG PET/CT as the reference. Rheumatology. (2020) 59:2062–73. doi: 10.1093/rheumatology/kez568
26. Gehlen M, Schaefer N, Schwarz-Eywill M, Maier A. Ultrasound to detect involvement of vertebral artery in giant cell arteritis. Clin Exp Rheumatol. (2018) 36( Suppl 111):169–70.
27. Czihal M, Zanker S, Rademacher A, Tatò F, Kuhlencordt PJ, Schulze-Koops H, et al. Sonographic and clinical pattern of extracranial and cranial giant cell arteritis. Scand J Rheumatol. (2012) 41:231–6. doi: 10.3109/03009742.2011.641581
28. Ješe R, Rotar Ž, Tomšič M, Hočevar A. The role of colour doppler ultrasonography of facial and occipital arteries in patients with giant cell arteritis: a prospective study. Eur J Radiol. (2017) 95:9–12. doi: 10.1016/j.ejrad.2017.07.007
29. Diamantopoulos AP, Haugeberg G, Hetland H, Soldal DM, Bie R, Myklebust G. Diagnostic value of color Doppler ultrasonography of temporal arteries and large vessels in giant cell arteritis: a consecutive case series. Arthritis Care Res. (2014) 66:113–9. doi: 10.1002/acr.22178
30. Zachrisson H, Svensson C, Dremetsika A, Eriksson P. An extended high-frequency ultrasound protocol for detection of vessel wall inflammation. Clin Physiol Funct Imaging. (2018) 38:586–94. doi: 10.1111/cpf.12450
31. Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med. (2005) 142:359–69. doi: 10.7326/0003-4819-142-5-200503010-00011
32. Arida A, Kyprianou M, Kanakis M, Sfikakis PP. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Dis. (2010) 11:44. doi: 10.1186/1471-2474-11-44
33. Ball EL, Walsh SR, Tang TY, Gohil R, Clarke JM. Role of ultrasonography in the diagnosis of temporal arteritis. Br J Surg. (2010) 97:1765–71. doi: 10.1002/bjs.7252
34. Seitz L, Christ L, Lötscher F, Scholz G, Sarbu AC, Bütikofer L, et al. Quantitative ultrasound to monitor the vascular response to tocilizumab in giant cell arteritis. Rheumatology. (2021) 60:5052–9. doi: 10.1093/rheumatology/keab484
35. Soares C, Costa A, Santos R, Abreu P, Castro P, Azevedo E. Clinical, laboratory and ultrasonographic interrelations in giant cell arteritis. J Stroke Cerebrovasc Dis. (2021) 30:105601. doi: 10.1016/j.jstrokecerebrovasdis.2021.105601
36. Sebastian A, Tomelleri A, Kayani A, Prieto-Pena D, Ranasinghe C, Dasgupta B. Probability-based algorithm using ultrasound and additional tests for suspected GCA in a fast-track clinic. RMD Open. (2020) 6:e001297. doi: 10.1136/rmdopen-2020-001297
37. Lauwerys BR, Puttemans T, Houssiau FA, Devogelaer JP. Color Doppler sonography of the temporal arteries in giant cell arteritis and polymyalgia rheumatica. J Rheumatol. (1997) 24:1570–4.
38. Schmid R, Hermann M, Yannar A, Baumgartner RW. [Color duplex ultrasound of the temporal artery: replacement for biopsy in temporal arteritis]. Ophthalmologica. (2002) 216:16–21. doi: 10.1159/000048291
39. Karahaliou M, Vaiopoulos G, Papaspyrou S, Kanakis MA, Revenas K, Sfikakis PP. Colour duplex sonography of temporal arteries before decision for biopsy: a prospective study in 55 patients with suspected giant cell arteritis. Arthritis Res Ther. (2006) 8:R116. doi: 10.1186/ar2003
40. Santoro L, D'Onofrio F, Bernardi S, Gremese E, Ferraccioli G, Santoliquido A. Temporal ultrasonography findings in temporal arteritis: early disappearance of halo sign after only 2 days of steroid treatment. Rheumatology. (2013) 52:622. doi: 10.1093/rheumatology/kes387
41. Pérez López J, Solans Laqué R, Bosch Gil JA, Molina Cateriano C, Huguet Redecilla P, Vilardell Tarrés M. Colour-duplex ultrasonography of the temporal and ophthalmic arteries in the diagnosis and follow-up of giant cell arteritis. Clin Exp Rheumatol. (2009) 27(1 Suppl 52):S77–82.
42. Aschwanden M, Kesten F, Stern M, Thalhammer C, Walker UA, Tyndall A, et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2x11 arterial regions. Ann Rheum Dis. (2010) 69:1356–9. doi: 10.1136/ard.2009.122135
43. Diamantopoulos AP, Myklebust G. Long-term inflammation in the temporal artery of a giant cell arteritis patient as detected by ultrasound. Ther Adv Musculoskelet Dis. (2014) 6:102–3. doi: 10.1177/1759720X14521109
44. Ford JA, DiIorio MA, Huang W, Sobiesczcyk P, Docken WP, Tedeschi SK. Follow-up vascular ultrasounds in patients with giant cell arteritis. Clin Exp Rheumatol. (2020) 38(Suppl 124):107–11.
45. De Miguel E, Roxo A, Castillo C, Peiteado D, Villalba A, Martín-Mola E. The utility and sensitivity of colour Doppler ultrasound in monitoring changes in giant cell arteritis. Clin Exp Rheumatol. (2012) 30(1 Suppl 70):S34–8.
46. Monti S, Floris A, Ponte CB, Schmidt WA, Diamantopoulos AP, Pereira C, et al. The proposed role of ultrasound in the management of giant cell arteritis in routine clinical practice. Rheumatology. (2018) 57:112–9. doi: 10.1093/rheumatology/kex341
47. Aschwanden M, Schegk E, Imfeld S, Staub D, Rottenburger C, Berger CT, et al. Vessel wall plasticity in large vessel giant cell arteritis: an ultrasound follow-up study. Rheumatology. (2019) 58:792–7. doi: 10.1093/rheumatology/key383
48. Ponte C, Monti S, Scirè CA, Delvino P, Khmelinskii N, Milanesi A, et al. Ultrasound halo sign as a potential monitoring tool for patients with giant cell arteritis: a prospective analysis. Ann Rheum Dis. (2021) 80:1475–82. doi: 10.1136/annrheumdis-2021-220306
49. Monti S, Ponte C, Pereira C, Manzoni F, Klersy C, Rumi F, et al. The impact of disease extent and severity detected by quantitative ultrasound analysis in the diagnosis and outcome of giant cell arteritis. Rheumatology (Oxford). (2020) 59:2299–307. doi: 10.1093/rheumatology/kez554
50. van der Geest KSM, Borg F, Kayani A, Paap D, Gondo P, Schmidt W, et al. Novel ultrasonographic Halo Score for giant cell arteritis: assessment of diagnostic accuracy and association with ocular ischaemia. Ann Rheum Dis. (2020) 79:393–9. doi: 10.1136/annrheumdis-2019-216343
51. Schmidt WA, Krause A, Schicke B, Kuchenbecker J, Gromnica-Ihle E. Do temporal artery duplex ultrasound findings correlate with ophthalmic complications in giant cell arteritis? Rheumatology. (2009) 48:383–5. doi: 10.1093/rheumatology/ken515
52. Gribbons KB, Ponte C, Craven A, Robson JC, Suppiah R, Luqmani R, et al. Diagnostic assessment strategies and disease subsets in giant cell arteritis: data from an international observational cohort. Arthritis Rheumatol. (2020) 72:667–76. doi: 10.1002/art.41165
53. Ponte C, Serafim AS, Monti S, Fernandes E, Lee E, Singh S, et al. Early variation of ultrasound halo sign with treatment and relation with clinical features in patients with giant cell arteritis. Rheumatology. (2020) 59:3717–26. doi: 10.1093/rheumatology/keaa196
54. Schmidt D, Hetzel A, Reinhard M, Auw-Haedrich C. Comparison between color duplex ultrasonography and histology of the temporal artery in cranial arteritis (giant cell arteritis). Eur J Med Res. (2003) 8:1–7.
55. Czihal M, Piller A, Schroettle A, Kuhlencordt P, Bernau C, Schulze-Koops H, et al. Impact of cranial and axillary/subclavian artery involvement by color duplex sonography on response to treatment in giant cell arteritis. J Vasc Surg. (2015) 61:1285–91. doi: 10.1016/j.jvs.2014.12.045
56. Bley TA, Reinhard M, Hauenstein C, Markl M, Warnatz K, Hetzel A, et al. Comparison of duplex sonography and high-resolution magnetic resonance imaging in the diagnosis of giant cell (temporal) arteritis. Arthritis Rheum. (2008) 58:2574–8. doi: 10.1002/art.23699
57. Ghinoi A, Zuccoli G, Nicolini A, Pipitone N, Macchioni L, Bajocchi GL, et al. 1T magnetic resonance imaging in the diagnosis of giant cell arteritis: comparison with ultrasonography and physical examination of temporal arteries. Clin Exp Rheumatol. (2008) 26(3 Suppl 49):S76–80.
58. Hauenstein C, Reinhard M, Geiger J, Markl M, Hetzel A, Treszl A, et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology. (2012) 51:1999–2003. doi: 10.1093/rheumatology/kes153
59. Yip A, Jernberg ET, Bardi M, Geiger J, Lohne F, Schmidt WA, et al. Magnetic resonance imaging compared to ultrasonography in giant cell arteritis: a cross-sectional study. Arthritis Res Ther. (2020) 22:247. doi: 10.1186/s13075-020-02335-4
60. Czihal M, Tatò F, Förster S, Rademacher A, Schulze-Koops H, Hoffmann U. Fever of unknown origin as initial manifestation of large vessel giant cell arteritis: diagnosis by colour-coded sonography and 18-FDG-PET. Clin Exp Rheumatol. (2010) 28:549–52.
61. Förster S, Tato F, Weiss M, Czihal M, Rominger A, Bartenstein P, et al. Patterns of extracranial involvement in newly diagnosed giant cell arteritis assessed by physical examination, colour coded duplex sonography and FDG-PET. VASA Zeitschrift fur Gefasskrankheiten. (2011) 40:219–27. doi: 10.1024/0301-1526/a000096
62. Imfeld S, Aschwanden M, Rottenburger C, Schegk E, Berger CT, Staub D, et al. [18F]FDG positron emission tomography and ultrasound in the diagnosis of giant cell arteritis: congruent or complementary imaging methods? Rheumatology. (2020) 59:772–8. doi: 10.1093/rheumatology/kez362
63. de Miguel E, Andreu JL, Naredo E, Möller I. Ultrasound in rheumatology: where are we and where are we going? Reumatol Clin. (2014) 10:6–9. doi: 10.1016/j.reuma.2013.04.005
64. Mahr A, Belhassen M, Paccalin M, Devauchelle-Pensec V, Nolin M, Gandon S, et al. Characteristics and management of giant cell arteritis in France: a study based on national health insurance claims data. Rheumatology. (2020) 59:120–8. doi: 10.1093/rheumatology/kez251
65. Ing E, Xu QA, Chuo J, Kherani F, Landau K. Practice preferences: temporal artery biopsy versus doppler ultrasound in the work-up of giant cell arteritis. Neuro-ophthalmology. (2020) 44:174–81. doi: 10.1080/01658107.2019.1656752
66. Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology. (2016) 55:66–70. doi: 10.1093/rheumatology/kev289
67. Chrysidis S, Terslev L, Christensen R, Fredberg U, Larsen K, Lorenzen T, et al. Vascular ultrasound for the diagnosis of giant cell arteritis: a reliability and agreement study based on a standardised training programme. RMD Open. (2020) 6:e001337. doi: 10.1136/rmdopen-2020-001337
68. Sundholm JKM, Paetau A, Albäck A, Pettersson T, Sarkola T. Non-invasive vascular very-high resolution ultrasound to quantify artery intima layer thickness: validation of the four-line pattern. Ultrasound Med Biol. (2019) 45:2010–8. doi: 10.1016/j.ultrasmedbio.2019.04.017
69. Bergner R, Splitthoff J, Wadsack D. Use of contrast-enhanced ultrasound sonography in giant cell arteritis: a proof-of-concept study. Ultrasound Med Biol. (2022) 48:143–8. doi: 10.1016/j.ultrasmedbio.2021.09.019
70. Schinkel AF, van den Oord SC, van der Steen AF, van Laar JA, Sijbrands EJ. Utility of contrast-enhanced ultrasound for the assessment of the carotid artery wall in patients with Takayasu or giant cell arteritis. Eur Heart J Cardiovasc Imaging. (2014) 15:541–6. doi: 10.1093/ehjci/jet243
71. Germanò G, Macchioni P, Possemato N, Boiardi L, Nicolini A, Casali M, et al. Contrast-enhanced ultrasound of the carotid artery in patients with large vessel vasculitis: correlation with positron emission tomography findings. Arthritis Care Res. (2017) 69:143–9. doi: 10.1002/acr.22906
72. Roncato C, Perez L, Brochet-Guégan A, Allix-Béguec C, Raimbeau A, Gautier G, et al. Colour doppler ultrasound of temporal arteries for the diagnosis of giant cell arteritis: a multicentre deep learning study. Clin Exp Rheumatol. (2020) 38(Suppl 124):120–5.
73. Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. (2018) 77:636–43. doi: 10.1136/annrheumdis-2017-212649
74. Mackie SL, Dejaco C, Appenzeller S, Camellino D, Duftner C, Gonzalez-Chiappe S, et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis: executive summary. Rheumatology. (2020) 59:487–94. doi: 10.1093/rheumatology/kez664
Keywords: giant cell (temporal) arteritis, ultrasound, large vessel vasculitis, biomarkers, temporal artery biopsy
Citation: Kirby C, Flood R, Mullan R, Murphy G and Kane D (2022) Evolution of ultrasound in giant cell arteritis. Front. Med. 9:981659. doi: 10.3389/fmed.2022.981659
Received: 29 June 2022; Accepted: 26 August 2022;
Published: 03 October 2022.
Edited by:
Andrea Di Matteo, Marche Polytechnic University, ItalyReviewed by:
Juan Molina Collada, Gregorio Marañón Hospital, SpainCopyright © 2022 Kirby, Flood, Mullan, Murphy and Kane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Colm Kirby, Y29sbWtpcmJ5MTFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.