- 1Department of Accident and Emergency, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
- 2Department of Neurology, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
- 3Department of Radiology, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
- 4Department of Emergency Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
Massive pulmonary embolism (MPE) is a high-risk medical emergency. Seizure as the clinical presentation of MPE is extremely rare, and to our knowledge, there have been no reports on successful percutaneous, catheter-based treatment of MPE presenting with new-onset seizures and cardiac arrest. In this report, we discuss the case of a 64-year-old woman who presented with an episode of seizure that lasted 5 h. Seizure occurred four times within 12 h after arrival at the hospital, and in the end, she sustained a cardiac arrest. The patient had no past history of seizure or cardiopulmonary disease. Bilateral MPE was detected by a computed tomography pulmonary angiogram, and she was successfully treated with percutaneous, catheter-directed anticoagulant therapy. Pulmonary embolism-related seizures are more difficult to diagnose and have higher mortality rates than seizures. MPE should be suspected in patients presenting with new-onset seizures and hemodynamic instability.
Introduction
Massive pulmonary embolism (MPE) is defined as an acute pulmonary embolism with hemodynamic instability (systolic blood pressure <90 mmHg for at least 15 min or the use of ionotropic support), cardiac arrest, or persistent bradycardia (heart rate <40 bpm with signs and symptoms of shock) without other apparent cause. It is a high-risk medical emergency, with a reported mortality rate ranging from 25 to 65% (1–3). Performing an early clinical diagnosis of MPE is challenging as there is a spectrum of phenotypes. Group 1 includes patients with tachycardia and tachypnea and those with the greatest risk of death (7.4%) and major bleeding (7.0%). Group 2 includes patients of younger age and those with no complications (mortality 1.4%; major bleed 1.6%). Group 3 includes older women, with a history of hypertensive and cerebrovascular disease (mortality 2.3%; major bleed 1.3%). Group 4 includes patients with recent surgery, trauma, and malignancy (mortality 2.5%; major bleed 1.9%) (4). Seizure commonly presents in emergency departments (EDs) (5). However, the incidence of MPE presenting as a seizure is <1%, but when it presents as a seizure, the reported mortality rate is 54.5% (6). To our knowledge, there has been no report of successful percutaneous catheter-directed treatment (CDT) of MPE presenting with new-onset seizures and cardiac arrest.
In this report, we present the case of a patient who presented with multiple episodes of seizure over 17 h and who subsequently sustained cardiac arrest. After a computed tomography pulmonary angiogram (CTPA), the patient was diagnosed with bilateral MPE and was successfully treated with CDT and anticoagulants.
Case description
A 64-year-old woman presented to the ED of a university hospital with a history of repeating seizures of short duration that had started 5 h prior to her arrival. She was brought to the hospital by emergency medical services (EMS).
During her brief generalized tonic seizure, she was unconscious, in a trismic state, and was incontinent. After the spontaneous termination of her seizure, she completely regained consciousness. She developed nausea, vomiting, and pallor. Less than 5 min after arrival in the ED, she had another seizure. She had no significant past medical history apart from a hysterectomy carried out 7 years ago. She also had no history of recent trauma, surgery, travel, use of illicit drugs, or smoking. She was afebrile and had a heart rate of 90 beats per minute and a blood pressure of 120/74 mmHg. She had good oxygen saturation in room air with a respiratory rate of 20 per min. Cardiorespiratory and neurological examinations were unremarkable otherwise. Her right lower limb was slightly swollen and discolored. An emergency complete blood count, liver and renal function tests, coagulation function, prothrombin time (PT) 14.4s, activated partial thromboplastin time (APTT) 31.6s, international normalized ratio (INR) 1.14, fibrinogen 2.78 g/L, thrombin time (TT) 16.2s), and troponin T (TNT) were normal. An electrocardiogram (ECG) showed sinus rhythm, with inversion of T-waves in II, III, aVF, and V1-V4 areas. A computed tomography (CT) scan of her brain was unremarkable. She was resuscitated and admitted to a neurology ward.
On admission to the ward, her heart rate was 98 beats per minute and blood pressure was 131/77 mmHg. The patient developed recurrent seizures with loss of consciousness two times in 3 h in the ward. Each episode lasted a few seconds, after which she regained consciousness within 2 min. A provisional diagnosis of an epileptic seizure was performed, and she was treated accordingly with antiepileptic treatment. After 30 min, she went into a coma (Glasgow Coma Score 3/15), had tachycardia, and then sustained a cardiac arrest. After a 15-min resuscitation with advanced cardiac life support, spontaneous circulation returned and she was transferred to the intensive care unit (ICU).
In the ICU, she had an abnormal coagulation function (D-dimer >20 ug/mL, PT 23.1 s, APTT 100.4 s, INR 2.09, and fibrinogen 0.97 g/L, TT to 38 s) and elevated cardiac markers (TNT 0.959 ng/mL and NT-BNP 3,054 pg/mL). Her arterial blood gas revealed lactic acidosis (pH 7.016, pCO2 6.74 kPa, pO2 22.1 kPa, lactate 14.8 mmol/L, and HCO3 12.3 mmol/L). A pulmonary embolism was suspected, and a subsequent CTPA confirmed a massive PE with bilateral pulmonary emboli (Figure 1A). A venous duplex ultrasound of the lower extremities showed extensive deep vein thrombosis (DVT) of the right saphenous vein, the superficial femoral vein, the popliteal vein, and the intramuscular vein. She then underwent a pulmonary angiogram with percutaneous mechanical thrombus removal and thrombectomy, an inferior vena cava angiogram, and filter placement (Figure 2). She did not receive an alteplase. An bedside echocardiography showed moderate tricuspid valve regurgitation (302 cm/s), normal pulmonary artery systolic pressure (about 41 mmHg), and normal left ventricular systolic function (ejection fraction 63%). An electroencephalography (EEG) was abnormal for epileptiform activity. Brain magnetic resonance imaging (MRI) with contrast demonstrated ischemic hypoxic encephalopathy, in which global hypoxic brain injury caused diffused edema with pronounced diffusion restriction of the perirolandic and occipital cortices (Figure 3). Additional anticardiolipin immunoglobulin G (IgG, <2 PL-IgG-U/ml) and IgM (3.42 PL-IgM-U/ml), protein C (70.7%), and protein S (21.4%) levels were normal. She received parenteral low-molecular weight heparin (LMWH) postoperatively and was prescribed oral rivaroxaban for 6 months as the anticoagulant therapy. In addition, she was treated with antiepileptic therapy (levetiracetam) for 9 months without any recurrence of seizures. As her neurological recovery was sub-optimal, she was intubated and had a tracheotomy. She remained seizure-free and regained consciousness (GCS E4VTM6). A CTPA showed that the PE had completely dissolved (Figure 1B) a month later. She was discharged in a good condition and was doing well at her follow-up visits.
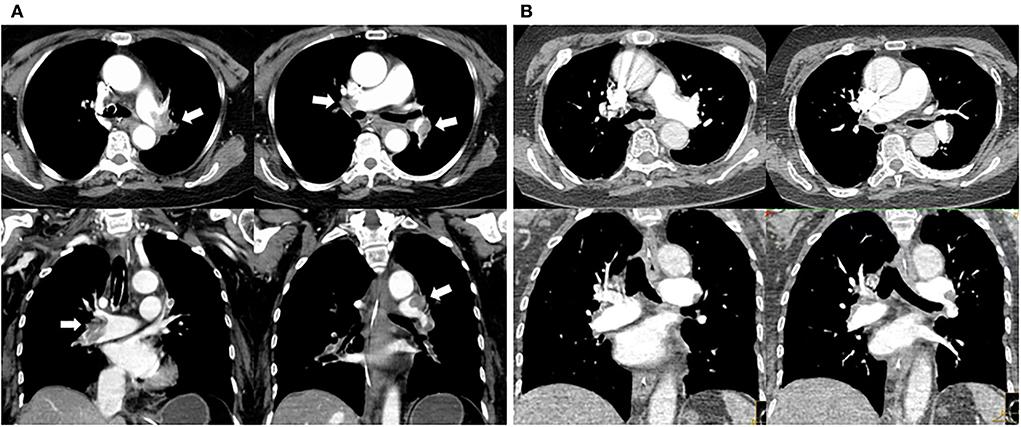
Figure 1. Changes in pulmonary embolism of CT pulmonary arteriography before and after percutaneous catheter-directed therapy. (A) Multiple filling defects in bilateral pulmonary artery trunks and their branches (white arrow) before CDT. (B) Thrombus eliminated 1 month after CDT and anticoagulation.
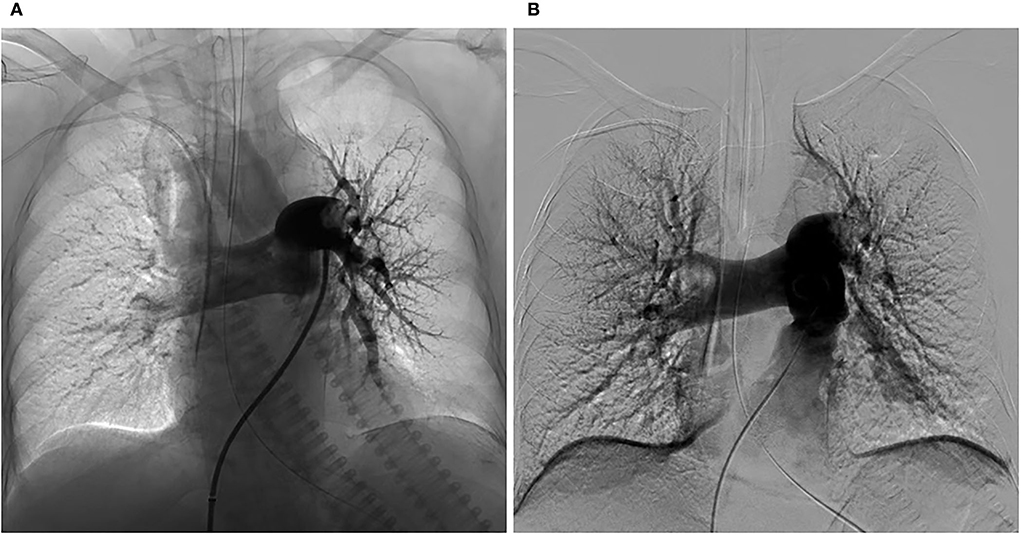
Figure 2. Changes in the pulmonary arteriography before and after CDT. (A) Pulmonary angiogram showing a mass filling defect in the distal end of bilateral pulmonary artery trunks and branches of the left lower lobes and poor perfusion before CDT. (B) Pulmonary angiogram showing the reduced defect of the thrombus in bilateral pulmonary artery trunks and improved perfusion after CDT. Pulmonary artery pressure before and after were 44/29 mmHg and 45/20 mmHg, respectively.
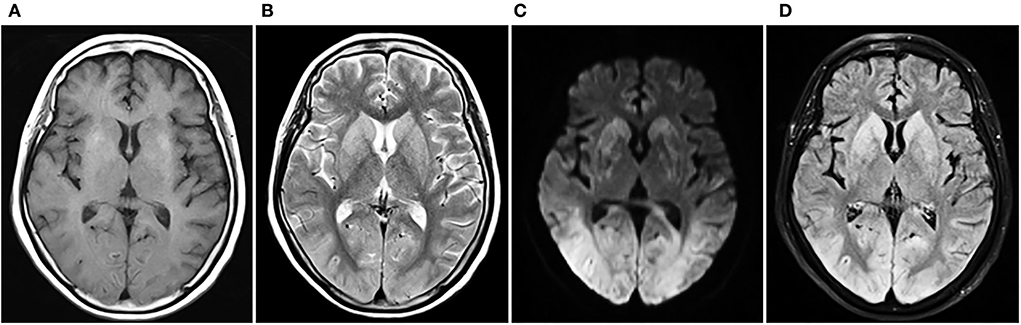
Figure 3. Cerebral MRI contrast of ischemic hypoxic encephalopathy. T2WI and T2-FLAIR show extensive gyral swelling and hyperintensity in basal ganglia bilaterally. DWI demonstrates bilateral symmetrical diffusion restriction throughout the cortex of both cerebral hemispheres, particularly involving the perirolandic and occipital cortices. (A) T1WI, (B) T2WI, (C) DWI, (D) T2-FLAIR. MRI, magnetic resonance imaging; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery.
Discussion
Pulmonary embolism (PE), a type of venous thromboembolism (VTE), is a life-threatening emergency. High morbidity and mortality are associated with acute cardiovascular disease, secondary to acute myocardial infarction and stroke (1, 7). MPE is a severe, acute form of PE with hemodynamic compromise, including refractory hypotension, pulselessness, or persistent profound bradycardia, resulting in mortality rates as high as 25 to 65% in the absence of an early diagnosis and timely intervention (2, 3).
Seizures are common clinical neurological manifestations of various pathologies. They have been noted in cardiopulmonary diseases such as long QT syndrome, severe bradycardia, systemic hypertension, aortic dissection, and cardiac arrest (8–11). However, new-onset seizure as the clinical presentation of MPE has rarely been reported; thus, recognition is more difficult, timely management is delayed, and consequently, it has a much higher risk of death and poor outcomes (3, 5, 6, 12, 13). We summarized the published cases of PE with seizure in Supplementary Table 1 (13–31). Among the patients with PE who presented with seizures (n = 23), 30% were hemodynamically unstable at admission and nearly 70% were hypoxic, and 40% of the cases (n = 9) developed cardiac arrest. With timely recognition and treatment, around half (n = 5) of these patients survived to discharge.
In our case, the patient was brought to the hospital by the EMS and was treated with supplementary oxygen during her brief generalized tonic seizures with impaired sensorium. Her hemodynamics and oxygen saturation were stable at admission, but later, she developed cardiac arrest. In our summarized data, 22% of the cases (n = 5) were not hypoxic on admission. Of these patients, only two cases developed cardiac arrest. Fortunately, our patient survived and gradually recovered to a good neurological state.
With the development of imaging and treatment, early diagnosis and severity assessment of acute PE should be highly feasible in the ED. Elevated levels of D-dimer assays have a low positive predictive value and do not accurately confirm a PE (1, 3). However, about 77% of the 13 published cases in the last 10 years had an elevated D-dimer level, including our patient. The pathognomonic S1Q3T3 pattern on the ECG supposedly suggests that PE (3) was observed in only a third of these patients. Most patients showed only sinus tachycardia.
In our case, the patient showed sinus rhythm, with inversion of T-waves in II, III, aVF, and V1-V4 regions on the ECG. The pattern is common and non-specific. The T-wave changes suggest abnormal repolarization of the right ventricular myocardium. In addition to pulmonary embolism, the etiology for the changes indicates myocardial disease such as myocardial ischemia or infarction, myocarditis, pericarditis, myocardial hypertrophy, right ventricular overload syndromes, and even other causes of fever, anemia, and normal variants. Therefore, during the interpretation of T-wave changes, we should consider the clinical history, symptoms, physical examination, and the presence of similar findings on previous ECGs (32, 33).
Transthoracic echocardiography (TTE) provides important information for stratifying risks in PE. In the situation of a patient with suspected PE who develops hemodynamic instability, the emergency beside TTE contributes to the rapid diagnosis of MPE (1, 2). Half of the patients have dilation of the right ventricle and/or the right atrium. In approximately 30–40% of cases, there is a D-shaped left ventricle, which predicts elevated pulmonary artery pressure. Compared with the gold standard of pulmonary angiography (PA), CTPA, which is highly sensitive (83%), specific (96%), and less invasive, is the first-line investigation for high-risk PE (2, 34, 35). Therefore, in about 85% of these 13 cases, MPE was confirmed by a CTPA.
Anticoagulation with unfractionated heparin, LMWH, or warfarin is regarded as the standard of care for all VTEs (1). Thrombolytics (e.g., rtPA), particularly emergency thrombolysis (within 48 h of symptom onset), is the most effective therapy for the treatment of PE, particularly in high-risk patients and patients whose condition deteriorated while receiving anticoagulation (1, 36). Percutaneous catheter-directed treatment (CDT) and surgical embolectomy are considered alternatives to systemic thrombolysis in patients with hemodynamic instability, refractory hypoxia, shock, or cardiac arrest (1, 3, 36, 37).
To our knowledge, this study is the first to report a case of a patient with MPE that presented with seizure and that was successfully managed with catheter-directed embolectomy and anticoagulant therapy soon after MPE was diagnosed. In our review of the literature, only two patients were treated surgically, and both survived the condition.
Seizure is believed to be secondary to ischemic hypoxic encephalopathy caused by MPE, which leads to right ventricular failure and respiratory failure. The former leads to transient cerebral hypoperfusion and metabolic acidosis due to decreased cardiac output and hypotension, and the latter leads to hypoxemia and respiratory acidosis probably due to ventilation-perfusion mismatch (1, 12, 13). PE is caused by multiple etiologies, including thromboembolism, air embolism, fat embolism, septic embolism, amniotic fluid embolism, foreign material pulmonary embolism, and tumor embolism. Moreover, the predisposing factors of PE included hereditary risk factors and environmental factors (Supplementary Table 2) (1, 38). In our reported case, DVT as well as PE was found. Thus, pulmonary arterial circulation was embolized by a DVT clot, which led to myocardial hypoxic injury and ischemic hypoxic encephalopathy. Finally, the patient presented with a secondary seizure.
The early diagnosis of PE poses a challenge to clinicians. A high clinical suspicion remains the cornerstone of timely diagnosis and treatment. Clinicians are advised to consider the risk of MPE among patients with new but refractory seizure and unilateral lower limb swelling. In developed health systems, clinicians may diagnose MPE with sophisticated imaging; in low- and middle-income countries, clinicians may resort to bedside ultrasonography and echocardiography for DVT and suspected MPE so that the patient can be treated promptly.
Data availability statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the (patients/participants OR patients/participants legal guardian/next of kin) was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
YZ and XH treated the patient. WL, YZ, YH, and YW made substantial contributions to the conception and design and drafted the manuscript. WL, YH, and ZL collected the clinical laboratory results and images. AW, TR, and YH revised the manuscript. All authors contributed to the writing of the manuscript, read, and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.980847/full#supplementary-material
Supplementary Table 1. Summary of patients' demographics and characteristics for the published cases of PE with seizure.
Supplementary Table 2. The etiologies and predisposing factors of PE.
References
1. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. (2019) 54:543–603. doi: 10.1183/13993003.01647-2019
2. Sista AK, Kuo WT, Schiebler M, Madoff DC. Stratification, imaging, and management of acute massive and submassive pulmonary embolism. Radiology. (2017) 284:5–24. doi: 10.1148/radiol.2017151978
3. Pollack CV, Schreiber D, Goldhaber SZ, Slattery D, Fanikos J, O'Neil BJ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. (2011) 57:700–6. doi: 10.1016/j.jacc.2010.05.071
4. Zhang S, Xu X, Ji Y, Yang Y, Yi Q, Chen H, et al. Clinical phenotypes with prognostic implications in pulmonary embolism patients with syncope. Front Cardiovasc Med. (2020) 15:836850. doi: 10.3389/fcvm.2022.836850
5. Shohet C, Yelloly J, Bingham P, Lyratzopoulos G. The association between the quality of epilepsy management in primary care, general practice population deprivation status and epilepsy-related emergency hospitalisations. Seizure. (2007) 16:351–5. doi: 10.1016/j.seizure.2007.02.005
6. Desai PV, Krepostman N, Collins M, De Sirkar S, Hinkleman A, Walsh K, et al. Neurological complications of pulmonary embolism: A literature review. Curr Neurol Neurosci Rep. (2021) 21:59. doi: 10.1007/s11910-021-01145-8
7. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. (2012) 379:1835–46. doi: 10.1016/S0140-6736(11)61904-1
8. Mendes LA, Davidoff R. Cardiogenic seizure with bradyarrhythmia: Documentation of the mechanism during asystole. Am Heart J. (1993) 125:1786–8. doi: 10.1016/0002-8703(93)90778-8
9. Ng SK, Hauser WA, Brust JC, Susser M. Hypertension and the risk of new-onset unprovoked seizures. Neurology. (1993) 43:425–8. doi: 10.1212/WNL.43.2.425
10. Gaul C, Dietrich W, Friedrich I, Sirch J, Erbguth FJ. Neurological symptoms in type A aortic dissections. Stroke. (2007) 38:292–7. doi: 10.1161/01.STR.0000254594.33408.b1
11. González A, Aurlien D, Larsson P, Berg KO, Dahl IT, Edvardsen T, et al. Seizure-like episodes and EEG abnormalities in patients with long QT syndrome. Seizure. (2018) 61:214–20. doi: 10.1016/j.seizure.2018.08.020
12. Zuin M, Rigatelli G, Zuliani G, Adami A, Zonzin P, Roncon LA-O. Seizures as the first clinical manifestation of acute pulmonary embolism: an underestimate issue in neurocritical care. Neurol Sci. (2020) 41:1427–36. doi: 10.1007/s10072-020-04275-y
13. Marine JE, Goldhaber SG. Pulmonary embolism presenting as seizures. Chest. (1997) 112:840–2. doi: 10.1378/chest.112.3.840
14. Hamilton M, Thompson EN. Unusual manifestations of pulmonary embolic disease. Postgrad Med J. (1963) 39:348–53. doi: 10.1136/pgmj.39.452.348
15. Fred HL, Willerson JT, Alexander JK. Neurological manifestations of pulmonary thromboembolism. Arch Intern Med. (1967) 120:33–7. doi: 10.1001/archinte.120.1.33
16. Fred HL, Yang M. Sudden loss of consciousness, dyspnea, and hypoxemia in a previously healthy young man. Circulation. (1995) 91:3017–9. doi: 10.1161/01.CIR.91.12.3017
17. Kupnik D, Grmec S. Pulmonary thromboembolism presenting as epileptiform generalized seizure. Eur J Emerg Med. (2004) 11:346–7. doi: 10.1097/00063110-200412000-00009
18. Chen KC, Chien DK, Tsai WD, Su YJ, Chang WH. Massive pulmonary embolism presenting as seizures. J Emerg Med Taiwan. (2008) 10:127–30.
19. Meyer MA. Seizure as the presenting sign for massive pulmonary embolism: Case report and review of the literature. Seizure. (2009) 18:76–8. doi: 10.1016/j.seizure.2008.06.008
20. Shah AK, Darwent M. Acute pulmonary embolism presenting as seizures. Emerg Med J. (2009) 26:299–300. doi: 10.1136/emj.2007.046151
21. Lam M, Jammal M, Tiev K, Toledano C, Kettaneh A. Pulmonary embolism revealed by a seizure: A case report and literature review. Rev Med Interne. (2012) 33:457–60. doi: 10.1016/j.revmed.2012.06.002
22. Wang JW, Xu MW, Luo BY. Pulmonary embolism presenting as recurrent transient loss of consciousness: syncope and seizure. Chin Med J. (2013) 126:193–4.
23. Kimura K, Mori H, Kitaguchi H, Yamao F, Shindo K. Pulmonary embolism as a cause of seizure. Am J Emerg Med. (2013) 31:1525–7. doi: 10.1016/j.ajem.2013.06.024
24. Volz EE, Jasani N. Seizure as a presentation of pulmonary embolism. J Emerg Med. (2014) 46:e1–4. doi: 10.1016/j.jemermed.2013.08.057
25. Park DH. Seizure caused by pulmonary embolism: two cases report and review of the literature. J Korean Soc Emerg Med. (2014) 25:115–9.
26. Ching YH, Alvey EF, Omar HR, Lynch CM, Mangar D, Camporesi EM. Pulmonary embolism presenting as a seizure in the immediate postpartum period. J Obstet Gynaecol. (2015) 35:92–3. doi: 10.3109/01443615.2014.936842
27. Nyquist A, Read C, Hilfiker J. Massive pulmonary embolism lurking on the neurology unit masquerading as a seizure. Chest. (2016) 150:450A. doi: 10.1016/j.chest.2016.08.463
28. Hashmani S, Tipoo Sultan FA, Kazmi M, Yasmeen A. Massive pulmonary embolism presenting as seizures. J Pak Med Assoc. (2016) 66:1656–8.
29. Lu SH, Wang YC, Wu YS, Huang SC, Lin CS. A rare cause of pulmonary embolism and seizure in a young man: antiphospholipid syndrome. Acta Cardiol Sin. (2016) 32:247–9. doi: 10.6515/acs20150413a
30. Pourshahid S, Dedhia S, Hakim S, Barakat M, Genin D. Seizure and pulmonary embolism: a differential that can save a life. Case Rep Pulmonol. (2017) 2017:1–2. doi: 10.1155/2017/3408795
31. Hu Z, Wen W, Wang X, Zhang X. Woman with seizures. Eur J Intern Med. (2021) 89:106–7. doi: 10.1016/j.ejim.2021.05.014
32. Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, et al. Prevalence and prognostic significance of T-wave inversions in right precordial leads of a 12-lead electrocardiogram in the middle-aged subjects. Circulation. (2012) 125:2572–7. doi: 10.1161/CIRCULATIONAHA.112.098681
33. Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the international society for computerized electrocardiology. J Am Coll Cardiol. (2009) 53:982–91. doi: 10.1161/CIRCULATIONAHA.108.191096
34. Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. (2006) 354:2317–27. doi: 10.1056/NEJMoa052367
35. Stein PD, Beemath A, Matta F, Weg JG, Yusen RD, Hales CA, et al. Clinical Characteristics of Patients with Acute Pulmonary Embolism: Data from PIOPED II. Am J Med. (2007) 120:871–9. doi: 10.1016/j.amjmed.2007.03.024
36. Paul JD, Cifu AS. Management of acute pulmonary embolism. JAMA. (2020) 324:597–8. doi: 10.1001/jama.2020.3905
37. Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani DJ, Desai ND, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the american heart association. Circulation. (2019) 140:e774–801. doi: 10.1161/CIR.0000000000000707
Keywords: anticoagulant therapy, massive pulmonary embolism, percutaneous catheter-directed treatment, seizure, surgical embolectomy, thromboembolic disease
Citation: Leong W, Zhang Y, Huang X, Luo Z, Wang Y, Rainer TH, Wai AKC and Huang Y (2022) Seizure as the clinical presentation of massive pulmonary embolism: Case report and literature review. Front. Med. 9:980847. doi: 10.3389/fmed.2022.980847
Received: 29 June 2022; Accepted: 25 October 2022;
Published: 21 November 2022.
Edited by:
David C. Rotzinger, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Carlos Jerjes-Sanchez, Escuela de Medicina y Ciencias de la Salud Tec Salud, Tecnológico de Monterrey, MexicoMansoor C. Abdulla, Sultan Qaboos Hospital, Oman
Copyright © 2022 Leong, Zhang, Huang, Luo, Wang, Rainer, Wai and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Huang, aHVhbmd5OUBoa3Utc3poLm9yZw==; Abraham K. C. Wai, d2VpamNAaGt1LXN6aC5vcmc=
†These authors have contributed equally to this work and share first authorship
 Waiian Leong1†
Waiian Leong1† Zhendong Luo
Zhendong Luo Yi Huang
Yi Huang