- 1Center for Reproductive Medicine, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 2Maternal and Child Health Hospital of Zoucheng, Zoucheng, Shandong, China
- 3Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, Shandong, China
Objective: The aim of this study was to investigate the effect of antibiotic treatment for chronic endometritis (CE) on reproductive outcomes.
Design: Systematic review and meta-analysis.
Patients: Women with reproductive failures, including recurrent implantation failure (RIF), and recurrent pregnancy loss (RPL).
Interventions: Literature searches were performed using three electronic databases (PubMed, Embase, and Web of Science) until 1 December 2021 (without date restriction). The following comparators were included: women with CE receiving antibiotics vs. untreated controls; women with cured CE vs. women with normal endometrial histology (negative for CE); and women with cured CE vs. women with persistent CE (PCE). The summary measures were indicated as odds ratio (OR) with a 95% confidence interval (CI).
Main outcome measures: These include on-going pregnancy rate/live birth rate (OPR/LBR), clinical pregnancy rate (CPR), and miscarriage rate/pregnancy loss rate (MR/PLR).
Results: A total of 2,154 women (from twelve studies) were enrolled. Compared with the control group, women with CE receiving antibiotics did not show a statistically significant difference in OPR/LBR (P = 0.09) and CPR (P = 0.36), although there was a lower MR (P = 0.03). Women with cured CE have higher OPR/LBR (OR 1.57) and CPR (OR 1.56) in comparison with those with non-CE. There was a statistically significantly higher OPR/LBR (OR 6.82, P < 0.00001) and CPR (OR 9.75, P < 0.00001) in women with cured CE vs. those with persistent CE.
Conclusion: While antibiotic treatment is a sensible option to cure CE, more well-designed prospective studies are needed to evaluate the reproductive impact of antibiotic treatment. Cured CE provides high-quality maternal conditions for subsequent embryo transfer and successful pregnancy.
Introduction
Chronic endometritis (CE) is an inflammatory disease characterized by the persistent presence of plasma cells in the endometrial stroma (1). CE often shows asymptomatic or subtle clinical disturbances, which consist of abnormal uterine bleeding (AUB), pelvic pain, and leukorrhea. Nevertheless, recent emerging studies demonstrate that CE may be associated with intrauterine pathological features such as polyps or fibroids and reproductive failures including recurrent pregnancy loss (RPL) and recurrent implantation failure (RIF) (2–8).
Chronic endometritis is a complex condition with many unresolved issues. Until today, no guideline or consent exists on how exactly to diagnose this condition, nor how best to treat it. Currently, the histological finding of infiltration of multiple plasmacytes into the endometrial stroma is considered the gold standard for CE diagnosis (9), but the amount of cells per sample/area or field remains unsettled (10). Based on the different diagnostic methods and investigated population, the prevalence of CE in infertile women varies considerably among different studies, from 2.8 to 86.5% (11–13). Interestingly, the incidence rate of CE was reported even higher, namely, ranging from 14 to 67.5% for women with RIF (5–7, 14–16) and 9.3–67.6% for recurrent miscarriage (RM) (3, 8, 12, 17, 18). Despite antibiotics being the primary prescription for CE, depending on the infectious agent detected and on the antibiogram result, the types, dosages, durations, and routes were inconsistent (19). Therefore, the cure rates of CE were reported to range from 52.94 to 100% after antibiotic therapy in previous studies (6, 8, 11, 12, 20). Some publications suggest that the administration of oral antibiotics could improve reproductive outcomes (6–8). The question of whether antibiotics are appropriate in the cure and relevant for pregnancy outcomes in patients with CE is important and still not completely clarified. For this reason, the aims of our systematic review and meta-analysis are to evaluate the reproductive effects of antibiotic treatment for chronic endometritis (CE) in women with RIF or RPL.
Materials and methods
Search strategy
Literature searches were performed using three electronic databases (PubMed, Embase, and Web of Science) until 1 December 2021 (without date restriction). Key search terms were as follows: (chronic endometritis OR endometrial inflammation OR CD138 OR plasma cells) AND (infertility OR repeated implantation failure OR repetitive implantation failure OR recurrent implantation failure OR recurrent pregnancy loss OR recurrent miscarriage OR recurrent spontaneous abortion). We also did a manual search to avoid missing relevant publications from the reference lists of key articles.
Eligibility criteria
The inclusion criteria were as follows: (1) experimental or observational studies in the English language; (2) participants who experienced reproductive failures, including infertility, recurrent implantation failure (RIF), and recurrent pregnancy loss (RPL); (3) all women who underwent diagnostic hysteroscopy and endometrial biopsy for histological analysis to confirm CE; and (4) all women who received assisted reproductive technology (ART) or attempted spontaneous pregnancy were monitored the reproductive outcomes.
The exclusion criteria were as follows: (1) studies without complete data; (2) studies such as case reports and reviews; and (3) studies evaluating other types of endometrial inflammation (e.g., acute, subacute, or tubercular endometritis).
Study selection and data extraction
Two investigators independently reviewed the inclusion criteria to select articles that qualified. Any disagreement was resolved through discussions with a third reviewer. Two investigators independently extracted the outcome data and study characteristics from eligible studies using piloted screening forms in Microsoft Office Excel. The results were examined repeatedly and discrepancies were discussed until a consensus was reached.
Comparators
Comparators were as follows: (1) Women with treated CE vs. untreated CE: defined as women receiving antibiotic treatment for CE vs. women with CE not receiving antibiotics. Control biopsy was not performed. (2) Women with cured CE vs. non-CE: defined as women with CE resolution (after antibiotic therapy) vs. women negative for CE (with normal endometrial histology). (3) Women with cured CE vs. persistent CE: defined as women in whom (after antibiotic therapy) a control biopsy showed the resolution of CE vs. those in which CE was still present.
Outcomes
Outcomes were on-going pregnancy or live birth rate [per patient (OPR/LBR)]: “on-going pregnancy” was defined as maintenance of pregnancy at 12 weeks or later of gestation; “live birth” was defined as a birth of at least one newborn after 24 weeks of gestation; clinical pregnancy rate [per patient (CPR)] was defined as the appearance of an intrauterine gestational sac with positive cardiac movement as documented by trans-vaginal ultrasonography (21); miscarriage rate or pregnancy loss rate [per clinical pregnancy (MR/PLR)] was defined as a pregnancy loss before 24 weeks of gestation.
Risk of bias
The quality assessment of all included studies was implemented by two reviewers based on the Newcastle-Ottawa Scale (NOS) for observational studies.
Statistical analysis
The meta-analysis was performed using Review Manager version 5.4.1 (Nordic Cochrane Centre, Cochrane Collaboration). All outcomes were compared, and any differences were discussed. Study outcomes were expressed using an odds ratio (OR) with a 95% confidence interval (95% CI). A P-value of <0.05 was defined as indicative of a statistically significant difference in results. Heterogeneity was assessed by presenting forest plots and by calculating the I2 value (>50% was considered extensive heterogeneity). If only I2 < 50%, heterogeneity was acceptable. When heterogeneity was high, a random-effects model was used to estimate study results; otherwise, the fixed-effects meta-analysis was performed. Potential publication bias was also illustrated qualitatively with a funnel plot using the Rev Man software if the distribution of CIs was significantly different.
Results
Study inclusion and basic characteristics
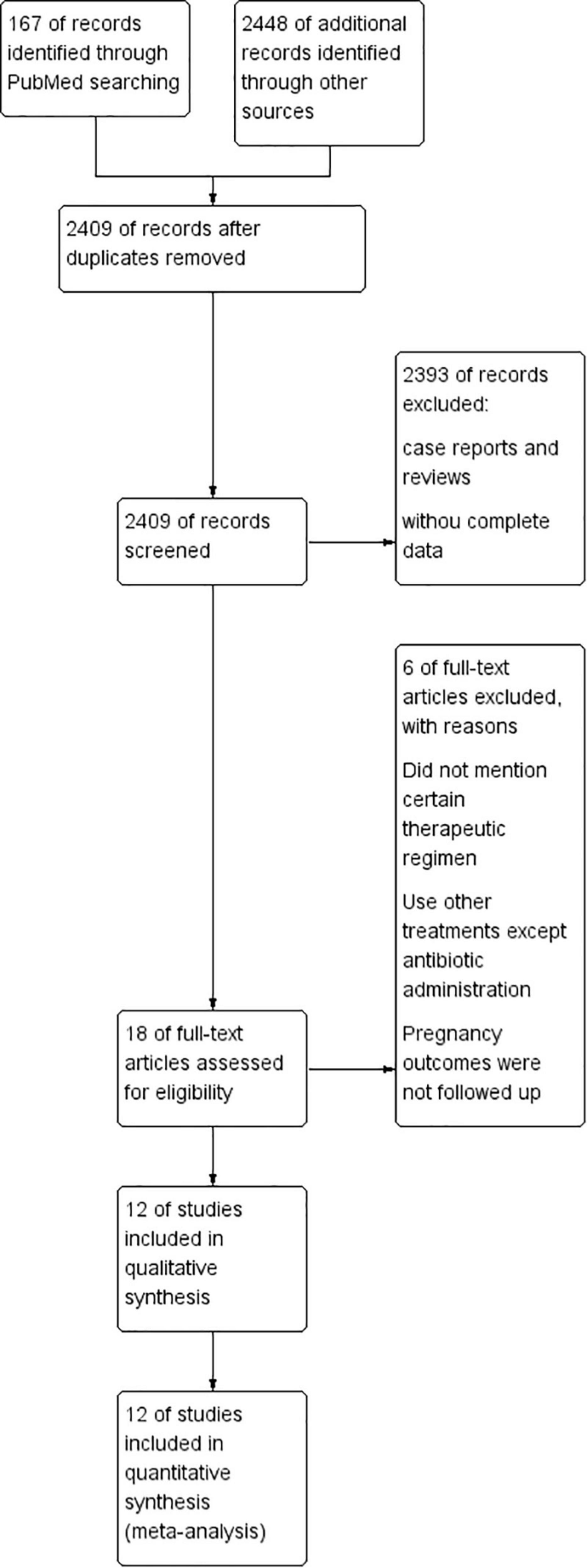
The search strategy initially retrieved 2,615 potentially relevant publications (PubMed: 167, EMBASE: 237, and Web of Science: 2,211). After removing duplicates, the titles and abstracts of the remaining 2,409 records were screened. Then, 18 studies were preselected for inclusion. After an assessment of the eligibility criteria, six articles were excluded (they did not mention certain therapeutic regimens; used other treatments except antibiotic administration; pregnancy outcomes were not well followed up). Finally, a total of 12 studies (3, 6–8, 12, 14–18, 20, 22) were included in the present meta-analysis (Figure 1).
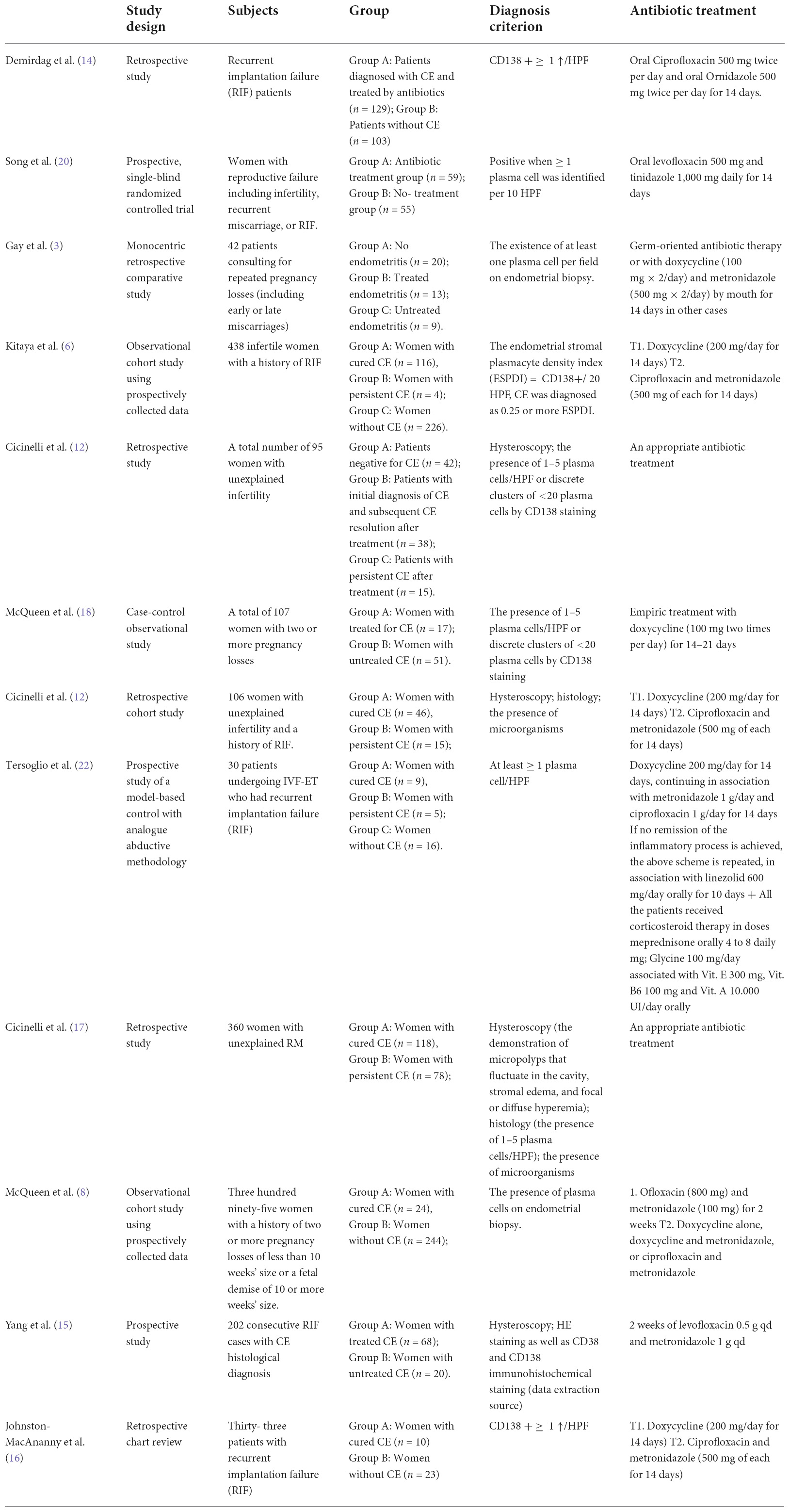
With respect to the study design, most studies included in this review were observational studies, of which six were retrospective studies, five were prospective studies, and one was a case-control study. The detailed baseline characteristics of the included studies are presented in Table 1.
Population
All studies enrolled 2,154 women with PRL/RM and RIF. Recurrent pregnancy loss (RPL) was defined as the loss of two or more clinically recognized pregnancies occurring before 20–24 weeks of gestation and includes embryonic and fetal losses (23). Recurrent implantation failure (RIF) was defined as failure to achieve a clinical pregnancy after the transfer of at least four good-quality embryos in a minimum of three fresh or frozen cycles in a woman under the age of 40 years (24).
Diagnosis of chronic endometritis
Currently, CE is diagnosed by endometrial biopsy, and the presence of plasma cells in the endometrial stroma is the generally accepted histological diagnostic criterion for CE. Plasma cells were identified in the stroma by traditional hematoxylin and eosin (H&E) staining alone. Thus, immunohistochemistry (IHC) for detection of the plasma cell marker CD138 (also known as syndecan-1) is used clinically to diagnose CE since it stains well on the surface of plasma cells. In most studies in this review, the diagnosis was based on the demonstration of at least one CD138-positive plasma cell/HPF. However, in Cicinelli’s studies, the diagnosis of CE was initially based on the demonstration of micropolyps that fluctuate in the cavity, stromal edema, and focal or diffuse hyperhemia, as previously published (7, 12, 17). In the follicular phase of the subsequent cycle following the treatment, all the patients were reevaluated uterine cavity by hysteroscopy for signs of CE and collected endometrial samples for histology and culture (25).
Treatment of chronic endometritis
To date, the first-line treatment protocol for CE was oral empiric antibiotics (doxycycline 100 mg two times a day for 14 days; ciprofloxacin and metronidazole 500 mg two times a day for 14 days). However, Cicinelli et al. (12, 17) also selected appropriate antibiotics according to the results of drug sensitivity and administered bacterium-sensitive antibiotics for 2 weeks as the second line. The detailed treatment regimens are presented in Table 1.
Quality assessment of the risk of study bias
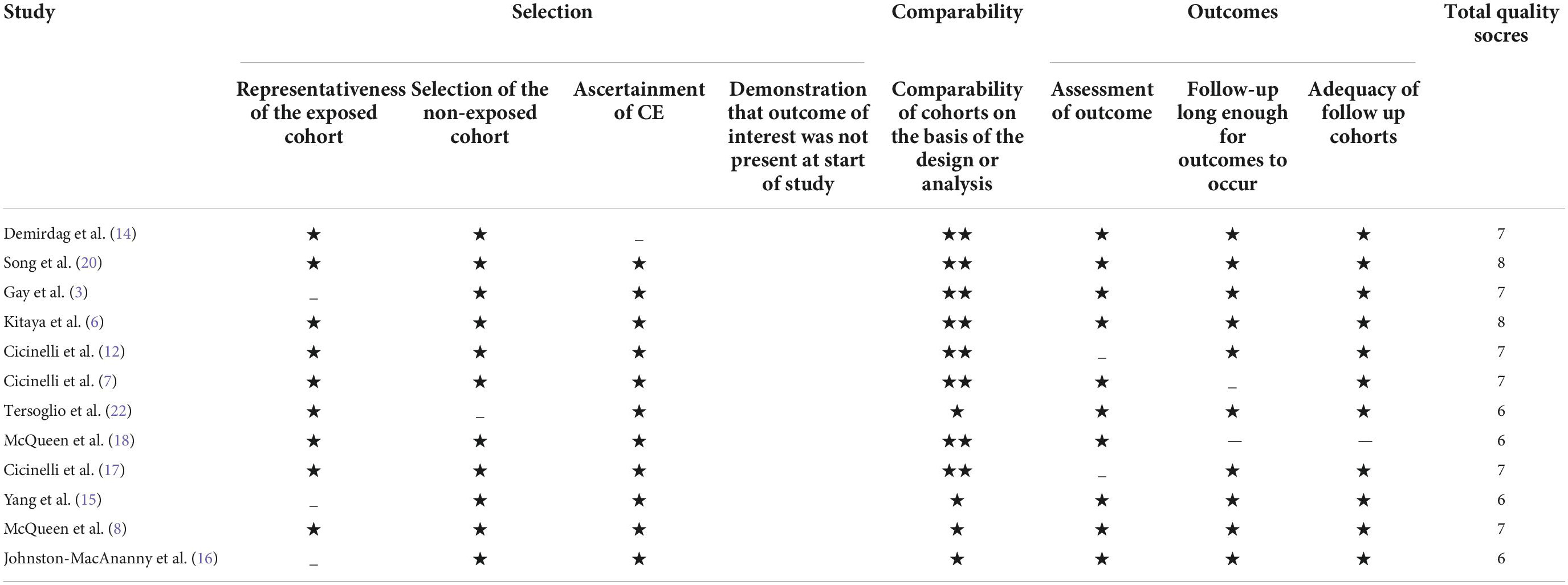
Half of the included studies (n = 12) were awarded seven stars, four studies were awarded six stars, and only two studies scored eight stars. The Newcastle-Ottawa Quality Assessment Scale is shown in Table 2.
Synthesis of results
Treated chronic endometritis versus untreated chronic endometritis
Compared with the control group, women with CE receiving antibiotics did not show a statistically significant difference in OPR/LBR (OR = 1.68, 95% CI = 0.93–3.03, I2 = 0%, P = 0.09) and CPR (OR = 1.33, 95% CI = 0.72–2.44, I2 = 0%, P = 0.36), although there was a lower MR (OR = 0.25, 95% CI = 0.07–0.90, I2 = 0%, P = 0.03; Figure 2). Sensitivity analysis was not performed due to minimal inconsistency (I2 = 0%).
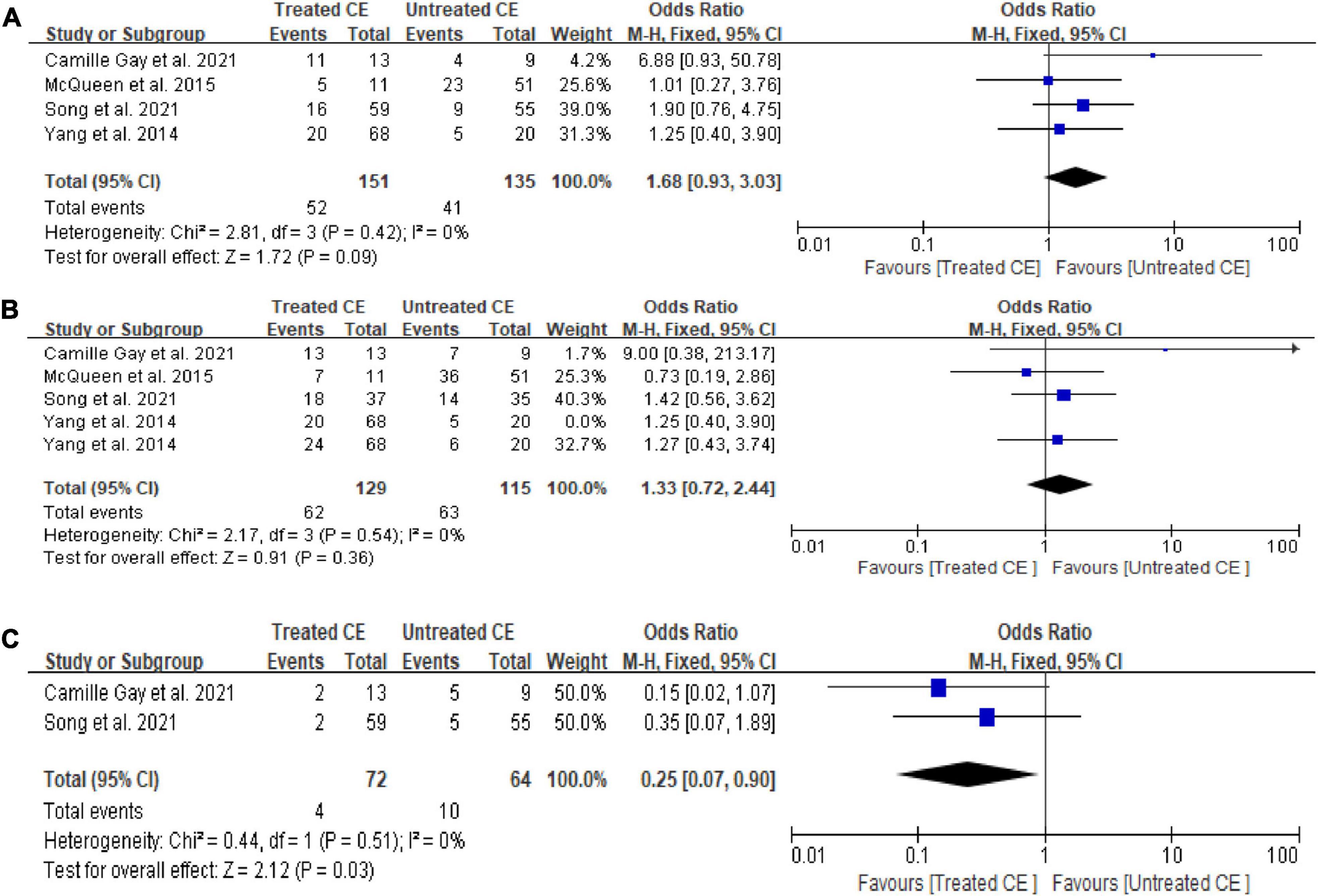
Figure 2. Forest plot of comparison: Treated chronic endometritis vs. Untreated: (A) on-going pregnancy/live birth rate; (B) clinical pregnancy rate; (C) miscarriage rate/pregnancy loss rate. M-H Mantal Haenszel.
Cured chronic endometritis versus non-chronic endometritis
We found higher OPR/LBR (OR = 1.57, 95% CI = 1.18–2.11, I2 = 81%, P = 0.002) and CPR (OR = 1.56, 95% CI = 1.15–2.12, I2 = 84%, P = 0.004) in women with cured CE in comparison with those with non-CE, with no difference in terms of MR/PLR (P = 0.73; Figure 3). The exclusion of the study by Cicinelli et al. (12) from the pooled analysis did yield significant changes to OPR/LBR (I2 = 68%, P = 0.17) and CPR (I2 = 42%, P = 0.40).
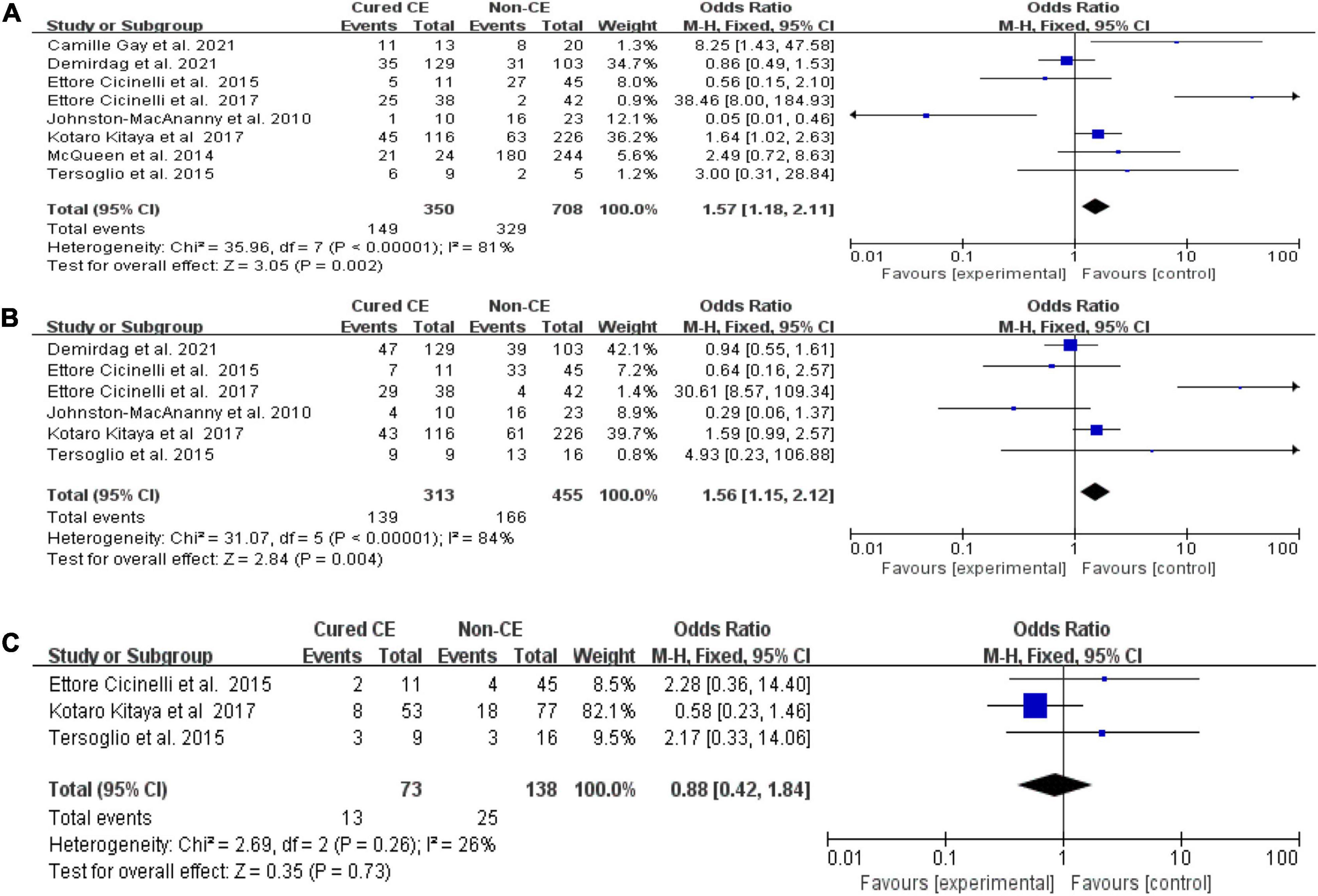
Figure 3. Forest plot of comparison: Cured chronic endometritis vs. Non-chronic endometritis. (A) on-going pregnancy/live birth rate; (B) clinical pregnancy rate; (C) miscarriage rate/pregnancy loss rate. M-H Mantal Haenszel.
Cured chronic endometritis versus persistent chronic endometritis
There was a statistically significantly higher OPR/LBR (OR = 6.82, 95% CI = 4.18–11.14, I2 = 0% P < 0.00001) and CPR (OR = 9.75, 95% CI = 4.11–23.13, I2 = 0%, P < 0.00001) in women with cured CE vs. those with persistent CE. No significant differences were found in MR/PLR (OR = 0.80, 95% CI = 0.30–2.14, I2 = 18%, P = 0.65; Figure 4).
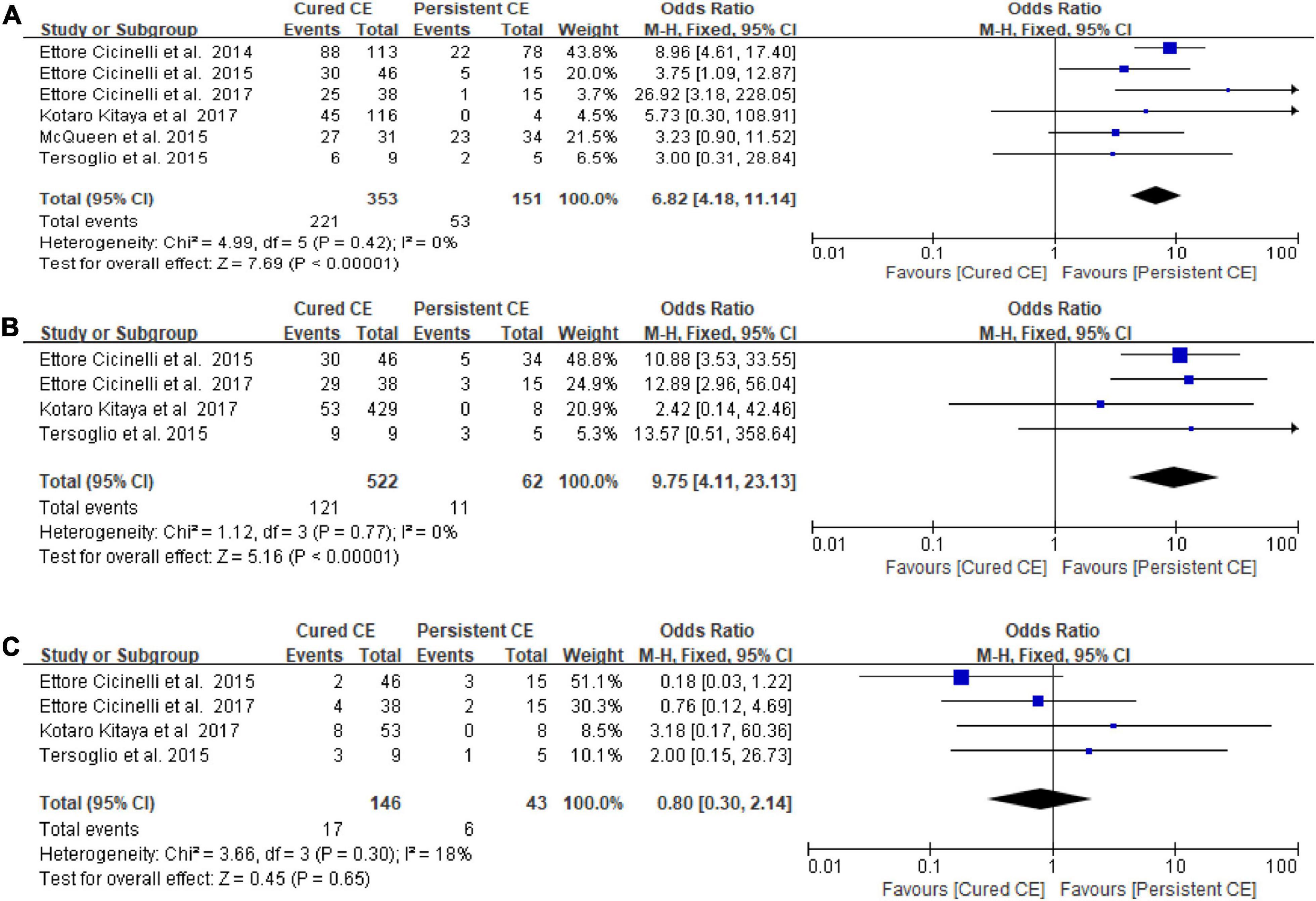
Figure 4. Forest plot of comparison: Cured chronic endometritis vs. Persistent chronic endometritis. (A) on-going pregnancy/live birth rate; (B) clinical pregnancy rate; (C) miscarriage rate/pregnancy loss rate. M-H Mantal Haenszel.
Discussion
Main findings
In this systematic review and meta-analysis, our results show that there was no statistically significant difference in OPR/LBR and CPR for women with CE receiving treatment vs. those not receiving therapy. Nevertheless, women with cured CE by effective treatment considerably improve in the clinical pregnancy rate and live birth rate/on-going pregnancy rate in comparison with those with persistent CE. Hence, we have considered that a repeat control biopsy should be performed to assess patients for CE resolution. The above findings suggest that CE is associated with adverse reproductive outcomes, such as RIF and RPL, whose accurate evaluation and effective treatment can promote the chance of successful pregnancy and live birth.
Interpretation and implications
A variety of studies in a population with a poor prognosis (repeated implantation failure and recurrent miscarriage) have suggested that a regimen of oral antibiotics for CE, which is a promising therapeutic strategy, could eliminate endometrium stromal plasma cells (ESPC) and improve reproductive outcomes to some extent (7, 8, 15, 17). However, two studies conclude that reproductive outcomes may not be improved after a single course of oral broad-spectrum antibiotics (14, 20). For one reason, in a study by Song et al. (20), the reproductive outcomes as subordinate endpoints were not found to have an adequately significant effect in distinct discrepancies between the groups (+17.5% OPR and 8.9% MR in the treatment arm vs. controls). Therefore, a further RCT with much larger sample size and a more homogeneous population is needed to be conducted based on a clinically oriented primary endpoint. In contrast, the diagnosis of CE depends on the immunohistochemical detection of plasma cells in endometrial biopsy samples, which produces a methodological bias in the assessment of CE cure. An assessment that was calculated as all CD138+ cell counts in an entire section evaluated divided by the account of the unit area could overcome the problem of local fluctuations in plasmacyte count as well as rectify the variation in results caused by sample size differences (10). Moreover, endometrial biopsy is actually a local scratch or injury to the endometrium, which has been found to improve IVF outcomes and subsequent clinical pregnancy and birth (26–28). In addition, there is a lack of consensus regarding optimal antibiotics, dose, and duration for the treatment of chronic endometritis. In clinical practice, even the microorganism causing the infection is frequently not identified, broad-spectrum antibiotics are usually prescribed, which can contribute to a high rate of recurrent infections after treatment, as well as side effects derived from the clearance of endogenous off-target microbiota in the uterine cavity and other body sites (29). If identification of microorganisms were carried out, antibiotic guidelines could be adapted to the pathogen found and to any possible allergy the patient might have to the antibiotics used (7, 12).
Interestingly, we found that the abortion rate decreased after antibiotic treatment, which may be related to the modification of the endometrial microenvironment. Recurrent pregnancy loss has been related to subclinical infection, endometrial inflammation status, and the abnormal endometrial microenvironment. The presence of CE can modify the receptivity of the endometrium with an abnormal microbiome environment that disturbs normal implantation (30). For successful implantation, mediators of inflammation such as leukocytes, cytokines, chemokines, and other endometrial factors (31–33), which play a crucial role in the regulation of immune status (34) and growth of the trophoblast, may modify endometrial receptivity. CE also alters uterine contractility in both the periovulatory and mid-luteal phases, which could help explain some symptoms such as pelvic pain, AUB, and implantation failure (35). Furthermore, the presence of CE may affect implantation and the establishment of pregnancy through disturbing decidualization in vitro and weakening the action of progesterone on endometrial stromal cells (ESC) (36). These findings may offer suggestions for the presence of chronic endometritis before pregnancy, which may be beneficial for future fertility treatment. Consequently, appropriate administration of antibiotics could not only decrease infectious agents for cured histopathologic CE but also be essential to improve endometrial receptivity.
Strength and limitations
The strength of this study comprises the rigorous design and comprehensive review, with a literature search completed by an information specialist. The characteristics of the included studies were summarized in detail. A particular novelty of our review is that we estimate the effects of therapy for CE in a population with RIF and RPL. In RIF and RPL cases, the accurate detection and therapy of chronic endometritis would avoid the excessive use of unnecessary assisted reproductive tests and could reduce financial uncertainty and shorten the time. Our study may open new cues in promoting future well-designed studies, providing essential information to scientists regarding the design of optimal management of CE diagnosis (and treatment).
There are also several limitations to be considered in this review. Initially, as no agreed gold standard or guidelines for the diagnosis and treatment of CE exists, it is very hard to group the trials according to similar procedures and standards. This also explains the large variety of prevalence presented in different studies. The inconsistent use of endometrial culture and antibiotic regimens (type of drug and duration) as well as different ovarian stimulation protocols and IVF-ET process may cause confounding bias in the results in evaluating the impacts of CE treatment on reproductive outcomes. Additionally, the ascertainment method of chronic endometritis resolution and the times of repeated hysteroscopy and biopsy for histopathologic CD138 immunohistochemical examination until the features were negatively varied among studies, potentially producing a deviation in CE detection. Furthermore, enrolled women with heterogeneous characteristics (Table 1) (i.e., suffering from repeated implantation failure and recurrent miscarriage would potentially lead to diverse estimates of the reproductive outcomes, but it can ensure the generality of results). Finally, what is effectively lacking are randomized clinical trials to improve the quality of analysis.
Conclusion
The present meta-analysis demonstrates that while antibiotic treatment is a sensible option to cure CE, more well-designed prospective studies are needed to evaluate the reproductive impact of antibiotic treatment. The control biopsy should be performed to confirm CE resolution (at histology). Cured CE provides high-quality maternal conditions for subsequent embryo transfer and successful pregnancy.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JL and LY independently reviewed the inclusion criteria to select articles qualified. LC resolved any disagreement through discussion. ZL and YL independently extracted the outcome data and study characteristics from eligible studies using piloted screening forms in Microsoft Office Excel. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (82071617).
Acknowledgments
The authors thank all participants in the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Crum CP, Egawa K, Fenoglio CM, Richart RM. Chronic endometritis: the role of immunohistochemistry in the detection of plasma cells. Am J Obstetr Gynecol. (1983) 147:812–5. doi: 10.1016/0002-9378(83)90045-5
2. Elder S, Bortoletto P, Romanski PA, Spandorfer S. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol. (2021) 86:e13410. doi: 10.1111/aji.13410
3. Gay C, Hamdaoui N, Pauly V, Rojat Habib M-C, Djemli A, Carmassi M, et al. Impact of antibiotic treatment for chronic endometritis on unexplained recurrent pregnancy loss. J Gynecol Obstetr Hum Reprod. (2021) 50:102034.
4. Kuroda K, Horikawa T, Moriyama A, Nakao K, Juen H, Takamizawa S, et al. Impact of chronic endometritis on endometrial receptivity analysis results and pregnancy outcomes. Immun Inflamm Dis. (2020) 8:650–8. doi: 10.1002/iid3.354
5. Zhang Y, Xu H, Liu Y, Zheng S, Zhao W, Wu D, et al. Confirmation of chronic endometritis in repeated implantation failure and success outcome in IVF-ET after intrauterine delivery of the combined administration of antibiotic and dexamethasone. Am J Reprod Immunol. (2019) 82:e13177. doi: 10.1111/aji.13177
6. Kitaya K, Matsubayashi H, Takaya Y, Nishiyama R, Yamaguchi K, Takeuchi T, et al. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am J Reprod Immunol (2017) 78:e12719. doi: 10.1111/aji.12719
7. Cicinelli E, Matteo M, Tinelli R, Lepera A, Alfonso R, Indraccolo U, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. (2015) 30:323–30. doi: 10.1093/humrep/deu292
8. McQueen DB, Bernardi LA, Stephenson MD. Chronic endometritis in women with recurrent early pregnancy loss and/or fetal demise. Fertil Steril. (2014) 101:1026–30.
9. Kitaya K, Matsubayashi H, Yamaguchi K, Nishiyama R, Takaya Y, Ishikawa T, et al. Chronic endometritis: potential cause of infertility and obstetric and neonatal complications. Am J Reprod Immunol. (2016) 75:13–22.
10. Liu Y, Chen X, Huang J, Wang C-C, Yu M-Y, Laird S, et al. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil Steril. (2018) 109:832–9. doi: 10.1016/j.fertnstert.2018.01.022
11. Xiong Y, Chen Q, Chen C, Tan J, Wang Z, Gu F, et al. Impact of oral antibiotic treatment for chronic endometritis on pregnancy outcomes in the following frozen-thawed embryo transfer cycles of infertile women: a cohort study of 640 embryo transfer cycles. Fertil Steril. (2021) 116:413–21. doi: 10.1016/j.fertnstert.2021.03.036
12. Cicinelli E, Matteo M, Trojano G, Mitola PC, Tinelli R, Vitagliano A, et al. Chronic endometritis in patients with unexplained infertility: prevalence and effects of antibiotic treatment on spontaneous conception. Am J Reprod Immunol. (2017) 79:e12782. doi: 10.1111/aji.12782
13. Kimura F, Takebayashi A, Ishida M, Nakamura A, Kitazawa J, Morimune A, et al. Review: chronic endometritis and its effect on reproduction. J Obstetr Gynaecol Res. (2019) 45:951–60.
14. Demirdag E, Guler I, Akdulum MF, Sahin E, Erdem O, Erdem A, et al. Subsequent IVF outcomes following antibiotic therapy for chronic endometritis in patients with recurrent implantation failure. J Obstet Gynaecol Res. (2021) 47:4350–6.
15. Yang R, Du X, Wang Y, Song X, Yang Y, Qiao J. The hysteroscopy and histological diagnosis and treatment value of chronic endometritis in recurrent implantation failure patients. Arch Gynecol Obstetr. (2014) 289:1363–9.
16. Johnston-MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. (2010) 93:437–41. doi: 10.1016/j.fertnstert.2008.12.131
17. Cicinelli E, Matteo M, Tinelli R, Pinto V, Marinaccio M, Indraccolo U, et al. Chronic endometritis due to common bacteria is prevalent in women with recurrent miscarriage as confirmed by improved pregnancy outcome after antibiotic treatment. Reprod Sci. (2014) 21:640–7. doi: 10.1177/1933719113508817
18. McQueen DB, Perfetto CO, Hazard FK, Lathi RB. Pregnancy outcomes in women with chronic endometritis and recurrent pregnancy loss. Fertil Steril. (2015) 104:927–31.
19. Huang W, Liu B, He Y, Xie Y, Liang T, Bi Y, et al. Variation of diagnostic criteria in women with chronic endometritis and its effect on reproductive outcomes: a systematic review and meta-analysis. J Reprod Immunol. (2020) 140:103146. doi: 10.1016/j.jri.2020.103146
20. Song D, He Y, Wang Y, Liu Z, Xia E, Huang X, et al. Impact of antibiotic therapy on the rate of negative test results for chronic endometritis: a prospective randomized control trial. Fertil Steril. (2021) 115:1549–56. doi: 10.1016/j.fertnstert.2020.12.019
21. Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. (2009) 92:1520–4.
22. Tersoglio AE, Salatino DR, Reinchisi G, Gonzalez A, Tersoglio S, Marlia C. Repeated implantation failure in oocyte donation. What to do to improve the endometrial receptivity? JBRA Assist Reprod. (2015) 19:44–52. doi: 10.5935/1518-0557.20150012
23. Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. (2018) 2018:hoy004.
24. Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod Biomed Online. (2014) 28:14–38.
25. Bouet P-E, El Hachem H, Monceau E, Gariépy G, Kadoch I-J, Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. (2016) 105:106–10. doi: 10.1016/j.fertnstert.2015.09.025
26. Günther V, von Otte S, Maass N, Alkatout I. Endometrial “Scratching” an update and overview of current research. J Turk German Gynecol Assoc. (2020) 21:124–9. doi: 10.4274/jtgga.galenos.2020.2019.0175
27. Bar G, Harlev A, Alfayumi-Zeadna S, Zeadna A, Bord I, Har-Vardi I, et al. Recurrent implantation failure: which patients benefit from endometrial scratching prior to IVF? Arch Gynecol Obstetr. (2020) 301:817–22.
28. Gürgan T, Kalem Z, Kalem MN, Ruso H, Benkhalifa M, Makrigiannakis A. Systematic and standardized hysteroscopic endometrial injury for treatment of recurrent implantation failure. Reprod Biomed Online. (2019) 39:477–83. doi: 10.1016/j.rbmo.2019.02.014
29. Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. (2006) 193:1478–86. doi: 10.1086/503780
30. Moreno I, Cicinelli E, Garcia-Grau I, Gonzalez-Monfort M, Bau D, Vilella F, et al. The diagnosis of chronic endometritis in infertile asymptomatic women: a comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am J Obstetr Gynecol. (2018) 218:602.e1–e16. doi: 10.1016/j.ajog.2018.02.012
31. Tortorella C, Piazzolla G, Matteo M, Pinto V, Tinelli R, Sabbà C, et al. Interleukin-6, interleukin-1β, and tumor necrosis factor α in menstrual effluents as biomarkers of chronic endometritis. Fertil Steril. (2014) 101:242–7. doi: 10.1016/j.fertnstert.2013.09.041
32. Di Pietro C, Cicinelli E, Guglielmino MR, Ragusa M, Farina M, Palumbo MA, et al. Altered transcriptional regulation of cytokines, growth factors, and apoptotic proteins in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol. (2013) 69:509–17. doi: 10.1111/aji.12076
33. Mishra K, Wadhwa N, Guleria K, Agarwal SER. PR and Ki-67 expression status in granulomatous and chronic non-specific endometritis. J Obstetr Gynaecol Res. (2008) 34:371–8. doi: 10.1111/j.1447-0756.2007.00700.x
34. Li Y, Yu S, Huang C, Lian R, Chen C, Liu S, et al. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil Steril. (2020) 113:187–96.e1. doi: 10.1016/j.fertnstert.2019.09.001
35. Pinto V, Matteo M, Tinelli R, Mitola PC, de Ziegler D, Cicinelli E. Altered uterine contractility in women with chronic endometritis. Fertil Steril. (2015) 103:1049–52. doi: 10.1016/j.fertnstert.2015.01.007
Keywords: chronic endometritis, infertility, antibiotic treatment, live birth rate, miscarriage rate
Citation: Liu J, Liu ZA, Liu Y, Cheng L and Yan L (2022) Impact of antibiotic treatment for chronic endometritis on pregnancy outcomes in women with reproductive failures (RIF and RPL): A systematic review and meta-analysis. Front. Med. 9:980511. doi: 10.3389/fmed.2022.980511
Received: 03 July 2022; Accepted: 21 September 2022;
Published: 03 November 2022.
Edited by:
Ursula Catena, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Shangrong Fan, Shenzhen Hospital, Peking University, ChinaNazan Yurtcu, Sivas Cumhuriyet University, Turkey
Copyright © 2022 Liu, Liu, Liu, Cheng and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Yan, eWFubGVpQHNkdS5lZHUuY24=; Lei Cheng, cWxma3FkQDE2My5jb20=
 Jingjing Liu1
Jingjing Liu1 Lei Yan
Lei Yan