- 1Department of Dermatology, University of Lübeck, Lübeck, Germany
- 2Manchester Sciences, University of Manchester, Manchester, United Kingdom
- 3Lübeck Institute of Experimental Dermatology, University of Lübeck, Lübeck, Germany
- 4Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel
Background: Cutaneous sarcoidosis is a relatively rare disease whose clinical manifestations include red-brown macules, plaques, papules and subcutaneous nodules. The skin changes may also be restricted to pre-existing scars. Cutaneous sarcoidosis can be associated with systemic organ involvement.
Objectives: Aim of this retrospective study was to longitudinally investigate clinical and laboratory findings in patients with cutaneous sarcoidosis.
Methods: Patients (>18 years) with histologically confirmed cutaneous sarcoidosis between January 2014 and December 2020 were included. Patient demographics, clinical features, laboratory and radiological findings, management, clinical outcomes and co-morbidities associated with cutaneous sarcoidosis were analyzed.
Results: Thirty-seven patients with cutaneous sarcoidosis were identified, of whom 57% were female. The most common clinical phenotype of cutaneous sarcoidosis was papular sarcoidosis (n = 16), while plaques and nodules were present in 9 patients. In contrast, subcutaneous (n = 1) and scar-associated sarcoidosis (n = 1) were rare. Of patients with systemic disease, the cutaneous disease followed, preceded, and coincided with the development of systemic sarcoidosis in 2, 9, and 12 patients, respectively. Levels of soluble interleukin (IL)-2 receptor, angiotensin converting enzyme (ACE), and C-reactive protein (CRP) were elevated, in 76%, 21%, and 50% of the tested patients respectively and predicted systemic involvement. Hypercalcemia was present in 6% of patients. Female sex and younger age (<54 years) were significantly associated with systemic manifestations.
Conlcusions: Cutaneous sarcoidosis was frequently associated with additional systemic involvement, particularly when present in young females. 24 % of patients with cutaneous sarcoidosis developed additional organ involvement during follow-up.
Introduction
Sarcoidosis is a multi-system disease that may involve almost any organ system (1). Cutaneous sarcoidosis lesions may be the only clinical manifestation, yet patients with sarcoid skin lesions may have involvements of other organs, including the lungs, lymph nodes, eye, liver, heart, joints and brain (1–3). Cutaneous sarcoidosis can manifest with sarcoid specific skin lesions, these changes typically show granuloma formation in the tissue, non-specific sarcoid skin lesions on the contrary present histologically with inflammation in the absence of granulomas (1, 3). Clinically, the disease can present with red, red-brown or violaceous colored macules, papules, plaques or nodules (Figures 1a–g). There may be single or multiple lesions, which may exhibit epidermal involvement or prominent telangiectasia (4, 5). Lupus pernio (bluish-red or violaceous nodules/plaques over nose, cheeks and ears), subcutaneous nodules (Darier-Roussy disease), and scar- and tattoo associated sarcoidosis, are recognized clinical presentations (4, 5). Of note, all these described skin lesions are sarcoid specific, whereas the typical non-specific sarcoid skin manifestation is erythema nodosum. Characterstically, erythema nodosum, manifests as tender erythematous nodules with subcutaneous infiltration (Figure 1h), commonly on the ventral aspect of the lower leg, showing the histological findings of panniculitis with septal inflammation (1, 2). Given that sarcoidosis is a multisystem disease, the diagnosis requires involvement of at least two organs. Thus, whether skin-only sarcoidosis can be classified as sarcoidosis or should be termed sarcoid-like granulomatous disease remains controversial (1, 2).
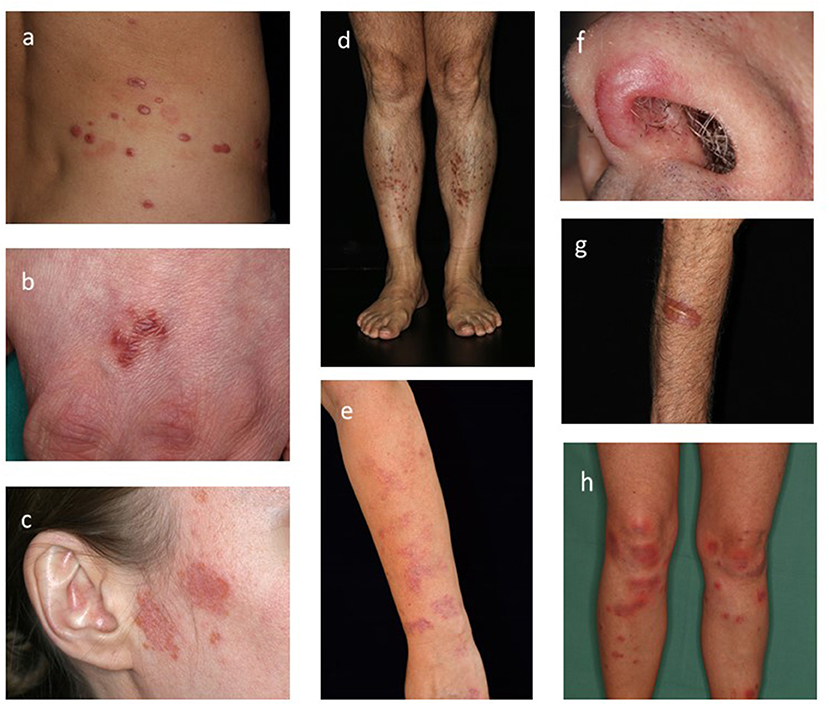
Figure 1. Clinical hallmarks of cutaneous sarcoidosis. (a–g) Specific sarcoid skin lesions. (a) Multiple violaceous nodules on the trunk. (b) Solitary red-brown plaques on the back of the hand. (c) red atrophic plaques with slight epidermal involvement. (d) Multiple small red-brown papules on the lower leg. (e) Bright red colored macules non the arm. (f) Violaceous plaque on the nose (Lupus pernio). (g) Red plaque associated to a scar. (h) Non-specific skin lesion: tender erythematous nodules with subcutaneous infiltration on the ventral lower leg (Erythema nodosum).
The treatment of cutaneous lesions can be challenging, but the application of potent/superpotent topical steroids remains the mainstay of treatment (1, 2). For patients with severe or progressive skin lesions and/or widespread cutaneous involvement, systemic corticosteroids may be required (1, 2). In addition, steroid-sparing agents such as hydroxychloroquine and methotrexate are used (6, 7). Although randomized, controlled trials are lacking in cutaneous sarcoidosis, multiple reports suggest efficacy of agents such as azathioprine, tetracylcine, thalidomide, and infliximab (1, 2).
To date, large multi-center cohort data on clinical phenotypes and therapeutic responses in cutaneous-dominant sarcoidosis, remain scant (8–18). The aim of this study was to analyzed the clinical features and non-skin organ involvement in patients with cutaneous sarcoidosis.
Methods
Study population and definition of eligible cases
The current retrospective cohort study included all patients diagnosed with cutaneous sarcoidosis between January 1st, 2014, and December 31th, 2020 in the Department of Dermatology, University of Lubeck, Lubeck, Germany. The study was approved by the institutional ethical committee (21–292) in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects. Cutaneous sarcoidosis was diagnosed based on compatible clinical manifestation in conjunction with typical histological features in all patients. Histopathological criteria for diagnosing cutaneous sarcoidosis were a granulomatous reaction pattern with discrete predominantly epithelioid granulomas without necrosis (non-caseating/naked granulomas), with few surrounding lymphocytes and often a rim of mild dermal fibrosis. Granuloma-forming infections (mycobacterial and fungal infections, leishmaniasis) as well as foreign body reactions, granulomatous rosacea, granuloma anunulare and granulomatosis with polyangiitis were exluded in all cases. Eligible patients were longitudinally followed from their diagnosis up to April 30th, 2021.
Definition of covariates
The medical records of eligible patients were systematically reviewed, and the following variables were retrieved: clinical and morphological features, utilized therapeutic modalities (local/systemic treatment), co-morbidities and routine laboratory and radiologic findings at baseline. Impaired lung function was defined by pathological pulmonary function tests. Severe hilar lymphadenopathy was considered in the radiologic stages I-II of the disease (19).
Exposure to immune-modulating drugs was defined in patients managed by any of the following agents: systemic corticosteroids, doxycycline, azathioprine, methotrexate, hydroxychloroquine, and infliximab. Complete blood count parameters, C-reactive protein (CRP), angiotensin-converting enzyme (ACE), and soluble interleukin 2 receptor (sIL-2R) levels were assessed prior to the administration of any systemic therapy. The globally acceptable cutoffs were adopted to define leukocytosis, neutrophilia, thrombocytosis, and elevated CRP (20, 21). Elevated levels of ACE, sIL-2R, and calcium were defined beyond the cutoffs of 82 U/L, 623 kU/L, and 2.55 mmol/L, respectively.
Statistical analysis
All continuous parameters were expressed as mean values (standard deviation [SD]). Percentages of different subgroups were compared by a Chi-square test. Normally distributed continuous variables were compared using the student t-test. To identify predictors of systemic involvement and acute disease course, a logistic regression model was employed to calculate odds ratios (ORs) and 95% (confidence intervals) CIs. SPSS software, version 25 (SPSS, Armonk, NY: IBM Corp), was used to perform all statistical analyses.
Results
Demographic and morphological features
Thirty-seven patients with cutaneous sarcoidosis were analyzed, of whom 21 (57%) were females and 16 (43%) were males. The mean (SD) and median (range) age of included patients was 52.2 (12.5) and 54.0 (22.0–77.0) years, respectively. The most frequently encountered manifestation of cutaneous sarcoidosis was papules (n = 16), whereas 9 patients displayed plaques and nodules. One patient had the subcutaneous variant (Darier-Roussy disease) and another had scar-associated sarcoidosis. Ten (27%) patients presented with erythema nodosum (Table 1). All patients were of the Caucasian ethnic group.
Systemic involvement and disease course
While 14 (38%) patients had isolated cutaneous disease, 23 (62%) patients demonstrated a multi-systemic disease with at least two organs involved during follow-up. Of patients with systemic disease, the cutaneous disease followed, preceded, and coincided with the development of systemic sarcoidosis in in 2 (9%), 9 (39%), and 12 (52%) patients, respectively. Subgroup analysis of patients with preceding skin involvement showed that patients with isolated cutaneous sarcoidosis displayed another organ involvement after a median (rage) of 12 (3–84) months. Bilateral hilar lymphadenopathy was the leading systemic manifestation of the disease (n = 23), followed by arthritis (n = 11), splenomegaly (n = 6), uveitis (n = 2), and neurosarcoidosis (n = 1). Eleven patients demonstrated impaired lung functions. Of note, stage I pulmonary sarcoidosis was present in 17 patients, stage II pulmonary sarcoidosis in 5 patients, and one patient was diagnosed with stage III pulmonary sarcoidosis. With regard to the disease course, 11 (30%) patients followed an acute course (sudden onset, non-recurrent course of disease), whereas 26 (70%) patients featured a chronic and indolent course (gradual disease course, often recurrent or progressive). Four Ten (27%) patients presented with Löfgren syndrome (Table 1).
Management, laboratory analyses, and comorbidities
The vast majority of study participants (n = 24; 65%) underwent topical corticosteroid treatment. Twelve (32%) patients were managed by systemic corticosteroids and six (16%) patients were placed on systemic immune-modulating drugs as delineated in Table 1. Five (13%) patients were treated by non-steroidal anti-inflammatory drugs (NSAIDs).
The laboratory analyses of eligible patients at baseline demonstrated in 21%, 76%, and 50% of patients increased levels of ACE, sIL-2R, and CRP, respectively. Less frequent laboratory findings included hypercalcemia (6%), leukocytosis (8%), neutrophilia (10%), and thrombocytosis (5%). Hypertension was present in 3 (8%) patients, whereas diabetes mellitus, asthma, and melanoma clustered with cutaneous sarcoidosis in two (5%) patients, each.
Predictors of systemic involvement and acute disease course
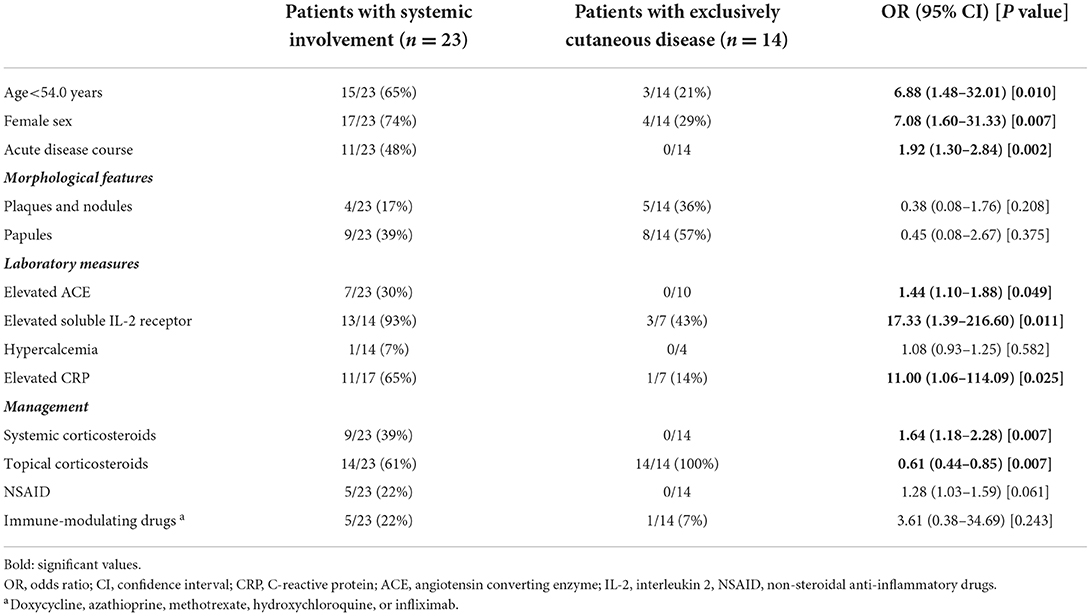
Table 2 demonstrates a logistic regression analysis aiming to identify predictors of systemic involvement. Female sex (OR, 7.08; 95% CI, 1.60–31.33; P = 0.007) and younger age (<54 years; OR, 6.88; 95% CI, 1.48–32.01; P = 0.010) at disease onset were significantly associated with systemic manifestation. Acute disease course (OR, 1.92; 95% CI, 1.30–2.84; P = 0.002) were positive predictors of systemic involvement. Expectedly, elevated levels of ACE (OR, 1.44; 95% CI, 1.10–1.88; P = 0.049), sIL-2R (OR, 17.33; 95% CI, 1.39–216.60; P = 0.011), and CRP (OR, 11.00; 95% CI, 1.06-114.09; P = 0.025) also predicted the presence of systemic manifestation.
Discussion
The most common clinical cutaneous manifestation in our cohort, were papular eruptions (43%). Comparing this data to cohort studies of different ethnic groups showed similiar results with papules (8, 9, 12, 16, 17) and plaques (11, 13, 14) being the most common skin features in cutaneous sarcoidosis. Our study demonstrated that sarcoid skin lesions were highly associated with additional systemic involvement. Specifically, 62% of the patients had systemic involvement. Of note, this is in line with previously published results (8). All our cutaneous sarcoidosis patients with systemic involvement showed bilateral hilar lymphadenopathy, of whom almost 50% also had an impaired lung function. Lung involvement in cutaneous sarcoidosis is frequent (8–10, 12, 13, 15–17, 22). The other organ manifestations were less pronounced, such as arthritis (30%), splenomegaly (16%) and uveitis (5%). Of note, the acute onset of erythema nodosum and/or periarticular inflammation or arthritis of the ankles, with bilateral hilar lymphadenopathy (and in some cases parenchymal infiltrates), accompanied by systemic symptoms such as fever and malaise (Löfgren's syndrome) represents systemic involvement (1, 2, 23, 24).
In more than half of the cases with systemic manifestation, cutaneous sarcoidosis occurred simultaneously with another organ involvement. Interestingly, nine patients developed a systemic disease after being diagnosed with an isolated cutaneous sarcoidosis. Indeed, organ involvement should be excluded in patients diagnosed with cutaneous sarcoidosis (5). Regular follow-up is also recommended to monitor disease activity and to allow early detection of systemic involvement in cases of sarcoidosis limited to the skin (1, 2). Expectedly, elevated serum levels of sIL-2R, ACE, and CRP predicted the presence of systemic manifestation (Table 2). It was already shown that sIL-2R is a sensitive biomarker for diagnosing sarcoidosis (and superior to ACE), moreover it was shown that elevated sIL-2R levels indicate systemic involvement (24). Furthermore, the correlation of sIL-2R levels and chest radiographic stage demonstrated that lower sIL-2R levels were present in patients without parenchymal lung disease, compared to those with parenchymal lung disease (25). This might be an explanation for the lower s-ILR levels in our study. Another possible explanation for the lower soluble IL-2R levels in patients with exclusively cutaneous sarcoidosis might be that patients with isolated skin lesions have a granulomatous inflammation pattern localized to the skin or no granulomatous reaction at all (non-specific sarcoid skin lesions), resulting in lower levels of sIL-2R. Further research, has to be performed to validate sIL-2R as a biomarker in isolated cutaneous sarcoidosis.
Hypercalcemia indicating a possible kidney involvement (26) or infrequently bone marrow sarcoidosis (27), was present in 6% of the tested patients. However, none of the patients in our cohort suffered from renal or bone marrow sarcoidosis. Furthermore, none of the patients showed endocrine or cardiac involvement (28). Of note, it was shown that the first-mentioned is under-diagnosed, and the latter is potentially occult and serious life-threatening, so patients should be proactively examined and investigated to exclude it (6).
In this analysis of Caucasians, cutaneous sarcoidosis was female-dominant (57%); this fact is in line with literature, most clinical studies suggest that cutaneous sarcoidosis is more common in women (8, 10–15, 17, 18). In addition, statistical analyzes showed that female patients and younger age of disease onset showed a higher tendency to additional organ involvement (Table 2).
Given the poor treatment response in localized cutaneous sarcoidosis, the frequent systemic involvement, and corticosteroids being still the mainstay of therapy, there is a pressing need for the development of new treatment strategies. Further research is required to the underlying cause of sarcoidosis and the path mechanisms driving the infiltration of non-caseating granulomas, leading to an often chronic and persistent inflammation.
Limitations of the study include the relatively small number of patients and the retrospective study design. This may have resulted in some degree of selection bias, given that the patients were attending a tertiary referral center.
In conclusion, this retrospective cohort study reveals that cutaneous sarcoidosis is frequently associated with additional systemic involvement, particularly when present in young females. Our findings underscore the importance of early recognition of cutaneous lesions of sarcoidosis, which may herald the development of systemic disease. Almost 25 % of patients displayed sarcoid skin lesions prior to systemic organ involvement.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Ethical Committee of the University of Lübeck (#21-292). The patients/participants provided their written informed consent to participate in this study.
Author contributions
KB: conception of the work, acquisition data, drafting the work, and writing manuscript. EL and DZ: critically revising, intellectual content, and final approval. RL: conception of the work, critically revising, and final approval. KK: data analysis, interpretation of data, critically revising, and final approval. All authors contributed to the article and approved the submitted version.
Funding
This work has been supported by the Cluster of Excellence Precision Medicine in Chronic Inflammation (EXC 2167) from the Deutsche Forschungsgemeinschaft and the Schleswig-Holstein Excellence-Chair Program from the State of Schleswig Holstein.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Drent M, Crouser ED, Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med. (2021) 385:1018–32. doi: 10.1056/NEJMra2101555
2. Grunewald J, Grutters JC, Arkema EV, Saketkoo LA, Moller DR, Müller-Quernheim J. Sarcoidosis. Nat Rev Dis Primers. (2019) 5:45. doi: 10.1038/s41572-019-0096-x
3. Haimovic A, Sanchez M, Judson MA, Prystowsky S. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. Extracutaneous disease. J Am Acad Dermatol. (2012) 66:719.e1–10. doi: 10.1016/j.jaad.2012.02.003
4. Haimovic A, Sanchez M, Judson MA, Prystowsky S. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. Cutaneous disease. J Am Acad Dermatol. (2012) 66:699.e1–18. doi: 10.1016/j.jaad.2011.11.965
5. Caplan A, Rosenbach M, Imadojemu S. Cutaneous Sarcoidosis. Semin Respir Crit Care Med. (2020) 41:689–99. doi: 10.1055/s-0040-1713130
6. Zic JA, Horowitz DH, Arzubiaga C. King LE. Treatment of cutaneous sarcoidosis with chloroquineReview of the literature. Arch Dermatol. (1991) 127:1034–40. doi: 10.1001/archderm.127.7.1034
7. Webster GF, Razsi LK, Sanchez M, Shupack JL. Weekly low-dose methotrexate therapy for cutaneous sarcoidosis. J Am Acad Dermatol. (1991) 24:451–4. doi: 10.1016/0190-9622(91)70071-9
8. Paolino A, Galloway J, Birring S, Brex P, Larkin G, Patel A, et al. Clinical phenotypes and therapeutic responses in cutaneous-predominant sarcoidosis: 6-year experience in a tertiary referral service. Clin Exp Dermatol. (2021) 46:1038–45. doi: 10.1111/ced.14614
9. García-Colmenero L, Sánchez-Schmidt JM, Barranco C, Pujol RM. The natural history of cutaneous sarcoidosis. Clinical spectrum and histological analysis of 40 cases. Int J Dermatol. (2019) 58:178–84. doi: 10.1111/ijd.14218
10. Mañá J, Rubio-Rivas M, Villalba N, Marcoval J, Iriarte A, Molina-Molina M, et al. Multidisciplinary approach and long-term follow-up in a series of 640 consecutive patients with sarcoidosis: cohort study of a 40-year clinical experience at a tertiary referral center in Barcelona, Spain. Medicine (Baltimore). (2017) 96:e7595. doi: 10.1097/MD.0000000000007595
11. Liu KL, Tsai WC, Lee CH. Cutaneous sarcoidosis: a retrospective case series and a hospital-based case-control study in Taiwan. Medicine (Baltimore). (2017) 96:e8158. doi: 10.1097/MD.0000000000008158
12. Wu MC, Lee JY. Cutaneous sarcoidosis in southern Taiwan: clinicopathologic study of a series with high proportions of lesions confined to the face and angiolupoid variant. J Eur Acad Dermatol Venereol. (2013) 27:499–505. doi: 10.1111/j.1468-3083.2012.04473.x
13. Torquato MF, Costa MKSD, Nico MMS. Cutaneous sarcoidosis: clinico-epidemiological profile of 72 patients at a tertiary hospital in São Paulo, Brazil. An Bras Dermatol. (2020) 95:57–62. doi: 10.1016/j.abd.2019.06.004
14. Ungprasert P, Wetter DA, Crowson CS, Matteson EL. Epidemiology of cutaneous sarcoidosis, 1976-2013: a population-based study from Olmsted County, Minnesota. J Eur Acad Dermatol Venereol. (2016) 30:1799–804. doi: 10.1111/jdv.13760
15. Mahabal GD, Peter DCV, George L, Thomas M, Pulimood SA. Cutaneous sarcoidosis: a retrospective clinico-pathological study from the indian subcontinent in patients attending a tertiary health care centre. Clin Exp Dermatol. (2019) 44:161–8. doi: 10.4103/idoj.IDOJ_606_20
16. Chong WS, Tan HH, Tan SH. Cutaneous sarcoidosis in Asians: a report of 25 patients from Singapore. Clin Exp Dermatol. (2005) 30:120–4. doi: 10.1111/j.1365-2230.2005.01729.x
17. Collin B, Rajaratnam R, Lim R, Lewis H, A. retrospective analysis of 34 patients with cutaneous sarcoidosis assessed in a dermatology department. Clin Exp Dermatol. (2010) 35:131–4. doi: 10.1111/j.1365-2230.2009.03400.x
18. Ben Jennet S, Benmously R, Chaâbane S, Fenniche S, Marrak H, Mohammed Z, et al. [Cutaneous sarcoidosis through a hospital series of 28 cases]. Tunis Med. (2008) 86:447–50.
19. Natalia Soto-Gomez N, Peters JI, Nambiar AM. Diagnosis and management of sarcoidosis. Am Fam Physician. (2016) 93:840–8.
20. Abramson N, Melton B. Leukocytosis: basics of clinical assessment. Am Fam Physician. (2000) 62:2053–60.
21. Oda E, Oohara K, Abe A, Veeraveedu PT, Watanabe K, Kato K, et al. The optimal cut-off point of C-reactive protein as an optional component of metabolic syndrome in Japan. Circ J. (2006) 70:384–8. doi: 10.1253/circj.70.384
22. Mana J, Marcoval J, Graells J, Salazar A, Peyri J, Pujol R. Cutaneous involvement in sarcoidosis. Relationship to systemic disease. Arch Dermatol. (1997) 133:882–8. doi: 10.1001/archderm.133.7.882
23. Lofgren S. Erythema nodosum: studies on etiology and pathogenesis in 185 adult cases. Acta Med Scand. (1946) (124) 1–197
24. Grunewald J, Eklund A. Löfgren's syndrome: human leukocyte antigen strongly influences the disease course. Am J Respir Crit Care Med. (2009) 179:307–12. doi: 10.1164/rccm.200807-1082OC
25. Eurelings LEM, Miedema JR, Dalm VASH, van Daele PLA, van Hagen PM, van Laar JAM, et al. Sensitivity and specificity of serum soluble interleukin-2 receptor for diagnosing sarcoidosis in a population of patients suspected of sarcoidosis. PLoS ONE. (2019) 14:e0223897. doi: 10.1371/journal.pone.0223897
26. Correia FASC, Marchini GS, Torricelli FC, Danilovic A, Vicentini FC, Srougi M, et al. Renal manifestations of sarcoidosis: from accurate diagnosis to specific treatment. Int Braz J Urol. (2020) 46:15–25. doi: 10.1590/s1677-5538.ibju.2019.0042
27. Saliba WR, Elias MS. Recurrent severe hypercalcemia caused by bone marrow sarcoidosis. Am J Med Sci. (2005) 330:147–9. doi: 10.1097/00000441-200509000-00010
Keywords: sarcoidosis, inflammation, skin, patient phenotyping, cutaneous sarcoidosis
Citation: Boch K, Langan EA, Zillikens D, Ludwig RJ and Kridin K (2022) Evaluation of clinical and laboratory characteristics of patients with cutaneous sarcoidosis: A single-center retrospective cohort study. Front. Med. 9:980507. doi: 10.3389/fmed.2022.980507
Received: 28 June 2022; Accepted: 26 September 2022;
Published: 10 October 2022.
Edited by:
Robert Gniadecki, University of Alberta, CanadaReviewed by:
Takashi Hashimoto, Osaka City University, JapanKlas Nordlind, Karolinska Institutet (KI), Sweden
Elaine Yacyshyn, University of Alberta, Canada
Copyright © 2022 Boch, Langan, Zillikens, Ludwig and Kridin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katharina Boch, S2F0aGFyaW5hLkJvY2hAdWtzaC5kZQ==
 Katharina Boch
Katharina Boch Ewan A. Langan
Ewan A. Langan Detlef Zillikens
Detlef Zillikens Ralf J. Ludwig
Ralf J. Ludwig Khalaf Kridin
Khalaf Kridin