
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 06 September 2022
Sec. Ophthalmology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.978107
Dry eye is a disorder of tear film and ocular surface characterized by ocular discomforts. It is associated with multiple causes and sometimes intractable. We investigated the effect of oral multivitamin supplementation (MVG) on dry eyes. Tear break-up time (TBUT), fluorescein ocular surface staining score, and tear secretion Schirmer test were measured in dry eye patients refractory to conventional topical treatment. The ocular surface disease index (OSDI), visual analog pain score (VAS), and modified standardized patient evaluation of eye dryness questionnaire were analyzed. In total, 42 eyes of 42 patients were included. TBUT increased at 1 and 3 months compared to baseline (p < 0.05). OSDI decreased at 1 and 3 months compared to baseline (p < 0.05). VAS score, impact on life, and frequency of total symptoms decreased at 3 months compared to baseline (p < 0.05). Oral administration of MVG, a vitamin complex formulation, was effective in stabilizing tear stability and alleviating symptoms in patients with intractable dry eye. Thus, it may be a viable treatment option for intractable dry eye.
Dry eye is an ocular surface disease that is characterized by symptoms including irritation and discomfort of the ocular surface caused by abnormal tear film and ocular surface inflammation (1). Tear film contains IgA, lysozyme, lactoferrin, lipocalin, phosphatidylcholine, phosphatidylethanolamine, cholesterol oleate, triglycerides, and free fatty acids (2). The composition of tears changes according to factors such as age, systemic diseases, and hormones (3). Dry eye is commonly treated with artificial tears, topical secretagogues, immunomodulators, or punctal occlusion (1). Although the treatment for dry eye syndrome is usually local, the risk factors for dry eye syndrome are generally systemic. Risk factors for dry eye syndrome include hormonal changes, aging, diabetes, thyroid disease, tobacco use, lack of sleep, depression, and drug use (3). It has been reported that vitamin D, vitamin A, vitamin B12, and antioxidants may be helpful as nutritional supplements related to dry eye (4). A lot of research has been performed for the treatment of dry eye, but the intractable dry eye, not responding to topical treatment, remains challenging (1, 5, 6). This may be due to a decrease in tear film quality resulting from changes in the composition of basally secreted tears or molecular changes in the ocular surface (7). In addition, nutritional deficiency may be one of the causes of intractable dry eye.
Oxidative stress has been reported as one of the mechanisms in dry eyes (8, 9). Oxidative stress is generated as a byproduct when energy is produced in mitochondria and normally plays a physiologic role in cell signaling (10). However, excessive oxidative stress damages DNA, RNA, or cell proteins, causing cell death and inducing inflammation although tears and ocular surfaces have antioxidant systems (11, 12). Oxidative stress increases with age and contributes to neuropathy and excitability (13). Resolving oxidative stress and neuropathy by oral administration of antioxidants or vitamins may improve ocular symptoms. Vitamins reduce dry eye symptoms. Tocopherol, ascorbic acid, and β-carotene are antioxidants that reduce oxidative stress and inflammation of the ocular surface (14). Thiamine nitrate (15) and pyridoxine (14) have been reported to ameliorate dry eye. Oral multivitamin drug (MVG; Greenwol soft cap, Kyungnam Pharm.) has been developed to reduce dry eye symptoms and includes tocopherol, ascorbic acid, thiamine, riboflavin, pyridoxine, β-carotene, zinc oxide, selenium, and ubidecarenone. In this study, we investigated the protective effect of MVG in intractable dry eyes.
This prospective study was approved by the Hallym University Medical Center Institutional Review Board and was carried out following the principles of the Declaration of Helsinki between January 2018 and May 2021, and patients with dry eye symptoms refractory to topical treatment were prescribed the oral administration of MVG. Informed consent was obtained from all patients involved in this study. Patients who met the following criteria were included in the study: older than 45 years of age; presence of clinical signs and symptoms of dry eye; presence of symptoms refractory to treatment with artificial tears, bioactive drugs, and punctal plug; and clinical examination variable assessments and survey completions within 1 month and/or 3 months after prescribing oral MVG. Topical treatment was continuously prescribed. MVG includes tocopherol acetate 200 mg, ascorbic acid 450 mg, thiamine nitrate 12.5 mg, riboflavin 6 mg, pyridoxine HCl 25 mg, β-carotene 30% suspension 10 mg, zinc oxide 17.43 mg, selenium 0.1% powder 25 mg, and ubidecarenone 5 mg. Oral MVG was prescribed twice daily.
The survey was conducted using the ocular surface disease index (OSDI), visual analog pain scale (VAS), and a modified standardized patient evaluation of eye dryness (SPEED) questionnaire before and after 1 and 3 months of MVG administration. Tear break-up time (TBUT), fluorescein staining score (FSS), eyelid margin hyperemia, and tear secretion were measured. OSDI was calculated after patient OSDI questionnaire completion for measuring dry eye symptoms severity (16). OSDI is scored on a scale of 0–100 with greater scores representing greater disability (16). VAS, which measured ocular pain intensity, was assessed using a segmental numerical technique (17), where 0 = no discomfort and 10 = maximal discomfort (18). A modified SPEED questionnaire was used to assess the frequency and severity of each symptom, including dryness, foreign body sensation (FBS), coldness, eye fatigue, pain, and photophobia (19). The SPEED questionnaire was scored for both frequency of symptoms (0 = no symptoms, 1 = sometimes, 2 = often, and 3 = constant) and severity of symptom/impact on daily life (0 = no symptoms, 1 = tolerable but not uncomfortable, 2 = uncomfortable but does not interfere with my day, 3 = bothersome and interferes with my day, and 4 = intolerable and unable to perform my daily tasks).
Tear break-up time, which indicates tear stability, was measured using fluorescein staining strips (Haag-Streit Strips, Haag-Streit, United States) and slit lamp microscopy without stimulation (18). Briefly, a drop of normal saline was instilled to the strip, and excessive liquid was removed by shaking strip. Then, the strip was touched to inferior conjunctiva. After blinking several times, the time between the last blinking and the first dry spot was measured as TBUT (20). Longer TBUT represents the stable tear film and shorter TBUT is considered as the unstable tear film (21). FSS was assessed after TBUT test under yellow filter and graded using the Oxford grading scale (22). FSS is defined as the degree of punctate keratitis and higher score is considered as more severe FSS (23). Eyelid margin hyperemia was graded using slit lamp microscopy. Eyelid margin hyperemia indicates the inflammation of eyelid and higher score represents the inflammation of eyelid (20). Tear secretion was measured using the Schirmer strip test without anesthesia. A fine strip paper was placed inside the lower conjunctival sac and the wet part was measured (20).
Data are expressed as the mean ± standard deviation. Statistical analyses were performed using unpaired Student’s t-test for two-group comparisons and one-way analysis of variance, followed by Tukey’s multiple comparison test for more than two groups using GraphPad Prism v.9 (GraphPad Software, San Diego, CA, United States).
In total, 42 eyes of 42 patients were included in the study. Seven (16.7%) men and 35 (83.3%) women, with a mean age of 53.64 ± 12.06 years were enrolled. Forty-two (100%) patients returned 1 and 3 months after prescribing oral MVG. At baseline, TBUT was 4.61 ± 2.10 s, FSS was 0.59 ± 0.92, and lid hyperemia was 1.59 ± 1.00. OSDI and VAS were 48.13 ± 22.78 and 3.17 ± 2.77, respectively. Impact on the day life and daily frequency were 2.62 ± 0.80 and 2.29 ± 1.04.
Tear break-up time increased at 1 and 3 months compared to baseline by 26.0 and 38.2%, respectively (5.81 ± 2.21 s at 1 month and 6.37 ± 2.64 at 3 months; p = 0.018 and 0.001; Figure 1A and Table 1). FSS and lid hyperemia did not change (Figures 1B,C). Tear secretion increased at 3 months compared to baseline (4.95 ± 2.73 mm at baseline vs. 6.78 ± 5.24 mm at 3 months; p = 0.030; Figure 1D). OSDI decreased at 1 and 3 months compared to baseline by 16.6 and 30.5% (40.16 ± 19.93 at 1 month and 33.44 ± 19.93 at 3 months; p = 0.011 and p < 0.001; Figure 2A). VAS, impact on life, and frequency of total symptoms were decreased at 3 months compared to baseline by 20.1, 19.8, and 18.8% (2.31 ± 2.31, 2.10 ± 0.82, and 1.86 ± 0.95; p = 0.029, 0.042, and <0.001; Figures 2B–D).
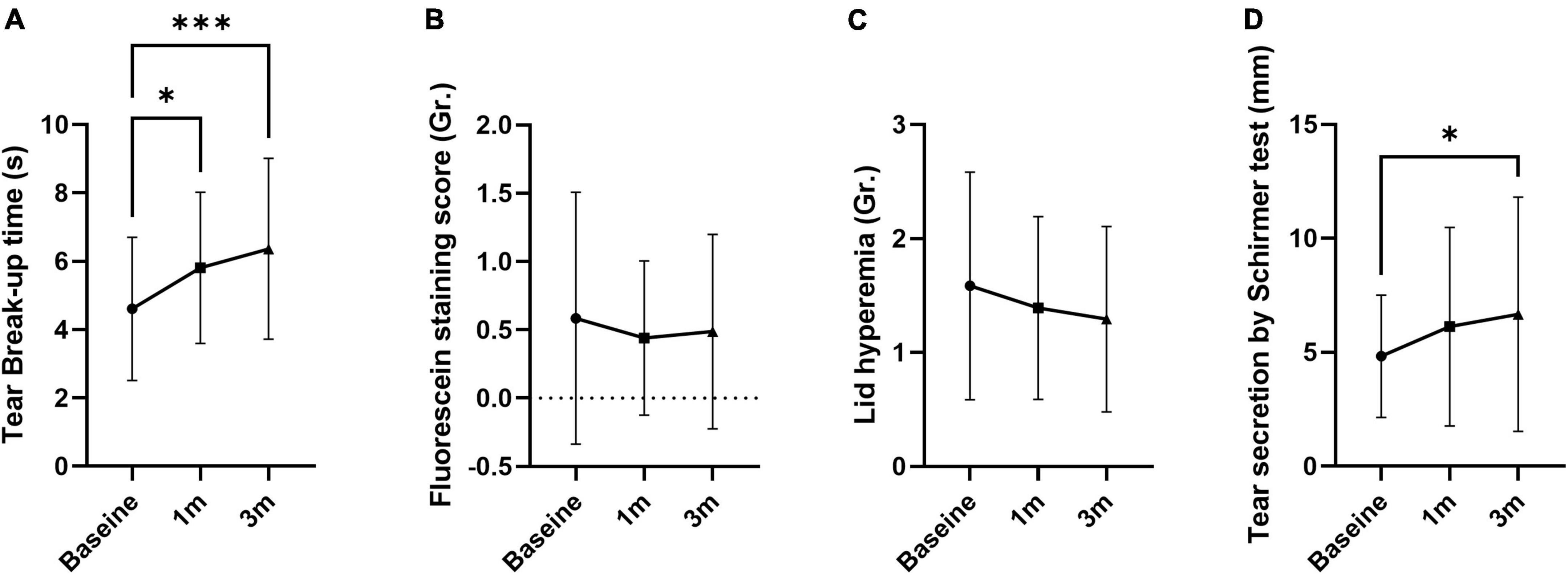
Figure 1. The effect of MVG on objective signs of dry eye. (A) Tear break-up time was improved. (B,C) Fluorescein staining score and lid hyperemia were not changed. (D) Tear secretion by Schirmer test was improved. Data presented as mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001.
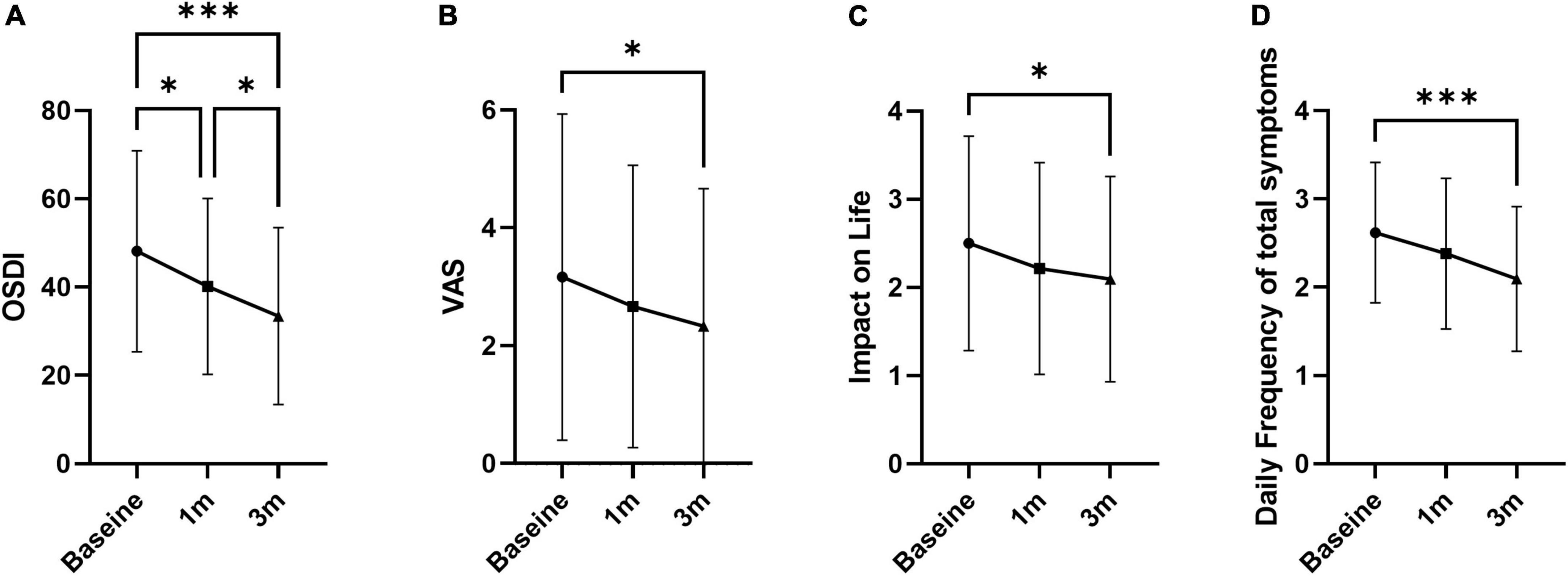
Figure 2. Overall symptoms of dry eye. (A) Ocular surface disease index. (B) Visual analog pain score. (C) Impact of symptoms on daily life. (D) Daily frequency of total symptoms. Data presented as mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001.
The frequency of individual symptoms was evaluated (Table 2). Dryness frequency decreased at 3 months compared to baseline by 12.7% (1.67 ± 0.87; p = 0.049; Figure 3A). FBS frequency decreased at 1 and 3 months compared to baseline by 30.4 and 26.5% (1.26 ± 0.91 and 1.33 ± 0.95; p = 0.005 and 0.012, respectively; Figure 3B). Coldness frequency decreased at 1 month compared to baseline by 25.0% (1.05 ± 0.91; p = 0.011; Figure 3C). The frequency of eye fatigue, pain, and photophobia did not change after oral MVG administration (Figures 3D–F). The severity of individual symptoms was also evaluated. The severity of dryness and FBS decreased at 3 months compared to baseline by 17.1 and 28.2% (1.40 ± 1.06; p = 0.049 and 0.004; Figures 4A,B). Coldness severity decreased at 1 month compared to baseline by 25.6% (1.19 ± 1.07; p = 0.005; Figure 4C). The frequency of eye fatigue, pain, and photophobia did not change after oral MVG administration (Figures 4D–F).
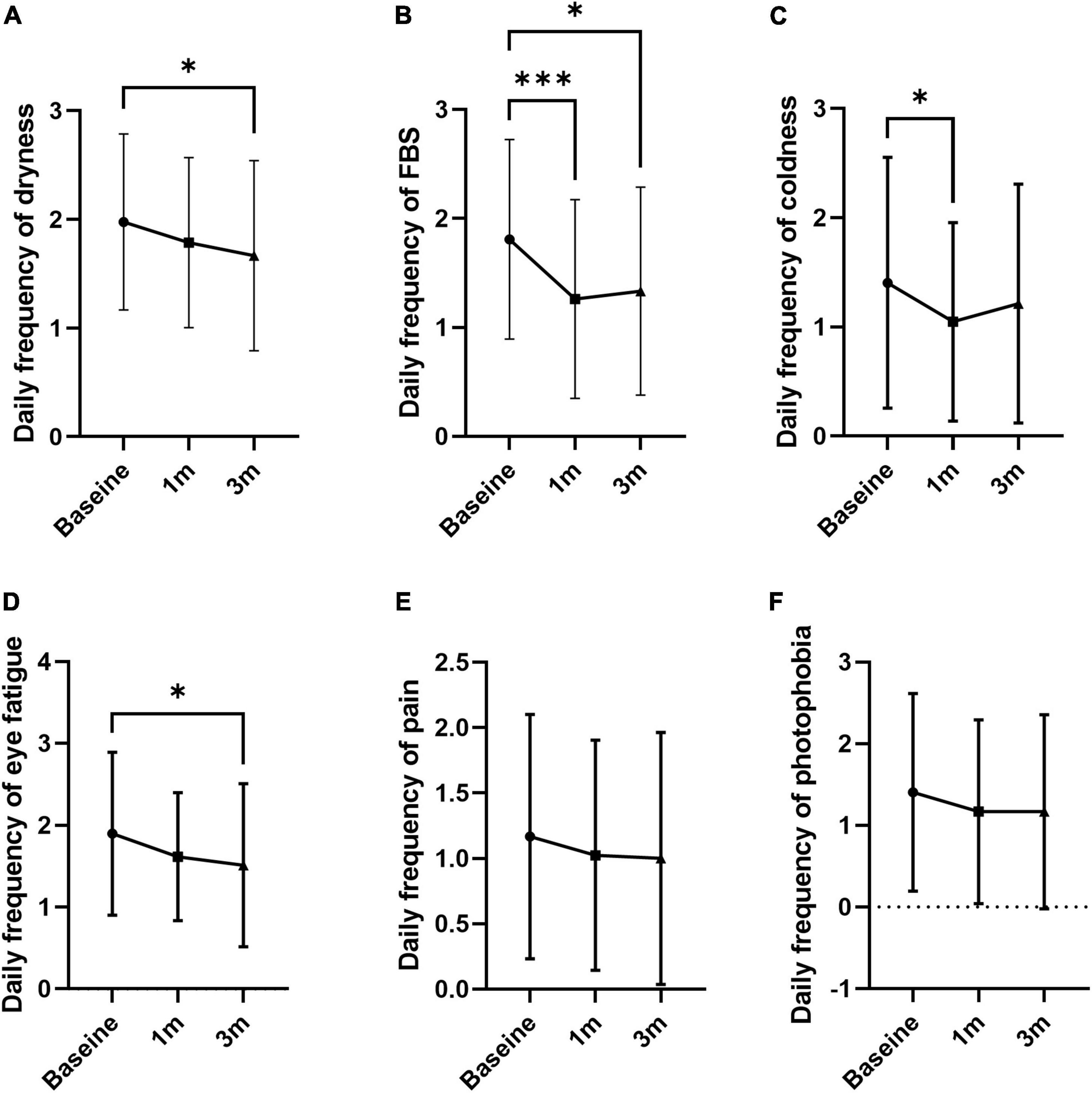
Figure 3. The effect of MVG on the frequency of each dry eye symptom. (A) Dryness. (B) Foreign body sensation. (C) Coldness. (D) Eye fatigue. (E) Pain. (F) Photophobia. * < 0.05, ** < 0.01, and ***p < 0.001.
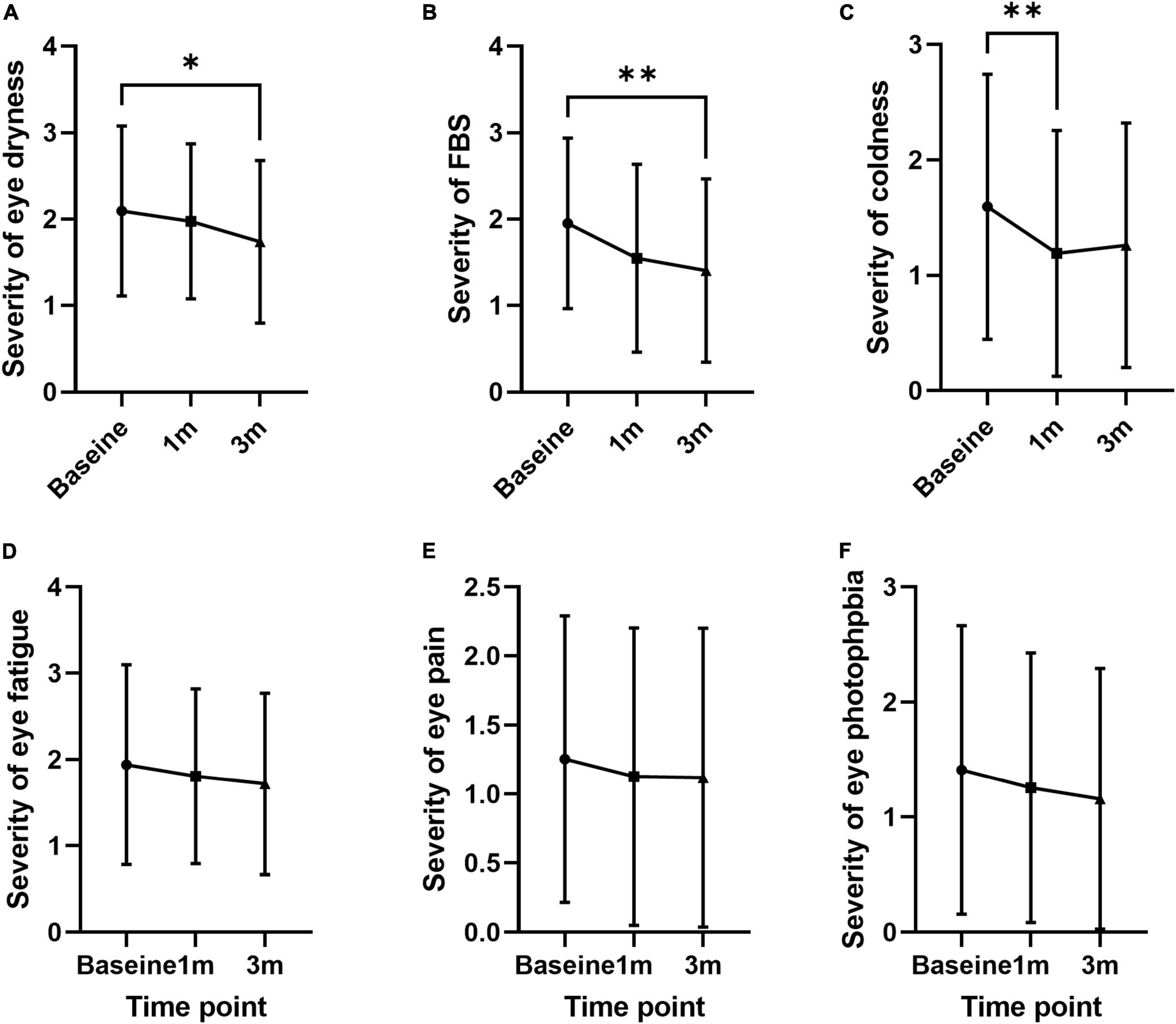
Figure 4. The effect of MVG on the severity of each dry eye symptom. (A) Dryness. (B) Foreign body sensation. (C) Coldness. (D) Eye fatigue. (E) Pain. (F) Photophobia. * < 0.05 and ** < 0.01.
Dry eye is a disease of the tear film and ocular surface affected by systemic nutritional status (4, 24). In this study, we investigated the effect of MVG on dry eye symptoms. The main mechanism of dry eye involves oxidative stress and inflammation (8, 25–27). Basic fibroblast growth factor (b-FGF) and L-cysteine supplementation has been suggested to promote the corneal epithelial healing and reduce the corneal haze (26–28). Oxidative stress causes inflammation through activation of nuclear factor-kappa B (NF-κB) signaling and secretion of senescence-associated secretary phenotypes (29). The imbalance between ROS and endogenous antioxidative system could result in neuropathic pain (30, 31). Neuropathic pain can be alleviated by inhibiting oxidative stress-induced NF-κB activation or by blocking protein kinase C (31, 32). Thus, the reduction of oxidative stress is necessary for minimizing inflammation and managing dry eye. The MVG used in this study consists of a vitamin supplement with antioxidant action that helps in dry eye syndrome (33). MVG includes tocopherol (vitamin E), ascorbic acid, thiamine (vitamin B1), riboflavin (vitamin B2), pyridoxine (vitamin B6), β-carotene (vitamin A precursor), zinc oxide, selenium, and ubidecarenone (coenzyme Q10). Oral administration of MVG improved the symptoms and signs of dry eye in this study.
Intractable dry eyes may be due to neuropathic pain or nutrition deficiency (34). Oxidative stress is critically involved in neuropathic pain and contributes to exaggeration of pain hypersensitivity during persistent pain (30, 34). Tocopherol, an antioxidant, has an analgesic effect by reducing central sensitization in neuropathic pain (35) and preventing peripheral neuropathy (36). Topical eyedrops of 0.1% crosslinked hyaluronic acid, coenzyme Q10, and vitamin E improve dry eye (37). Ascorbic acid, one of the antioxidants contained in tear films, protects the ocular surface against the oxidative stress (38). Supplementation of ascorbic acid shows antinociceptive effects (39, 40). It has been reported that the combination of vitamin C and vitamin E attenuates the neuropathic pain behavior by synergistic antinociceptive effects, although vitamin C or vitamin E given alone failed to reduce the nociceptive behavior (39). Thiamine, which reduces peripheral neuropathic pain (41), attenuates symptoms of dry eye (15). Riboflavin supplementation reduces hyperalgesia and inflammation (42) and inhibits histamine-dependent itch by modulating transient receptor potential vanilloid 1 (43). Pyridoxine is used for the management of sicca syndrome (14) and improves neuropathic pain, such as allodynia and hyperalgesia, via suppression of oxidative–nitrosative stress, pro-inflammatory cytokines secretion, and apoptosis (44). β-Carotene is a precursor of vitamin A, deficiency of which leads to xerophthalmia and ocular surface inflammation (45, 46). Topical and oral vitamin A supplementation reduces dry eye signs and symptoms, also promoting goblet cell proliferation (47–49). Zinc is a part of many essential enzymes, including superoxide dismutase (50). The absence of zinc or zinc deficiency is associated with apoptosis, DNA damage, and changes immune function, partly due to the important role of zinc in countering oxidative stress (51). Topical zinc-hyaluronate improves ocular surface symptoms by decreasing corneal mechano- and polymodal receptor excitability (52). Selenium protects the ocular surface from oxidative stress by reducing matrix metallopeptidase-9, interleukin-6, and 8-hydroxy-2′-deoxyguanosine (53, 54). Coenzyme Q10 prevents peripheral neuropathy and neuropathic pain and attenuates neuron loss (55, 56). Eyedrops containing crosslinked hyaluronic acid and CoQ10 restore the health of ocular surface (57), and coenzyme Q10 protects lacrimal glands against the oxidative stress (58).
Conventional treatments often fail to improve dry eye symptoms, which may be associated with oxidative stress or systemic causes. Oral administration of MVG, a vitamin complex formulation, was effective in stabilizing tear stability and alleviating symptoms in patients with intractable dry eye syndrome. Thus, it may be a viable treatment strategy for intractable dry eye.
Datasets are available from the corresponding author on reasonable request.
This prospective study was approved by the Hallym University Kangnam Sacred Heart Hospital Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Acquisition of data: SP, and YS. Analysis and/or interpretation of data: SP, JH, and YS. Drafting the manuscript: SP, JH, and YS. Revising the manuscript critically for relevant intellectual content: YS. Approval of the version of the manuscript to be published: SP, JH, and YS.
This study was supported by the National Research Foundation (NRF) grant (NRF-2018R1A2B6002251) funded by the Korean Government, Hallym University Medical Center Research Fund, and Hallym University Research Fund.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Milner MS, Beckman KA, Luchs JI, Allen QB, Awdeh RM, Berdahl J, et al. Dysfunctional tear syndrome: Dry eye disease and associated tear film disorders – new strategies for diagnosis and treatment. Curr Opin Ophthalmol. (2017) 27:3–47.
2. Willcox MDP, Argueso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, et al. TFOS DEWS II tear film report. Ocul Surf. (2017) 15:366–403.
3. Nebbioso M, Del Regno P, Gharbiya M, Sacchetti M, Plateroti R, Lambiase A. Analysis of the pathogenic factors and management of dry eye in ocular surface disorders. Int J Mol Sci. (2017) 18:1764. doi: 10.3390/ijms18081764
4. Pellegrini M, Senni C, Bernabei F, Cicero AFG, Vagge A, Maestri A, et al. The role of nutrition and nutritional supplements in ocular surface diseases. Nutrients. (2020) 12:952.
5. Agarwal P, Craig JP, Rupenthal ID. Formulation considerations for the management of dry eye disease. Pharmaceutics. (2021) 13:207.
6. Simsek C, Dogru M, Kojima T, Tsubota K. Current management and treatment of dry eye disease. Turk J Ophthalmol. (2018) 48:309–13.
7. O’Neil EC, Henderson M, Massaro-Giordano M, Bunya VY. Advances in dry eye disease treatment. Curr Opin Ophthalmol. (2019) 30:166–78.
8. Dogru M, Kojima T, Simsek C, Tsubota K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Invest Ophthalmol Vis Sci. (2018) 59:DES163–8.
9. Wang B, Zeng H, Zuo X, Yang X, Wang X, He D, et al. TLR4-dependent DUOX2 activation triggered oxidative stress and promoted HMGB1 release in dry eye. Front Med. (2021) 8:781616. doi: 10.3389/fmed.2021.781616
10. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: Harms and benefits for human health. Oxid Med Cell Longev. (2017) 2017:8416763.
11. Lemos CN, da Silva L, Faustino JF, Fantucci MZ, Murashima AAB, Adriano L, et al. Oxidative stress in the protection and injury of the lacrimal gland and the ocular surface: Are there perspectives for therapeutics? Front Cell Dev Biol. (2022) 10:824726. doi: 10.3389/fcell.2022.824726
12. Deng R, Hua X, Li J, Chi W, Zhang Z, Lu F, et al. Oxidative stress markers induced by hyperosmolarity in primary human corneal epithelial cells. PLoS One. (2015) 10:e0126561. doi: 10.1371/journal.pone.0126561
13. Latini A, Pereira PJS, Couture R, Campos MM, Talbot S. Oxidative Stress: Neuropathy, Excitability, and Neurodegeneration. Oxid Med Cell Longev. (2019) 2019:2715326.
14. Horrobin DF, Campbell A, McEwen CG. Treatment of the Sicca syndrome and Sjogren’s syndrome with E.F.A., pyridoxine and vitamin C. Prog Lipid Res. (1981) 20:253–4. doi: 10.1016/0163-7827(81)90048-5
15. Ren X, Chou Y, Jiang X, Hao R, Wang Y, Chen Y, et al. Effects of oral vitamin B1 and mecobalamin on dry eye disease. J Ophthalmol. (2020) 2020:9539674. doi: 10.1155/2020/9539674
16. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. (2000) 118:615–21.
17. Klimek L, Bergmann KC, Biedermann T, Bousquet J, Hellings P, Jung K, et al. Visual analogue scales (VAS): Measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care: Position paper of the German society of allergology (AeDA) and the German Society of Allergy and Clinical Immunology (DGAKI), ENT Section, in collaboration with the working group on Clinical Immunology, Allergology and Environmental Medicine of the German Society of Otorhinolaryngology, Head and Neck Surgery (DGHNOKHC). Allergo J Int. (2017) 26:16–24. doi: 10.1007/s40629-016-0006-7
18. Friedman NJ, Butron K, Robledo N, Loudin J, Baba SN, Chayet A. A nonrandomized, open-label study to evaluate the effect of nasal stimulation on tear production in subjects with dry eye disease. Clin Ophthalmol. (2016) 10:795–804. doi: 10.2147/OPTH.S101716
19. Salsman JM, Schalet BD, Andrykowski MA, Cella D. The impact of events scale: A comparison of frequency versus severity approaches to measuring cancer-specific distress. Psychooncology. (2015) 24:1738–45. doi: 10.1002/pon.3784
20. Wu Y, Wang C, Wang X, Mou Y, Yuan K, Huang X, et al. Advances in dry eye disease examination techniques. Front Med. (2021) 8:826530. doi: 10.3389/fmed.2021.826530
21. Tsubota K. Short tear film breakup time-type dry eye. Invest Ophthalmol Vis Sci. (2018) 59:DES64–70.
22. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. (2003) 22:640–50. doi: 10.1097/00003226-200310000-00008
23. Amparo F, Wang H, Yin J, Marmalidou A, Dana R. Evaluating corneal fluorescein staining using a novel automated method. Invest Ophthalmol Vis Sci. (2017) 58:BIO168–73.
24. Rand AL, Asbell PA. Nutritional supplements for dry eye syndrome. Curr Opin Ophthalmol. (2011) 22:279–82.
25. de Souza RG, Yu Z, Hernandez H, Trujillo-Vargas CM, Lee A, Mauk KE, et al. Modulation of oxidative stress and inflammation in the aged Lacrimal Gland. Am J Pathol. (2021) 191:294–308. doi: 10.1016/j.ajpath.2020.10.013
26. Meduri A, Scalinci SZ, Morara M, Ceruti P, Grenga PL, Zigiotti GL, et al. Effect of basic fibroblast growth factor in transgenic mice: Corneal epithelial healing process after excimer laser photoablation. Ophthalmologica. (2009) 223:139–44.
27. Meduri A, Scorolli L, Scalinci SZ, Grenga PL, Lupo S, Rechichi M, et al. Effect of the combination of basic fibroblast growth factor and cysteine on corneal epithelial healing after photorefractive keratectomy in patients affected by myopia. Indian J Ophthalmol. (2014) 62:424–8. doi: 10.4103/0301-4738.119420
28. Meduri A, Bergandi L, Perroni P, Silvagno F, Aragona P. Oral l-cysteine supplementation enhances the long term-effect of topical basic fibroblast growth factor (bFGF) in reducing the corneal haze after photorefractive keratectomy in myopic patients. Pharmaceuticals. (2020) 13:67. doi: 10.3390/ph13040067
29. Kratsovnik E, Bromberg Y, Sperling O, Zoref-Shani E. Oxidative stress activates transcription factor NF-kB-mediated protective signaling in primary rat neuronal cultures. J Mol Neurosci. (2005) 26:27–32. doi: 10.1385/jmn:26:1:027
30. Carrasco C, Naziroglu M, Rodriguez AB, Pariente JA. Neuropathic pain: Delving into the oxidative origin and the possible implication of transient receptor potential channels. Front Physiol. (2018) 9:95. doi: 10.3389/fphys.2018.0009
31. Xu J, Wu S, Wang J, Wang J, Yan Y, Zhu M, et al. Oxidative stress induced by NOX2 contributes to neuropathic pain via plasma membrane translocation of PKCepsilon in rat dorsal root ganglion neurons. J Neuroinflammation. (2021) 18:106. doi: 10.1186/s12974-021-02155-6
32. Zhang X, Guan Z, Wang X, Sun D, Wang D, Li Y, et al. Curcumin alleviates oxaliplatin-induced peripheral neuropathic pain through inhibiting oxidative stress-mediated activation of NF-kappaB and mitigating inflammation. Biol Pharm Bull. (2020) 43:348–55. doi: 10.1248/bpb.b19-00862
33. de Souza RG, de Paiva CS, Alves MR. Age-related autoimmune changes in Lacrimal Glands. Immune Netw. (2019) 19:e3.
34. Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain: An important yet underevaluated feature of dry eye. Eye. (2015) 29:301–12. doi: 10.1038/eye.2014.263
35. Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, et al. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. (2006) 122:53–62. doi: 10.1016/j.pain.2006.01.013
36. Heiba MA, Ismail SS, Sabry M, Bayoumy WAE, Kamal KA. The use of vitamin E in preventing taxane-induced peripheral neuropathy. Cancer Chemother Pharmacol. (2021) 88:931–9. doi: 10.1007/s00280-021-04347-6
37. Serrano-Morales JM, De-Hita-Cantalejo C, Sanchez-Gonzalez MC, Bautista-Llamas MJ, Sanchez-Gonzalez JM. Efficacy of 0.1% crosslinked hyaluronic acid, coenzyme Q10 and vitamin E in the management of dry eye disease in menopause patients receiving antidepressants. Eur J Ophthalmol. (2020) 32:1120672120972026. doi: 10.1177/1120672120972026
38. Venkata SJ, Narayanasamy A, Srinivasan V, Iyer GK, Sivaramakrishnan R, Subramanian M, et al. Tear ascorbic acid levels and the total antioxidant status in contact lens wearers: A pilot study. Indian J Ophthalmol. (2009) 57:289–92. doi: 10.4103/0301-4738.53054
39. Lu R, Kallenborn-Gerhardt W, Geisslinger G, Schmidtko A. Additive antinociceptive effects of a combination of vitamin C and vitamin E after peripheral nerve injury. PLoS One. (2011) 6:e29240. doi: 10.1371/journal.pone.0029240
40. Carr AC, McCall C. The role of vitamin C in the treatment of pain: New insights. J Transl Med. (2017) 15:77. doi: 10.1186/s12967-017-1179-7
41. Onk D, Mammadov R, Suleyman B, Cimen FK, Cankaya M, Gul V, et al. The effect of thiamine and its metabolites on peripheral neuropathic pain induced by cisplatin in rats. Exp Anim. (2018) 67:259–69. doi: 10.1538/expanim.17-0090
42. Granados-Soto V, Teran-Rosales F, Rocha-Gonzalez HI, Reyes-Garcia G, Medina-Santillan R, Rodriguez-Silverio J, et al. Riboflavin reduces hyperalgesia and inflammation but not tactile allodynia in the rat. Eur J Pharmacol. (2004) 492:35–40. doi: 10.1016/j.ejphar.2004.03.043
43. Lee K, Choi YI, Im ST, Hwang SM, Lee HK, Im JZ, et al. Riboflavin inhibits histamine-dependent itch by modulating transient receptor potential Vanilloid 1 (TRPV1). Front Mol Neurosci. (2021) 14:643483. doi: 10.3389/fnmol.2021.643483
44. Kandhare AD, Mukherjee AA, Bodhankar SL. Neuroprotective effect of Azadirachta indica standardized extract in partial sciatic nerve injury in rats: Evidence from anti-inflammatory, antioxidant and anti-apoptotic studies. EXCLI J. (2017) 16:546–65. doi: 10.17179/excli2017-161
45. Alam J, Yu Z, de Paiva CS, Pflugfelder SC. Retinoid regulation of ocular surface innate inflammation. Int J Mol Sci (2021) 22:1092. doi: 10.3390/ijms22031092
46. Chakraborty U, Chandra A. Bitot’s spots, dry eyes, and night blindness indicate vitamin A deficiency. Lancet. (2021) 397:e2.
47. Alanazi SA, El-Hiti GA, Al-Baloud AA, Alfarhan MI, Al-Shahrani A, Albakri AA, et al. Effects of short-term oral vitamin A supplementation on the ocular tear film in patients with dry eye. Clin Ophthalmol. (2019) 13:599–604. doi: 10.2147/OPTH.S198349
48. Fogagnolo P, De Cilla S, Alkabes M, Sabella P, Rossetti L. A review of topical and systemic vitamin supplementation in ocular surface diseases. Nutrients. (2021) 13:1998. doi: 10.3390/nu13061998
49. Kim EC, Choi JS, Joo CK. A comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am J Ophthalmol. (2009) 147:206–13e3. doi: 10.1016/j.ajo.2008.08.015
50. Ei HH, Zheng T, Farooq MU, Zeng R, Su Y, Zhang Y, et al. Impact of selenium, zinc and their interaction on key enzymes, grain yield, selenium, zinc concentrations, and seedling vigor of biofortified rice. Environ Sci Pollut Res Int. (2020) 27:16940–9. doi: 10.1007/s11356-020-08202-8
51. Song Y, Leonard SW, Traber MG, Ho E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J Nutr. (2009) 139:1626–31.
52. Perenyi K, Dienes L, Kornafeld A, Kovacs B, Kiss HJ, Szepessy Z, et al. The effect of tear supplementation with 0.15% preservative-free zinc-hyaluronate on ocular surface sensations in patients with dry eye. J Ocul Pharmacol Ther. (2017) 33:487–92. doi: 10.1089/jop.2016.0194
53. Higuchi A, Inoue H, Kawakita T, Ogishima T, Tsubota K. Selenium compound protects corneal epithelium against oxidative stress. PLoS One. (2012) 7:e45612. doi: 10.1371/journal.pone.0045612
54. Higuchi A, Inoue H, Kaneko Y, Oonishi E, Tsubota K. Selenium-binding lactoferrin is taken into corneal epithelial cells by a receptor and prevents corneal damage in dry eye model animals. Sci Rep. (2016) 6:36903. doi: 10.1038/srep36903
55. Shi TJ, Zhang MD, Zeberg H, Nilsson J, Grunler J, Liu SX, et al. Coenzyme Q10 prevents peripheral neuropathy and attenuates neuron loss in the db-/db- mouse, a type 2 diabetes model. Proc Natl Acad Sci U.S.A. (2013) 110:690–5. doi: 10.1073/pnas.1220794110
56. Zhang YP, Eber A, Yuan Y, Yang Z, Rodriguez Y, Levitt RC, et al. Prophylactic and antinociceptive effects of coenzyme Q10 on diabetic neuropathic pain in a mouse model of type 1 diabetes. Anesthesiology. (2013) 118:945–54. doi: 10.1097/ALN.0b013e3182829b7b
57. Tredici C, Fasciani R, Villano A, Gambini G, Caporossi A. Efficacy of eye drops containing crosslinked hyaluronic acid and CoQ10 in restoring ocular health exposed to chlorinated water. Eur J Ophthalmol. (2020) 30:430–8. doi: 10.1177/1120672120907311
58. Yakin M, Eksioglu U, Sadic M, Koca G, Ozkan-Uney G, Yumusak N, et al. Coenzyme Q10 for the protection of lacrimal gland against high-dose radioiodine therapy-associated oxidative damage: Histopathologic and tissue cytokine level assessments in an animal model. Curr Eye Res. (2017) 42:1590–6. doi: 10.1080/02713683.2017.1362006
Keywords: dry eye symptoms, dryness, intractable dry eye, multivitamin drug, nutrition
Citation: Park SH, Hwang JS and Shin YJ (2022) Effect of multivitamin drug on intractable dry eye symptoms. Front. Med. 9:978107. doi: 10.3389/fmed.2022.978107
Received: 25 June 2022; Accepted: 25 July 2022;
Published: 06 September 2022.
Edited by:
Alessandro Meduri, University of Messina, ItalyReviewed by:
Laura De Luca, University of Messina, ItalyCopyright © 2022 Park, Hwang and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Young Joo Shin, c2NoaW5uQGhhbm1haWwubmV0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.