
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 13 September 2022
Sec. Intensive Care Medicine and Anesthesiology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.976963
This article is part of the Research TopicClinical Teaching and Practice in Intensive Care Medicine and AnesthesiologyView all 17 articles
 Peiling Chen1,2†
Peiling Chen1,2† Jingqi Gao1,2†
Jingqi Gao1,2† Jun Li1,2
Jun Li1,2 Rongguo Yu1,2
Rongguo Yu1,2 Ling Wang1,3
Ling Wang1,3 Fangqin Xue1,4
Fangqin Xue1,4 Xiaochun Zheng1,5
Xiaochun Zheng1,5 Ling Gao1,2
Ling Gao1,2 Xiuling Shang1,2*
Xiuling Shang1,2*Objective: To establish an early warning scoring system for septic shock in patients with digestive tract perforation (DTP) and evaluate its diagnostic efficacy.
Methods: Patients with surgically confirmed or clinically diagnosed DTP admitted to the Department of Intensive Care Medicine of Fujian Provincial Hospital from June 2012 to October 2021 were retrospectively analyzed. General demographic characteristics, perforation-related information, vital signs, common laboratory indicators, and common ICU scores (Glasgow Coma Scale score, Acute Physiology and Chronic Health Evaluation-II score,Sequential Organ Failure Assessment score) were collected. The patients were divided into shock group and non-shock group according to whether the patients had septic shock during hospitalization. The risk factors of septic shock were screened by basic statistical analysis and multivariate Logistic regression analysis. The receiver operating characteristic curve was drawn to determine the cut-off value of the continuous indicators and discretized with reference to clinic, and the corresponding score was set according to the β regression coefficient of each variable.
Results: A total of 176 patients with DTP were included. The average age of the patients was 64.13 ± 14.67 years old, and 74.40% were males. The incidence of septic shock was 30.11% (53/176). Multivariate Logistic regression analysis showed that the highest heart rate≥105 beats/min, Glasgow Coma Scale score≤14 points, lactic acid≥5.75 mmol/L, procalcitonin≥41.47 ug/L, C-reactive protein≥222.5 mg/L were independent risk factors for septic shock in patients with DTP. The total score of clinical diagnostic scoring system of septic shock in patients with DTP was 6 points, including the highest heart rate≥105 beats/min (1 point), lactic acid≥5.75 mmol/L (two points), procalcitonin≥41.47 ug/L (one point), C-reactive protein≥222.5 mg/L (1 point), and Glasgow Coma Scale score≤14 points (1 point). The area under ROC curve (AUC) of this scoring system was 0.789 and the 95% confidence interval was 0.717–0.860 (P < 0.001); when the optimal cut-off value was 2.5, the sensitivity and specificity were 54.70 and 87.80%, respectively.
Conclusion: This new score system has its certain clinical value and has important guiding significance for clinicians to judge the prognosis of patients with DTP in time.
Digestive tract perforation (DTP) is a potentially devastating complication that may result from various disease processes and is an important indication of emergency surgery. The most common conditions that cause gastrointestinal perforation are peptic ulcer, gastrointestinal tumor, trauma, and inflammatory bowel disease. If left untreated, it leads to death. Although the incidence of DTP has decreased significantly over the past 30 years, especially due to the development of intensive care technology, the advancement of treatment concepts, and the development of various new drugs, the mortality rate is still high (1, 2). According to statistics, the average 30-day mortality rate of peptic ulcer perforation is 23.75% (3). Another report pointed out that gastrointestinal perforation accounted for 40% of peptic ulcer-related deaths, and its 90-day mortality rate was as high as 30% (4–6). Multiple factors, including advanced age, use of non-steroidal anti-inflammatory drugs, diabetes, and use of glucocorticoids, have been associated with increased mortality in patients with DTP (7–10).
Septic shock is a major risk factor for increased mortality in patients with DTP (2, 4). Five studies in Europe, Asia, and Africa reported a significant increase in shock-related mortality in patients with DTP (11–15). Also, septic shock after DTP is a common critical illness in intensive care units (ICU) (16). It is estimated that 30–35% of patients with DTP have sepsis before they arrive in the operating room, and 25% of patients develop septic shock within 30 days of surgery (17).
Recent studies indicated that early recognition and appropriate management of the first few hours after septic shock could significantly improve the prognosis of patients (18). However, so far, no early identification methods have been proposed for screening patients with DTP. Therefore, based on quantitative clinical data, in this study, we analyzed risk factors of septic shock in the patients with DTP and established an early warning scoring system for septic shock in patients with gastrointestinal perforation, aiming to assist clinicians in early identification and intervention of patients with DTP, so as to reduce the occurrence of adverse outcomes.
Patients with surgically confirmed or clinically diagnosed DTP admitted to the Department of Intensive Care Medicine of Fujian Provincial Hospital from June 2012 to October 2021 were retrospectively analyzed. DTP was defined as the destruction of the integrity of the digestive tract, i.e., complete non-invasive penetration of the wall of the esophagus, stomach, small intestine, or large intestine (19). The clinical diagnostic criteria were the presence of free gas under the diaphragm by the plain abdominal film in a vertical position, or the presence of gas-liquid coexistence by abdominal ultrasound, or the presence of free gas in the abdominal cavity by abdominal computed tomography (CT) (20, 21). The diagnostic criteria for septic shock were in line with sepsis-3.0 (22), i.e., patients with sepsis had persistent hypotension after adequate volume resuscitation and needed vasoconstrictor drugs to maintain mean arterial pressure (MAP) ≥ 65 mmHg and serum lactate level > 2 mmol/L.
Patients were divided into shock group and non-shock group according to whether septic shock occurred during hospitalization. Clinical data, including demographic characteristics, perforation-related information, vital signs, common laboratory indicators, ICU common scores (Glasgow Coma Scale score, GCS score;Acute Physiology and Chronic Health Evaluation-II score,APACHE-II score;Sequential Organ Failure Assessment score,SOFA score), and mortality rate within 28 days were collected and compared.
The study protocol was approved by the ethics committee (K2021-09-043), and since it was a retrospective study, the informed consent of patients was exempted from ethical approval.
Inclusion criteria were: (1) patients admitted to the Intensive Care Unit of our Hospital from June 2012 to October 2021; (2) patient aged > 18 years at the date of admission; (3) patients with surgically confirmed or clinically diagnosed DTP based on the above criteria (see section Study design). Exclusion criteria were: (1) patients aged <18 years; (2) those with missing electronic medical records. All selected patients were routinely treated by the same associate chief physician with 10 years of experience in the field.
The general data of patients were collected, including gender, age, past medical history, perforation area, and perforation-operation time interval. Within 24 h of admission to ICU, the heart rate (HR), respiratory rate (RR), mean arterial pressure (MAP), oxygenation index (PaO2/FiO2), 24-h urine volume, serum sodium (Na+), serum potassium (K+), total bilirubin (TBIL), serum creatinine (SCr), platelet count (PLT), albumin (Alb), procalcitonin (PCT), C-reactive protein (CRP), the potential of hydrogen (pH), lactic acid (Lac), GCS score, APACHE-II score, SOFA score and the mortality rate within 28 days were collected and analyzed.
Data were analyzed using SPSS 25.0 statistical software. Quantitative data conforming to a normal distribution were expressed as mean ± standard deviation (x̄± s), and the unpaired t-test was used for comparison between groups. The quantitative data with skewness distribution were expressed as median (quartile) [M (QL, QU)], and the Wilcoxon Mann-Whitney test was used for comparison between groups. Categorical data were expressed as percentages, and the chi-square test was used for comparison. Variables with P ≤ 0.05 (bilateral) were considered to be statistically significant and variables with P > 0.05 were excluded. The receiver operator characteristic curve (ROC curve) was used to analyze the retained continuous indicators to determine the cutoff value and were discretized into discrete indicators by referring to clinic. Taking the occurrence of septic shock as the dependent variable, the independent variables were screened by the method of forwarding stepwise regression (LR), and the independent risk factors of shock in patients with DTP were determined by multivariate Logistic regression analysis. Relative risk was expressed by odds ratio (OR) and 95% confidence interval (95%CI). The corresponding score was set according to the β coefficient of each risk factor, and the sum of each risk factor's scores was the patient's total risk score. The diagnostic efficiency of the scoring system was evaluated by area under ROC Curve (AUC). AUC ranged from 0.5 to 1.0, with <0.7 indicating low diagnostic value, 0.7–0.9 indicating moderate diagnostic value, and >0.9 indicating high diagnostic value.
A total of 290 patients with gastrointestinal perforation were screened. Then, 22 patients who were <18 years old and 92 patients with serious missing electronic medical records were excluded (Figure 1). Finally, 176 patients were included in the analysis, including 53 patients with septic shock and 123 patients without septic shock (Table 1). The average age of the patients was 64.13 ± 14.67 years, and 74.40% were male. The median perforation area was 2.13 (0.64, 3.09) cm2, and the median time interval from perforation to operation was 4.5(2.37, 53.02) h. The mortality rate within 28 days was 11.40%. APACHE-II score, SOFA score, highest HR, highest K+, Lac, PCT, CRP, and SCr in the shock group were higher than those in the non-shock group, while GCS score, Alb, pH, PaO2/FiO2 were lower than those in the non-shock group (all P < 0.05). However, there were no significant differences in gender, age, perforation area, the time interval from perforation to operation, 24-h urine volume, PLT, TBIL, and other indicators between the two groups.
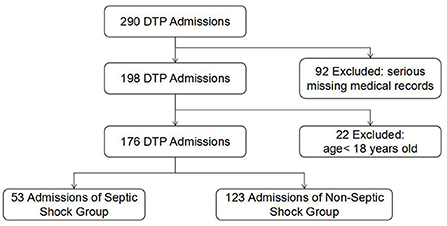
Figure 1. Flowchart of DTP cohorts analyzed showing the inclusion and exclusion criteria. DTP, digestive tract perforation.
ROC curve was used to analyze the continuous indicators, including highest HR, GCS score, Lac, SCr, PCT, CRP, highest K+, Alb, pH, SOFA score, and APACHE-II score (Table 2). The cut-off value corresponding to the maximum value of the Jorden index was taken as the diagnostic cut-off point to determine the optimal cut-off value (highest HR: 105 beats/min, Lac: 5.75 mmol/L, SCr: 116.5 umol/L, PCT: 41.47 ug/L, CRP: 222.5 mg/L, highest K+: 4.35 mmol/L, Alb: 18.15 g/L, pH: 7.28, PaO2/FiO2: 171.75 mmHg, GCS score: 14.5 points, SOFA score: 8.88 points, APACHE -II score: 22.5 points), and transformed into dichotomous data according to clinic.
Univariate Logistic regression analysis was used to screen the risk factors affecting the occurrence of septic shock (Table 3): With the occurrence of septic shock as the factor variable, univariate Logistic regression analysis was performed on the above discrete indicators (highest HR ≥ 105 beats/min, GCS score ≤14 points, Lac ≥5.75 mmol/L, SCr ≥116.5 umol/L, PCT ≥ 41.47 ug/L, CRP ≥ 222.5 mg/L, highest K+ ≥ 4.35 mmol/L, Alb ≤ 18.15 g/L, pH ≤ 7.28,PaO2/FiO2 ≤ 171.75 mmHg, SOFA score ≥8.88 points, APACHE-II score ≥22.5 points), and the results were all statistically significant indicators (all P < 0.05).
With the occurrence of septic shock as the dependent variable, the above discrete indicators (highest HR ≥ 105 beats/min, GCS score≤14 points, Lac ≥ 5.75 mmol/L, SCr ≥ 116.5 umol/L, PCT ≥ 41.47 ug/L, CRP ≥ 222.5 mg/L, highest K+ ≥ 4.35 mmol/L, Alb ≤ 18.15 g/L, pH ≤ 7.28,PaO2/FiO2 ≤ 171.75 mmHg, SOFA score ≥8.88 points, APACHE-II score ≥22.5 points) were included in the multivariate Logistic regression equation for analysis (Table 4). The independent variables were screened by Forward stepwise regression (LR). Independent risk factors of septic shock were: highest HR ≥ 105 beats/min (odds ratio (OR) = 2.977, 95% confidence interval (95% CI) was 1.405~6.311, P = 0.004), GCS score≤14 points (OR = 2.494, 95% CI was 1.127~5.522, P = 0.024), Lac ≥ 5.75 mmol/L (OR = 4.907, 95%CI was 1.490~16.165), PCT ≥ 41.47 ug/L (OR = 2.821, 95%CI was 1.321~6.028, P = 0.007), CRP ≥ 222.5 mg/L (OR = 3.298, 95% CI was 1.401–7.760, P = 0.006).
The regression coefficient of the β value obtained by Logistic regression analysis was assigned to calculate the ratio of the β value of the screened variables to the minimum β value and determine the score of the calculated ratio (Table 5). Finally, the clinical diagnostic score system of shock in patients with DTP was successfully constructed: the highest HR ≥ 105 beats /min (one point), GCS score ≤14 points (one point), Lac ≥ 5.75 mmol/L (2 points), PCT ≥ 41.47 ug/L (1 point), CRP ≥ 222.5 mg/L (one point), and the total score was 6 points.
The scores of all patients were calculated according to the above-established scoring system for the clinical diagnosis of shock in patients with DTP; the clinical diagnostic value of shock was evaluated by the ROC curve (Figure 2). The results showed that the AUC of the scoring system for the septic shock diagnosis was 0.789, the 95% CI was 0.717–0.860 (P < 0.001). When the optimal cut-off value was 2.5 points, its sensitivity and specificity were 54.70 and 87.80%, respectively.
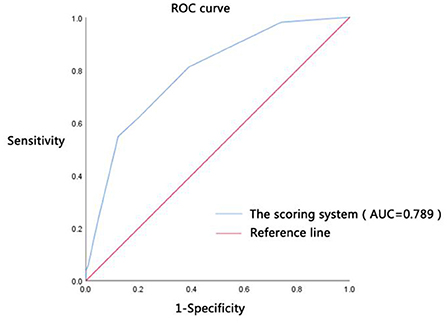
Figure 2. Diagnostic efficacy. ROC curve was the receiver operating characteristic curve; AUC was the area under the ROC curve; the scoring system was constructed by the highest HR ≥ 105 beats/min (1 point), Lac ≥ 5.75 mmol/L (two points), PCT ≥ 41.47 ug/L (one point), CRP ≥ 222.5 mg/L (one point), and GCS score ≤14 points (one point).
In this study, an early warning scoring system for septic shock in patients with DTP was successfully established based on general demographic data, perforation-related information, vital signs, common laboratory indicators, GCS score, APACHE-II score, and SOFA score. Early warning score of septic shock included: DTP = highest HR ≥ 105 beats/min (one point) + GCS score ≤14 points (one point) + Lac≥5.75 mmol/L (two points) + PCT ≥ 41.47 ug/L (one point)+CRP ≥ 222.5 mg/L (one point). When the optimal cut-off value was 2.5 points, the sensitivity and specificity were 54.70 and 87.80%, respectively. The early warning scoring system for septic shock in DTP will provide more possibilities for the treatment and diagnosis of patients with DTP.
Tachycardia is a warning sign of internal metabolic stress (23). Persistent tachycardia often suggests a poor prognosis in patients with septic shock (24). Songne et al. (25) found that HR > 94 beats/min was a significant predictor of failure of non-surgical treatment in patients with perforated peptic ulcers. Moreover, Møller et al. (26) suggested that tachycardia was one of the poor prognostic factors in patients with perforated peptic ulcers. Our study showed that the highest HR ≥ 105 beats/min was an independent risk factor for septic shock in patients with DTP, which was consistent with previous findings (24–26). Therefore, for patients with DTP, the HR should be closely monitored after admission.
Consciousness change is one of the three major clinical windows for assessing organ perfusion in patients with septic shock (27). Various scores associated with sepsis prognosis, including the SOFA score (28), APAPHE-II score (29), and the National Early Warning Score (NEWS) (30), have been used to assessing patient sanity with GCS score. Multiple studies have confirmed that GCS scores are associated with poor prognosis in patients with sepsis. In 1993, Basto et al. (31) found that lower GCS scores associated with sepsis were associated with higher mortality. A recent study by Wu et al. (32) confirmed that the GCS score was an important risk factor for predicting death in patients with sepsis. However, in the diagnostic criteria for septic shock proposed in Sepsis-3.0 (22), the GCS score is not a necessary condition for the diagnosis of septic shock but one of the detection items of SOFA score and has an auxiliary diagnostic value for septic shock. This study confirmed that a GCS score ≤14 points can be used as an independent risk factor for septic shock in patients with DTP, suggesting that consciousness change has a stronger early predictive value for patients with DTP. In addition, consciousness change was the manifestation of insufficient central perfusion in patients with sepsis, which was easily observed in clinical practice and had good timeliness and promotion. Therefore, compared with the diagnostic criteria of Sepsis-3.0 (22), the early warning scoring system of septic shock established in this study could assist clinicians in identifying patients with DTP combined with shock, thus guiding clinical diagnosis and treatment strategies.
Elevated arterial lactate is a manifestation of tissue hypoperfusion. In sepsis-3.0 (22), Lac ≥ 2.0 mmol/L is listed as one of the diagnostic criteria for septic shock. As an indicator of tissue hypoperfusion in patients with severe infection associated with patient prognosis, elevated lactate levels could assist clinicians in early predicting outcomes in patients with septic shock (33). In a retrospective study of 1,043 patients with septic shock, Oh et al. found a poorer prognosis in patients with high lactic acid compared to those with low lactic acid, suggesting that arterial lactic acid is a very reliable diagnostic and prognostic predictor of septic shock (34). Moreover, Bakker et al. (35) suggested that Lac >2 mmol/L is an independent risk factor for death in patients with septic shock. However, this study showed that Lac≥5.75 mmol/L was an independent risk factor for septic shock in patients with DTP, and Lac accounted for a high percentage of the early warning scoring system constructed in this study, suggesting that hyperlactatemia had a good early prediction value for septic shock in DTP patients. However, the optimal cut-off value of lactate in this study was significantly higher than the cut-off value of lactate in sepsis-3.0 (22), and the lactate level in the non-shock group was also significantly higher than the normal range which might be related to the combination of stress hyperlactatemia in patients with DTP. When the digestive tract is perforated, stress factors such as inflammation, pain, and surgical trauma could stimulate the secretion of catecholamines, leading to stress hyperlactatemia. Therefore, in a clinical setting, in addition to actively improving the microcirculation perfusion state, stress factors should also be actively controlled to reduce stress injury and avoid secondary injury caused by excessive resuscitation in patients with DTP complicated with hyperlactatemia.
As rapid and reliable markers of inflammation, serum procalcitonin (PCT) and C-reactive protein (CRP) play irreplaceable roles in diagnosing infectious diseases (36–38) and have a good clinical diagnosis and prognostic value for patients with sepsis and septic shock (39). A prospective study (40) of 78 patients with suspected sepsis admitted to the ICU suggested that PCT had good diagnostic and prognostic value in sepsis and septic shock. In a study of 423 patients with DTP, Grupp et al. (41) confirmed that elevated CRP had a predictive value for adverse outcomes in patients with DTP. In this study, we found that PCT ≥ 41.47 ug/L and CRP≥222.5 mg/L are independent risk factors for septic shock in patients with DTP, which further confirms that elevated levels of PCT and CRP could predict septic shock in patients with DTP. In this study, specific cut-off values of PCT and CRP levels were given, which were higher than those of other site infections. Therefore, it suggested that PCT and CRP might respond differently to infection at different sites, and enteric-borne infection might cause higher levels of PCT and CRP. In addition, it should be noted that since CRP was less specific, surgical trauma and other factors could also affect CRP levels, and reducing the interference of other factors on CRP might help obtain more valuable results.
This study has a few limitations. First, this study was a retrospective single-center study with relatively small sample size. Thus, a large-scale multi-center study is needed for further verification. Second, all patients included in this study were admitted to ICU, and those not admitted to ICU were excluded, which might lead to selection bias and affect the clinical characteristics of the non-shock group. Third, patients receiving antibiotics were not excluded in this study, which might affect the experimental results. However, since most patients with DTP in our hospital received emergency surgical treatment immediately after admission, the effect of antibiotic treatment on the experimental results should be relatively small based on the actual situation. Fourth, due to the limited sample size, this study has not been validated, which will limit the diagnostic efficiency and clinical application of the early warning scoring system. We will make further improvements in future larger studies.
In conclusion, the highest HR ≥ 105 beats/min, GCS score ≤14 points, Lac ≥ 5.75 mmol/L, PCT≥ 41.47 ug/L, CRP≥222.5 mg/L were independent risk factors for septic shock in patients with DTP. The early warning scoring system of septic shock in patients with DTP constructed based on these risk factors showed its certain clinical value, providing early warning indicators for clinicians to identify patients with DTP complicated with shock, which might improve the prognosis of patients. These indicators are easy to obtain in clinical practice and have been used in clinical practice for a long time, so they have a good promotion. The establishment of a scoring system based on common indicators may improve the prognosis of patients with septic shock in DTP, which is expected to contribute to the standardization of clinical teaching and practice of intensive care medicine and anesthesiology.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Fujian Provincial Hospital, Fuzhou, China. The Ethics Committee waived the requirement of written informed consent for participation.
PC and JG collected study data and drafted the present manuscript. XS revised the manuscript. All authors contributed to the article and approved the submitted version.
The study was supported by Natural Science Foundation of Fujian [No. 2020J011086], Fujian Provincial Horizontal Issues grants [No. 2020-YC-001], and the high-level hospital foster grants from Fujian Provincial Hospital, Fujian Province, China [No. (2020) HSJJ14].
We are grateful to all patients who participated in this study and the staff of Fujian Provincial Hospital for their cooperation and contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen H, Zhang H, Li W, Wu S, Wang W. Acute gastrointestinal injury in the intensive care unit: a retrospective study. Ther Clin Risk Manag. (2015) 11:1523–9. doi: 10.2147/TCRM.S92829
2. Tarasconi A, Coccolini F, Biffl WL, Tomasoni M, Ansaloni L. Picetti E, et al. Perforated and bleeding peptic ulcer: WSES guidelines. World J Emerg Surg. (2020) 15:019–0283. doi: 10.1186/s13017-019-0283-9
3. Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. (2011) 84:102–13. doi: 10.1159/000323958
4. Søreide K, Thorsen K, Harrison EM, Bingener J, Møller MH, Ohene-Yeboah M, et al. Perforated peptic ulcer. Lancet. (2015) 386:1288–98. doi: 10.1016/S0140-6736(15)00276-7
5. Daniel VT, Wiseman JT, Flahive J, Santry HP. Predictors of mortality in the elderly after open repair for perforated peptic ulcer disease. J Surg Res. (2017) 215:108–13. doi: 10.1016/j.jss.2017.03.052
6. Søreide K, Thorsen K, Søreide JA. Strategies to improve the outcome of emergency surgery for perforated peptic ulcer. Br J Surg. (2014) 101:51–64. doi: 10.1002/bjs.9368
7. Xie F, Yun H, Bernatsky S, Curtis JR. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. (2016) 68:2612–7. doi: 10.1002/art.39761
8. Gisbert JP, Legido J, García-Sanz I, Pajares JM. Helicobacter pylori and perforated peptic ulcer prevalence of the infection and role of non-steroidal anti-inflammatory drugs. Dig Liver Dis. (2004) 36:116–20. doi: 10.1016/j.dld.2003.10.011
9. Kujath P, Schwandner O, Bruch HP. Morbidity and mortality of perforated peptic gastroduodenal ulcer following emergency surgery. Langenbecks Arch Surg. (2002) 387:298–302. doi: 10.1007/s00423-002-0331-9
10. Hermansson M, Staël von Holstein C, Zilling T. Peptic ulcer perforation before and after the introduction of H2-receptor blockers and proton pump inhibitors. Scand J Gastroenterol. (1997) 32:523–9. doi: 10.3109/00365529709025093
11. Chan WH, Wong WK, Khin LW, Soo KC. Adverse operative risk factors for perforated peptic ulcer. Ann Acad Med Singap. (2000) 29:164–7.
12. Rajesh V, Chandra SS, Smile SR. Risk factors predicting operative mortality in perforated peptic ulcer disease. Trop Gastroenterol. (2003) 24:148–50.
13. Kocer B, Surmeli S, Solak C, Unal B, Bozkurt B, Yildirim O, et al. Factors affecting mortality and morbidity in patients with peptic ulcer perforation. J Gastroenterol Hepatol. (2007) 22:565–70. doi: 10.1111/j.1440-1746.2006.04500.x
14. Deus Fombellida J, Gil Romea I, Moreno Mirallas MJ, Urieta Carpi A. Risk factors in the surgical management of perforated duodeno-pyloric ulcer. Rev Esp Enferm Dig. 1998.90:503-13.
15. Madiba TE, Nair R, Mulaudzi TV, Thomson SR. Perforated gastric ulcer–reappraisal of surgical options. S Afr J Surg. (2005) 43:58–60.
16. Du B, An Y, Kang Y, Yu X, Zhao M, Ma X, et al. Characteristics of critically ill patients in ICUs in mainland China. Crit Care Med. (2013) 41:84–92. doi: 10.1097/CCM.0b013e31826a4082
17. Møller MH, Adamsen S, Thomsen RW, Møller AM. Multicentre trial of a perioperative protocol to reduce mortality in patients with peptic ulcer perforation. Br J Surg. (2011) 98:802–10. doi: 10.1002/bjs.7429
18. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock:2016. Intensive Care Med. (2017) 43:304–77. doi: 10.1007/s00134-017-4683-6
19. Arora R, Campbell JP, Simon G, Sahni N. Does serum procalcitonin aid in the diagnosis of bloodstream infection regardless of whether patients exhibit the systemic inflammatory response syndrome? Infection. (2017) 45:291–8. doi: 10.1007/s15010-016-0965-0
20. Kim SH, Shin SS, Jeong YY, Heo SH, Kim JW, Kang HK. Gastrointestinal tract perforation: MDCT findings according to the perforation sites. Korean J Radiol. (2009) 10:63–70. doi: 10.3348/kjr.2009.10.1.63
21. Hollerweger A, Maconi G, Ripolles T, Nylund K, Higginson A, Serra C, et al. Gastrointestinal Ultrasound (GIUS) in intestinal emergencies - An EFSUMB position paper. Ultraschall Med. (2020) 41:646–57. doi: 10.1055/a-1147-1295
22. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus definitions for sepsis and septic shock. Jama. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
23. DellaVolpe JD, Moore JE, Pinsky MR. Arterial blood pressure and heart rate regulation in shock state. Curr Opin Crit Care. (2015) 21:376–80. doi: 10.1097/MCC.0000000000000239
24. Datta PK, Rewari V, Ramachandran R, Singh PM, Ray BR, Aravindan A, et al. Effectiveness of enteral ivabradine for heart rate control in septic shock: a randomized controlled trial. Anaesth Intensive Care. (2021) 49:366–78. doi: 10.1177/0310057X211009913
25. Songne B, Jean F, Foulatier O, Khalil H, Scotté M. Non operative treatment for perforated peptic ulcer: results of a prospective study. Ann Chir. (2004) 129:578–82. doi: 10.1016/j.anchir.2004.06.012
26. Møller MH, Adamsen S, Thomsen RW, Møller AM. Preoperative prognostic factors for mortality in peptic ulcer perforation: a systematic review. Scand J Gastroenterol. (2010) 45:785–805. doi: 10.3109/00365521003783320
27. Corradi F, Via G, Tavazzi G. What's new in ultrasound-based assessment of organ perfusion in the critically ill: expanding the bedside clinical monitoring window for hypoperfusion in shock. Intensive Care Med. (2020) 46:775–9. doi: 10.1007/s00134-019-05791-y
28. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
29. Giamarellos-Bourboulis EJ, Norrby-Teglund A, Mylona V, Savva A, Tsangaris I, Dimopoulou I, et al. Risk assessment in sepsis: a new prognostication rule by APACHE II score and serum soluble urokinase plasminogen activator receptor. Crit Care. (2012)16:1. doi: 10.1186/cc11463
30. Royal College of Physicians National Early Warning Score (NEWS) 2: Standardizing the Assessment of Acute-Illness Severity in the NHS. Updated Report of a Working Party London: RCP (2017).
31. Bastos PG, Sun X, Wagner DP, Wu AW, Knaus WA. Glasgow Coma Scale score in the evaluation of outcome in the intensive care unit: findings from the Acute Physiology and Chronic Health Evaluation III study. Crit Care Med. (1993) 21:1459–65. doi: 10.1097/00003246-199310000-00012
32. Wu Y, Huang S, Chang X. Understanding the complexity of sepsis mortality prediction via rule discovery and analysis: a pilot study. BMC Med Inform Decis Mak. (2021) 21:334. doi: 10.1186/s12911-021-01690-9
33. Trzeciak S, Dellinger RP, Chansky ME, Arnold RC, Schorr C, Milcarek B, et al. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. (2007) 33:970–7. doi: 10.1007/s00134-007-0563-9
34. Oh DH, Kim MH, Jeong WY, Kim YC, Kim EJ, Song JE, et al. Risk factors for mortality in patients with low lactate level and septic shock. J Microbiol Immunol Infect. (2019) 52:418–25. doi: 10.1016/j.jmii.2017.08.009
35. Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. (1996) 171:221–6. doi: 10.1016/S0002-9610(97)89552-9
36. Schlattmann P, Brunkhorst FM. Procalcitonin as a diagnostic marker for sepsis. Lancet Infect Dis. (2014) 14:189. doi: 10.1016/S1473-3099(13)70325-6
37. Walker C. Procalcitonin-guided antibiotic therapy duration in critically ill adults. AACN Adv Crit Care. (2015) 26:99–106. doi: 10.4037/NCI.0000000000000079
38. Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. (2004) 30:261–77. doi: 10.1385/IR:30:3:261
39. Cui N, Zhang H, Chen Z, Yu Z. Prognostic significance of PCT and CRP evaluation for adult ICU patients with sepsis and septic shock: retrospective analysis of 59 cases. J Int Med Res. (2019) 47:1573–9. doi: 10.1177/0300060518822404
40. Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. (2001) 164:396–402. doi: 10.1164/ajrccm.164.3.2009052
Keywords: septic shock, gastrointestinal tract, perforation, risk factors, early warning score
Citation: Chen P, Gao J, Li J, Yu R, Wang L, Xue F, Zheng X, Gao L and Shang X (2022) Construction and efficacy evaluation of an early warning scoring system for septic shock in patients with digestive tract perforation: A retrospective cohort study. Front. Med. 9:976963. doi: 10.3389/fmed.2022.976963
Received: 23 June 2022; Accepted: 16 August 2022;
Published: 13 September 2022.
Edited by:
Longxiang Su, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Alfredo N. C. Santana, Escola Superior de Ciências da Saúde, BrazilCopyright © 2022 Chen, Gao, Li, Yu, Wang, Xue, Zheng, Gao and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuling Shang, emtzeGxpbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.