
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 26 August 2022
Sec. Rheumatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.972586
This article is part of the Research Topic Molecular Markers in Rheumatic Diseases and Their Comorbidities View all 7 articles
Objectives: Previous studies showed conflicting results regarding peripheral vitamin D levels in ankylosing spondylitis (AS). We performed this systemic review and meta-analysis to explore whether vitamin D may influence AS process.
Methods: Articles published until March 2022 were searched in databases as follows: PubMed, Web of Science, and Google Scholar. The present study included cross-sectional and case-control studies regarding vitamin D levels in patients with AS. Studies were excluded according to the following exclusion criteria: (1) we excluded studies which did not provide sufficient information regarding the comparison of vitamin D levels in AS patients and healthy controls (HC). Vitamin D levels in the two group studies should be reported or could be calculated in included studies; (2) meta-analysis, reviews and case reports. STATA 12.0 software was used to make a meta-analysis. Standard mean differences (SMDs) and 95% confidence intervals (CIs) were computed as effect size.
Results: The present meta-analysis showed no significant difference in peripheral 1,25-dihydroxyvitamin D3 (1,25OHD) levels between AS and healthy controls (HCs) in Caucasians with a random effects model [SMD: −0.68, 95% CI (−1.90, 0.54)]. Patients with AS had lower peripheral 25-hydroxyvitamin D (25OHD) levels compared with HC with a random effects model [SMD: −0.45, 95% CI: (−0.70, −0.20)]. Patients with AS had higher peripheral C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels compared with HC in Caucasian population with random effects models [CRP: SMD: 1.08, 95% CI: (0.78, 1.37); ESR: SMD: 0.86, 95% CI: (0.39, 1.34)]. However, no significant difference in alkaline phosphatase (ALP), parathyroid hormone (PTH) or calcium levels were indicated between AS and HC in Caucasian with random effects models [ALP: SMD: 0.07, 95% CI: (−0.41, 0.55); PTH: SMD: −0.15, 95% CI: (−0.56, 0.26); calcium: SMD: −0.06, 95% CI: (−0.39, 0.26)].
Conclusion: In conclusion, the study showed an inverse association between 25OHD and AS, which suggests that vitamin D may have a protective effect on AS. ESR and C-reactive protein (CRP) are important biomarkers for AS.
Ankylosing spondylitis (AS) is a chronic immune-mediated progressive rheumatic disease that mainly affects the axial spine, especially, the axial spine and sacroiliac joints (1). AS is characterized by progressive spinal ankylosis and chronic pain resulting in joint dysfunction and long-term disability (2). It was estimated that the prevalence of AS ranged from 7.4 to 23.8 per 10,000 (3). Up to now, the pathogenesis of AS has not been well-clarified. However, an increasing body of evidence suggests that AS is an inherited disease, particularly, closely genetic associated with HLA-B27 (4). Issue data reported that AS could lead to a series of complications, namely, iritis, osteoporosis, spinal compression fractures, and cardiovascular disease (5). AS leads to a life-long impact on patients and increases society burden because of the common comorbidities and chronic progressive course of work disability (6).
Vitamin D is a fat-soluble that is important in mineral and bone metabolism. The natural way to obtain vitamin D is through safe sunlight exposure and daily diet supplement (7). Vitamin D has multiple biological functions, such as stimulating calcium and phosphate absorption in the intestine, regulating parathyroid hormone (PTH) secretion (8). Vitamin D plays a role in other beneficial actions, namely, improving immune function, reducing the risk of cancer death, and preventing cardiovascular complications (9, 10).
There are increasing data linking vitamin D and the immune system. Vitamin D deficiency has been described as a risk factor in the progression of several autoimmune diseases such as multiple sclerosis and Crohn's disease, generally attributed to the potent immunomodulatory effects of 1,25-dihydroxyvitamin D3 (1,25OHD) (11, 12). Klingberg et al. reported that serum 25-hydroxyvitamin D (25OHD) in patients with AS was not different from healthy controls (HCs), and not associated with disease activity (13), whereas Fotoh et al. reported that there was a significant difference in serum 25OHD between patients with HC and AS (14). Because of these conflicting results, we performed this systemic review and meta-analysis to explore whether vitamin D may influence AS process.
The present study was conducted drawing on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (15). The present study is a meta-analysis, ethical approval was not applicable.
Articles published until March 2022 were searched in databases as follows: PubMed, Web of Science, and Google Scholar. Search terms (“ankylosing spondylitis” OR “AS”) AND (“vitamin D” OR “25OHD” OR “1,25OHD”) were used.
The present study included cross-sectional and case-control studies regarding vitamin D levels in patients with AS. Studies were excluded according to the following exclusion criteria: (1) we excluded studies which did not provide sufficient information regarding the comparison of vitamin D levels in patients with AS and HC. Vitamin D levels in the two group studies should be reported or could be calculated in included studies; (2) meta-analysis, reviews, and case reports.
In total, two investigators extracted data from finally included studies independently. Extracted data showed as follows: author, publication year, study location, sample size, gender, mean age, and results. STATA 12.0 software was used to make meta-analysis. Standard mean differences (SMDs) and 95% confidence intervals (CIs) were computed as effect size. Q-test and I2 were used to assess heterogeneity among studies. In addition, meta-regression analysis was conducted to detect source of the heterogeneity. With high heterogeneity (p-value of Q test ≤ 0.05 and I2 ≥ 50%), random effects models were used to compute results; with low heterogeneity (p-value of Q test > 0.05 and I2 <50%), fixed effects models were used. Subgroup analyses (for different ethnicities) were used to investigate the effect of ethnicities on results. In addition, the stability of the meta-analysis was evaluated with sensitivity analysis. Moreover, the Begg's test, Egger's test, and funnel plots were made to evaluate publication bias.
Selection procedures were illustrated in Supplementary Figure 1. Supplementary Table 1 showed study characteristics of 15 finally included studies (13, 14, 16–28). These studies included 2,703 patients with AS and 6,145 HC.
The present meta-analysis showed no significant difference in peripheral 1,25OHD levels between AS and HC in Caucasian with a random effects model [SMD: −0.68, 95% CI (−1.90, 0.54); p-value of Q test <0.001, I2 = 96.2%; Figure 1]. Patients with AS had lower peripheral 25OHD levels compared with HC with a random effects model [SMD: −0.45, 95% CI: (−0.70, −0.20); p-value of Q test <0.001, I2 = 94.3%; Figure 2]. Subgroup studies showed no significant difference in peripheral 25OHD levels between patients with AS and HC in the Caucasian population [SMD: −0.41, 95% CI: (−0.67, −0.16); Supplementary Figure 2]. No significant difference in alkaline phosphatase (ALP) levels were indicated between AS and HC in Caucasian with a random effects model [SMD: 0.07, 95% CI: (−0.41, 0.55); p-value of Q test <0.001, I2 = 85.0%; Figure 3]. Patients with AS had higher peripheral C-reactive protein (CRP) levels compared with HC in the Caucasian population with a random effects model [SMD: 1.08, 95% CI: (0.78, 1.37); p-value of Q test <0.001, I2 = 85.3%; Figure 4]. In addition, patients with AS had higher peripheral erythrocyte sedimentation rate (ESR) levels compared with the HC in the Caucasian with random effects models [SMDs: 0.86, 95%CI: (0.39, 1.34); p-value of Q test <0.001, I2 = 95.1%; Figure 5], whereas no significant difference in PTH or peripheral calcium levels was showed between AS and HC in the Caucasian with a random effects model [PTH: SMD: −0.15, 95% CI: (−0.56, 0.26); p-value of Q test <0.001, I2 = 91.7%; Figure 6; calcium: SMD: −0.06, 95% CI: (−0.39, 0.26); p-value of Q test = 0.002, I2 = 76.8%; Figure 7].
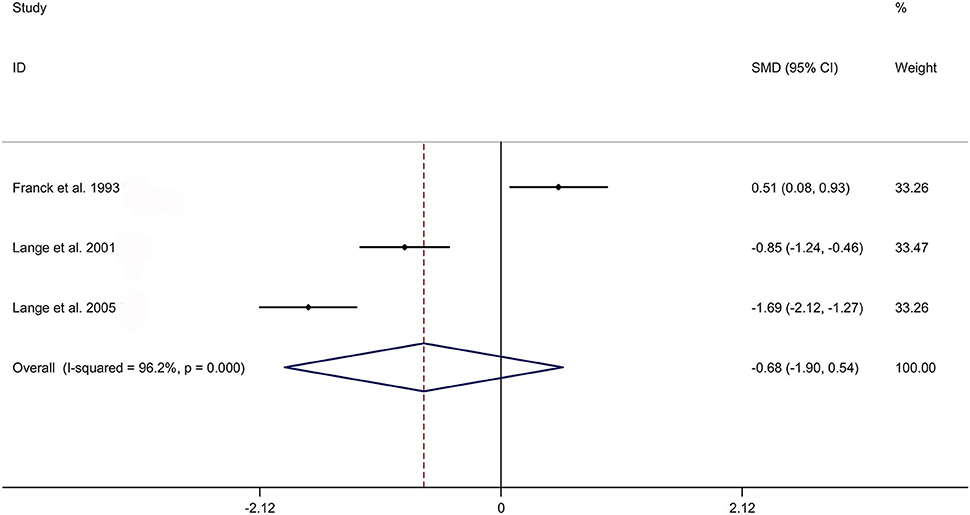
Figure 1. Forest plot regarding comparison in peripheral 1,25OHD levels between AS and HC. AS, ankylosing spondylitis; HC, healthy controls; 1,25OHD, 1,25-dihydroxyvitamin D3.
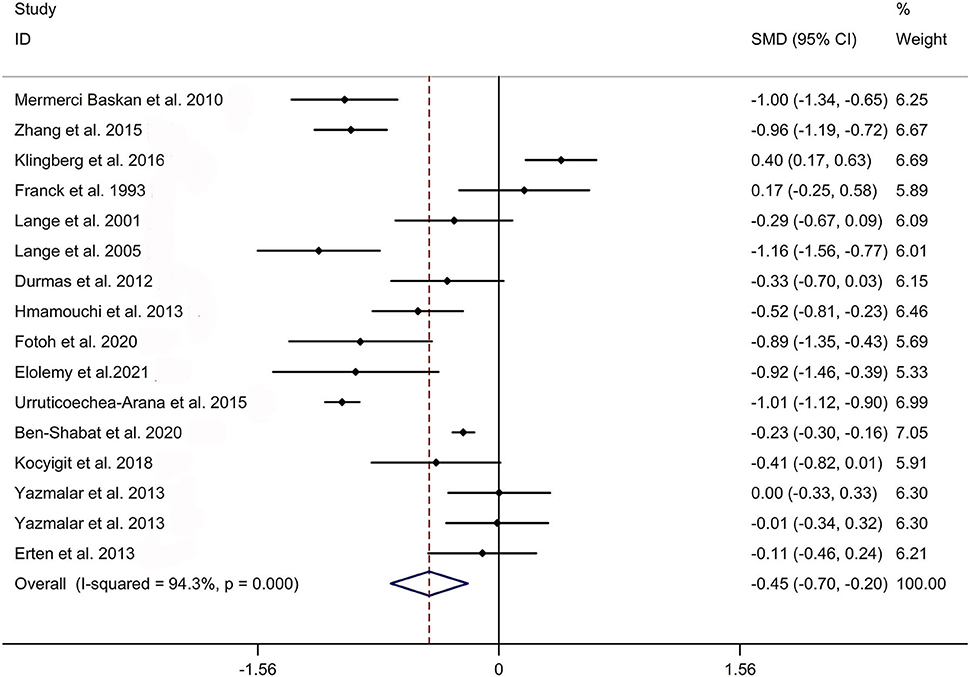
Figure 2. Forest plot regarding comparison in peripheral 25OHD levels between AS and HC. AS, ankylosing spondylitis; HC, healthy controls; 25OHD, 25-hydroxyvitamin D.
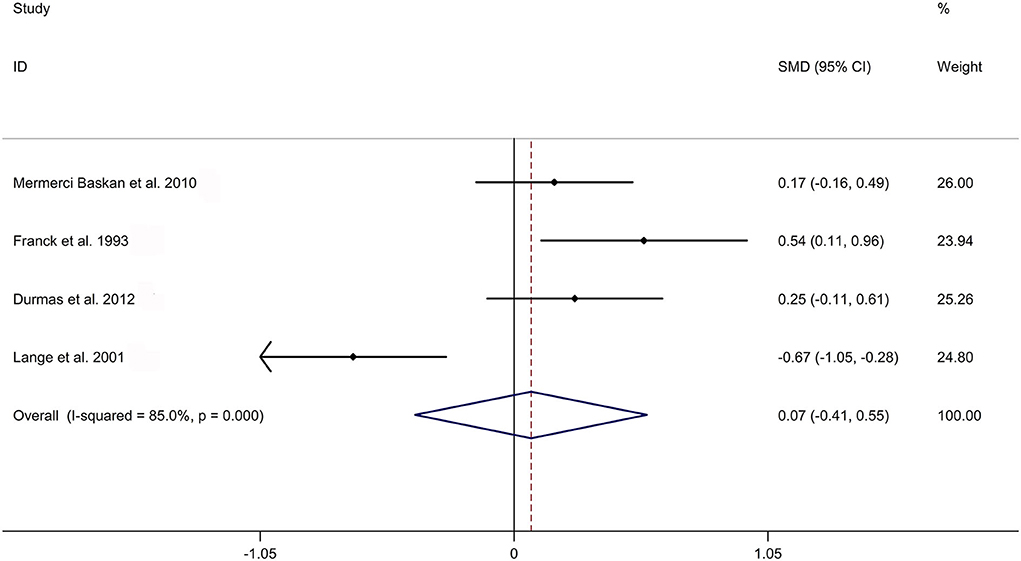
Figure 3. Forest plot regarding comparison in peripheral ALP levels between AS and HC. ALP, alkaline phosphatase; AS, ankylosing spondylitis; HC, healthy controls.
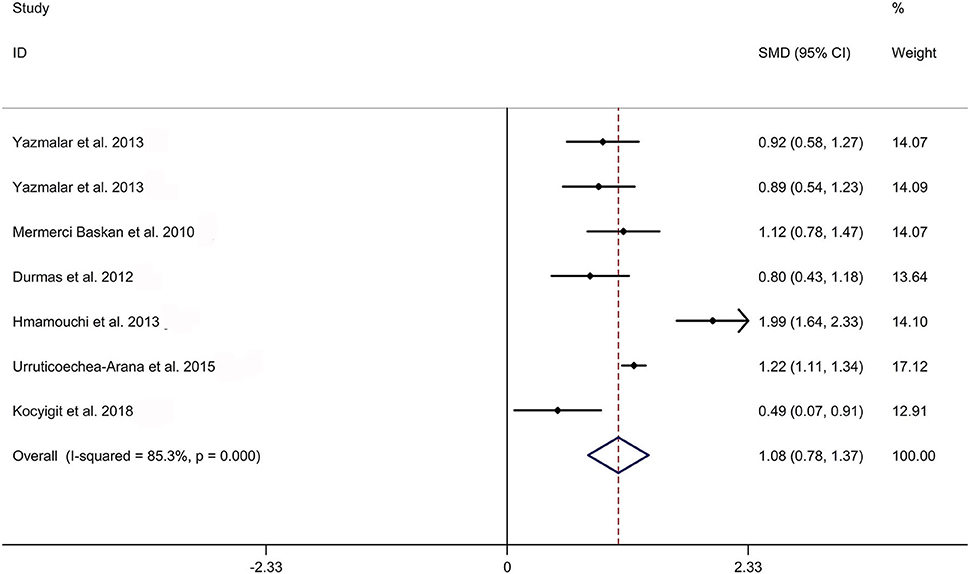
Figure 4. Forest plot regarding comparison in peripheral CRP levels between AS and HC. AS, ankylosing spondylitis; CRP, C-reactive protein; HC, healthy controls.
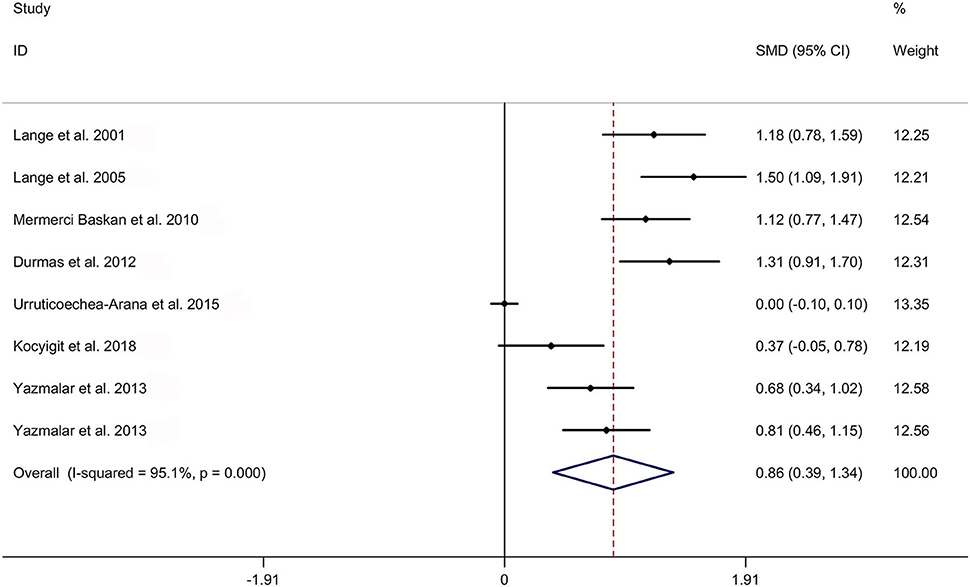
Figure 5. Forest plot regarding comparison in peripheral ESR levels between AS and HC. AS, ankylosing spondylitis; ESR, erythrocyte sedimentation rate; HC, healthy controls.

Figure 6. Forest plot regarding comparison in peripheral PTH levels between AS and HC. AS, ankylosing spondylitis; HC, healthy controls; PTH, parathyroid hormone.
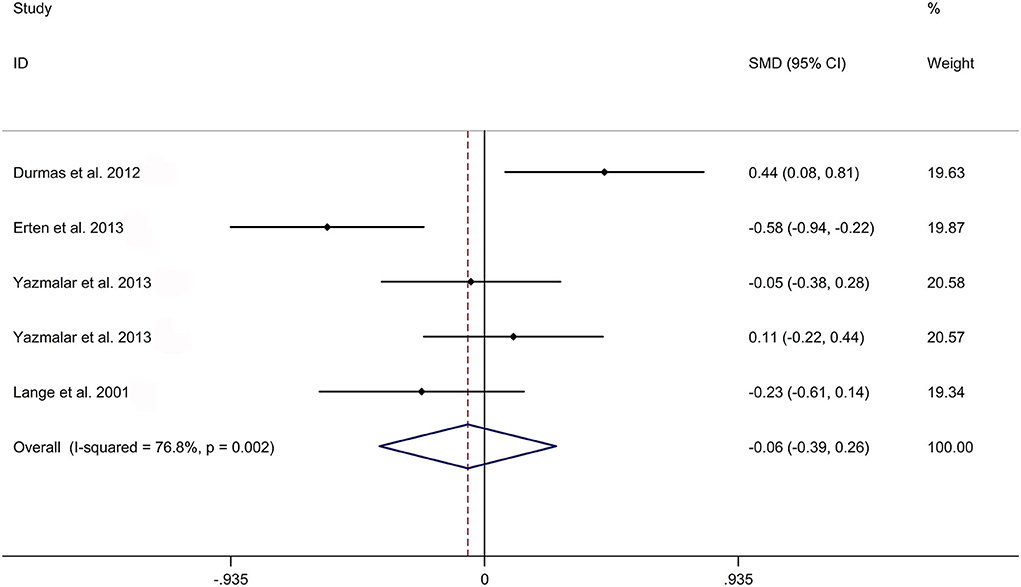
Figure 7. Forest plot regarding comparison in peripheral calcium levels between AS and HC. AS, ankylosing spondylitis; HC, healthy controls.
Meta-regression analysis showed that ages and gender were not responsible for heterogeneity across studies regarding comparison of peripheral 25OHD, ALP, CRP, ESR, PTH, and calcium levels between patients with AS and HC (Supplementary Table 2).
Sensitivity analyses showed no changes in the direction of effect when any one study was excluded for all meta-analyses (Supplementary Figure 3). Supplementary Table 3 and Supplementary Figure 4 showed results of publication bias. The Begg's test, Egger's tests, and funnel plots showed no significant risks of publication bias for meta-analyses regarding comparison of peripheral 1,25OHD, 25OHD, ALP, CRP, PTH, and calcium between patients with AS and HC (Supplementary Table 3 and Supplementary Figures 4A–G), whereas the Begg's test, Egger's tests, and funnel plots showed significant risks of publication bias for meta-analyses regarding comparison of ESR between patients with AS and HC (Supplementary Table 3 and Supplementary Figure 4E)
In this systemic review and meta-analysis, we found that there was a significant difference between HC and patients with AS in the level of serum 25OHD, whereas no difference in 1,25OHD. This result was consistent with the article published by Cai et al. in 2015 which reported that the SMD of 25OHD between patients with AS and controls was −0.66 (−1.08, −0.24), and 1,25OHD was −0.72 (−1.79, 0.35) (29). The conversion of vitamin D into the active hormone 1,25OHD needs two hydroxylation steps. The concentration of 1,25OHD, which is in the order of pg/ml, is much lower than 25OHD (order of ng/ml) in the blood, thus 25OHD is more easily to be detected than 1,25OHD (30). Moreover, the conversion rate of 1,25OHD is regulated by many paracrine and autocrine factors including PTH concentrations (31). In addition, 1,25OHD is with a short half-life between 4 and 6 h. Therefore, 1,25OHD is not associated with patients with AS, which may be because of the instability of 1,25OHD in serum. This result needs more convincing evidence to be confirmed.
In total, 1,25OHD and vitamin D are recognized as important immune system regulators that is widely accepted and the vitamin D receptor (VDR) has been found in the immune system (32). Murdaca et al. reported the important role of vitamin D and the gut microbiome in the efficiency of the immune response (33). Interactions between vitamin D, gut microbiome, and the immune system may happen at several levels and can include both the innate and the adaptive immune systems (33). Immune system and the microbiome are interconnected, and vitamin D plays a critical role in this dynamic (33). In total, 1,25OHD works mainly by means of VDR (34). VDR is a transcription factor and nuclear hormone receptor expressed in some tissues, namely, the intestines, liver, adipose tissue, and most immune cells. VDR modulates procedures of metabolic and immune system (35). Because VDR expresses in most immune cells (including CD4+ and CD8+ T cells, B cells, antigen-presenting cells (APCs), and neutrophils), it plays a critical role in the modulation of the immune response (36). In addition, VDR is also highly expressed in the small intestine and colon, where it plays a critical role in immunity, host-microbial interactions, and susceptibility to pathogenic infection. VDR expressed in the intestines is critical for maintaining a healthy microbiome (37). Changes in vitamin D/VDR signaling are related to microbiome dysbiosis, which in turn has been associated with both intestinal inflammatory processes and extra-intestinal conditions such as AS. A previous study supported that vitamin D and 1,25OHD can effectively treat animal of T-cells-mediated diseases such as multiple sclerosis (38). A low level of 25OHD was associated with increasing risk of multiple sclerosis among Caucasians (39). In addition, several recently published articles provided novel evidence to support that vitamin D associated gene polymorphisms were in connection with AS. Zhang et al. showed that in Han Chinese the haplotypes (TG) of rs11168266-rs11168267 in the VDR gene was associated with AS susceptibility (40). A case-control study indicated that haplotypes of rs1544410 and rs731236 in the VDR gene significantly conferred the risk of AS (41). Furthermore, serum VDR levels have been linked to disease activity and clinical parameters among patients with AS, which may be a potential marker of AS (42). Jung et al. reported that vitamin D-binding protein gene polymorphisms were related to the risk of peripheral arthritis and uveitis among patients with AS (43).
Previous studies have concluded that there is a tightly regulated feedback cycle between vitamin D and PTH secretion (44). PTH stimulates the conversion of 25OHD to 1,25OHD in the kidney, and enhances renal calcium reabsorption (45). Thus, it is considered that serum PTH levels were inversely associated with 25OHD. In our study, no significant difference in PTH levels was showed between AS and HC in Caucasian (SMD: −0.15, 95% CI: −0.56–0.26). ESR and CRP are non-specific indicators for systemic inflammation. Our research showed that both CRP and ESR were higher in patients with AS than in controls (CRP: SMD = 1.08, 95% CI: 0.78, 1.37; ESR: SMD = 0.86, 95% CI: 0.39, 1.34).
We discussed the associations between activity of AS and several critical factors and found there were significant differences in 25OHD, ESR, and CRP between patients with AS and HC. However, there were some limitations in this meta-analysis. At first, most of included studies were based on Caucasians, and only one on yellow race. Second, because of the limitation of data resources, we could not evaluate the impact of several factors (such as diet and sunshine duration in the area where the sample population is located, and the season of blood sampling) on the association between AS and vitamin D.
In conclusion, the study showed an inverse association between 25OHD and AS, which suggests that vitamin D may have protective effect on AS. However, whether vitamin D supplement decreases the risk of AS needs further research. More well-designed studies are needed to confirm the relationship between vitamin D and AS.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
MD planned the study, made data collection, and manuscript writing. JP made study search, data collection, and data analysis. DW made study search and data analysis. HW submitted the study. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.972586/full#supplementary-material
1. Wenker KJ, Quint JM. Ankylosing Spondylitis. Treasure Island, FL: StatPearls Publishing LLC. (2021).
2. Liang H, Xu L, Tian X, Wang S, Liu X, Dai Y, et al. The comparative efficacy of supervised- versus home-based exercise programs in patients with ankylosing spondylitis: a meta-analysis. Medicine. (2020) 99:e19229. doi: 10.1097/MD.0000000000019229
3. Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology. (2014) 53:650–7. doi: 10.1093/rheumatology/ket387
4. Simone D, Al Mossawi MH, Bowness P. Progress in our understanding of the pathogenesis of ankylosing spondylitis. Rheumatology. (2018) 57:vi4–9. doi: 10.1093/rheumatology/key001
5. Voruganti A, Bowness P. New developments in our understanding of ankylosing spondylitis pathogenesis. Immunology. (2020) 161:94–102. doi: 10.1111/imm.13242
6. Lee JS, Oh BL, Lee HY, Song YW, Lee EY. Comorbidity, disability, and healthcare expenditure of ankylosing spondylitis in Korea: a population-based study. PLoS ONE. (2018) 13:e0192524. doi: 10.1371/journal.pone.0192524
7. Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. (2015) 55:1193–205. doi: 10.1080/10408398.2012.688897
8. Mazzaferro S, Goldsmith D, Larsson TE, Massy ZA, Cozzolino M. Vitamin D metabolites and/or analogs: which D for which patient? Curr Vasc Pharmacol. (2014) 12:339–49. doi: 10.2174/15701611113119990024
9. Urena-Torres P, Souberbielle JC. Pharmacologic role of vitamin D natural products. Curr Vasc Pharmacol. (2014) 12:278–85. doi: 10.2174/15701611113119990020
10. Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. (2019) 366:l4673. doi: 10.1136/bmj.l4673
11. Harrison SR, Li D, Jeffery LE, Raza K, Hewison M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif Tissue Int. (2020) 106:58–75. doi: 10.1007/s00223-019-00577-2
12. Illescas-Montes R, Melguizo-Rodríguez L, Ruiz C, Costela-Ruiz VJ. Vitamin D and autoimmune diseases. Life Sci. (2019) 233:116744. doi: 10.1016/j.lfs.2019.116744
13. Klingberg E, Oleröd G, Hammarsten O, Forsblad-d'Elia H. The vitamin D status in ankylosing spondylitis in relation to intestinal inflammation, disease activity, and bone health: a cross-sectional study. Osteoporos Int. (2016) 27:2027–33. doi: 10.1007/s00198-016-3489-7
14. Fotoh DS, Serag DM, Badr IT, Saif DS. Prevalence of subclinical carotid atherosclerosis and vitamin D deficiency in Egyptian ankylosing spondylitis patients. Arch Rheumatol. (2020) 35:335–42. doi: 10.46497/ArchRheumatol.2020.7694
15. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151, 264–269, w264. doi: 10.7326/0003-4819-151-4-200908180-00135
16. Franck H, Keck E. Serum osteocalcin and vitamin D metabolites in patients with ankylosing spondylitis. Ann Rheum Dis. (1993) 52:343–6. doi: 10.1136/ard.52.5.343
17. Lange U, Jung O, Teichmann J, Neeck G. Relationship between disease activity and serum levels of vitamin D metabolites and parathyroid hormone in ankylosing spondylitis. Osteoporos Int. (2001) 12:1031–5. doi: 10.1007/s001980170013
18. Lange U, Teichmann J, Strunk J, Müller-Ladner U, Schmidt KL. Association of 125 vitamin D3 deficiency, disease activity and low bone mass in ankylosing spondylitis. Osteoporos Int. (2005) 16:1999–2004. doi: 10.1007/s00198-005-1990-5
19. Mermerci Başkan B, Pekin Dogan Y, Sivas F, Bodur H, Ozoran K. The relation between osteoporosis and vitamin D levels and disease activity in ankylosing spondylitis. Rheumatol Int. (2010) 30:375–81. doi: 10.1007/s00296-009-0975-7
20. Durmus B, Altay Z, Baysal O, Ersoy Y. Does vitamin D affect disease severity in patients with ankylosing spondylitis? Chin Med J (Engl). (2012) 125:2511–5. doi: 10.3760/cma.j.issn.0366-6999.2012.14.021
21. Erten S, Kucuksahin O, Sahin A, Altunoglu A, Akyol M, Koca C. Decreased plasma vitamin D levels in patients with undifferentiated spondyloarthritis and ankylosing spondylitis. Intern Med. (2013) 52:339–44. doi: 10.2169/internalmedicine.52.9047
22. Hmamouchi I, Allali F, El Handaoui B, Amine H, Rostom S, et al. The relation between disease activity, vitamin D levels and bone mineral density in men patients with ankylosing spondylitis. Rheumatol Rep. (2013) 5:3. doi: 10.4081/rr.2013.e3
23. Yazmalar L, Ediz L, Alpayci M, Hiz O, Toprak M, Tekeoglu I. Seasonal disease activity and serum vitamin D levels in rheumatoid arthritis, ankylosing spondylitis and osteoarthritis. Afr Health Sci. (2013) 13:47–55. doi: 10.4314/ahs.v13i1.7
24. Urruticoechea-Arana A, Martín-Martínez MA, Castañeda S, Piedra CA, González-Juanatey C, Llorca J, et al. Vitamin D deficiency in chronic inflammatory rheumatic diseases: results of the cardiovascular in rheumatology [CARMA] study. Arthritis Res Ther. (2015) 17:211. doi: 10.1186/s13075-015-0704-4
25. Zhang P, Li Q, Wei Q, Liao Z, Lin Z, Fang L, et al. Serum vitamin D and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen in patients with ankylosing spondylitis. Biomed Res Int. (2015) 2015:543806. doi: 10.1155/2015/543806
26. Kocyigit BF, Akyol A. Vitamin D levels in patients with ankylosing spondylitis: is it related to disease activity? Pak J Med Sci. (2018) 34:1209–14. doi: 10.12669/pjms.345.15739
27. Ben-Shabat N, Watad A, Shabat A, Bragazzi NL, Comaneshter D, Cohen AD, et al. Low vitamin D levels predict mortality in ankylosing spondylitis patients: a nationwide population-based cohort study. Nutrients. (2020) 12:1400. doi: 10.3390/nu12051400
28. Elolemy G, Hassan W, Nasr M, Baraka E. Hypovitaminosis D in patients with ankylosing spondylitis: frequency and consequences. Curr Rheumatol Rev. (2021) 17:365–72. doi: 10.2174/1573397117666210308122515
29. Cai G, Wang L, Fan D, Xin L, Liu L, Hu Y, et al. Vitamin D in ankylosing spondylitis: review and meta-analysis. Clin Chim Acta. (2015) 438:316–22. doi: 10.1016/j.cca.2014.08.040
30. Saponaro F, Saba A, Zucchi R. An update on vitamin D metabolism. Int J Mol Sci. (2020) 21:6573. doi: 10.3390/ijms21186573
31. Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. (2012) 95:1357–64. doi: 10.3945/ajcn.111.031070
32. Grant WB, Holick MF. Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev. (2005) 10:94–111. doi: 10.1016/j.jep.2005.01.062
33. Murdaca G, Gerosa A, Paladin F, Petrocchi L, Banchero S, Gangemi S. Vitamin D and microbiota: is there a link with allergies? Int J Mol Sci. (2021) 22:4288. doi: 10.3390/ijms22084288
34. Bakke D, Sun J. Ancient nuclear receptor VDR with new functions: microbiome and inflammation. Inflamm Bowel Dis. (2018) 24:1149–54. doi: 10.1093/ibd/izy092
35. Sun J. Dietary vitamin D, vitamin D receptor, and microbiome. Curr Opin Clin Nutr Metab Care. (2018) 21:471–4. doi: 10.1097/MCO.0000000000000516
36. Malaguarnera L. Vitamin D and microbiota: two sides of the same coin in the immunomodulatory aspects. Int Immunopharmacol. (2020) 79:106112. doi: 10.1016/j.intimp.2019.106112
37. Wong M. What has happened in the last 50 years in immunology. J Paediatr Child Health. (2015) 51:135–9. doi: 10.1111/jpc.12834
38. Gu SG, Wang CJ, Zhao G, Li GY. Role of vitamin D in regulating the neural stem cells of mouse model with multiple sclerosis. Eur Rev Med Pharmacol Sci. (2015) 19:4004–11.
39. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. (2006) 296:2832–8. doi: 10.1001/jama.296.23.2832
40. Zhang P, Li Q, Qi J, Lv Q, Zheng X, Wu X, et al. Association between vitamin D receptor gene polymorphism and ankylosing spondylitis in Han Chinese. Int J Rheum Dis. (2017) 20:1510–6. doi: 10.1111/1756-185X.12949
41. Cai G, Zhang X, Xin L, Wang L, Wang M, Yang X, et al. Associations between vitamin D receptor gene polymorphisms and ankylosing spondylitis in Chinese Han population: a case-control study. Osteoporos Int. (2016) 27:2327–33. doi: 10.1007/s00198-016-3500-3
42. Kültür T, Öztaş D, Keskin D, Keskin G, Inal A, Kara H. The relationship of serum vitamin D receptor levels with disease activity and clinical parameters in patients with ankylosing spondylitis. Turk J Phys Med Rehabil. (2019) 65:389–93. doi: 10.5606/tftrd.2019.3296
43. Jung KH, Kim TH, Sheen DH, Lim MK, Lee SK, Kim JY, et al. Associations of vitamin d binding protein gene polymorphisms with the development of peripheral arthritis and uveitis in ankylosing spondylitis. J Rheumatol. (2011) 38:2224–9. doi: 10.3899/jrheum.101244
44. Khundmiri SJ, Murray RD, Lederer E. PTH and vitamin D. Compr Physiol. (2016) 6:561–601. doi: 10.1002/cphy.c140071
Keywords: ankylosing spondylitis, meta-analysis, vitamin D, systematic review, 1,25-dihydroxyvitamin D3
Citation: Diao M, Peng J, Wang D and Wang H (2022) Peripheral vitamin D levels in ankylosing spondylitis: A systematic review and meta-analysis. Front. Med. 9:972586. doi: 10.3389/fmed.2022.972586
Received: 18 June 2022; Accepted: 05 August 2022;
Published: 26 August 2022.
Edited by:
Efrain Chavarria-Avila, University of Guadalajara, MexicoReviewed by:
Napoleon Bellua Sam, University for Development Studies, GhanaCopyright © 2022 Diao, Peng, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongbo Wang, eXVzMnNxMDEyOTFAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.