- Department of Emergency, The Third Affiliated Hospital of Chongqing Medical University, Chongqing, China
Objective: The aim of this meta-analysis was to determine the role of an aggressive intravenous hydration protocol of Lactated Ringer’s solution in patients with mild acute pancreatitis (MAP).
Methods: A systematic search was conducted in PubMed, EMBASE, Cochrane Library, and China National Knowledge Infrastructure (CNKI) to identify randomized controlled trials (RCTs) published before August 19, 2022. The clinical outcomes were evaluated using the standard mean difference (SMD), mean difference (MD), risk ratio (RR), and 95% confidence interval (CI). The primary outcome was clinical improvement, while the secondary outcomes were the development of systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS), relief of epigastric abdominal pain, and length of hospital stay (LoH). Statistical analysis was performed with RevMan 5.4. Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) Working Group system was used to determine the quality of evidences.
Results: There were five RCTs with 370 MAP patients included, and the overall methodological quality was moderate. Aggressive hydration protocol was comparable to standard hydration protocol in terms of clinical improvement (RR = 1.33, 95%CI = 0.95–1.87, P = 0.10; very low evidence). Fewer events of SIRS (RR = 0.48, 95%CI = 0.31–0.72, P < 0.001; low evidence) and MODS (RR = 0.34, 95%CI = 0.13–0.91, P = 0.03; moderate evidence) were reported in patients receiving aggressive hydration protocol. Meanwhile, aggressive hydration protocol also significantly relieved epigastric abdominal pain (SMD = −0.53, 95%CI = −0.81 to −0.25, P < 0.001; low evidence) and shorten the LoH (MD = −2.36, 95%CI = −3.17 to −1.55, P < 0.001; low evidence) compared with standard hydration protocol.
Conclusion: For patients with MAP, aggressive hydration protocol may be more effective than standard hydration protocol at lowering SIRS and MODS rates, relieving epigastric abdominal pain, and shortening the LoH. Due to the small number of studies that are eligible and poor methodological quality of eligible studies, further studies are required to validate our findings.
Introduction
Acute pancreatitis (AP) is an inflammation of the pancreas that typically manifests as sudden onset of epigastric abdominal pain, nausea, and epigastric tenderness to palpation (1). AP has also been one of the most common causes of gastrointestinal-related hospitalization (2). Issued data showed that approximately 30 were confirmed to have AP out from per 100,000 persons annually (3), with an increasing trend in the incidence (4). There are three subtypes of AP are classified based in severity: mild, moderately severe, and severe (5). Although the majority of patients with AP were in mild stage (6, 7), if local inflammation is not effectively treated, patients could develop local complications or even progress to systemic inflammation (8). More importantly, uncontrolled systemic inflammation is a major role in the emergence of organ failure, a condition that was previously associated with a higher risk of mortality (9). Unfortunately, there were currently no approved pharmaceutical treatments that could change how AP developed (10).
Findings from experimental studies showed that AP impairs splanchnic perfusion and the pancreatic microcirculation (11). As a result of the potential loss of the fluid in the third space, fluid resuscitation was suggested for the maintenance of hemodynamics in patients with AP (10, 12). Studies have also shown that fluid resuscitation is beneficial for lowering mortality and the occurrence of complications such as pancreatic necrosis and organ failure (10). For fluid resuscitation in AP, crystalloid solutions are preferred over colloid solutions among the available solutions (13). Numerous studies have investigated the efficacy and safety of Lactated Ringer’s (LR’s) solution as fluid resuscitation solutions, and meta-analyses recently suggested that LR’s solution might be superior to normal saline solution for the management of AP (14–16).
Traditional recommendations for fluid resuscitation varied (10, 17), but some urgently proposed concerns about aggressive hydration have been made (18). A recent meta-analysis (19) also suggested that early aggressive intravenous fluid therapy may increase the risk of pulmonary edema and acute kidney injury while not improving mortality. It should be noted that although patients of any severity and different solutions were simultaneously considered in this meta-analysis, no subgroup analysis was designed to explore how these factors affected the pooled results. It is unknown whether patients with mild AP (MAP) would benefit from an aggressive hydration protocol. Therefore, we perform this meta-analysis to determine the role of aggressive hydration of LR’s solution in the management of MAP patients.
Materials and methods
The present meta-analysis was carried out based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (20). No institutional ethical approval and patients’ informed consent was necessary because this was a meta-analysis of previous studies. In addition, we did not previously register the formal protocol of our meta-analysis; however, we strictly followed the Cochrane handbook for systematic reviews of interventions (21).
Literature identification
Two independent authors (FW and DS) searched four electronic databases, including PubMed, EMBASE, and the Cochrane library, and China National Knowledge Infrastructure (CNKI) databases, for relevant studies published before December 31, 2021. The latest search was updated on August 19, 2021. Medical subject headings terms and text words were used for development of the search strategy with the Boolean operators. The third senior author was brought into the conversation to help resolve any disagreements between the two authors. Additionally, the reference lists of the included studies were also manually screened to find additional studies. Details of search strategies for target databases are documented in Supplementary Table 1.
Study selection
Based on the study titles, abstracts, and full texts, two independent authors (FW and QA) assessed the eligibility of the studies. Studies were included if they met the following criteria: (a) randomized controlled trials (RCTs), (b) studies of adult patients confirmed with MAP based on revised Atlanta classification (5); (c) patients in aggressive hydration group received LR’s solution 15–20 ml/kg bolus followed by infusion at 3 ml/kg/h, regardless of supplementary treatments such as probiotics; (d) patients in standard hydration group received LR’s solution 10 ml/kg bolus followed by infusion at 1.5 ml/kg/h; (e) studies reporting at least one of clinical improvement, the development of systemic inflammatory response syndrome (SIRS), the development of multiple organ dysfunction syndrome (MODS), the relief of abdominal pain, and the length of hospital stay (LoH).
According to the following criteria, we excluded ineligible studies: (a) studies that investigated fluid hydration in AP after ERCP, pediatric patient populations, animal studies, and cell lines were disregarded; (b) studies did not provide enough raw data that could be used to calculate effect estimates; (c) duplicate studies, abstracts, case reports, narrative reviews, or commentary. In case of any disagreements between the two investigators, the third investigator was consulted.
Data extraction
Data from the included studies, including first author’s name, publication year, country, sample size, the proportion of male patients, age, and clinical outcomes, were extracted by two independent investigators (FW and SZ). The third author was consulted if there were any disagreements between the two authors. If the outcome was presented as a median with a range, we calculated the corresponding mean and standard deviation using a recognized formula (22).
Outcomes of interesting
The primary outcome was clinical improvement within 36 h. Clinical improvement was deemed to have occurred if all of the following criteria were satisfied, including a decrease in hematocrit, BUN, and serum creatinine from baseline, a reduction in epigastric abdominal pain as measured by the visual analog scale and oral intake tolerance. We defined the development of SIRS (defined as meeting at least two of the following four criteria: heart rate > 90/min, white blood count > 12,000 or < 4,000 cells/mm3; respiratory rate > 20/min, partial pressure of carbon dioxide < 32 mmHg on room air, or T > 38°C or < 36°C), the development of MODS, the relief of epigastric abdominal pain [measured by visual analog scale (VAS) or numerical rating scale (NRS)], and the LoH as the secondary outcomes.
Risk of bias assessment and the quality of evidence
Using a tool developed by the Cochrane Collaboration’s tool (23), the included studies’ quality was evaluated using seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other risk. Each bias item would be labeled with “high,” “unclear,” or “low” risk according to the assessment criteria. In case of any disagreements between the two investigators (FW and JL), the third investigator was consulted (DS). Moreover, we determined the quality of evidence using Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) Working Group system (24).
Statistical analysis
We utilized Review Manager version 5.4 (the Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, 2014) to analyze the data. Standard mean difference (SMD) with the corresponding 95% confidence interval (CI) was used to express the estimate of epigastric abdominal pain because two different instruments were used for the pain assessment. On the contrary, mean difference (MD) with 95% CI was used to express the estimate of LoH. Risk ratio (RR) with 95% CI was used for expressing the estimates of dichotomous variables. Statistical heterogeneity was quantified using I2 statistic (25). Significant statistical heterogeneity was determined if the I2 value was ≥ 50%, and therefore, a random-effects model was used for meta-analysis. Otherwise, a fixed-effects model was selected for meta-analysis. Meanwhile, we compared the pooled result of the fixed-effects model and that of the random-effects model for sensitivity analysis if substantial statistical heterogeneity was detected. Publication bias test was not reported because the number of eligible studies was less than 10 (26). If p < 0.05, the result of statistics was significant.
Results
Literature retrieval
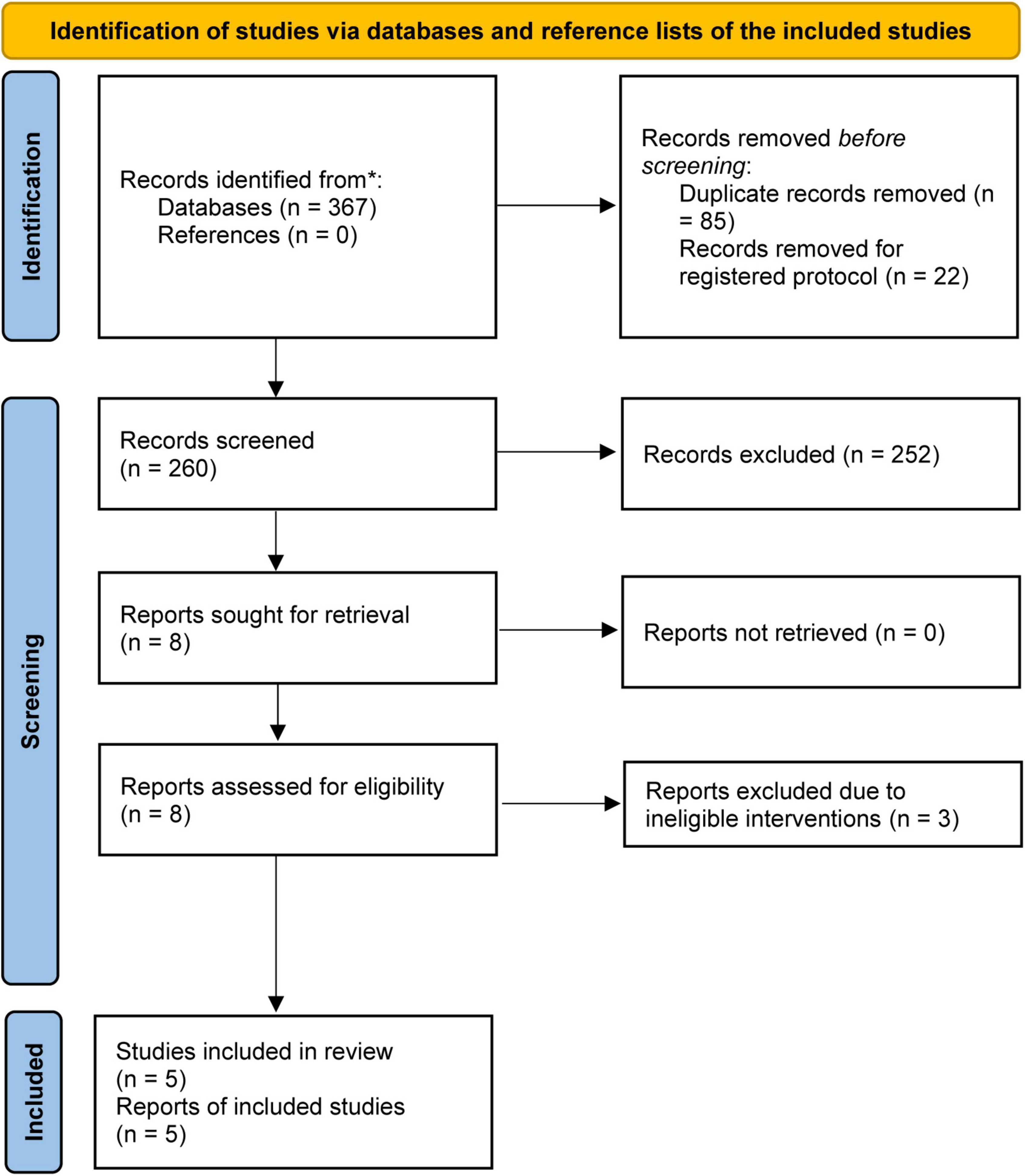
The initial literature search yielded a total of 367 relevant studies, of which 85 duplicate studies and 22 registered study protocols were removed. After checking the titles and abstracts of the remaining studies, 8 articles were retained for assessment based on full-text screening. After removing 3 studies because they used colloid solution for fluid resuscitation (27–29), five studies (30–34) were included for statistical analysis. The process of study selection is displayed in Figure 1.
Characteristics of studies
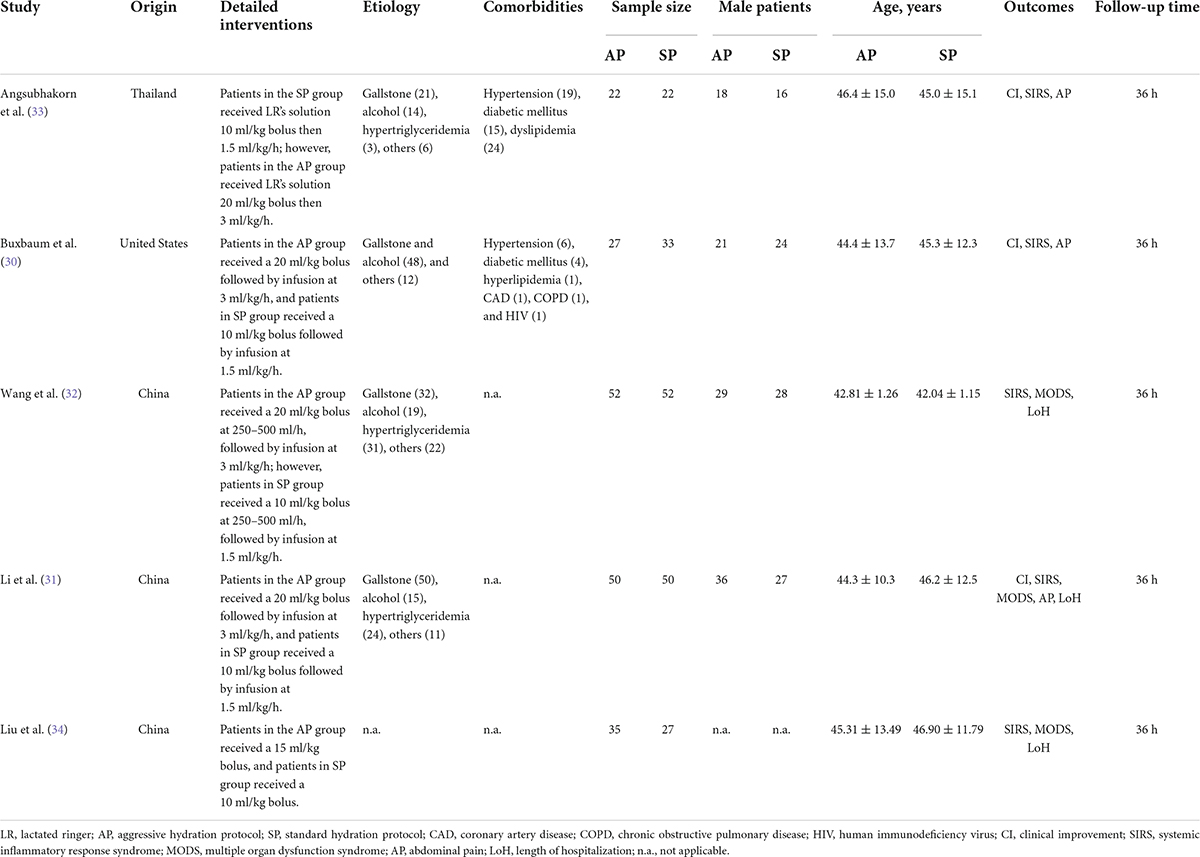
A total of 5 studies published between 2017 and 2021 were included in this meta-analysis. Three studies (31, 32, 34) published in China, and the remaining two studies published in Thailand (33) and United States (30), respectively. The sample size of individual study ranged from 44 to 104, with a total number of 370. Four studies (30–33) used 20 ml/kg bolus in aggressive hydration group, but one study (31) used 15 ml/kg bolus as aggressive hydration protocol. In addition, one study (32) supplemented probiotics in the aggressive hydration group. Other characteristics of the included studies were summarized in Table 1.
Risk of bias
Details of risk of bias assessment of all eligible studies were displayed in Figure 2. All studies (30–34) correctly generated and concealed random sequence and therefore were labeled as “low” risk at selection bias domain. Two studies (30, 33) reported to blind participants and personnel, and therefore labeled as “low” risk at performance bias. Detection bias was considered as “low” risk in only one study (30). Incomplete outcome data and selective reporting data were considered as “low” risk in all eligible studies (30–34). Three studies (30, 33, 34) were labeled as “high” risk in other risk domain due to small sample size.
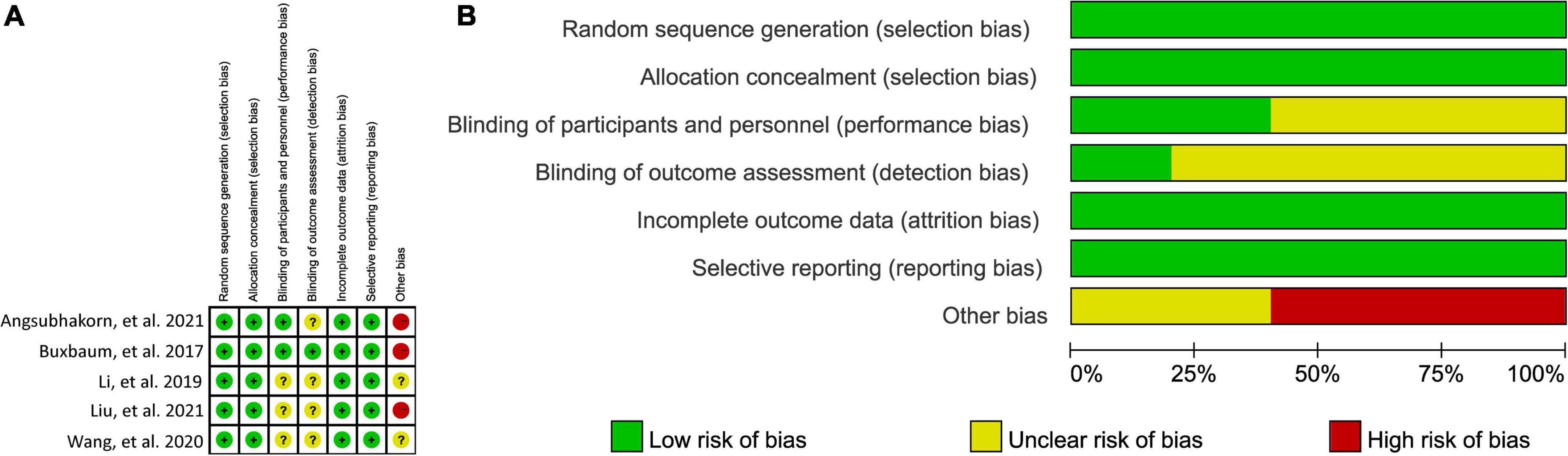
Figure 2. Risk of bias summary (A) and graph (B). “green (+),” “yellow (?),” and “red (−),” indicates “low,” “unclear,” and “high” risk of bias, respectively.
Clinical improvement
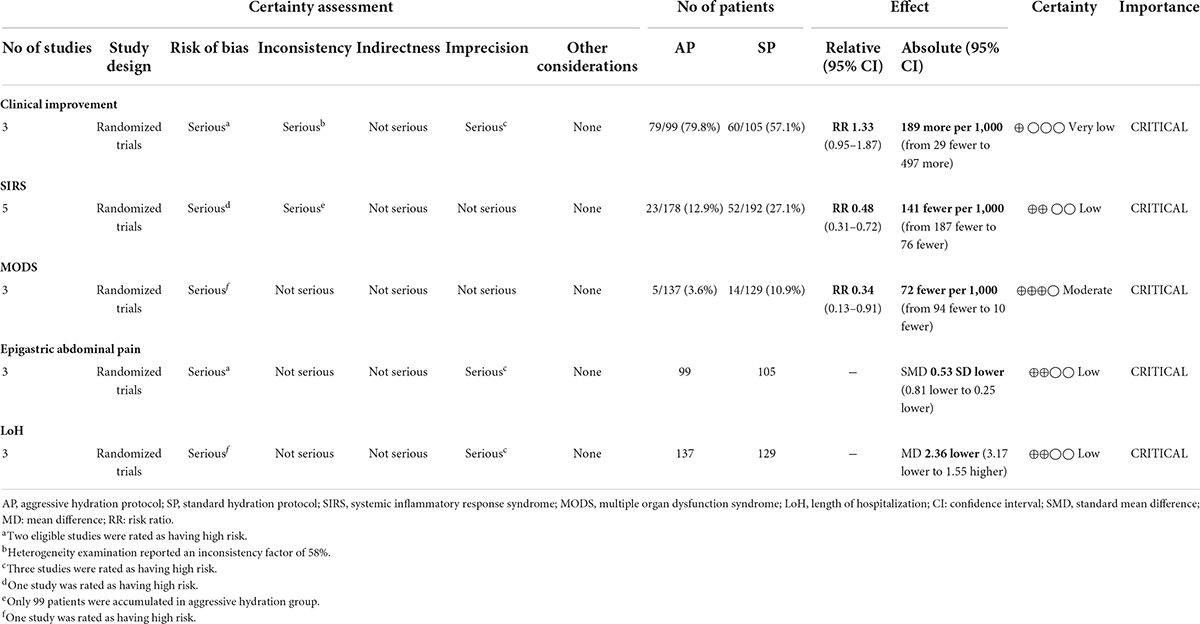
A total of 3 studies involving 204 patients (30, 31, 33) reported the data of clinical improvement. The random-effects model was used because there was significant heterogeneity across studies (P = 0.09, I2 = 58%). As shown in Figure 3, the pooled results showed no statistically significant difference in clinical improvement between aggressive hydration protocol and standard hydration protocol (79.8% vs. 57.1%; RR = 1.33; 95% CI = 0.85–1.87; P = 0.10), which was only supported by the very low evidence (Table 2).
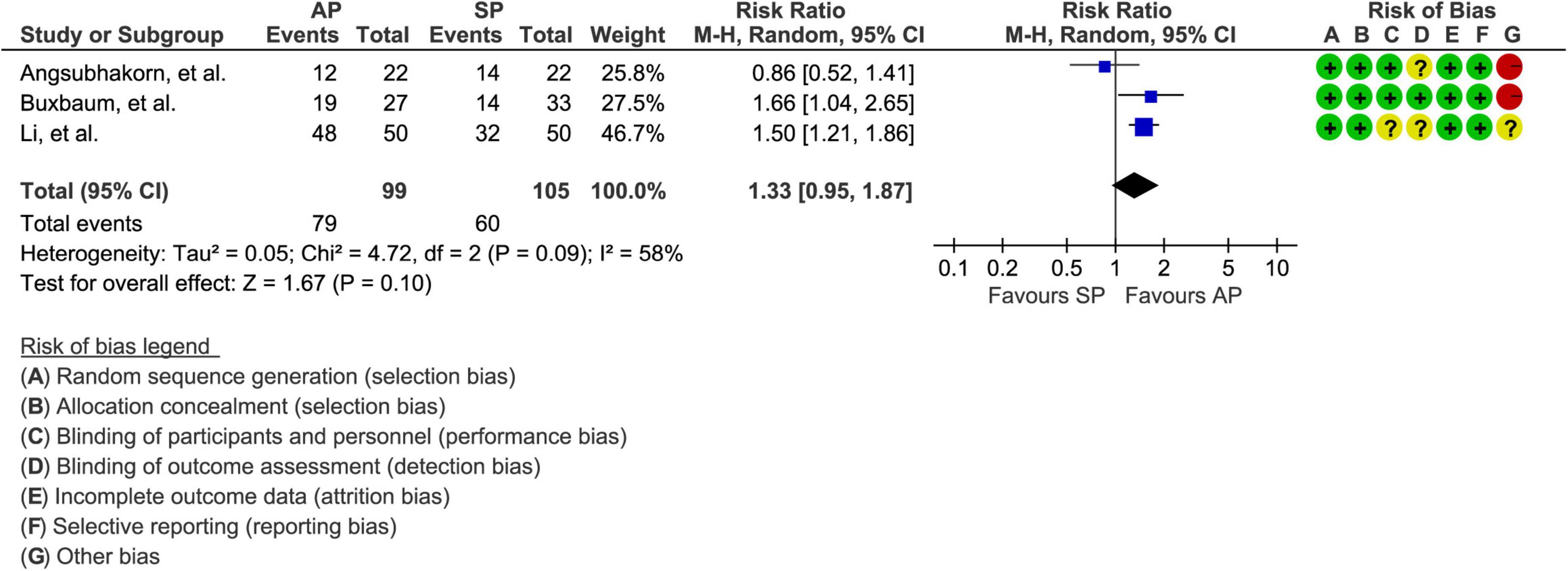
Figure 3. Forest plot of clinical improvement. AP, aggressive hydration protocol; SP, standard hydration protocol; CI, confidence interval.
Systemic inflammatory response syndrome
The events of SIRS were reported in all eligible studies (30–34) involving 370 patients. Because there was no evidence of statistical heterogeneity between studies (P = 0.27, I2 = 22%), the fixed-effects model was therefore used in meta-analysis. As shown in Figure 4A, compared with standard hydration protocol, fewer patients suffered from SIRS in the aggressive hydration protocol group (12.9% vs. 27.1%; RR = 0.48; 95% CI = 0.31–0.72; P < 0.001), which was supported by the low evidence (Table 2).
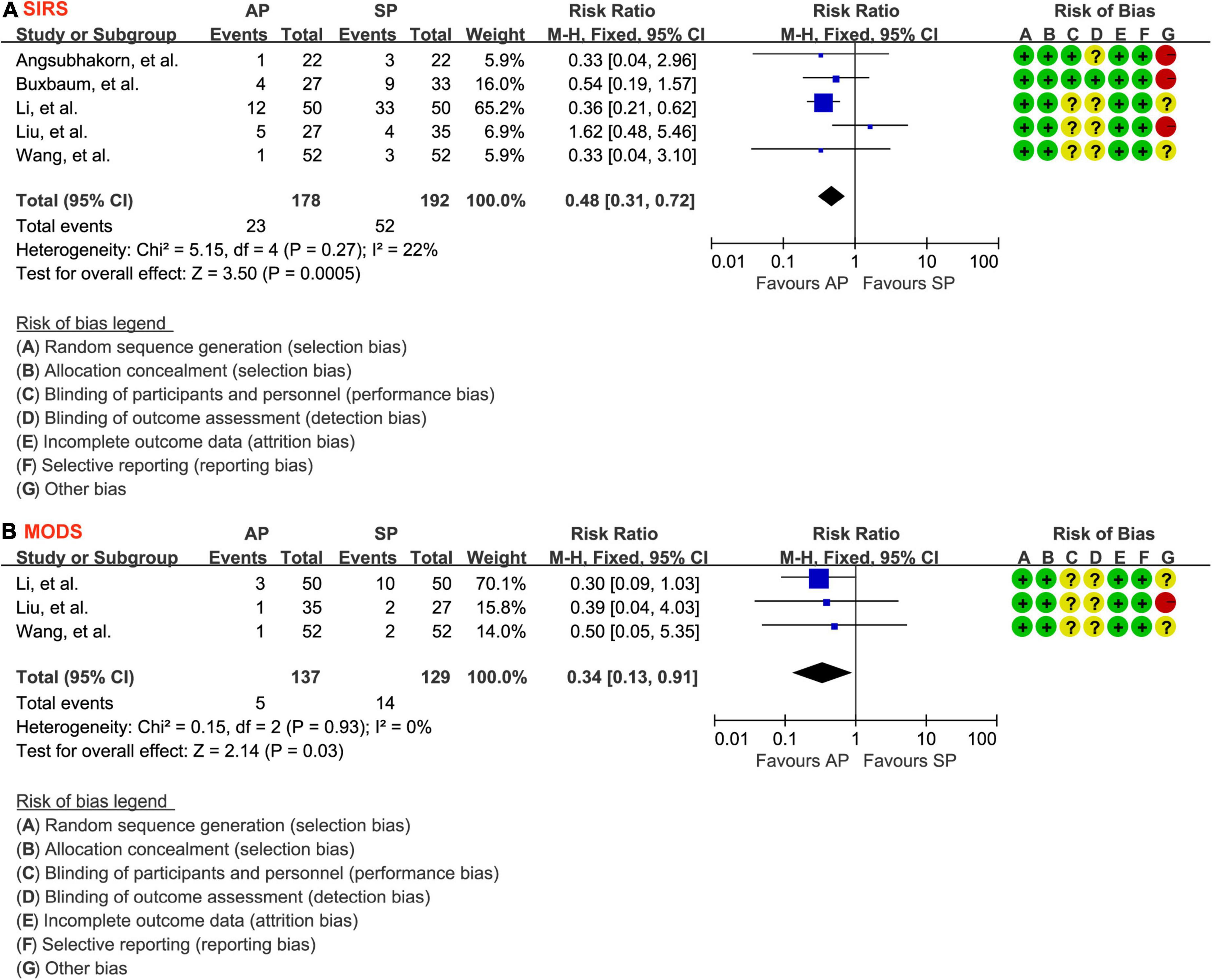
Figure 4. Forest plot of the development of SIRS (A) and MODS (B). SIRS, systemic inflammatory response syndrome; MODS, multiple organ dysfunction syndrome; AP, aggressive hydration protocol; SP, standard hydration protocol; CI, confidence interval; M-H, Mantel-Haenszel.
Multiple organ dysfunction syndrome
The events of MODS were reported in 3 studies (31, 32, 34) involving 266 patients. The fixed-effects model was chosen because significant statistical heterogeneity between studies was not detected (P = 0.93, I2 = 0.0%), so a fixed-effect model was selected. As shown in Figure 4B, aggressive hydration protocol was associated with lower incidence of MODS compared with standard hydration protocol (3.6% vs. 10.9%; RR = 0.34; 95% CI = 0.13–0.91; P = 0.03), which was supported by the moderate evidence (Table 2).
Epigastric abdominal pain
A total of 3 studies (30, 31, 33) involving 204 patients reported the data of the relief of epigastric abdominal pain and a fixed-effects model was selected because there is insignificant heterogeneity between studies (P = 0.57, I2 = 0.0%). As shown in Figure 5A, aggressive hydration protocol significantly relieved the epigastric abdominal pain compared with standard hydration protocol (SMD = −0.53; 95% CI = −0.81 to −0.25; P < 0.001), which was supported by the low evidence (Table 2).
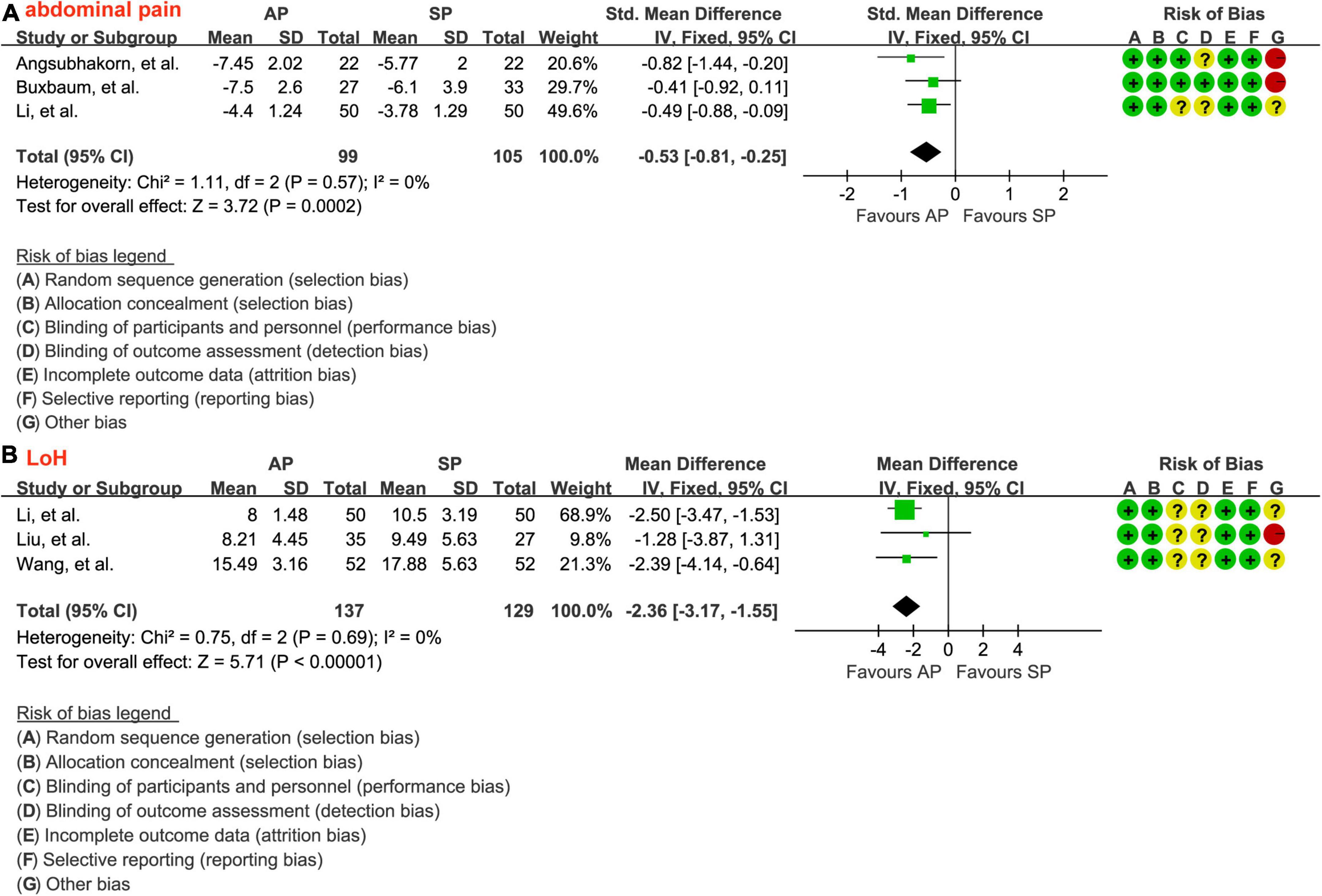
Figure 5. Forest plot of the relief of epigastric abdominal pain (A) and the LoH (B). LoH, length of hospital stay; AP, aggressive hydration protocol; SP, standard hydration protocol; SD, standard deviation; CI, confidence interval; IV, inverse variance.
Length of hospital stay
The data of LoH after treatment were reported by a total of 3 studies involving 266 patients, and there was no significant heterogeneity across studies (P = 0.69, I2 = 0.0%). The result from the fixed-effects model suggested that, as shown in Figure 5B, patients who received aggressive hydration protocol had shorter LoH than those patients who received standard hydration protocol (MD = −2.36; 95% CI = −3.17 to −1.55; P < 0.001), which was supported by the low evidence (Table 2).
Discussion
Main findings
Although a previous meta-analysis suggested that aggressive hydration protocol did not reduce mortality and might increase the risk for acute kidney injury and pulmonary edema; however, the benefit of aggressive hydration protocol has not yet been determined in patients with MAP. This is the first meta-analysis, to the best of our knowledge, which evaluated the efficacy and safety of aggressive intravenous hydration protocol of LR’s solution in patients with MAP. Very low evidence suggested a comparable clinical improvement between aggressive hydration protocol and standard protocol of LR’s solution. However, low to moderate evidences suggested that aggressive hydration protocol of LR’s solution also significantly decreased the risk of developing SIRS and MODS, accelerated the relief of epigastric abdominal pain, and shorten the LoH among patients with MAP.
Comparison with previous meta-analyses
One meta-analysis (19) has been done to date to determine whether aggressive intravenous fluid therapy helps patients with AP have fewer mortality and better clinical outcomes. The authors of this meta-analysis, which included 11 eligible studies (4 RCTs and 7 cohort studies), came to the conclusion that early aggressive intravenous fluid therapy did not reduce mortality but might increase the risk of acute kidney injury and pulmonary edema. It should be noted that patients of any severity were not separately analyzed in this meta-analysis, which simultaneously included studies with diverse designs into individual analysis. As a result, previous meta-analysis could not generate a firm conclusion for a specific population. We significantly improve the homogeneity of studies when compared to previous meta-analysis by only including patients who were diagnosed with MAP and then received an aggressive hydration protocol of LR’s solution. Additionally, this meta-analysis only included RCTs with full texts, greatly increasing the reliability of conclusions.
Strengths and limitations
There were some advantages to the present meta-analysis. First, this meta-analysis included the most comprehensive RCTs on the “head-to-head” comparison of aggressive hydration protocol and standard hydration protocol in the management of patients with mild AP. Second, we were able to better capture all potentially eligible studies because there were no restrictions on publication language or status in this meta-analysis. Third, clinical practitioners may use the strong evidence from this meta-analysis to guide their selection of fluid resuscitation strategies.
This meta-analysis had some limitations. First, only a small number of eligible studies with small sample sizes were included in this meta-analysis, which could have weakened the validity of all pooled results. Second, because there were so few eligible studies included, the publication bias test was not conducted. As a result, we were unable to eliminate the negative impact of publication bias on all pooled results. Third, due to sparse data, this meta-analysis did not evaluate laboratory parameters such as serum creatinine, inflammatory markers, and procalcitonin level. Forth, most eligible studies (80%) did not clearly describe details of blinding of participants, personnel, and outcome assessment. Therefore, RCTs with high-quality and adequate sample size are still urgently needed to confirm our findings. Fifth, the aggressive protocol was defined differently in one study (31) than it was in the other, and probiotics were added in the aggressive hydration group in one study (32). The variations may therefore introduce bias into the pooled results. Sixth, mortality was not a reported outcome in all studies. Future studies should take this outcome into account when comparing the efficacy and safety of different hydration protocols. Seventh, because most eligible studies were conducted in China, caution should be used when interpreting the applicability of our findings. Finally, although we conducted this meta-analysis in strict accordance with the Cochrane handbook for systematic reviews of interventions, we did not register the formal protocol on any public platform, which may introduce potential bias to impair the reliability of the pooled results.
Conclusion
In contrast to standard hydration protocol, aggressive hydration protocol may effectively reduce the rates of SIRS and MODS, relieve epigastric abdominal pain, and shorten the LoH among patients with MAP more efficaciously than standard hydration protocol. However, given the small number of eligible studies, inadequate sample sizes, and poor overall methodological quality that were included in this meta-analysis, more studies are required to validate our findings.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
FW and DS: conceptualization, methodology, writing original draft preparation, and formal analysis. FW: software and visualization. QA and SZ: validation. FW and JL: investigation and resources. DS and JL: data curation and writing review and editing. DS: supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
DS was supported by the Technological Innovation and Demonstrational Application Project of Chongqing Science and Technology Bureau, with approval number of cstc2020jscx-msxmX0160.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.966824/full#supplementary-material
Supplementary Table 1 | Search strategy.
References
1. Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. (2004) 291:2865–8. doi: 10.1001/jama.291.23.2865
2. Kozak LJ, Lees KA, DeFrances CJ. National hospital discharge survey: 2003 annual summary with detailed diagnosis and procedure data. Vital Health Stat. (2006) 13:1–206.
3. Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: A systematic review. Pancreas. (2006) 33:323–30. doi: 10.1097/01.mpa.0000236733.31617.52
4. Krishna SG, Kamboj AK, Hart PA, Hinton A, Conwell DL. The changing epidemiology of acute pancreatitis hospitalizations: A decade of trends and the impact of chronic pancreatitis. Pancreas. (2017) 46:482–8. doi: 10.1097/mpa.0000000000000783
5. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: Revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. doi: 10.1136/gutjnl-2012-302779
6. Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. (2013) 13(Suppl. 2):e1–15. doi: 10.1016/j.pan.2013.07.063
7. Garber A, Frakes C, Arora Z, Chahal P. Mechanisms and management of acute pancreatitis. Gastroenterol Res Pract. (2018) 2018:6218798.
8. Sternby H, Bolado F, Canaval-Zuleta HJ, Marra-López C, Hernando-Alonso AI, Del-Val-Antoñana A, et al. Determinants of severity in acute pancreatitis: A nation-wide multicenter prospective cohort study. Ann Surg. (2019) 270:348–55. doi: 10.1097/sla.0000000000002766
9. Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. (2019) 156:2008–23. doi: 10.1053/j.gastro.2018.12.041
10. Tenner S, Baillie J, DeWitt J, Vege SS. American college of gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol. (2013) 108:1400–15; 1416. doi: 10.1038/ajg.2013.218
11. Gardner TB, Vege SS, Pearson RK, Chari ST. Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol. (2008) 6:1070–6. doi: 10.1016/j.cgh.2008.05.005
12. Di Martino M, Van Laarhoven S, Ielpo B, Ramia JM, Manuel-Vázquez A, Martínez-Pérez A, et al. Systematic review and meta-analysis of fluid therapy protocols in acute pancreatitis: Type, rate and route. HPB (Oxford). (2021) 23:1629–38. doi: 10.1016/j.hpb.2021.06.426
13. Myburgh JA. Fluid resuscitation in acute medicine: What is the current situation? J Intern Med. (2015) 277:58–68. doi: 10.1111/joim.12326
14. Aziz M, Ahmed Z, Weissman S, Ghazaleh S, Beran A, Kamal F, et al. Lactated Ringer’s vs normal saline for acute pancreatitis: An updated systematic review and meta-analysis. Pancreatology. (2021) 21:1217–23. doi: 10.1016/j.pan.2021.06.002
15. Gu YY, Wang YL, Chen ZN, Wang S, Liu XZ. Meta-analysis of lactated Ringer’s solution and normal saline for management of acute pancreatitis. Shijie Huaren Xiaohua Zazhi. (2021) 29:1292–7. doi: 10.11569/wcjd.v29.i22.1292
16. Vedantam S, Tehami N, de-Madaria E, Barkin JA, Amin S. Lactated ringers does not reduce SIRS in acute pancreatitis compared to normal saline: An updated meta-analysis. Dig Dis Sci. (2021) 67:3265–74. doi: 10.1007/s10620-021-07153-5
17. de-Madaria E, Soler-Sala G, Sánchez-Payá J, Lopez-Font I, Martínez J, Gómez-Escolar L, et al. Influence of fluid therapy on the prognosis of acute pancreatitis: A prospective cohort study. Am J Gastroenterol. (2011) 106:1843–50. doi: 10.1038/ajg.2011.236
18. Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology. (2018) 154:1096–101. doi: 10.1053/j.gastro.2018.01.032
19. Gad MM, Simons-Linares CR. Is aggressive intravenous fluid resuscitation beneficial in acute pancreatitis? A meta-analysis of randomized control trials and cohort studies. World J Gastroenterol. (2020) 26:1098–106. doi: 10.3748/wjg.v26.i10.1098
20. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
21. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.2. Cochrane, 2021. (2021). Available online at: http://www.training.cochrane.org/handbookwww.training.cochrane.org/handbook (accessed February 2021).
22. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
23. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
24. Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. (2014) 349:g5630. doi: 10.1136/bmj.g5630
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
26. Palma Perez S, Delgado Rodriguez M. Practical considerations on detection of publication bias. Gac Sanit. (2006) 20(Suppl. 3):10–6. doi: 10.1157/13101085
27. Yang GM, Wiu WQ. Effect of limited fluid resuscitation on blood lactic acid value and gastrointestinal function in patients with acute pancreatitis. Int Med Health Guidance News. (2017) 23:690–2. doi: 10.3760/cma.j.issn.1007-1245.2017.05.024
28. Chen QM, Xiong HH, Zhu SQ, Ye LF. Clinical effects of early appropriate fluid resuscitation in patients with acute pancreatitis. Chin J Gastroenterol Hepatol. (2019) 25:223–5. doi: 10.3969/j.issn.1006-5709.2016.02.029
29. Karki B, Thapa S, Khadka D, Karki S, Shrestha R, Khanal A, et al. Intravenous Ringers lactate versus normal saline for predominantly mild acute pancreatitis in a Nepalese Tertiary Hospital. PLoS One. (2022) 17:e0263221. doi: 10.1371/journal.pone.0263221
30. Buxbaum JL, Quezada M, Da B, Jani N, Lane C, Mwengela D, et al. Early aggressive hydration hastens clinical improvement in mild acute pancreatitis. Am J Gastroenterol. (2017) 112:797–803. doi: 10.1038/ajg.2017.40
31. Li H, Gao M, Ge WW, Yin CL, Sun YS, Wang ZH, et al. The value of early aggressive hydration in patients with mild acute pancreatitis. Chin J Emerg Med. (2019) 28:794–7. doi: 10.3760/cma.j.issn.1671-0282.2019.06.030
32. Wang SY, Wang F, Tang YH. The application value of early aggressive hydration among patients with mild acute pancreatitis. Guangdong Med J. (2020) 41:844–8. doi: 10.13820/j.cnki.gdyx.20192776
33. Angsubhakorn A, Tipchaichatta K, Chirapongsathorn S. Comparison of aggressive versus standard intravenous hydration for clinical improvement among patients with mild acute pancreatitis: A randomized controlled trial. Pancreatology. (2021) 21:1224–30. doi: 10.1016/j.pan.2021.06.004
Keywords: acute pancreatitis, intravenous fluid resuscitation, aggressive intravenous hydration, Lactated Ringer, meta-analysis
Citation: Wu F, She D, Ao Q, Zhang S and Li J (2022) Aggressive intravenous hydration protocol of Lactated Ringer’s solution benefits patients with mild acute pancreatitis: A meta-analysis of 5 randomized controlled trials. Front. Med. 9:966824. doi: 10.3389/fmed.2022.966824
Received: 11 June 2022; Accepted: 22 August 2022;
Published: 08 September 2022.
Edited by:
Mathieu Jozwiak, Centre Hospitalier Universitaire de Nice, FranceReviewed by:
Carlos Diaz-Arocutipa, Saint Ignatius of Loyola University, PeruXu Tian, University of Rovira i Virgili, Spain
Diansan Su, Shanghai Jiao Tong University, China
Copyright © 2022 Wu, She, Ao, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong She, NTI0MzUyN0BxcS5jb20=
 Fei Wu
Fei Wu Dong She
Dong She