- 1Department of Clinical Dermatology, San Gallicano Dermatological Institute, IRCCS, Rome, Italy
- 2Biostatistics Unit, San Gallicano Dermatological Institute IRCCS, Rome, Italy
- 3General Surgery and Liver Transplantation, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
- 4Microbiology and Virology Unit, Dermatological Clinical and Research Department, San Gallicano Dermatological Institute, IRCCS, Rome, Italy
- 5Department Otolaryngology Head and Neck Surgery, IRCCS Regina Elena National Cancer Institute, Rome, Italy
- 6Medical Direction, IRCCS Regina Elena National Cancer Institute, Rome, Italy
- 7Scientific Direction, San Gallicano Dermatological Institute, IRCCS, Rome, Italy
Introduction: Psoriasis has not been directly linked to a poor prognosis for COVID-19, yet immunomodulatory agents used for its management may lead to increased vulnerability to the dangerous complications of SARS-CoV-2 infection, as well as impair the effectiveness of the recently introduced vaccines. The three-dose antibody response trend and the safety of BNT162b2 mRNA vaccine in psoriasis patients treated with biologic drugs have remained under-researched.
Materials and methods: Forty-five psoriatic patients on biologic treatment were enrolled to evaluate their humoral response to three doses of BNT162b2. IgG titers anti-SARS-CoV-2 spike protein were evaluated at baseline (day 0, first dose), after 3 weeks (second dose), four weeks post-second dose, at the time of the third dose administration and 4 weeks post-third dose. Seropositivity was defined as IgG ≥15 antibody-binding units (BAU)/mL. Data on vaccine safety were also collected by interview at each visit.
Results: A statistically significant increase in antibody titers was observed after each dose of vaccine compared with baseline, with no significant differences between patients and controls. Methotrexate used in combination with biologics has been shown to negatively influence the antibody response to the vaccine. On the contrary, increasing body mass index (BMI) positively influenced the antibody response. No adverse effects were reported, and no relapses of psoriasis were observed in the weeks following vaccine administration in our study population.
Conclusions: Our data are largely consistent with the recent literature on this topic confirming the substantial efficacy and safety of BNT162b2 mRNA vaccine on psoriatic patients treated with biologics of different types and support the recommendation to perform additional doses in this specific subgroup of patients.
Introduction
The 2019 coronavirus disease (COVID-19) significantly impacted patients with chronic autoimmune diseases also because of their concomitant immunomodulating treatments. Psoriasis and immunomodulators have been linked to an increased risk of serious infections, including viral pneumonias (1–7).
In addition, psoriasis patients frequently suffer from cardio-metabolic comorbidities, now considered as strong risk factors for acute respiratory distress syndrome (ARDS) and poor COVID-19 prognosis (8–10).
However, preliminary data from several large cohort studies assessing the risk of hospitalization, intensive care unit admission, and mortality due to COVID-19 in psoriasis patients treated with systemic immunomodulating therapies showed only minimal increased risk of COVID-19-related complications and poor prognosis mainly in individuals on conventionals immunomodulators such as Methotrexate (11–16).
Previous evidence suggests immunomodulators may prevent the hyperactivity of the innate immune system underlying the cytokine storm and subsequent multi-organ damage induced by SARS-CoV-2 infection (17–19).
Nevertheless, several scientific societies and the Italian Ministry of Health, in view of a potential immunological vulnerability, included psoriasis patients currently on immunomodulatory treatment, among the high-priority categories for COVID-19 vaccination.
To date, four COVID-19 vaccines are authorized by EMA for public use (20–25): two mRNA vaccines (Pfizer–BioNTech BNT162b2 and Moderna mRNA-1273), and two viral vector vaccines (Oxford–AstraZeneca AZD1222 and Johnson & Johnson Ad26.CoV2.S).
Our institution implemented a priority-based vaccination plan with Pfizer-BioNTech vaccine for obese or immunocompromised patients. BNT162b2 is a nucleoside-modified mRNA vaccine encoding the SARS-CoV-2 spike glycoprotein and it is administered intramuscularly as two doses given 21 days apart.
Although BNT162b2 was found to be 95% effective in preventing COVID-19 in global phase 2 and 3 studies (26), several concerns have been raised and investigated in several clinical trials and reports around impaired protective response (27–37) and psoriasis relapses after vaccine administration (38–54).
National Psoriasis Foundation COVID-19 Task Force provides guidance for the management of patients with psoriatic disease during the pandemic and strongly encourages patients who do not have contraindications to vaccination, to receive an mRNA-based COVID-19 regardless of the concurrent use of therapies for psoriasis and/or psoriatic arthritis (55). Conversely, the American College of Rheumatology, recommended to withhold methotrexate 1 week after each dose of vaccine for patients with well-controlled disease, basing this recommendation on data from influenza and pneumococcal vaccines (56).
Although testing for vaccine immune response is not currently recommended, many studies have estimated the vaccine's protective effectiveness on the basis of its ability to induce a humoral response (57–59).
We assume that post-vaccine IgG titer might be used as a reliable surrogate to predict vaccine efficacy.
At present, only limited data are available on the antibody response to COVID-19 mRNA vaccines in psoriasis patients, particularly regarding their trend after each dose. The present study aims to assess the humoral responses to three doses of SARS-CoV-2 mRNA vaccine BNT162b2 in a cohort of psoriasis patients on biologic treatment.
Materials and methods
A prospective single-center cohort study was conducted to assess the immunogenicity and safety of three doses of BNT162b2 on psoriatic patients treated with biologic drugs. From April 1 to May 15, 2021, 45 psoriasis patients receiving biologics, obese (BMI ≥30 kg/m2) or with HIV infection as additional risk factor for immune impairment, were enrolled and followed prospectively for nine months. A group of 45 healthy controls matched by age, sex, and BMI were recruited from the staff of the Istituti Fisioterapici Ospitalieri (IFO), Rome, Italy for comparison of the antibody responses.
The vaccination schedule was based on two intramuscular injections of 30 μg per dose of BNT162b2 vaccine 3 weeks apart, followed by a third dose administered 5 months apart from the second dose. Neither suspension nor dose modification of the biologic therapy schemes was planned in any treatment set, whereas in accordance with the recommendations of the American College of Rheumatology, patients were advised to discontinue methotrexate for 1 week after each dose of vaccine.
Neutralizing IgG titers anti-SARS-CoV-2 spike protein were evaluated at baseline (day 0, first injection, time point TP0), after 3 weeks (day 21, second injection, TP1), four weeks post-second dose (day 51, TP2), at the time of the third dose administration (day 200, TP3) and four weeks post-third dose (day 230, TP4).
Dermatologic and rheumatologic assessments were carried out at baseline and data on vaccine safety were also collected on TP1, TP2 and TP4 by interview. Local and systemic side effects as well as any flare-up of the underlying disease occurred after vaccine administration were recorded. All participants were asked to provide nose and throat swabs on each defined TP but no antibody tests to detect any asymptomatic SARS-CoV-2 infections were performed on the study population.
The study was approved by the IRCCS Central Ethical Committee of Regione Lazio in January 2021 (Prot. N-1463/21) and conducted in compliance with the Helsinki Declaration and Good Clinical Practice. All subjects signed a specific written informed consent before study enrollment.
Serological test and definition LIAISON® SARS-CoV-2 S1/S2 IgG by DiaSorin®, Saluggia, Italy. The LIAISON® SARS-CoV-2 S1/S2 IgG test is a quantitative chemiluminescent immunoassay (CLIA), fully automated on LIAISON® XL platform, for the detection of IgG antibodies against the subunits S1 and S2 of SARS-CoV-2 spike protein. The subunits S1 and S2 are responsible for binding and fusion of virus to the host cell, respectively, and are both targets of neutralizing antibodies. According to the manufacturer technical manual1, the result of a LIAISON® SARS-CoV-2 S1/S2 IgG test has to be reported as positive with a signal of 15 AU/mL or higher, equivocal between 12 and 15 AU/mL and negative and <12 AU/mL.
Clinical and analytic performance of this automated serological test identifying SARS-CoV-2 S1/S2-neutralizing IgG in a semi-quantitative manner was published in September 2020 (60).
For the analysis' purposes, the result of 15 AU/mL indicated by DiaSorin® was considered as the cutoff to discriminate responders from non-responders to vaccination.
Statistical analysis
To control for potential confounders that could affect the outcomes of interest, propensity score matching (PSM) was employed (61, 62) to generate two different groups of patients (i.e., affected and not affected by psoriasis) with balanced distribution of baseline features. Presence of psoriasis was considered as dependent variable, with the absence of psoriasis as control group. Covariates included in the analysis were age, gender and BMI. Patients and controls were matched 1:1 with the nearest-neighbor method and using no caliper distance of the standard deviation of the logit of the estimated propensity score to ensure good matches. Balance between the two groups was assessed using the relative multivariate imbalance measure L1 proposed by Iacus, King and Porro (63, 64).
We reported the categorical variables through absolute and relative frequencies, whereas the continuous variables through means with standard deviations (SD). Geometric mean of AU/mL concentration (GMC) and its 95% confidence interval (95%CI) was reported for all time points and for each group. TP4/TP3 and TP2/TP1 ratio were computed and reported using the geometric mean (GM) and its 95%CI. Kolmogorov-Smirnov normality test was calculated for all the continuous variables. To explore the differences between continuous variables, Mann-Whitney or the T-Student test were utilized, according to the nature of data distribution. The relationships between categorical variables were analyzed using the Pearson's Chi-square. The Wilcoxon test was applied to compare the IgG titer at TP4, TP3, T2 and T1 with the baseline value in psoriasis patients and control subjects. A generalized univariate and multivariate linear model (GLM) was implemented to evaluate the correlation between logarithm of IgG titer at TP4 and covariates. P-values < 0.05 were considered significant. All statistical analyses were performed using SPSS statistical software version 21 (SPSS inc., Chicago IL, USA).
Propensity score matched analysis
PSM was identified in 90 (45 per group) matched patients at a 1:1 ratio out of a total of 128 patients. The L1 test measure was larger in the unmatched sample (0.931) than in the matched sample (0.911), indicating that the two groups were well balanced across all variables considered. The absence of differences between the two groups regarding patient gender confirmed the success of the matching for this variable. BMI and age remained statistically different due to the substantial gap in values between the two groups before the matching.
Results
Enrollment and characteristics of the study population
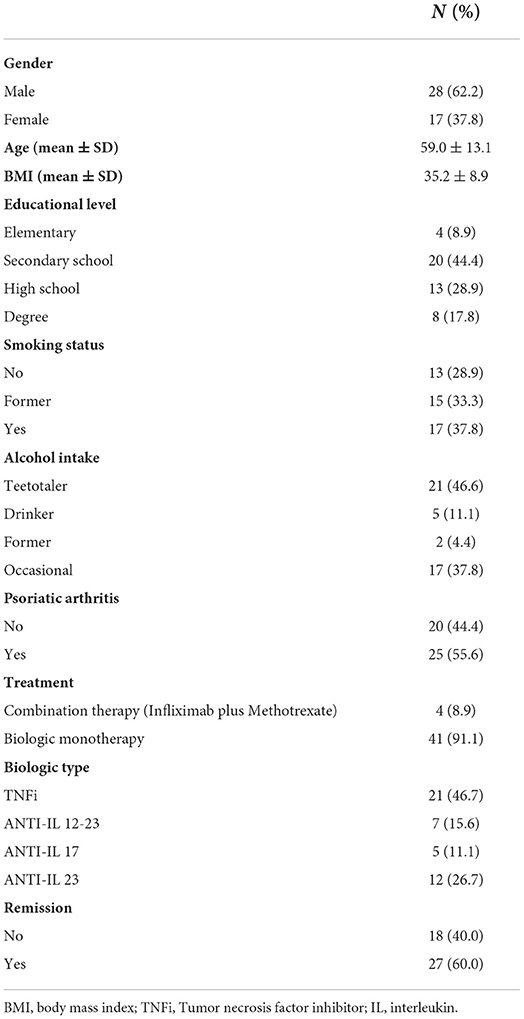
Out of 700 psoriasis patients on biologic treatment followed at our Psoriasis Clinic, 45 patients (28 males and 17 females) matched the inclusion criteria and were enrolled in the study. Baseline characteristics of the psoriasis patients are summarized in Table 1. Twenty patients had only skin involvement and 25 were diagnosed with psoriatic arthritis (PsA). Forty-one patients had obesity (BMI ≥30 kg/m2) and 4 patients were normal weight but HIV-infected. Twenty-three (51.1%) patients had BMI>35 kg/m2, with a mean BMI 41.7 (SD, ±7.2). At the moment of the first dose of vaccine 41 patients (91.1%) were being treated with subcutaneous biologic as monotherapy and 4 patients (8.9%) were on intravenous infliximab in combination with methotrexate. Twenty-one patients (46.7%) were on TNF inhibitors (TNFi) while 24 (53.3%) were on anti-interleukin (ANTI-IL) drugs.
Sixty percent of enrolled patients were in complete remission defined by PASI index <3 for only skin psoriasis patients and by minimal disease activity (MDA) criteria for PsA patients (MDA = 5 of the 7 following criteria were met: tender joint count < or = 1; swollen joint count < or = 1; Psoriasis Activity and Severity Index < or = 1 or body surface area < or = 3; patient pain visual analog score (VAS) < or = 15; patient global disease activity VAS < or = 20; health assessment questionnaire < or = 0.5; tender entheseal points < or = 1).
The control cohort consisted of 45 age, sex and BMI matched non psoriasis controls recruited from the staff of Istituti Fisioterapici Ospitalieri (IFO), Rome, Italy.
Serological response to BNT162b2 at different time points
Table 2 reports the comparative analysis of anti SARS-CoV-2 spike protein GMC of IgG on TP0, TP1, TP2, TP3 and TP4.
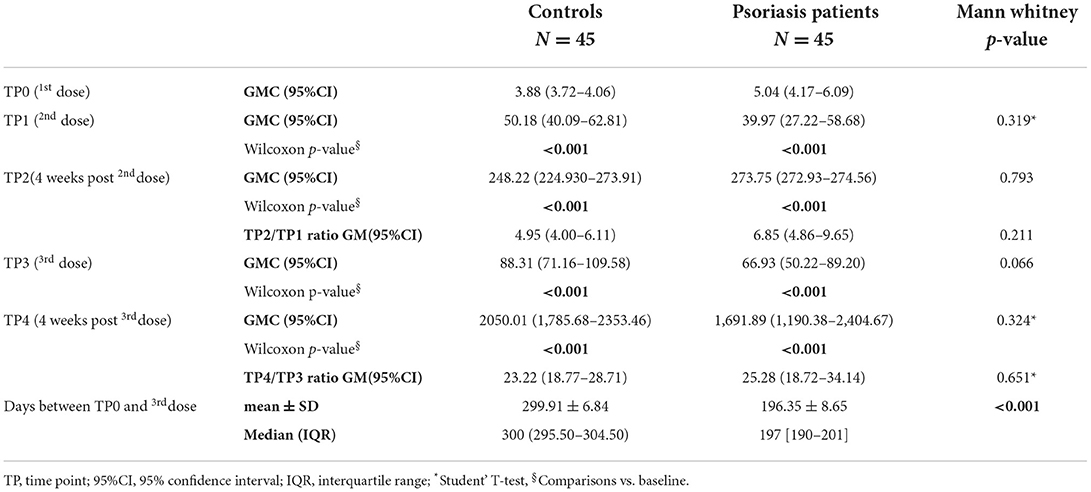
Table 2. Geometric mean of concentration (GMC) and GM ratios of anti SARS-CoV-2 spike protein IgG at different time points, in psoriasis patients and control group.
A statistically significant increase in antibody titer to TP1 and TP2, compared to baseline, was observed in both psoriasis patients and control group. No statistically significant difference was found when comparing the antibody response on TP1(p = 0.319) and TP2 (p = 0.793) of psoriasis patients vs. controls.
The median time elapsed between administration of the first and third dose of vaccine was 300 days for controls (IQR: 295.50–304.50) and 197 (IQR: 190–201) for psoriatic patients.
The GMC declined moderately 5 months after the booster dose (TP3), being 66.93 in psoriatic patients and 88.31 in controls. The comparison between patients and controls did not show statistically significant differences. No cases of seroconversion loss have been observed between the second dose of vaccine and the day of the third dose. The detection performed 1 month after the third dose administration showed a marked increase of the GMC in both patients (1,691.89; 95%CI: 1,190.38–2,404.67) and controls (2,050.01; 95%CI: 1,785.68–2,353.46). The comparison between patients and controls showed GMC levels about 17% lower in patients vs. controls, but this difference was not statistically significant. Moreover, the two groups were not statistically different even in the TP2/TP1 RATIO value (p = 0.211).
The four HIV patients showed an antibody response identical to the other psoriasis patients. Only one female patient (Age = 72; BMI = 37,2 kg/m2) on combination therapy (infliximab plus methotrexate) did not respond to the double dose of vaccine. Her antibody titer increased from 3.79 AU/mL on TP0 to 5.9 AU/mL on TP2. Nevertheless, the detection performed 4 weeks after the third dose showed a response slightly above the cut-off of 15 AU/mL indicated by DiaSorin® to discriminate responders from non-responders to vaccination. Interestingly, antibody titers on the TP4 detection were significantly lower in patients receiving combination therapy compared to those on biologic monotherapy (Table 3; p = 0.037).
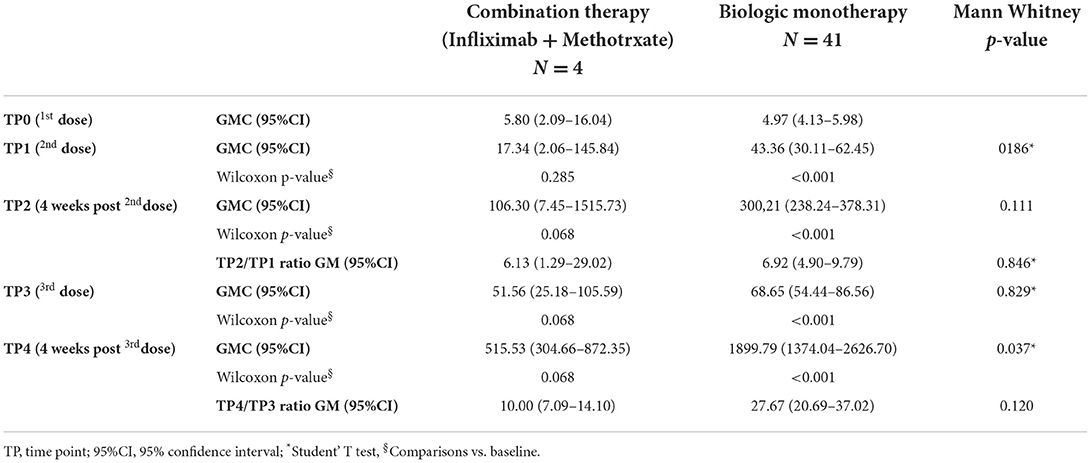
Table 3. Geometric mean of concentration (GMC) and GM ratios of anti SARS-CoV-2 spike protein IgG at different time points according to different treatment regimens.
Four subjects (all psoriasis patients) showed a positivity to the serological test performed at baseline probably due to asymptomatic COVID-19 infection occurred before the study began, however, a sensitivity analysis excluding the four positive patients did not reveal statistically significant differences in the results obtained (Supplementary Table S2).
Predictors of antibody response to BNT162b2
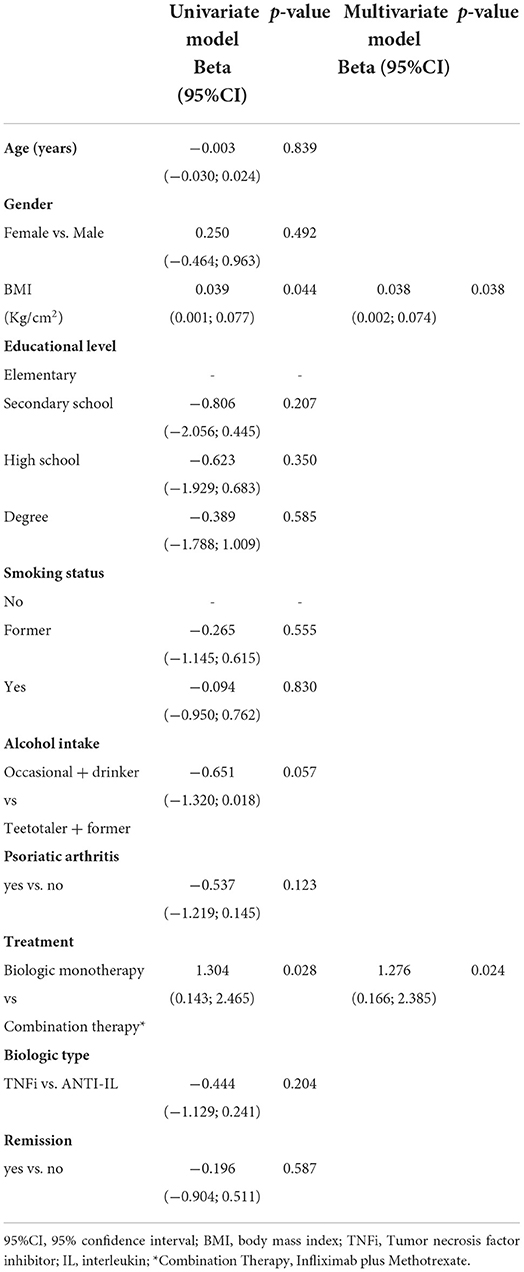
The impact of age, gender, BMI and diagnosis of psoriasis on TP4 antibody titers was investigated in all subjects (psoriasis patients and controls) with the GLM (Supplementary Table S3). No variables were significantly associated with the antibody response.
The GLM of antibody titers of psoriasis patients on TP4, identified higher BMI as a significant predictor of a greater antibody response to the third dose of the vaccine (Table 4).
The same statistical model also demonstrated a stronger antibody response in patients on monotherapy as compared with patients on combination therapy (biologic plus methotrexate). Conversely, no differences were observed when comparing patients on TNFi and those on ANTI-IL treatment.
The impact of additional variables (smoking status, alcohol intake, education level, diagnosis of psoriatic arthritis, remission, type of biologic used) also was assessed on the TP1 response rate by a univariate logistic regression model in the psoriasis patients. None of the included variables was shown to influence the response rate (data not shown).
BNT162b2 vaccine safety
Although a detailed safety analysis has not been carried out on the study cohort, no local or systemic adverse effects have been reported on the three TPs except for mild pain on the injection site and mild fever mainly after the second dose. No worsening of skin or joint inflammation was observed among the herein reported cases during the weeks following the three doses of vaccine.
SARS-CoV-2 infection during the study period
No enrolled patients tested positive for nose and throat swabs collected on each TP and none had symptomatic infections during the observational period.
Discussion
The BNT162b2 vaccine is highly effective and safe, as demonstrated in phase 3 clinical trials, reaching a clinical efficacy of 95% in preventing severe forms of COVID-19 (26). However, real-world data on the efficacy and safety of mRNA vaccines are limited, especially in those patients with chronic inflammatory diseases treated with immunomodulators.
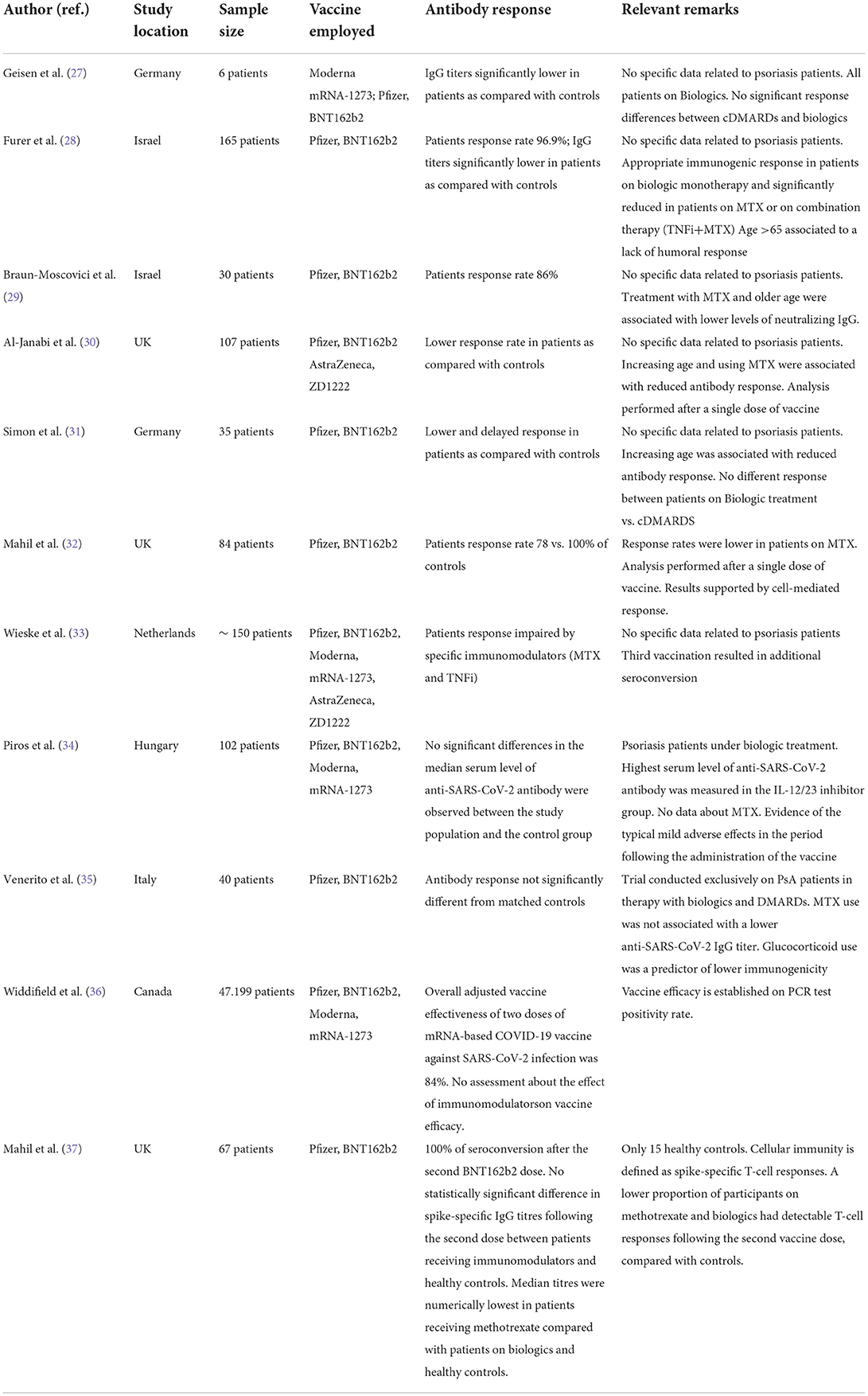
Although patients with psoriasis and psoriatic arthritis do not show an increased vulnerability to SARS-CoV-2, any immune system alteration makes the clinical course of COVID-19 largely unpredictable. In addition, several immunomodulators used in inflammatory disorders have been associated with impaired rates of humoral response to the SARS-CoV-2 vaccine (Table 5).
Two core topics are driving the scientific debate around the use of COVID-19 vaccines in patients with chronic inflammatory diseases on immunomodulating treatment.
Risk of psoriasis relapse in response to the vaccine-induced immune stimulation
In this study, we observed no cases of significant worsening of joint and skin manifestations of psoriasis, although at the time of vaccination not all of our patients were in complete remission. Shoenfeld et al. suggested a possible role of vaccines (including COVID-19 vaccines) as triggers for relapse or onset of autoimmune diseases, due to the potential agonism of vaccines on TLRs 7/8 or 9 (65). Although extensive real-world experience is lacking, to date 46 cases of psoriasis relapses following vaccination with COVID-19 have been described in the literature (38–54).
In some rare cases, relapses progressed to particularly severe forms such as erythrodermic or generalized pustular psoriasis. The immunopathogenic mechanisms underlying these exacerbations after vaccination with COVID-19 mRNA vaccines are not yet understood, however according to a recent focus by Watad et al., most of these events should be considered rare and usually responsive to therapy (66).
Lack of appropriate protective immune response after vaccination
We considered the neutralizing IgG titer anti-SARS-CoV-2 as a proper index of the immune response to BNT162b2.
The present study examined a cohort of patients with at least two factors potentially impairing immune response to vaccines: an immune-mediated disorder characterized by an imbalance of cytokines and lymphocytes such as psoriasis and the treatment with different types of immunomodulators.
Our study population showed an appropriate humoral response to the BNT162b2 vaccine when compared to controls. This is partly in contrast with previous studies reporting impaired response rates and lower antibody titers in patients with various immune-rheumatologic diseases (27–33). However, broad agreement exists around biologics used in psoriasis and psoriatic arthritis minimally affecting vaccine response (34–37).
Importantly, methotrexate negatively affected the antibody response, although firm conclusions around its role as a single agent cannot be drawn, as it was used only in four patients in combination with infliximab and discontinued for 1 week after the vaccination. Nevertheless, methotrexate impairment of the antibody response to vaccines has been widely demonstrated and temporary discontinuation during vaccination is recommended by most scientific societies (67–76).
Approximately 5 months after the second dose we observed a significant decline in antibody titer. Whether that was significant in terms of protection from viral infection cannot be assessed due to the small sample size and the limited follow-up. Nevertheless, none of our patients reported COVID-19 symptoms during the study time. Mahil et al. showed not all patients on immunomodulators were able to develop a T cell response after the second dose vaccination, suggesting a possible faster decay of cell-mediated immunity in this type of patients (37). Conversely, according to Wiskle et al., the reduced antibody response observed in patients receiving immunomodulators may not result in a clinically significant short-term loss of protection, being these medications unable to affect memory B-cell function. Nevertheless, the same authors reported an increased rate of seroconversion after the third dose of vaccine in patients treated with specific immunomodulators (33).
Four weeks after the third dose, psoriasis patients showed antibody levels ~10-fold higher than those achieved after the second dose, with no significant differences compared to control subjects. In accordance with the results of Wiskle et al., our GLM model showed methotrexate used in combination with TNFi to have a slight negative impact on the antibody response even after the third dose of vaccine (33).
Obesity is an extensively studied metabolic disorder characterized by chronic low-grade inflammation potentially impairing both humoral and cell-mediated immune function.
The combination of these phenomena could explain both the poor prognosis of obese patients in COVID 19 infection, but also an abnormal response to the vaccine (76, 77). There is ample evidence to support a lack of efficacy in the antibody response of obese people to common vaccines such as influenza, rabies and hepatitis B (78, 79).
Diversely, the multivariate analysis we performed revealed increased BMI to favorably influence the antibody response. In line with this interesting finding, we have previously demonstrated that obesity was unable to influence the antibody response to BNT162b2 in psoriatic patients after two doses of BNT162b2 vaccine as also highlighted by Cristaudo et al. (59) and Pellini et al. (80).
Interestingly, neither the type of biological treatment (TNFi vs. ANTI-IL) nor the clinical condition at the time of vaccination (remission vs. active disease) influenced the humoral response to vaccine. Patients with an additional immunological risk factor, such as HIV infection, also had satisfactory antibody responses to the vaccine. Our study has limitations, mainly owing to the small number of patients enrolled and the duration of follow-up, therefore, the multivariate analyses we performed could not have provided significant results. Nevertheless, we confirmed the safety of BNT162b2, both in terms of typical vaccine adverse effects and risk of psoriasis relapse. Three doses of mRNA vaccine elicited a vigorous anti-Spike response, presumably providing effective protection from SARS-CoV-2 infection, as highlighted by the lack of symptomatic COVID-19 in our study population. In conclusion, the risks-benefits balance of BNT162b2 vaccine in a psoriatic population treated with immunomodulators is strongly in favor of the benefits. Thus, we strongly support the implementation of the third vaccination for selected patients with immune-mediated inflammatory disorders receiving immunomodulators as a useful measure to improve immune response to the vaccine and supposedly the level of protection against SARS-CoV-2. Further targeted studies will be needed in order to assess in patients treated with immunomodulators the persistence of antibody protection and the need for subsequent booster vaccinations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and the study was approved by the IRCCS Central Ethical Committee of Regione Lazio in January 2021 (Prot. N-1463/21). The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This research was funded by the Italian Ministry of Health (RC 2022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.961904/full#supplementary-material
Footnotes
1. ^https://www.diasorin.com/en/immunodiagnostic-solutions/clinical-areas/infectious-diseases/covid-19
References
1. Li X, Andersen KM, Chang HY, Curtis JR, Alexander GC. Comparative risk of serious infections among real-world users of biologics for psoriasis or psoriatic arthritis. Ann Rheum Dis. (2020) 79:285–91. doi: 10.1136/annrheumdis-2019-216102
2. Kao LT, Lee CZ, Liu SP, Tsai MC, Lin HC. Psoriasis and the risk of pneumonia: a population-based study. PLoS ONE. (2014) 9:e116077. doi: 10.1371/journal.pone.0116077
3. Takeshita J, Shin DB, Ogdie A, Gelfand JM. Risk of serious infection, opportunistic infection, and herpes zoster among patients with psoriasis in the United Kingdom. J Invest Dermatol. (2018) 138:1726–35. doi: 10.1016/j.jid.2018.01.039
4. Wakkee M, de Vries E, van den Haak P, Nijsten T. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population-based cohort. J Am Acad Dermatol. (2011) 65:1135–44. doi: 10.1016/j.jaad.2010.08.036
5. Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. (2020) 82:1217–8. doi: 10.1016/j.jaad.2020.03.031
6. Dávila-Seijo P, Dauden E, Descalzo M, Carretero G, Carrascosa J, Vanaclocha F, et al. Infections in moderate to severe psoriasis patients treated with biological drugs compared to classic systemic drugs: findings from the BIOBADADERM registry. J Invest Dermatol. (2017) 137:313–21. doi: 10.1016/j.jid.2016.08.034
7. Wan MT, Shin DB, Winthrop KL, Gelfand JM. The risk of respiratory tract infections and symptoms in psoriasis patients treated with interleukin 17 pathway-inhibiting biologics: A meta-estimate of pivotal trials relevant to decision making during the COVID-19 pandemic. J Am Acad Dermatol. (2020) 83:677–9. doi: 10.1016/j.jaad.2020.05.035
8. Azfar RS, Seminara NM, Shin DB, Troxel AB, Margolis DJ, Gelfand JM. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. (2012) 148:995–1000. doi: 10.1001/archdermatol.2012.1401
9. Aksentijevich M, Lateef SS, Anzenberg P, Dey AK, Mehta NN. Chronic inflammation, cardiometabolic diseases and effects of treatment: psoriasis as a human model. Trends Cardiovasc Med. (2020) 30:472–8. doi: 10.1016/j.tcm.2019.11.001
10. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. (2006) 296:1735–41. doi: 10.1001/jama.296.14.1735
11. Yiu ZZN, Harding-Oredugba G, Griffiths CEM, Warren RB, McMullen E, Hunter HJA. Risk of COVID-19 infection in adult patients with atopic eczema and psoriasis: a single-centre cross-sectional study. Br J Dermatol. (2021) 185:441–3. doi: 10.1111/bjd.20062
12. Raiker R, Pakhchanian H, Patel VA. 254 COVID-19 related outcomes in psoriasis psoriasis arthritis patients. J Invest Dermatol. (2021) 141:S45–S45. doi: 10.1016/j.jid.2021.02.276
13. Piaserico S, Gisondi P, Cazzaniga S, Naldi L. Lack of evidence for an increased risk of severe COVID-19 in psoriasis patients on biologics: a cohort study from northeast Italy. Am J Clin Dermatol. (2020) 21:749–51. doi: 10.1007/s40257-020-00552-w
14. Damiani G, Pacifico A, Bragazzi NL, Malagoli P. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. (2020) 33:e13475. doi: 10.1111/dth.13475
15. Holcomb ZE, Santillan MR, Morss-Walton PC, Salian P, Her MJ, Giannotti NM, et al. Risk of COVID-19 in dermatologic patients receiving long-term immunomodulatory therapy. J Am Acad Dermatol. (2020) 83:1215–8. doi: 10.1016/j.jaad.2020.06.999
16. Yendo TM, Sato MN, Branco ACCC, Pietrobon AJ, Teixeira FME, Ramos YÁL, et al. Impact of inflammatory immune dysfunction in psoriasis patients at risk for COVID-19. Vaccines (Basel). (2021) 9:478. doi: 10.3390/vaccines9050478
17. Schoot TS, Kerckhoffs APM, Hilbrands LB, van Marum RJ. Immunosuppressive drugs and COVID-19: a review. Front Pharmacol. (2020) 11:1333. doi: 10.3389/fphar.2020.01333
18. Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S, et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. (2020) 14:1022. doi: 10.3332/ecancer.2020.1022
19. Lai Q, Spoletini G, Bianco G, Graceffa D, Agnes S, Rossi M, et al. SARS-CoV2 and immunosuppression: a double-edged sword. Transpl Infect Dis. (2020) 22:e13404. doi: 10.1111/tid.13404
20. Speeckaert R, Lambert J, Puig L, Speeckaert M, Lapeere H, De Schepper S, et al. Vaccinations in patients receiving systemic drugs for skin disorders: what can we learn for SARS-CoV-2 vaccination strategies? Drugs R D. (2021) 9:1–10. doi: 10.1007/s40268-021-00349-0
21. EMA Recommends First COVID-19 Vaccine for Authorisation in the EU | European Medicines Agency (europa.eu) (2020). Available online at: https://www.ema.europa.eu/en/news/ema−recommends−first−covid−19−vaccine−authorisation−eu#:\sim:text=EMA%20recommends%20first%20COVID%2D19%20vaccine%20for%20authorisation%20in%20the%20EU,−Share&text=Comirnaty%20is%20now%20authorised%20across%20the%20EU.&text=EMA%20has%20recommended%20granting%20a,from%2016%20years%20of%20age.%20 (accessed August 21, 2022).
22. EMA Recommends COVID-19 Vaccine Moderna for Authorisation in the EU | European Medicines Agency (europa.eu) (2021).Available online at:https://www.ema.europa.eu/en/news/ema−recommends−covid−19−vaccine−moderna−authorisation−eu(accessed August21, 2022).
23. EMA Receives Application for Conditional Marketing Authorisation of COVID-19 Vaccine AstraZeneca | European Medicines Agency (europa.eu) (2021). Available online at: https://www.ema.europa.eu/en/news/ema-receives-application-conditional-marketing-authorisation-covid-19-vaccine-astrazeneca (accessed August 21, 2022).
24. EMA Recommends COVID-19 Vaccine Janssen for authorisation in the EU | European Medicines Agency (europa.eu) (2021). Available online at: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-janssen-authorisation-eu (accessed August 21, 2022).
25. McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. (2021) 6:74. doi: 10.1038/s41541-021-00336-1
26. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
27. Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. (2021) 80:1306–11. doi: 10.1136/annrheumdis-2021-220272
28. Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. (2021) 80:1330–8. doi: 10.1136/annrheumdis-2021-220647
29. Braun-Moscovici Y, Kaplan M, Braun M, Markovits D, Giryes S, Toledano K, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis. (2021) 80:1317–21. doi: 10.1136/annrheumdis-2021-220503
30. Al-Janabi A, Littlewood Z, Griffiths CEM, Hunter HJA, Chinoy H, Moriarty C, et al. Antibody responses to single-dose SARS-CoV-2 vaccination in patients receiving immunomodulators for immune-mediated inflammatory disease. Br J Dermatol. (2021) 185:646–8. doi: 10.1111/bjd.20479
31. Simon D, Tascilar K, Fagni F, Krönke G, Kleyer A, Meder C, et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis. (2021) 80:1312–6. doi: 10.1136/annrheumdis-2021-220461
32. Mahil SK, Bechman K, Raharja A, Domingo-Vila C, Baudry D, Brown MA, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. (2021) 3:e627–37. doi: 10.1016/S2665-9913(21)00212-5
33. Wieske L, van Dam KPJ, Steenhuis M, Stalman EW, Kummer LYL, van Kempen ZLE, et al. T2B! Immunity against SARS-CoV-2 study group. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. (2022) 4:e338–50. doi: 10.1016/S2665-9913(22)00034-0
34. Piros ÉA, Cseprekál O, Görög A, Hidvégi B, Medvecz M, Szabó Z, et al. Seroconversion after anti-SARS-CoV-2 mRNA vaccinations among moderate-to-severe psoriatic patients receiving systemic biologicals-Prospective observational cohort study. Dermatol Ther. (2022) 35:e15408. doi: 10.1111/dth.15408
35. Venerito V, Stefanizzi P, Fornaro M, Cacciapaglia F, Tafuri S, Perniola S, et al. Immunogenicity of BNT162b2 mRNA SARS-CoV-2 vaccine in patients with psoriatic arthritis on TNF inhibitors. RMD Open. (2022) 8:e001847. doi: 10.1136/rmdopen-2021-001847
36. Widdifield J, Kwong JC, Chen S, Eder L, Benchimol EI, Kaplan GG, et al. Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes among individuals with immune-mediated inflammatory diseases tested between March 1 and Nov 22, 2021, in Ontario, Canada: a population-based analysis. Lancet Rheumatol. (2022) 4:e430–40. doi: 10.1016/S2665-9913(22)00096-0
37. Mahil SK, Bechman K, Raharja A, Domingo-Vila C, Baudry D, Brown MA, et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol. (2022) 4:e42–52. doi: 10.1016/S2665-9913(21)00333-7
38. Tran TB, Pham NTU, Phan HN, Nguyen HT. Generalized erythrodermic psoriasis triggered by vaccination against severe acute respiratory syndrome Coronavirus 2. Dermatol Ther. (2022) 20:e15464. doi: 10.1111/dth.15464
39. Frioui R, Chamli A, Zaouak A, Hlel I, Khanchel F, Fenniche S, et al. case of new-onset acute generalized pustular psoriasis following Pfizer-BioNTech COVID-19 vaccine. Dermatol Ther. (2022) 13:e15444. doi: 10.1111/dth.15444
40. Lopez ED, Javed N, Upadhyay S, Shekhar R, Sheikh AB. Acute exacerbation of psoriasis after COVID-19 Pfizer vaccination. Proc (Bayl Univ Med Cent). (2021) 35:199–201. doi: 10.1080/08998280.2021.2003681
41. Kabbani M, Poskin M, Benhadou F. Psoriasis exacerbation after COVID-19 vaccination in high-risk group: How to manage it? Dermatol Ther. (2022) 35:e15368. doi: 10.1111/dth.15368
42. Phuan CZY, Choi EC, Oon HH. Temporary exacerbation of pre-existing psoriasis and eczema in the context of COVID-19 messenger RNA booster vaccination: a case report and review of the literature. JAAD Int. (2022) 6:94–6. doi: 10.1016/j.jdin.2021.11.004
43. Brazão C, Alpalhão M, Aguado-Lobo M, Antunes J, Soares-de-Almeida L, Filipe P. Is there a link between guttate psoriasis and SARS-CoV-2? A series of three cases. An Bras Dermatol. (2022) 97:271–3. doi: 10.1016/j.abd.2021.07.006
44. Koumaki D, Krueger-Krasagakis SE, Papadakis M, Katoulis AC, Gkiaouraki I, Zografaki K, et al. Psoriasis flare-up after AZD1222 and BNT162b2 COVID-19 mRNA vaccines: report of twelve cases from a single centre. J Eur Acad Dermatol Venereol. (2022) 36:e411–5. doi: 10.1111/jdv.17965
45. Durmaz I, Turkmen D, Altunisik N, Toplu SA. Exacerbations of generalized pustular psoriasis, palmoplantar psoriasis, and psoriasis vulgaris after mRNA COVID-19 vaccine: a report of three cases. Dermatol Ther. (2022) 35:e15331. doi: 10.1111/dth.15331
46. Wei N, Kresch M, Elbogen E, Lebwohl M. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. (2022) 19:74–7. doi: 10.1016/j.jdcr.2021.11.016
47. Musumeci ML, Caruso G, Trecarichi AC, Micali G. Safety of SARS-CoV-2 vaccines in psoriatic patients treated with biologics: a real life experience. Dermatol Ther. (2022) 35:e15177. doi: 10.1111/dth.15177
48. Pesqué D, Lopez-Trujillo E, Marcantonio O, Giménez-Arnau AM, Pujol RM. New-onset and exacerbations of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. (2022) 36:e80–1. doi: 10.1111/jdv.17690
49. Pacifico A, d'Arino A, Pigatto PDM, Malagoli P. Young Dermatologists Italian Network, Damiani G. COVID-19 vaccines do not trigger psoriasis flares in patients with psoriasis treated with apremilast. Clin Exp Dermatol. (2021) 46:1344–6. doi: 10.1111/ced.14723
50. Damiani G, Allocco F, Young Dermatologists Italian Network, Malagoli P. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. (2021) 46:1106–8. doi: 10.1111/ced.14631
51. Quattrini L, Verardi L, Caldarola G, Peluso G, De Simone C, D'Agostino M. New onset of remitting seronegative symmetrical synovitis with pitting oedema and palmoplantar psoriasis flare-up after SARS-CoV-2 vaccination. J Eur Acad Dermatol Venereol. (2021) 35:e727–9. doi: 10.1111/jdv.17502
52. Krajewski PK, Matusiak Ł, Szepietowski JC. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J Eur Acad Dermatol Venereol. (2021) 35:e632–4. doi: 10.1111/jdv.17449
53. Mieczkowska K, Kaubisch A, McLellan BN. Exacerbation of psoriasis following COVID-19 vaccination in a patient previously treated with PD-1 inhibitor. Dermatol Ther. (2021) 6:e15055. doi: 10.1111/dth.15055
54. Onsun N, Kaya G, Işik BG, Güneş B. A generalized pustular psoriasis flare after CoronaVac COVID-19 vaccination: case report. Health Promot Perspect. (2021) 11:261–2. doi: 10.34172/hpp.2021.32
55. Gelfand JM, Armstrong AW, Bell S, Anesi GL, Blauvelt A, Calabrese C, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: Version 2-Advances in psoriatic disease management, COVID-19 vaccines, and COVID-19 treatments. J Am Acad Dermatol. (2021) 84:1254–68. doi: 10.1016/j.jaad.2020.12.058
56. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients With Rheumatic and Musculoskeletal Diseases: Version 2. Arthritis Rheumatol. (2021) 73:1093–07. doi: 10.1002/art.41734
57. Antibody Testing Is Not Currently Recommended to Assess Immunity After COVID-19 Vaccination: FDA Safety Communication | FDA (2021). Available online at: https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety (accessed August 21, 2022).
58. Test for Past Infection | CDC (2022). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html?CDC_AA_refVal=https%3A%2F%2F (accessed August 21, 2022).
59. Cristaudo A, Graceffa D, Pimpinelli F, Sperati F, Spoletini G, Bonifati C, et al. Immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in psoriasis patients treated with biologic drugs. J Eur Acad Dermatol Venereol. (2022) 36:e266–8. doi: 10.1111/jdv.17861
60. Bonelli F, Sarasini A, Zierold C, Calleri M, Bonetti A, Vismara C, et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J Clin Microbiol. (2020) 58:e01224–20. doi: 10.1128/JCM.01224-20
61. Austin PC. Comparing paired vs. non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat Med. (2011) 30:1292–301. doi: 10.1002/sim.4200
62. Ben B, Hansen. Jake bowers covariate balance in simple, stratified and clustered comparative studies. Statist Sci. (2008) 23:219–36. doi: 10.1214/08-STS254
63. Iacus SM, King G, Porro G. CEM Coarsened exact matching software. J Stat Softw. (2009) 30:1–27. doi: 10.18637/jss.v030.i09
64. Iacus SM, King G, Porro G. Causal Inference without Balance checking: coarsened exact matching. Political Analysis. (2011) 20:1–24. doi: 10.1093/pan/mpr013
65. Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. (2021) 20:102792. doi: 10.1016/j.autrev.2021.102792
66. Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines (Basel). (2021) 9:435. doi: 10.3390/vaccines9050435
67. Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). (2014) 66:1016–26. doi: 10.1002/acr.22246
68. Fomin I, Caspi D, Levy V, Varsano N, Shalev Y, Paran D, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis. (2006) 65:191–4. doi: 10.1136/ard.2005.036434
69. Elkayam O, Bashkin A, Mandelboim M, Litinsky I, Comaheshter D, Levartovsky D, et al. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum. (2010) 39:442–7. doi: 10.1016/j.semarthrit.2008.12.002
70. Rondaan C, Furer V, Heijstek MW, Agmon-Levin N, Bijl M, Breedveld FC, et al. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open. (2019) 9:e001035. doi: 10.1136/rmdopen-2019-001035
71. Brodmerkel C, Wadman E, Langley RG, Papp KA, Bourcier M, Poulin Y, et al. Immune response to pneumococcus and tetanus toxoid in patients with moderate-to-severe psoriasis following long-term ustekinumab use. J Drugs Dermatol. (2013) 12:1122–9.
72. Kapetanovic MC, Nagel J, Nordström I, Saxne T, Geborek P, Rudin A. Methotrexate reduces vaccine-specific immunoglobulin levels but not numbers of circulating antibody-producing B cells in rheumatoid arthritis after vaccination with a conjugate pneumococcal vaccine. Vaccine. (2017) 35:903–8. doi: 10.1016/j.vaccine.2016.12.068
73. Ribeiro AC, Laurindo IM, Guedes LK, Saad CG, Moraes JC, Silva CA, et al. Abatacept and reduced immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). (2013) 65:476–80. doi: 10.1002/acr.21838
74. Ribeiro AC, Guedes LK, Moraes JC, Saad CG, Aikawa NE, Calich AL, et al. Reduced seroprotection after pandemic H1N1 influenza adjuvant-free vaccination in patients with rheumatoid arthritis: implications for clinical practice. Ann Rheum Dis. (2011) 70:2144–7. doi: 10.1136/ard.2011.152983
75. Haberman RH, Herati R, Simon D, Samanovic M, Blank RB, Tuen M, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. (2021) 80:1339–44. doi: 10.1136/annrheumdis-2021-220597
76. Muscogiuri G, Bettini S, Boschetti M, Barrea L, Savastano S, Colao A. Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Low-grade inflammation, CoVID-19, and obesity: clinical aspect and molecular insights in childhood and adulthood. Int J Obes (Lond). (2022) 7:1–8. doi: 10.1038/s41366-022-01111-5
77. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. (2020) 21:e13128. doi: 10.1111/obr.13128
78. Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond). (2012) 36:1072–7. doi: 10.1038/ijo.2011.208
79. Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA. (1985) 254:3187–9. doi: 10.1001/jama.1985.03360220053027
Keywords: psoriasis, vaccine, immunogenicity, biologics, COVID-19
Citation: Graceffa D, Sperati F, Bonifati C, Spoletini G, Lora V, Pimpinelli F, Pontone M, Pellini R, Di Bella O, Morrone A and Cristaudo A (2022) Immunogenicity of three doses of anti-SARS-CoV-2 BNT162b2 vaccine in psoriasis patients treated with biologics. Front. Med. 9:961904. doi: 10.3389/fmed.2022.961904
Received: 05 June 2022; Accepted: 15 August 2022;
Published: 06 September 2022.
Edited by:
Giovanni Genovese, University of Milan, ItalyReviewed by:
Luis Puig, Universitat Autònoma de Barcelona, SpainVito Di Lernia, Azienda USL-IRCCS di Reggio Emilia, Italy
Maria Sole Chimenti, Rheumatology, Allergology and Clinical Immunology University of Rome Tor Vergata, Italy
Copyright © 2022 Graceffa, Sperati, Bonifati, Spoletini, Lora, Pimpinelli, Pontone, Pellini, Di Bella, Morrone and Cristaudo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dario Graceffa, ZGFyaW8uZ3JhY2VmZmFAaWZvLml0
†These authors have contributed equally to this work and share first authorship
 Dario Graceffa
Dario Graceffa Francesca Sperati
Francesca Sperati Claudio Bonifati1
Claudio Bonifati1 Gabriele Spoletini
Gabriele Spoletini Fulvia Pimpinelli
Fulvia Pimpinelli