
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 17 August 2022
Sec. Intensive Care Medicine and Anesthesiology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.960847
This article is part of the Research Topic New Advances in Bedside Assessment and Monitoring of Acute Respiratory Failure Patients View all 5 articles
 Sheng-En Chu1,2†
Sheng-En Chu1,2† Jian-Xun Lu3,4†
Jian-Xun Lu3,4† Shi-Chuan Chang2,5
Shi-Chuan Chang2,5 Kuang-Hung Hsu4,6,7,8,9
Kuang-Hung Hsu4,6,7,8,9 Zhong Ning Leonard Goh10
Zhong Ning Leonard Goh10 Chen-Ken Seak10
Chen-Ken Seak10 Joanna Chen-Yeen Seak10
Joanna Chen-Yeen Seak10 Chip-Jin Ng3,4
Chip-Jin Ng3,4 Chen-June Seak3,4,11* on behalf of SPOT investigators
Chen-June Seak3,4,11* on behalf of SPOT investigatorsBackground: Early recognition of patients with community-acquired pneumonia (CAP) at risk of poor outcomes is crucial. However, there is no effective assessment tool for predicting the development of respiratory failure in patients with CAP. Diaphragmatic ultrasonography (DUS) is a novel technique developed for evaluating diaphragmatic function via measurements of the diaphragm thickening fraction (DTF) and diaphragm excursion (DE). This study evaluated the accuracy of DUS in predicting the development of respiratory failure in patients with CAP, as well as the feasibility of its use in the emergency department (ED) setting.
Materials and methods: This was a single-center prospective cohort study. We invited all patients with ED aged ≥ 20 years who were diagnosed with CAP of pneumonia severity index (PSI) SIe diagnosed with CAP of pneumonia severe with respiratory failure or septic shock were excluded. Two emergency physicians performed DUS to obtain DTF and DE measurements. Data were collected to calculate PSI, CURB-65 score, and Infectious Diseases Society of America/American Thoracic Society severity criteria. Study endpoints were taken at the development of respiratory failure or 30 days post-ED presentation. Continuous variables were analyzed using T-tests, while categorical variables were analyzed using chi-square tests. Further logistic regression and receiver operating characteristic curve analyses were performed to examine the ability to predict the development of respiratory failure. Intra- and inter-rater reliability was examined with intraclass correlation coefficients (ICCs).
Results: In this study, 13 of 50 patients with CAP enrolled developed respiratory failure. DTF was found to be an independent predictor (OR: 0.939, p = 0.0416). At the optimal cut-off point of 23.95%, DTF had 69.23% of sensitivity, 83.78% of specificity, 88.57% of negative predictive value, and 80% of accuracy. Intra- and inter-rater analysis demonstrated good consistency (intra-rater ICC 0.817, 0.789; inter-rater ICC 0.774, 0.781).
Conclusion: DUS assessment of DTF may reliably predict the development of respiratory failure in patients with CAP presenting to the ED. Patients with DTF > 23.95% may be considered for outpatient management.
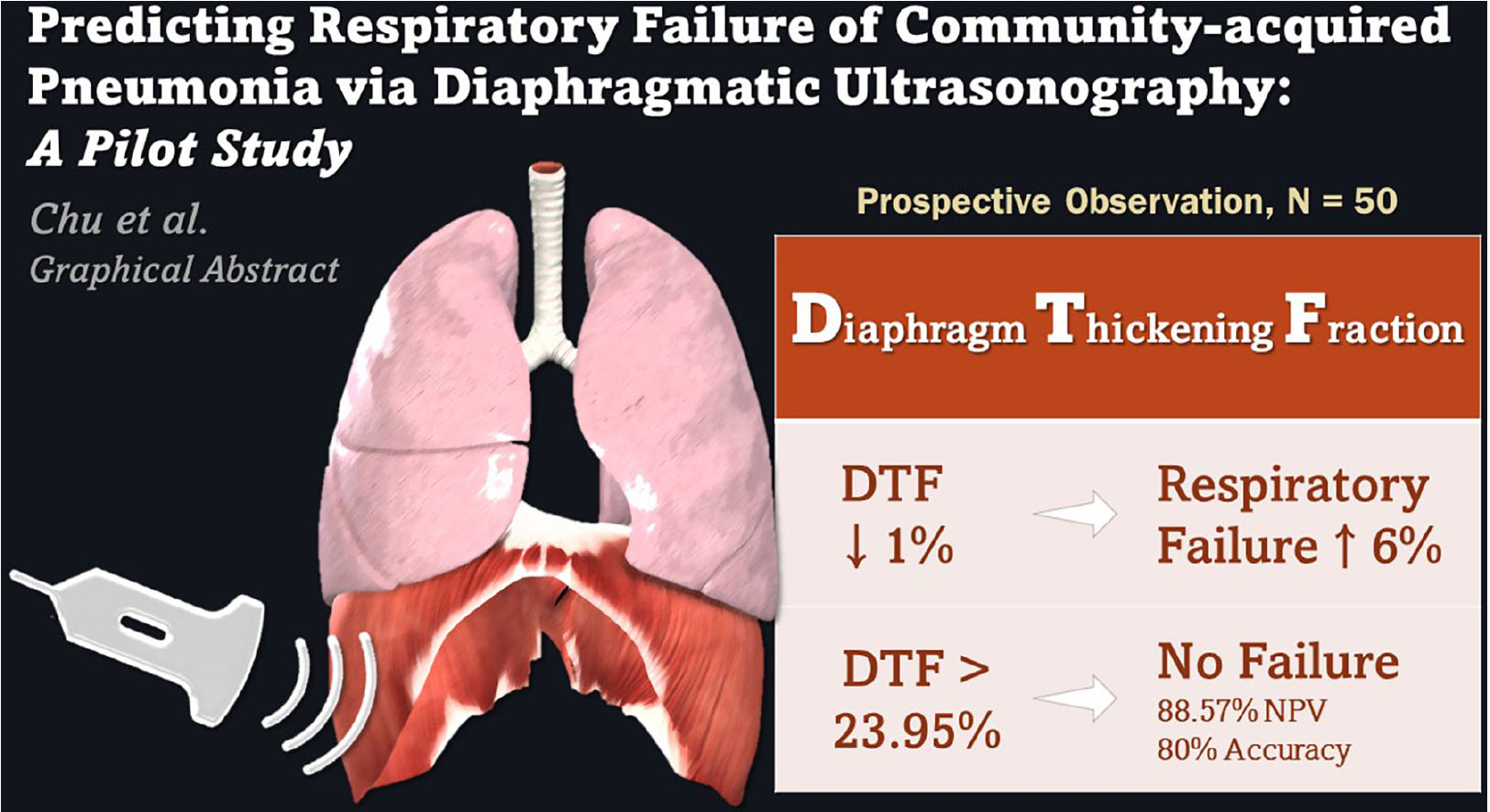
GRAPHICAL ABSTRACT. Diaphragmatic ultrasonography assessment of diaphragm thickening fraction (DTF) may reliably predict the development of respiratory failure in community-acquired pneumonia patients presenting to the emergency department.
Pneumonia, an infectious disease of the pulmonary parenchyma, is a major cause of morbidity and mortality worldwide. It has been found to be the deadliest communicable disease and the 4th leading cause of death globally in 2016, accounting for 3 million deaths (1, 2).
Community-acquired pneumonia (CAP) refers to pneumonia that is contracted by the patient in his own community environment, independently from healthcare facilities. Patients with CAP have a wide-ranging clinical presentation, varying from fever and cough to severe respiratory distress, acute respiratory distress syndrome, and even multiple organ dysfunction syndromes.
Early recognition of patients with the potential to deteriorate during their admission is important in the emergency department (ED) (3). To this end, various scoring tools have been evaluated in predicting the prognoses of patients with CAP, with the results being used to determine subsequent management (4–17). The pneumonia severity index (PSI) (7) has been recommended by the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) for use in the management of CAP (18), due to its well-validated, strong discriminatory power in risk stratification. PSI is, however, too complicated to calculate quickly and thus has limited clinical application in the ED. The CURB-65 score is another popular prediction tool (6) that is recommended by the British Thoracic Society and the National Institute for Health and Care Excellence (19). Despite its ease of use, the safety and effectiveness of CURB-65 in guiding clinical decision-making have not been determined.
Other scoring systems have been developed to identify patients with CAP who are critically ill and consequently require admission to the intensive care unit (ICU). These scores include the severe community-acquired pneumonia score (4), SMART-COP (16), and the IDSA/ATS major and minor severity criteria (17). These scoring systems are, however, not suitable for use in the ED, due to either their reliance on laboratory data that is not readily available in all ED settings or the time-consuming complex calculations required – these attributes would be a hindrance in the fast-paced ED where prompt decision-making is necessary.
Risk stratification via the abovementioned prognostic scores was performed by evaluating the risks of mortality of patients with CAP. Nevertheless, given that CAP imposes significant morbidity in addition to mortality, such risk assessment can also be performed through consideration of the morbidity risks of developing severe disease sequelae. One important complication of CAP would be respiratory failure, though there has yet to be any established evaluation method to predict its development in patients with CAP.
The diaphragm is a major inspiratory muscle and as such plays a critical role in the work of respiration (20). Diaphragmatic dysfunction has been proposed to be a marker of severe CAP, infection, and sepsis (21, 22). It is thought to represent a variant of organ failure (22) and is associated with failure to wean off mechanical ventilation as well as prolonged ICU stays (23).
Diagnosing diaphragmatic dysfunction was difficult and complicated previously (24–27), though the advent of point-of-care ultrasonography has led to huge strides in this aspect (28–31). The diaphragmatic function can currently be evaluated via 2 main ultrasonography techniques. The first technique, diaphragm thickening fraction (DTF), is a measurement of the difference in end-inspiratory and end-expiratory diaphragmatic thickness, expressed as a fraction with the denominator of the end-expiratory thickness. The second method is to measure diaphragm excursion (DE), the diaphragmatic altitude difference between expiration and inspiration (20).
Both DTF and DE were originally developed for use in the ICU to assess patients’ suitability to be weaned off mechanical ventilation. A few studies have assessed the practicality and effectiveness of diaphragmatic ultrasonography (DUS) outside the ICU, demonstrating good intra- and inter-rater reproducibility of right-sided DE measurements in the ED (32–34). Its utility in predicting the need for mechanical ventilation has, however, not been fully examined.
In this study, we aimed to evaluate the two DUS techniques of DTF and DE in predicting the development of respiratory failure in patients with CAP presenting to the ED. These results will assist emergency physicians (EPs) and intensivists in the early identification of patients with CAP at risk of deteriorating rapidly and subsequently enable clinicians to make necessary arrangements in advance for mechanical ventilation and ICU admission.
This was a single-center, prospective cohort study of all patients who visited the ED of Linkou Chang Gung Memorial hospital from December 2018 to March 2019 and were diagnosed with CAP. Linkou Chang Gung Memorial Hospital is one of the largest tertiary medical centers in the world with 3,406 beds and approximately 15,000 monthly ED visits in 2019 (35). This study was approved by the Institutional Review Board of the Chang Gung Memorial Hospital (IRB no: 201801855B0).
All patients aged 20 years or older who visited our ED and were diagnosed with CAP of moderate-to-high severity (PSI class ≥ 4) were invited to participate in this study, except for those who fulfilled the following exclusion criteria: [1] history of prior admission to any healthcare facility in the preceding 48 h; [2] patients who meet the IDSA/ATS severity major criteria (17) on ED presentation, i.e., already in respiratory failure requiring immediate mechanical ventilation or in septic shock requiring vasopressor support; or [3] patients visiting the ED more than once with the same diagnosis. Eligible participants were counseled regarding the objectives and details of our study prior to informed consent being obtained.
All patients diagnosed with CAP in our ED were managed by EP-led teams in accordance with the 2018 Taiwanese guidelines, endorsed by the Infectious Diseases Society of Taiwan and the Taiwan Society of Pulmonary and Critical Care Medicine (36). Appropriate initial treatments were commenced in line with our hospital’s standard treatment protocols approved by the hospital’s ED committee, based on each patient’s history, initial clinical evaluation, and vital signs on presentation. PSI, CURB-65 scores, and IDSA/ATS severity criteria were assessed to assist the attending EP in deciding the need for hospital admission and/or intensive care.
After eligible patients had consented to participate in our study, DUS was performed immediately by two board-certified EPs (raters A and B) who had been trained in emergency ultrasonography and are accredited by both the Taiwan Society of Emergency Medicine and the Taiwan Society of Ultrasound in Medicine. These two raters were blinded to the patients’ full diagnoses and treatment courses, while patients and attending clinical teams were blinded to the ultrasonography findings.
Diaphragmatic ultrasonography was performed at the bedside using the Mindray M7 portable ultrasound machine. Each examination comprised measurements, which were repeated for three consecutive respiratory cycles and subsequently averaged. Both raters took turns performing the DUS examination twice on the right hemidiaphragm. The right hemidiaphragm was opted over the left hemidiaphragm since the latter was often obscured by gastric contents with a less-favorable spleen window (28, 32, 37). All DTF and DE measurements obtained were recorded accordingly for statistical analysis.
The clinical progress of all recruited patients was followed. Study endpoints were taken at either development of respiratory failure or 30 days post-ED presentation.
The diaphragm thickening fraction is calculated using the following formula:
It was assessed via ultrasound through the 7th to 9th intercostal spaces at the right mid-axillary line using M-mode while the patients lay in the supine position. The evaluation was performed using a high-frequency linear probe (Mindray L12-4s probe, 6–10 MHz) in the longitudinal plane (Figure 1A).
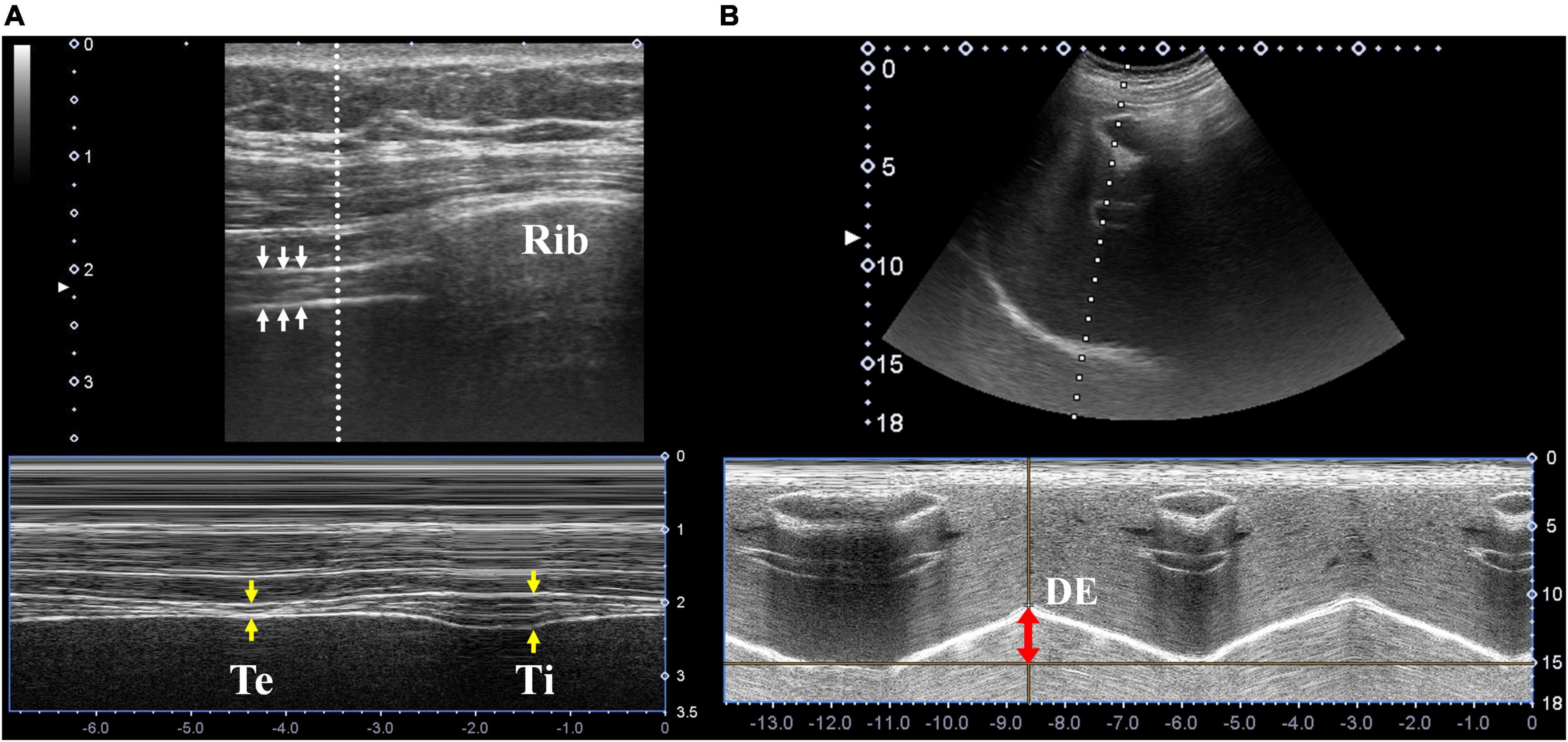
Figure 1. Diaphragmatic ultrasonography. (A) Ultrasonographic images of the diaphragm (white arrows) in the longitudinal plane were located in the right 7th intercostal space along the mid-axillary line. The thickness at end expiration (yellow arrows, labeled as “Te”) and end inspiration (labeled as “Ti”) were measured using M-mode ultrasonography. (B) Ultrasonographic images of the diaphragm in the sagittal plane were located in the right subcostal area between the mid-clavicular and anterior axillary lines. Diaphragm excursion was measured using M-mode ultrasonography (red double-head arrow).
Diaphragm excursion was assessed using the right subcostal view (below the right costal margin between mid-clavicular and anterior axillary lines) while the patients lay in the supine position. The evaluation was performed using a low-frequency linear probe (Mindray C5-2s probe, 2.5–6 MHz) in the sagittal plane, with cranial angulation to produce a perpendicular cross-sectional image of the posterior diaphragm using the liver as an acoustic window (Figure 1B).
Continuous variables were recorded as means ± standard deviation and analyzed using T-tests, while categorical variables were expressed as frequencies with their corresponding percentages and evaluated via chi-square tests. The averages of the 4 DTF and 4 DE measurements recorded for each patient with CAP were used for statistical analysis between patients who developed respiratory failure vs. those who did not. Logistic regression models were also constructed to assess the association between the variables and study outcomes. Receiver operating characteristic (ROC) curve analysis with c-statistics was performed, with optimal cut-off points identified for DTF and DE. Areas under the ROC curve (AUROC) were plotted for DTF, DE, PSI, CURB-65, and the IDSA/ATF minor criteria to compare their abilities to predict the development of respiratory failure in patients with CAP.
To assess the intra- and inter-rater reliability of DUS, intraclass correlation coefficients (ICCs) with 95% confidence intervals were calculated, based on a mean-rating (k = 2), single-measure, and 2-way mixed effects model (38). All statistical analyses were performed using Statistical Analysis Software version 9.4 and Statistical Package for the Social Sciences version 24.0. Statistical significance was taken at 0.05.
In this study, 50 patients with CAP who presented to the ED from December 2018 to March 2019 were recruited for this study. Post-hoc analysis found this sample size to be sufficiently powered at 86.9%. These patients had a mean age of 78 years, with a majority of them being men (56%). Notably, 13 of these 50 patients with CAP (26%) eventually developed respiratory failure during hospitalization, approximately 7 days after presenting to the ED (165.6 ± 143.3 h). In comparison with patients who did not develop respiratory failure, patients with CAP who progressed into respiratory failure were found to be more anemic (p = 0.0087) with higher blood urea nitrogen levels (p = 0.0264). There were no statistically significant differences in patient demographics, underlying comorbidities, initial vital signs, and other laboratory investigation results between both groups (Table 1).
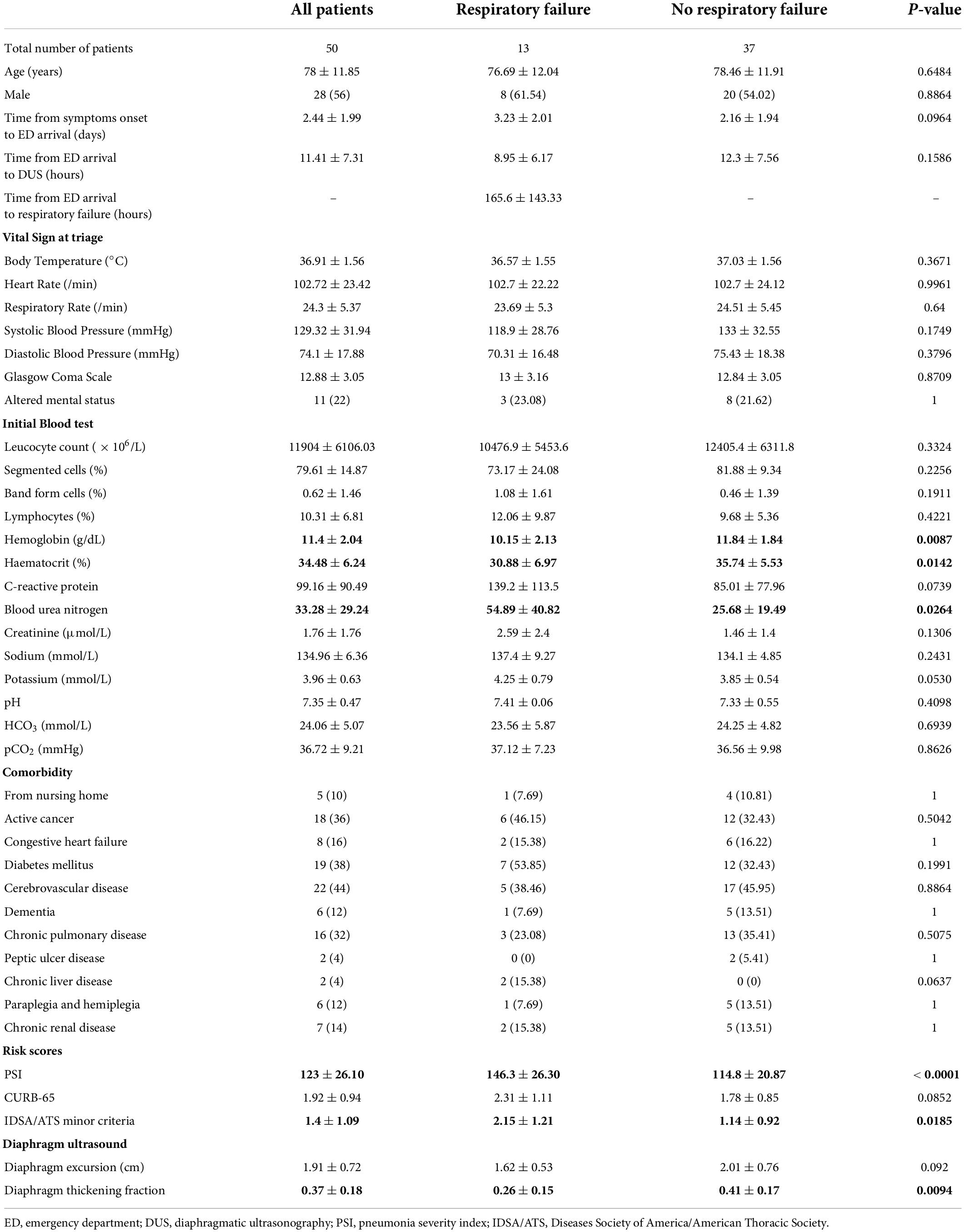
Table 1. Characteristics of community-acquired pneumonia (CAP) patients who developed respiratory failure versus those who did not.
In terms of risk stratification tools assessed in the ED, patients with CAP who developed respiratory failure were found to have higher PSI scores (p < 0.0001) and fulfilled more IDSA/ATS minor criteria (p = 0.0185). DUS examination revealed that DTF in patients who developed respiratory failure was lower at 26 ± 15% vs. 41 ± 17% in those who did not (p = 0.0094), while there were no significant differences in DE measurements for both groups (Table 1).
Further logistic regression analyses were performed for each risk stratification tool. Univariate analysis found that DTF (OR 0.934, p = 0.0178), PSI (OR 1.050, p = 0.0012), and IDSA/ATS minor criteria (OR 2.585, p = 0.0078) had significant correlation with the development of respiratory failure. Subsequent multivariate analysis revealed that only DTF and PSI were independent predictors. Each reduction of 1% in DTF increased the odds of developing respiratory failure by 6.1% (Table 2).
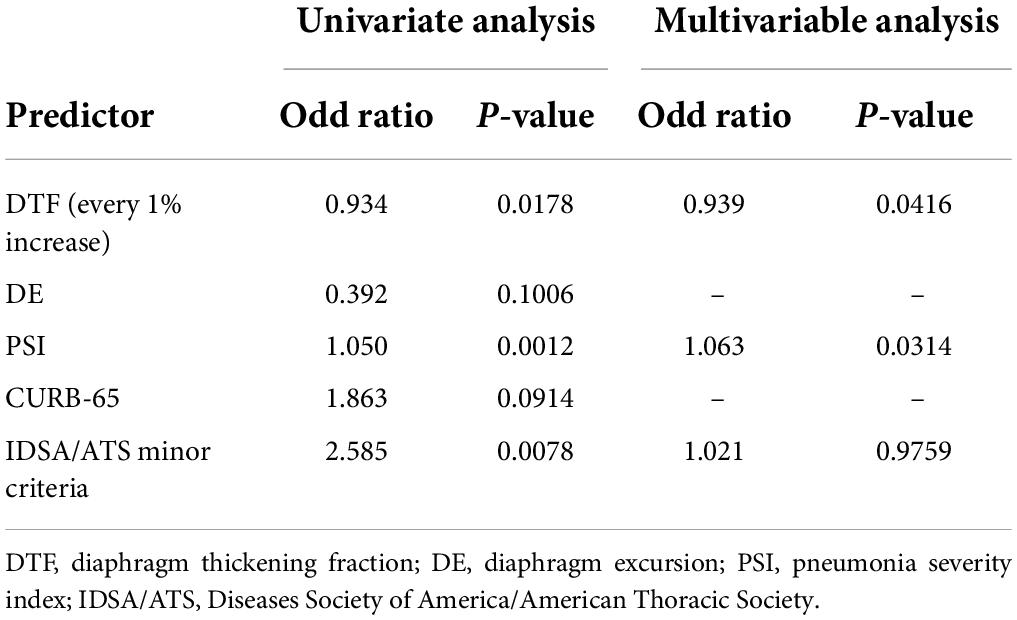
Table 2. Logistic regression of risk stratification tools for predicting respiratory failure in patients with community-acquired pneumonia.
Receiver operating characteristic (ROC) curve analysis of the studied risk stratification tools found PSI to be the best performing tool with an area under ROC curve (AUROC) of 0.8565, while DTF ranked second with an AUROC of 0.7796 (Figure 2). Further comparison of both ROC curves revealed that both PSI and DTF were comparable in their abilities to predict the development of respiratory failure (Pr > ChiSq = 0.3841) (Table 3). The optimal cut-off point, identified via maximizing Youden’s index, was found to be 23.95% for DTF, with a corresponding sensitivity of 69.23%, specificity of 83.78%, negative predictive value of 88.57%, and accuracy of 80% (Table 4).
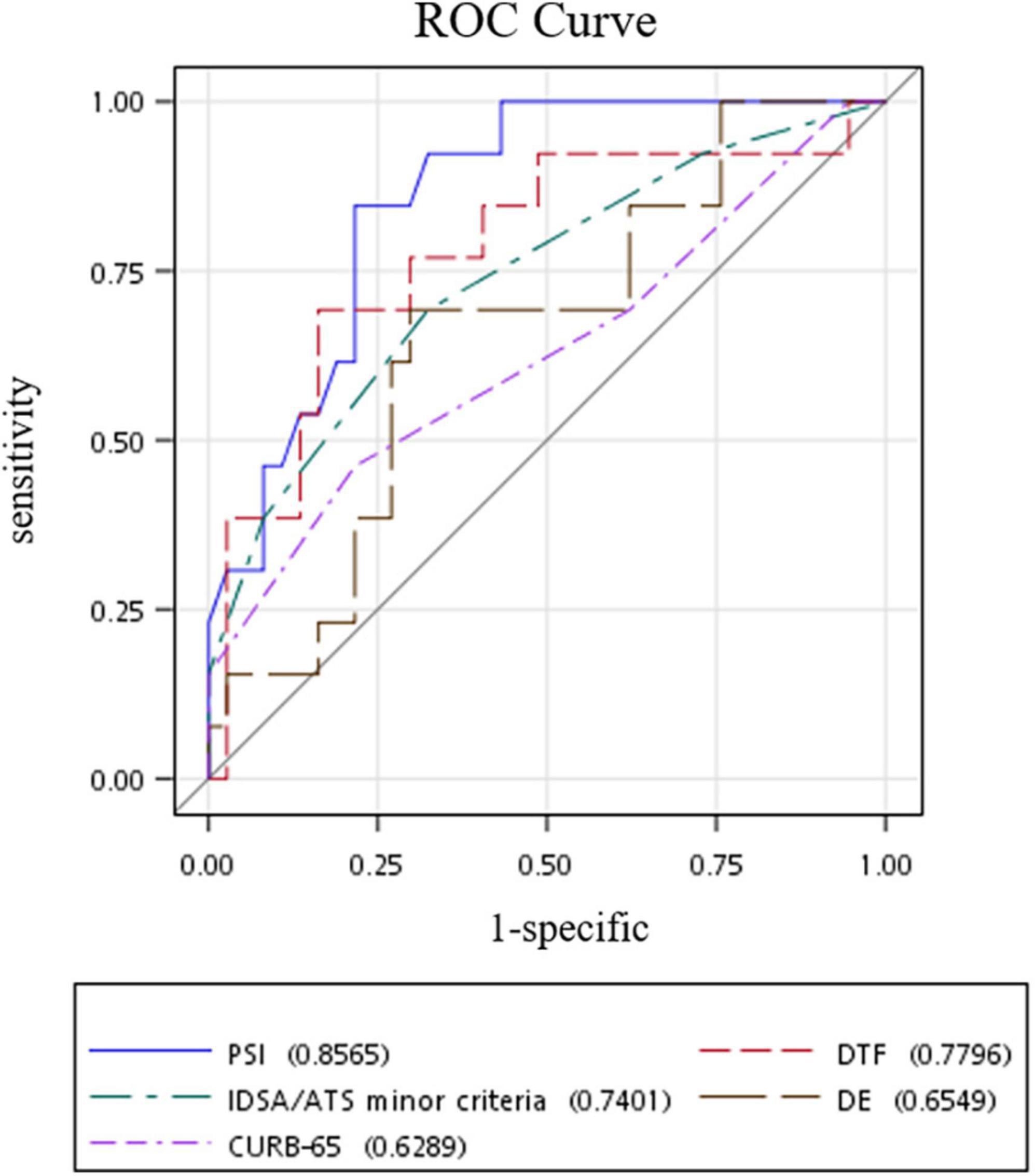
Figure 2. ROC curves of risk stratification tools in predicting respiratory failure in patients with community-acquired pneumonia. ROC, receiver operating characteristic; DTF, diaphragm thickening fraction; DE, diaphragm excursion; PSI, pneumonia severity index; IDSA/ATS, Diseases Society of America/American Thoracic Society.
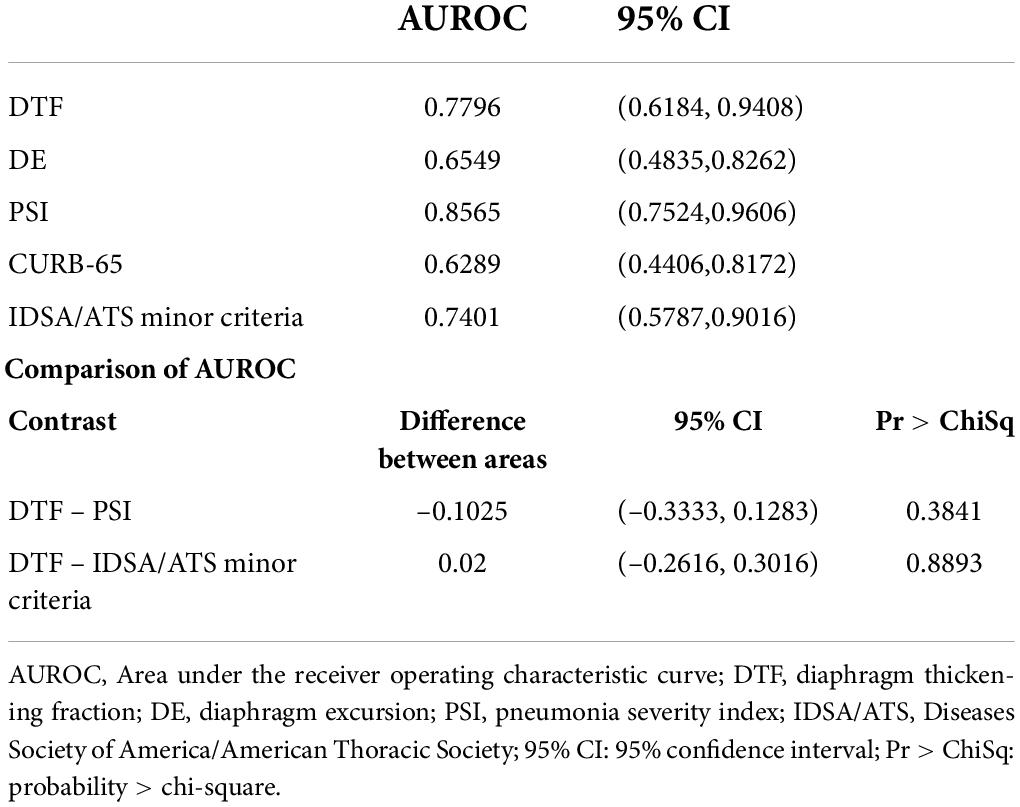
Table 3. Area under the receiver operating characteristic curve (AUROC) analysis of risk stratification tools in predicting development of respiratory failure.
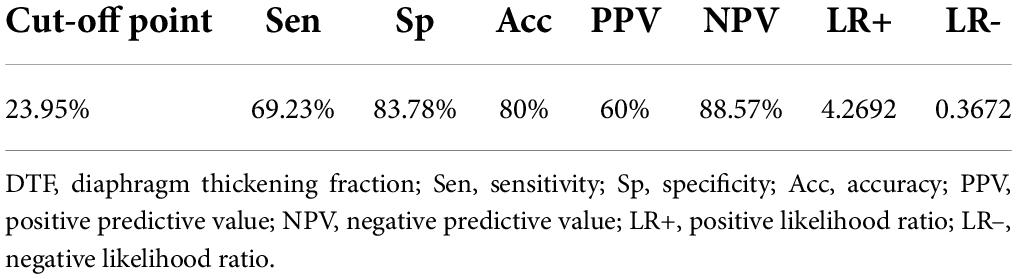
Table 4. Optimal cut-off point for diaphragm thickening fraction (DTF) with its corresponding sensitivity, specificity, and accuracy.
Evaluation of DTF’s intra- and inter-rater reliabilities demonstrated good consistency, with 0.774 (95% CI: 0.633 – 0.865) and 0.781 (95% CI: 0.644 – 0.870) inter-rater intraclass correlation coefficients (ICCs) and 0.817 (95% CI: 0.700 – 0.892) and 0.789 (95% CI: 0.657 – 0.874) intra-rater ICCs (Table 5).
Our study demonstrates that the DTF technique of DUS can reliably predict the development of respiratory failure in patients with CAP presenting to the ED. We also found that DE is a poor predictor of the development of respiratory failure. This study is, to the best of our knowledge, the first to describe the association of critical illness-associated diaphragm dysfunction with the development of respiratory failure in patients with CAP.
Early identification of patients with CAP at risk of deteriorating rapidly via point-of-care ultrasonography would enable clinicians to make necessary arrangements in advance for mechanical ventilation and ICU admission. This prognostication and risk stratification tool may also allow clinicians to prioritize and optimize the care of these critically ill patients with CAP and facilitate counseling and communication with patients and their families from the very beginning of their hospital admissions. Conversely, patients with a low risk of developing respiratory failure can be considered for outpatient management.
During the respiratory cycle, the diaphragm thickens in the inspiratory phase while moving caudally to create negative intrathoracic pressure for drawing air into the lungs; conversely, the diaphragm flattens out during expiration while moving cranially in the expiratory phase to create a positive intrathoracic pressure to expel air out of the lungs. Diaphragmatic weakness is highly prevalent in critically ill patients. Often referred to as “ventilator-induced diaphragmatic dysfunction,” it is a well-recognized pathological entity in the ICU (39). Recent findings of its occurrence even before ICU admission have led to the coining of a new term – “critical illness-associated diaphragm weakness” (40). The DTF is a measurement of the changing diaphragmatic thickness during respiration and, therefore, reflects the magnitude of active diaphragmatic contraction (41). The lower the DTF, the poorer the diaphragmatic contractility, i.e., the greater the extent of diaphragmatic dysfunction.
This direct representation of the diaphragm’s function is perhaps the reason why we found a strong correlation between DTF and the development of respiratory failure in patients with CAP presenting to the ED. Since it provides a preview of diaphragmatic dysfunction, DTF may be used to enable early risk stratification and rapid decision-making at the bedside in the ED – future follow-up studies can be performed to evaluate and validate its clinical application.
Stroke, neuromuscular disease, age, and cardiopulmonary diseases such as heart failure and chronic obstructive pulmonary disease have been associated with diaphragmatic dysfunction (24, 42–46). This is most probably due to the poor physiological reserves of patients with these comorbidities, manifesting as decreased respiratory function and correspondingly decreased DTFs. The significant correlation between DTF and development of respiratory failure in our study is, however, unlikely to be accounted for by this phenomenon, as comparisons of underlying comorbidities between both patient groups found no statistically significant differences (Table 1). A probable explanation is that the patients with these comorbidities severe enough to cause an impaired respiratory function at their baselines would have presented in a bad enough shape to have fulfilled our exclusion criteria and, consequently, were not included in this study. Further studies can consider collecting data on such patients with CAP who are already in respiratory failure on ED presentation to test this hypothesis.
While the DTF was found to be a valuable risk stratification tool in predicting the development of respiratory failure in patients with CAP at the ED, the same does not hold true for the DE. This is perhaps because DTF directly evaluates the extent of diaphragmatic contractility (and therefore function) by measuring the variability of its thickness during the respiratory cycle, while DE is an indirect evaluation of this contractility by measuring the altitude displacement of the diaphragm between inspiration and expiration. Not only is this DE measurement of altitude displacement influenced by active diaphragmatic contractions, it is also affected by other forces external to the diaphragm (41). During inspiration, the negative pressure generated by the accessory inspiratory muscles pulls the diaphragm cranially; conversely, the positive pressure generated by the accessory expiratory muscles during expiration pushes the diaphragm cranially. When there is diaphragmatic dysfunction, these accessory muscles all work in conjunction to compensate for the weak diaphragm to maintain a similar respiratory volume and therefore resulting in a similar DE on DUS, albeit with a paradoxical pattern (41, 47). Our finding that DE is a poor predictor of the development of respiratory failure echoes those of previous studies (32, 33).
Univariate logistic regression analysis of the various risk stratification tools found that PSI, IDSA/ATS minor criteria, and DTF were significantly associated with the development of respiratory failure in patients with CAP, while multivariate analysis confirmed that PSI and DTF were independent predictors. Further AUROC analysis revealed that PSI and DTF were comparable in their risk stratification abilities. While statistical analysis had demonstrated that DTF was noninferior to the more established PSI, the former is more clinically feasible and applicable in the ED environment. This is especially so with the increasing ubiquity of point-of-care ultrasonography in the ED, which would allow rapid visualization of the diaphragm and DTF measurements within minutes. In stark contrast, calculating the PSI would require a much longer time to collate the required data on the patient’s comorbidities, clinical history, physical examination, laboratory investigation results, and radiological imaging.
At a cut-off point of 23.95%, DTF was found to be 80% accurate in predicting the development of respiratory failure in patients with CAP, with a sensitivity of 69.23% and a specificity of 83.78%. Its high NPV of 88.57% and negative likelihood ratio of 0.3672 mean that patients with DTF > 23.95% are very unlikely to develop respiratory failure; these patients can be considered for outpatient management and follow-up. Patients with DTF < 23.95% have a high likelihood of developing respiratory failure (positive likelihood ratio 4.2692) and therefore would benefit from hospitalization and monitoring by healthcare professionals, especially in the first week of illness (mean time from ED arrival to respiratory failure of 165 h).
Our study is a single-center observational study with a relatively small sample size. Therefore, further studies should be performed to confirm our findings and validate the use of DUS and DTF assessment in patients with CAP. As with any other ultrasonography-based evaluation, DUS is operator-dependent (48). Nevertheless, our study demonstrated high intra- and inter-rater reliabilities when DUS was performed by properly trained operators well-versed in ultrasonography. Future studies can be performed to evaluate the feasibility of this assessment modality in the hands of junior doctors with less experience, to determine if adequate intra- and inter-rater reliability can still be maintained after proper training in line with the EXODUS consensus statement (49, 50).
Diaphragmatic ultrasonography assessment of DTF in patients with CAP may be a reliable and accurate risk stratification tool to predict the development of respiratory failure. The short time required by a trained operator to obtain these measurements makes it ideal for the ED setting. Patients with DTF > 23.95% may be considered for outpatient management – further studies are, however, required to validate its use in the clinical management of CAP in the ED.
The SPOT includes the following members: Johan Seak, Jia-Qi Xu, Yi-Zhen Chen, Hao-Tsai Cheng, Hsien-Yi Chen, Yu-Shao Chou, Chih-Huang Li, Tzu-Heng Cheng, Chen-Bin Chen, Chia-Hau Chang, Chien-Lin Chen, Wei-Chun Lin, and Chiao-Hsuan Hsieh.
The datasets generated and/or analyzed during this current study are available from the corresponding author on reasonable request.
The studies involving human participants were reviewed and approved by Institutional Review Board of the Chang Gung Memorial Hospital (IRB no: 201801855B0). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
K-HH: approve the formal analysis. S-EC, J-XL, and C-JS: conceptualization. S-EC and J-XL: data curation. K-HH and C-JS: formal analysis and supervision. C-JS: funding acquisition and resources. S-CC, ZG, C-KS, JS, and C-JS: methodology. S-EC, J-XL, C-JN, and C-JS: investigation. C-JS and ZG: validation, visualization, and writing – review and editing. S-EC, J-XL, ZG, C-KS, JS, and C-JS: writing – original draft. All authors contributed to the article and approved the submitted version.
This study was supported by the Ministry of Science and Technology of Taiwan (MOST 109-2314-B-182A-102-) and Chang Gung Memorial Hospital in Taiwan (CORPG3H0231, CORPG3H0191, CPRPG3D0012, CMRPG3J1721, and CMRPVVL0071). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank the Stratification to Prevent Overcrowding Taskforce (SPOT) from the Department of Emergency Medicine, Lin-Kou Medical Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan and the Department of Emergency Medicine, New Taipei Municipal Tucheng Hospital, New Taipei City, Taiwan for their assistance in the investigation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CAP, community-acquired pneumonia; CI, confidence interval; DUS, diaphragmatic ultrasonography; DTF, diaphragm thickening fraction; DE, diaphragm excursion; ED, emergency department; PSI, pneumonia severity index; ATS, American Thoracic Society; IDSA, Infectious Diseases Society of America; ICU, intensive care unit; EP, emergency physician; ROC, receiver operating characteristic; AUROC, area under the receiver operating characteristic; RF, respiratory failure; ICC, intraclass correlation coefficients.
1. Global Health Estimates 2016. Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Geneva: World Health Organization (2018).
3. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. (1992) 101:1644–55. doi: 10.1378/chest.101.6.1644
4. Espana PP, Capelastegui A, Gorordo I, Esteban C, Oribe M, Ortega M, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. (2006) 174:1249–56.
5. Carratala J, Fernandez-Sabe N, Ortega L, Castellsagué X, Rosón B, Dorca J, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med. (2005) 142:165–72.
6. Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. (2003) 58:377–82. doi: 10.1136/thorax.58.5.377
7. Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. (1997) 336:243–50.
8. Porath A, Schlaeffer F, Lieberman D. Appropriateness of hospitalization of patients with community-acquired pneumonia. Ann Emerg Med. (1996) 27:176–83.
9. Kurashi NY, Al-Hamdan A, Ibrahim EM, Al-Idrissi HY, Al-Bayari TH. Community acquired acute bacterial and atypical pneumonia in Saudi Arabia. Thorax. (1992) 47:115–8.
10. Torres A, Serra-Batlles J, Ferrer A, Jiménez P, Celis R, Cobo E, et al. Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. (1991) 144:312–8.
11. Black ER, Mushlin AI, Griner PF, Suchman AL, James RL Jr., Schoch DR. Predicting the need for hospitalization of ambulatory patients with pneumonia. J Gen Intern Med. (1991) 6:394–400.
12. Ortqvist A, Hedlund J, Grillner L, Jalonen E, Kallings I, Leinonen M, et al. Aetiology, outcome and prognostic factors in community-acquired pneumonia requiring hospitalization. Eur Respir J. (1990) 3:1105–13.
13. Fine MJ, Smith DN, Singer DE. Hospitalization decision in patients with community-acquired pneumonia: a prospective cohort study. Am J Med. (1990) 89:713–21.
14. Daley J, Jencks S, Draper D, Lenhart G, Thomas N, Walker J. Predicting hospital-associated mortality for Medicare patients. A method for patients with stroke, pneumonia, acute myocardial infarction, and congestive heart failure. JAMA. (1988) 260:3617–24. doi: 10.1001/jama.260.24.3617
15. Labarere J, Schuetz P, Renaud B, Claessens YE, Albrich W, Mueller B. Validation of a clinical prediction model for early admission to the intensive care unit of patients with pneumonia. Acad Emerg Med. (2012) 19:993–1003. doi: 10.1111/j.1553-2712.2012.01424.x
16. Charles PG, Wolfe R, Whitby M, Fine MJ, Fuller AJ, Stirling R, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. (2008) 47:375–84.
17. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. (2007) 44(Suppl 2):S27–72. doi: 10.1086/511159
18. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. (2019) 200:e45–67. doi: 10.1164/rccm.201908-1581ST
19. Lim WS, Smith DL, Wise MP, Welham SA, British Thoracic S. British Thoracic Society community acquired pneumonia guideline and the NICE pneumonia guideline: how they fit together. Thorax. (2015) 70:698–700.
20. Supinski GS, Morris PE, Dhar S, Callahan LA. Diaphragm dysfunction in critical illness. Chest. (2018) 153:1040–51.
21. Supinski GS, Alimov AP, Wang L, Song XH, Callahan LA. Calcium-dependent phospholipase A2 modulates infection-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol. (2016) 310:L975–84. doi: 10.1152/ajplung.00312.2015
22. Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med. (2013) 188:213–9. doi: 10.1164/rccm.201209-1668OC
23. Llamas-Alvarez AM, Tenza-Lozano EM, Latour-Perez J. Diaphragm and lung ultrasound to predict weaning outcome: systematic review and meta-analysis. Chest. (2017) 152:1140–50.
24. Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. (2003) 168:10–48.
25. Mills GH, Kyroussis D, Hamnegard CH, Wragg S, Polkey MI, Moxham J, et al. Cervical magnetic stimulation of the phrenic nerves in bilateral diaphragm paralysis. Am J Respir Crit Care Med. (1997) 155:1565–9.
26. Multz AS, Aldrich TK, Prezant DJ, Karpel JP, Hendler JM. Maximal inspiratory pressure is not a reliable test of inspiratory muscle strength in mechanically ventilated patients. Am Rev Respir Dis. (1990) 142:529–32.
27. Mier A, Brophy C, Moxham J, Green M. Twitch pressures in the assessment of diaphragm weakness. Thorax. (1989) 44:990–6.
28. Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF, Zangrillo A. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med. (2017) 43:29–38.
29. Gursel G, Inci K, Alasgarova Z. Can diaphragm dysfunction be reliably evaluated with pocket-sized ultrasound devices in intensive care unit? Crit Care Res Pract. (2018) 2018:5192647. doi: 10.1155/2018/5192647
30. Dube BP, Dres M, Mayaux J, Demiri S, Similowski T, Demoule A. Ultrasound evaluation of diaphragm function in mechanically ventilated patients: comparison to phrenic stimulation and prognostic implications. Thorax. (2017) 72:811–8. doi: 10.1136/thoraxjnl-2016-209459
31. Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation. impact of inspiratory effort. Am J Respir Crit Care Med. (2015) 192:1080–8.
32. Bobbia X, Clement A, Claret PG, Bastide S, Alonso S, Wagner P, et al. Diaphragmatic excursion measurement in emergency patients with acute dyspnea: toward a new diagnostic tool? Am J Emerg Med. (2016) 34:1653–7. doi: 10.1016/j.ajem.2016.05.055
33. Clément A, Zieleskiewicz L, Bonnec J-M, Occéan BV, Bastide S, Muller L, et al. Diaphragmatic excursion measurement in emergency department patients with acute dyspnea to predict mechanical ventilation use. Am J Emerg Med. (2020) 38:2081–7. doi: 10.1016/j.ajem.2020.06.044
34. Cammarota G, Sguazzotti I, Zanoni M, Messina A, Colombo D, Vignazia GL, et al. Diaphragmatic ultrasound assessment in subjects with acute hypercapnic respiratory failure admitted to the emergency department. Respir Care. (2019) 64:1469–77. doi: 10.4187/respcare.06803
35. Seak CJ, Liu YT, Ng CJ Spot investigators. Rapid responses in the emergency department of Linkou Chang Gung Memorial Hospital, Taiwan effectively prevent spread of COVID-19 among healthcare workers of emergency department during outbreak: lessons learnt from SARS. Biomed J. (2020) 43:388–91. doi: 10.1016/j.bj.2020.06.002
36. Chou CC, Shen CF, Chen SJ, Chen HM, Wang YC, Chang WS, et al. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J Microbiol Immunol Infect. (2019) 52:172–99.
37. Vetrugno L, Guadagnin GM, Barbariol F, Langiano N, Zangrillo A, Bove T. Ultrasound imaging for diaphragm dysfunction: a narrative literature review. J Cardiothorac Vasc Anesth. (2019) 33:2525–36.
38. Koo TK, Li MYA. Guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. (2016) 15:155–63.
39. Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. (2004) 169:336–41.
40. Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med. (2017) 43:1441–52.
41. Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. (2013) 39:801–10.
43. van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barre syndrome. Lancet Neurol. (2008) 7:939–50.
44. Similowski T, Catala M, Rancurel G, Derenne JP. Impairment of central motor conduction to the diaphragm in stroke. Am J Respir Crit Care Med. (1996) 154(2 Pt 1):436–41. doi: 10.1164/ajrccm.154.2.8756819
45. Ottenheijm CA, Jenniskens GJ, Geraedts MC, Hafmans T, Heunks LM, van Kuppevelt TH, et al. Diaphragm dysfunction in chronic obstructive pulmonary disease: a role for heparan sulphate? Eur Respir J. (2007) 30:80–9. doi: 10.1183/09031936.00125106
46. Kelley RC, Ferreira LF. Diaphragm abnormalities in heart failure and aging: mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail Rev. (2017) 22:191–207.
47. Gerscovich EO, Cronan M, McGahan JP, Jain K, Jones CD, McDonald C. Ultrasonographic evaluation of diaphragmatic motion. J Ultrasound Med. (2001) 20:597–604.
48. Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, Brace D, et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med. (2015) 41:642–9.
49. Haaksma ME, Smit JM, Boussuges A, Demoule A, Dres M, Ferrari G, et al. EXpert consensus on diaphragm ultrasonongraphy in the critically ill (EXODUS): a Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Crit Care. (2022) 26:99. doi: 10.1186/s13054-022-03975-5
Keywords: diaphragm, community-acquired pneumonia, respiratory failure, ultrasonography, point-of-care, stratification to prevent overcrowding taskforce (SPOT)
Citation: Chu S-E, Lu J-X, Chang S-C, Hsu K-H, Goh ZNL, Seak C-K, Seak JC-Y, Ng C-J and Seak C-J (2022) Point-of-care application of diaphragmatic ultrasonography in the emergency department for the prediction of development of respiratory failure in community-acquired pneumonia: A pilot study. Front. Med. 9:960847. doi: 10.3389/fmed.2022.960847
Received: 03 June 2022; Accepted: 18 July 2022;
Published: 17 August 2022.
Edited by:
Giuseppe Accurso, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, ItalyReviewed by:
Cesare Gregoretti, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, ItalyCopyright © 2022 Chu, Lu, Chang, Hsu, Goh, Seak, Seak, Ng and Seak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen-June Seak, anVsaWFuc2Vha0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.