
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 30 August 2022
Sec. Pulmonary Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.960689
Objective: Hilar and lung lymph node metastases (N1) are defined as ipsilateral bronchial and intrapulmonary lymph nodes. However, the cleaning standards for ipsilateral bronchial lymph nodes in different lobes and segments within the same lobe in segmentectomy are not clearly defined.
Materials and methods: Sixty-six patients undergoing pulmonary resection for the treatment of lung cancer were evaluated. Intraoperatively visible non-tumor-bearing lobe (NTBL) and post-operatively non-tumor-bearing segment (NTBS) lymph nodes were removed and analyzed. The associations between the NTBL LNs and clinicopathological characteristics were analyzed.
Results: Non-tumor-bearing lobe LNs metastases were found in 8 (12.1%) of the 66 patients, NTBS LNs metastasis were not found (0/13). The presence of NTBL metastases was significantly associated with age (<60 years vs. ≥60 years, P = 0.037), differentiation (Grade 1 well differentiated vs. Grade 2 moderately differentiated vs. Grade 3 poorly differentiated, P = 0.012), CAT-scan-findings of Mediastinal and hilar lymph nodes metastasis (node-positive vs. node-negative, P = 0.022), pN stage (N0 vs. N1 vs. N2, P = 0.003) and p stage (I vs. II vs. III, P = 0.009). Multivariate logistic analysis showed that tumor differentiation (P = 0.048, HR 6.229; 95% CI 1.016–38.181) and pN (P = 0.024, HR 5.099; 95% CI 1.245–20.878) were statistically significant predictors.
Conclusions: Lobar lymph node metastasis of NTBL occurs frequently in patients with NSCLC, but lymph node metastases in NTBS LNs are rare. Advanced age, poorly differentiated and N1 and N2 status of CAT-scan-findings were independent risk factors for the involvement of the NTBL lobar lymph nodes. Although lymph node metastases in NTBS are rare, further investigation of the need to dissect is required.
Lung cancer has the highest morbidity and mortality of all neoplastic diseases worldwide (1). Over two million new cases were diagnosed in 2018, making it the most common type of cancer. In China, a 40% increase in mortality is predicted between 2015 and 2030.
The standard surgical treatment for early stage non-small cell lung cancer (NSCLC) is lobectomy accompanied by systematic lymph node dissection (2, 3). The lymph node (LN) stage is critical for prognosis (4). In the National Comprehensive Cancer Network (NCCN) guidelines, systemic mediastinal LN (N2) clearance plays an important role in NSCLC surgical treatment, however, upgrading the staging after discovery of unexpected metastases in intrapulmonary LNs (N1), especially in non-primary tumor lobes and segments (NTBL/NTBS), has been reported (5–8). There are few reports on lobar lymph node metastases in NTBL/NTBS, for the following possible reasons: (1) The technique of intraoperative NTBL/NTBS LNs extraction is difficult with an increased likelihood of post-operative complications; (2) randomized trials have not demonstrated that LN resection provides more survival benefit than sampling (9, 10); and (3) As NTBL/NTBS LNs analysis is dependent on post-operative pathological examination rather than intraoperative assessment by the surgeons, incomplete resection may result (11). We hypothesized that patients undergoing lobectomy, anatomic segmentectomy, or wedge resection may have metastasized N1 LNs in NTBL/NTBS, affecting the post-operative pathological N staging. Our aim was to study the features of NTBL/NTBS LNs metastases and their correlation with clinicopathological features and clinical significance, to explore the necessity of NTBL/NTBS LN clearance.
From March 2015 to March 2017, 66 consecutive patients undergoing pulmonary resection for lung cancer were evaluated. The study was approved by the Shandong Provincial Tumor Hospital Ethics Committee, and all patients signed prior informed consent. Inclusion criteria: (1) NSCLC patients requiring anatomical lung resection and mediastinal LNs dissection; (2) Lymph node dissection of at least 3 mediastinal lymph nodes, including subcarinal nodes, and simultaneous removal of intrapulmonary and hilar lymph nodes; and (3) For patients who have undergone NTBS LNs resection, the tumor size on CT was ≤3 cm. Exclusion criteria:(1) Patients with a previous history of other tumors or distant metastases; (2) Patients receiving preoperative chemotherapy, radiotherapy, or other anti-cancer treatment; (3) Patients undergoing sublobar resection who did not receive the systematic mediastinum and pulmonary LNs dissection; (4) Ipsilateral lung multiple lesions in patients; and (5) Small cell lung cancer patients. If any one of the above five exclusion criteria were met, the patient was not included in the group. The tumor size was divided into 4 groups (≤3 cm, 3∼≤5 cm, 6∼≤7 cm, and >7 cm) in accordance with the 8th Tumor Node Metastasis (TNM) classification for Lung Cancer (12).
Histopathologic analysis was performed by following the 2015 World Health Organization classification of tumors of the lung (13). The specific steps of lung LN clearance are as follows: first, we clear the hilar and mediastinal LNs 2–9 (at least 3 stations) mediastinal LNs cleaning is routinely implemented for cancer in the left and right lung cancer, respectively, paying special attention to the complete resection of LNs in Groups 10–11 of the lobes. The intraoperatively visible NTBL/NTBS LNs were removed and marked. The post-operative specimens were processed by experienced thoracic surgeons and pathologists, and intrapulmonary 12–14 LNs and NTBL/NTBS lymph nodes visible to the naked eye were identified and harvested (Figures 1, 2). The excised lobe’s bronchus was dissected along the proximal to the telecentric end to the segment bronchus; after determining the bronchial boundaries at all levels together with the inter-venous veins, visible intrapulmonary LNs were removed along the direction of the bronchial tree. The group, anatomical location, and numbers of removed LNs were recorded. All specimens were fixed in 10% formalin solution.

Figure 1. Lung bronchial tree specimens after lymph node dissection in right upper lobe and right middle lobe.

Figure 2. Lung bronchial tree specimens after intrapulmonary lymph node dissection in the right middle/lower lobe and right lower lobe.
Data were analyzed using SPSS (Version 22.0; IBM Corp., Armonk, NY, United States) and Excel. Continuous variables were expressed as mean ± standard deviation and differences analyzed using the t-test. The chi-square (χ2) test or Fisher’s exact test were used to investigate relationships between NTBL LNs and clinicopathological variables. P < 0.05 was considered statistically significant. Logistic regression models were built using a stepwise selection method. Multivariate analysis was used to identify independent predictors among the variables. The heat map is made by excel.
The patient characteristics including age, sex, smoking history, serum carcinoembryonic antigen (CEA) levels, NSE levels, Cyfra21-1 levels, tumor differentiation cN2 stage, tumor size, lymph node metastasis, and staging are shown in Table 1.
Among the 66 patients, VATS was performed on 45 patients and open thoracotomy was performed on 21 patients. NTBL metastases were found in 8 (12.1%) cases. The presence of NTBL metastasis was significantly associated with age (<60 years vs. ≥60 years, P = 0.037), differentiation (Grade 1 well differentiated vs. Grade 2 moderately differentiated vs. Grade 3 poorly differentiated, P = 0.012), CAT-scan-findings of Mediastinal and hilar lymph nodes (node-positive vs. node-negative, P = 0.022), pN stage (N0 vs. N1 vs. N2, P = 0.003), and p stage (I vs. II vs. III, P = 0.009) (Table 2). NTBL/NTBS metastases were not significantly associated with the patients’ sex, smoking history, CEA, NSE, Cyfra21-1, tumor size, or pT stage.
The frequencies of lymph node metastases did not differ significantly according to the primary site, left or right lung, or upper lobe vs. middle/lower lobe tumor (P = 0.619) (Table 3).
Univariate and multivariate logistic analyses were used to determine the impacts of the potential predictors of lymph node metastasis. Univariate analysis identified tumor differentiation (P = 0.013, HR 8.111; 95% CI 1.569–41.92), cN2 (P = 0.015, HR 7.286; 95% CI 1.478–35.915), pN (P = 0.008, HR 6.075; 95% CI 1.603–23.025), and p stage (P = 0.014, HR 5.485; 95% CI 1.42–21.184) as statistically significant predictors (Table 4). Further analysis with multivariate analysis showed that tumor differentiation (P = 0.048, HR 6.229; 95% CI 1.016–38.181) and pN (P = 0.024, HR 5.099; 95% CI 1.245–20.878) were statistically significant predictors.

Table 4. Univariate and multivariate logistic analyses the impacts of the potential predictors of lymph node metastasis.
The lymphatic drainage system of the lung was first described by Rouvière in 1932 (14, 15). In 1951, Cahan suggested that pneumonectomy together with hilar and mediastinal lymph node dissection should be a standard surgery for patients with lung cancer, which he termed “radical pneumonectomy” and, 9 years later, reported 48 cases of “radical lobectomy”, namely, lobectomy with regional lymph node dissection. In 1978, Naruke et al. (15) described a map numbering the lymph node stations from 1 to 9 for mediastinal (N2) stations and 10–14 for N1 stations. In 1996, after wide-ranging discussion, the term “Systematic Nodal Dissection” was accepted by the International Association for the Study of Lung Cancer (IASLC) (16). Subsequently, the accepted surgical treatment for NSCLC has been radical lobectomy, in other words, lobectomy combined with systematic lymph node dissection.
Lymph nodes may be assessed intraoperatively by one of five methods (17–19). The first is selected lymph node biopsy, which is usually used to prove N1 or N2 disease in cases, such as exploratory thoracotomy, where resection is not possible. Secondly, the Sampling-Sampling method involves the removal of one or more lymph nodes under the guidance of preoperative or intraoperative findings. In this case, the selection of lymph node stations is predetermined by the surgeon. Thirdly, systematic nodal dissection dissects and removes all the mediastinal tissue containing the lymph nodes. Fourthly, lobe-specific systematic node dissection involves the excision of the mediastinal tissue and lymph nodes, depending on the lobar position of the primary tumor, and, fifthly, extended lymph node dissection in which the bilateral mediastinal and cervical lymph nodes are removed.
There are various guidelines for the extent of lymph node resection. The NCCN guidelines stipulate sampling a minimum of three N2 stations or total mediastinal dissection (20). The Commission on Cancer (CoC) recommends the removal and analysis of at least 10 lymph nodes (21) while the European Society of Thoracic Surgeons recommends mediastinal dissection of all lymph nodes (22). According to the International Association for the Study of Lung Cancer (IASLC) Staging Committee recommendations, there must be no extracapsular extension of the tumor and the highest mediastinal node must test negative after removal. (23).
Despite the differences in current intraoperative lymph node assessments, systematic lymph node dissection is the gold standard. Using this, some scholars have found unexpected metastases in intrapulmonary LNs (N1) especially in NTBL/NTBS LNs (24). The metastatic characteristics and impacts on the patient prognosis of NTBL/NTBS LNs are unknown.
In our study, NSCLC patients requiring anatomical lung resection and mediastinal LNs dissection. Lymph node dissection of at least 3 mediastinal lymph nodes, including subcarinal nodes, and simultaneous removal of intrapulmonary and hilar lymph nodes. Clinicopathological features and lymph node metastasis of disease in 66 lung cancer patients are shown in Figures 3, 4.
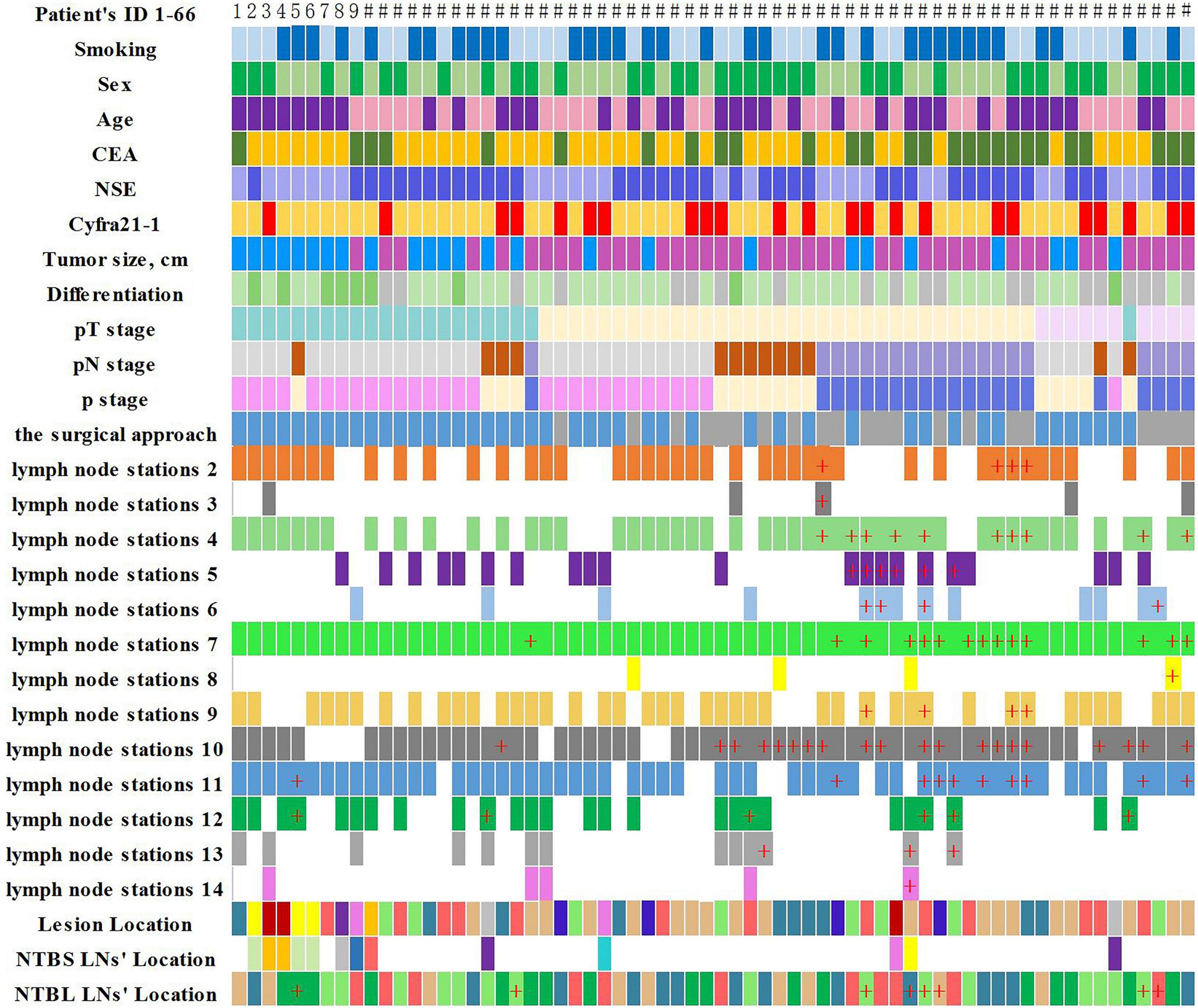
Figure 3. Clinicopathological features and lymph node metastasis of the 66 lung cancer patients (“+” represents positive lymph node metastasis).
NTBL LNs metastases were found in 8 (12.1%) of the 66 patients. Yamanaka et al. (9) and Liu et al. (24) reported that the incidence of lobar lymph node metastases in NTBL LNs were 10.13 and 12.6%. Liu et al. inferred that these extended lymph node metastases were mainly secondary to lymphatic congestion (24). It is worth noting that one patient in our study was found to have an isolated NTBL LNs metastasis, and his pathological stage was upgraded from I to II b, which has an important impact on his treatment strategy. This patient’s preoperative CT showed a solid component-based nodule, and the post-operative pathology showed poorly differentiated squamous cell carcinoma of the lung. Robinson et al. (25). reported the frequent occurrence of occult nodal disease in peripheral N1 stations (11–13) in NSCLC patients with peripheral tumors less than 2 cm diameter and no apparent metastatic spread (T1a-bN0M0). Combined with our research, this suggests that lymph node metastasis in clinical T1a-bN0M0 patients can occur not only in the primary tumor N1, N2 station but also in NTBL LNs. It is well known that the number of positive LNs is an independent prognostic factor of survival in patients with N1 NSCLC (26). NTBL can increase the detection rate of positive lymph nodes, which not only can reduce local recurrence rate but also predict patient prognosis. Yamanaka et al. (9). suggested dissection of all lobar lymph nodes in the remaining NTBL LNs in patients with lobar-hilar or multistation mediastinal lymph node metastases, particularly those on the right side.
No patients (0/13) with NTBS LNs metastasis were found. Sakairi et al. (27). and Lin (28) reported incidences of lymph node metastases in NTBS LNs of 1.6 and 1.2%, respectively. In clinical stage IA, the incidence of peripheral lung cancer lymph node metastases in NTBS LNs was found to be 1.0% (28). These research data are consistent with our findings regardless of the differences in staging. Metastasized lymph nodes occur infrequently in NTBS LNs and the necessity of the cleaning of NTBS LNs lymph nodes during surgery requires further research.
Interestingly, our statistical analyses showed that NTBL LNs metastases are associated with CAT-scan-findings of Mediastinal and hilar lymph nodes metastasis and not associated with location. NTBL lobar lymph node metastasis was most often observed among patients with larger tumor size, N1/N2 nodal involvement, with lymph vascular invasion (LVI), and visceral pleural invasion (VPI) (24). Because of this, we consider NTBL LNs resection to be of vital importance for these patients in terms of accurate staging and reduction of the post-operative local recurrence rate. In addition, NTBL LNs showed a significant correlation with tumor tissue differentiation (P = 0.013), which is of great significance for guiding the patients’ prognosis and post-operative management.
Our research shows that lobar lymph node metastasis of NTBL occurs frequently in patients with NSCLC, but lymph node metastases in NTBS LNs are rare. Advanced age, poorly differentiated and N1 and N2 status of CAT-scan-findings were independent risk factors for the involvement of the NTBL lobar lymph nodes. 18F-FDG PET/CT provides high sensitivity and accuracy, it has overtaken CT as the imaging source of choice for preoperative staging (29). In our study, PET-CT is performed in 8 of 66 patients, but unfortunately, none of these 8 patients have NTBS/NTBL lymph nodes metastasis in post-operative pathology, otherwise, we can further analyze the guiding significance of PET-CT in NTBL/NTBS lymphadenectomy. This is very important. Further investigation into the necessity of NTBL/NTBS dissection is required. We will continue to follow up patients to explore the clinical significance of NTBL/NTBS LNs.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
This study was approved by the Shandong Provincial Tumor Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HJ was in charge of project design. LH collected, analyzes data, and wrote the manuscripts. PS and XL performed the operation and analyzes the data. ZW and DZ used software to analyze data. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
2. Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg. (1960) 39:555–72. doi: 10.1016/S0022-5223(20)31797-9
3. Network Ncc. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 1.2022. Amsterdam: NCC (2022).
4. Riquet M, Mordant P. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. Transl Lung Cancer Res. (2013) 2:1–2.
5. Ramirez RA, Wang CG, Miller LE, Adair CA, Berry A, Yu X, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol. (2012) 30:2823–8. doi: 10.1200/JCO.2011.39.2589
6. Yamanaka A, Hirai T, Takahashi A, Konishi F. Analysis of lobar lymph node metastases around the bronchi of primary and nonprimary lobes in lung cancer: risk of remnant tumor at the root of the nonprimary lobes. Chest. (2002) 121:112–7. doi: 10.1378/chest.121.1.112
7. Maeshima AM, Tsuta K, Asamura H, Tsuda H. Prognostic implication of metastasis limited to segmental (level 13) and/or subsegmental (level 14) lymph nodes in patients with surgically resected nonsmall cell lung carcinoma and pathologic N1 lymph node status. Cancer. (2012) 118:4512–8. doi: 10.1002/cncr.27424
8. Li N, Tan F, Li J, Shao K, Zhao J, Mu J, et al. Blind spot in lung cancer lymph node metastasis: crosslobe peripheral lymph node metastasis in early stage patients. Thorac Cancer. (2018) 9:480–5. doi: 10.1111/1759-7714.12620
9. Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. (2011) 141:662–70. doi: 10.1016/j.jtcvs.2010.11.008
10. Izbicki JR, Passlick B, Pantel K, Pichlmeier U, Hosch SB, Karg O, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg. (1998) 227:138–44. doi: 10.1097/00000658-199801000-00020
11. Gephardt GN, Baker PB. Lung carcinoma surgical pathology report adequacy: a College of American Pathologists Q-Probes study of over 8300 cases from 464 institutions. Arch Pathol Lab Med. (1996) 120:922–7.
12. Asamura H, Chansky K, Crowley J, Crowley J, Goldstraw P, Rusch VW, et al. The International association for the study of lung cancer lung cancer staging project: proposals for the revision of the n descriptors in the forthcoming 8th edition of the tnm classification for lung cancer. J Thorac Oncol. (2015) 10:1675–84. doi: 10.1097/JTO.0000000000000678
13. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. 4th ed. In: WD Travis, E Brambilla, AP Burke editors. WHO Classification of Tumours. Lyon: International Agency for Research on Cancer (2015). 7 p.
14. Nohl HC. An investigation into the lymphatic and vascular spread of carcinoma of the bronchus. Thorax. (1956) 11:172–85. doi: 10.1136/thx.11.3.172
15. Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thoracic Cardiovasc Surg. (1978) 76:832–9. doi: 10.1016/S0022-5223(19)39559-5
16. Goldstraw P. Report on the international workshop on intrathoracic staging. Lung Cancer. (1997) 18:107–11. doi: 10.1016/S0169-5002(97)00051-2
17. Wo Y, Li H, Zhang Y, Peng Y, Wu Z, Liu P, et al. The impact of station 4L lymph node dissection on short-term and long-term outcomes in non-small cell lung cancer. Lung Cancer. (2022) 170:141–7. doi: 10.1016/j.lungcan.2022.06.018
18. Erickson CJ, Fernandez FG, Reddy RM Minimally invasive and open approaches to mediastinal nodal assessment. Ann Surg Oncol. (2018) 25:64–67. doi: 10.1245/s10434-016-5677-2
19. Dezube AR, Mazzola E, Bravo-Iniguez CE, De León LE, Rochefort MM, Bueno R, et al. Analysis of lymph node sampling minimums in early stage non-small-cell lung cancer. Semin Thorac Cardiovasc Surg. (2021) 33:834–45. doi: 10.1053/j.semtcvs.2020.11.007
20. National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 1.2017). Jen kintown, PA: National Comprehensive Cancer Network (2006).
21. American College of Surgeons CoC Quality of Care Measures. Lung measure specififications. (n.d.). Available online at https://www.facs.org/w/media/fifiles/quality%20programs/cancer/ncdb/measure%20specs%20nscl.ashx. (accessed October 21, 2016).
22. Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. (2006) 30:787–92. doi: 10.1016/j.ejcts.2006.08.008
23. Rami-Porta R, Wittekind C, Goldstraw P. International association for the study of lung cancer (IASLC) staging committee: complete resection in lung cancer surgery: proposed definition. Lung Cancer. (2005) 49:25–33. doi: 10.1016/j.lungcan.2005.01.001
24. Liu J, Li J, Lin G, Long Z, Li Q, Liu B, et al. Risk factors of lobar lymph node metastases in non-primary tumor-bearing lobes among the patients of non-small-cell lung cancer. PLoS One. (2020) 15:e0239281. doi: 10.1371/journal.pone.0239281
25. Robinson EM, Ilonen IK, Tan KS, Plodkowski AJ, Bott M, Bains MS. Prevalence of occult peribronchial N1 nodal metastasis in peripheral clinical N0 <2 cm NSCLC. Ann Thorac Surg. (2020) 109:270–6. doi: 10.1016/j.athoracsur.2019.07.037
26. Jonnalagadda S, Smith C, Mhango G, Wisnivesky JP. The number of lymph node metastases as a prognostic factor in patients with N1 non-small cell lung cancer. Chest. (2011) 140:433–40. doi: 10.1378/chest.10-2885
27. Sakairi Y, Yoshino I, Yoshida S, Suzuki H, Tagawa T, Iwata T, et al. Pattern of metastasis outside tumor-bearing segments in primary lung cancer: rationale for segmentectomy. Ann Thorac Surg. (2014) 97:1694–700. doi: 10.1016/j.athoracsur.2013.12.015
28. Wang L, Jiang W, Zhan C, Shi Y, Zhang Y, Lin Z, et al. Lymph node metastasis in clinical stage IA peripheral lung cancer. Lung Cancer. (2015) 90:41–6. doi: 10.1016/j.lungcan.2015.07.003
Keywords: lung cancer, lymph node metastases (LN metastases), non-tumor-bearing lobe (NTBL) lymph nodes, non-tumor-bearing segment (NTBS) lymph nodes, outside tumor-bearing lobes and/or segments
Citation: Han L, Jia H, Song P, Liu X, Wang Z and Zhang D (2022) Lymph node metastases outside tumor-bearing lobes and/or segments in non–small cell lung cancer. Front. Med. 9:960689. doi: 10.3389/fmed.2022.960689
Received: 03 June 2022; Accepted: 12 August 2022;
Published: 30 August 2022.
Edited by:
Michel Gonzalez, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandCopyright © 2022 Han, Jia, Song, Liu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Jia, emhvbmdsaXV5aXl1YW4xMUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.