
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Med., 08 July 2022
Sec. Pathology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.958840
This article is part of the Research TopicGlobal Excellence in Pathology: AfricaView all 12 articles
 Lisa M. Bebell1*
Lisa M. Bebell1* Joseph Ngonzi2
Joseph Ngonzi2 Frederick A. Meier3
Frederick A. Meier3 Chrystalle Katte Carreon4,5
Chrystalle Katte Carreon4,5 Abraham Birungi6
Abraham Birungi6 Vanessa B. Kerry7,8
Vanessa B. Kerry7,8 Raymond Atwine6
Raymond Atwine6 Drucilla J. Roberts9
Drucilla J. Roberts9Introduction: Over two million stillbirths and neonatal deaths occur in sub-Saharan Africa (sSA) annually. Despite multilateral efforts, reducing perinatal mortality has been slow. Although targeted pathologic investigation can often determine the cause of perinatal death, in resource-limited settings, stillbirths, early neonatal deaths, and placentas are rarely examined pathologically. However, the placenta is a key source of diagnostic information and is the main determinant of fetal growth and development in utero, influencing child health outcomes.
Methods: In 2016, our collaborative intercontinental group began investigating infectious perinatal death and adverse child health outcomes in Uganda. We developed and initiated a 4-day combined didactic/practical curriculum to train health workers in placental collection, gross placental examination, and tissue sampling for histology. We also trained a local technician to perform immunohistochemistry staining.
Results: Overall, we trained 12 health workers who performed gross placental assessment for > 1,000 placentas, obtaining > 5,000 formalin-fixed tissue samples for research diagnostic use. Median placental weights ranged from 425 to 456 g, and 33.3% of placentas were < 10th percentile in weight, corrected for gestational age. Acute chorioamnionitis (32.3%) and maternal vascular malperfusion (25.4%) were common diagnoses.
Discussion: Through a targeted training program, we built capacity at a university-affiliated hospital in sSA to independently perform placental collection, gross pathologic examination, and placental tissue processing for histology and special stains. Our training model can be applied to other collaborative research endeavors in diverse resource-limited settings to improve research and clinical capacity and competency for diagnostics and management of stillbirth, neonatal death, and child health outcomes.
There are at least 5.5 million stillbirths and neonatal deaths annually (1), and > 40% occur in sub-Saharan Africa (sSA). Despite multilateral efforts, reducing global perinatal morbidity and mortality has been slow, especially in sSA (2). The placenta is the main determinant of fetal growth and development in utero (3) and mediates maternal adaptations and maladaptions to pregnancy (4–6), reflecting or influencing a multitude of fetal and child health outcomes (7). One poor outcome is fetal growth restriction. Fetal growth restriction is diagnosed when the fetus fails to meet its full growth potential due to pathological factors in utero. Growth restriction most often results from placental dysfunction (8). Globally, fetal growth restriction is a leading cause of stillbirth, neonatal mortality, and childhood and long-term morbidity (8). For these reasons, the International Federation of Gynecology and Obstetrics (FIGO) strongly recommends sending placentas from pregnancies with fetal growth restriction for histopathological examination, and reporting results according to the Amsterdam consensus diagnostic nosology (9). The FIGO recommendation recognizes that placental examination improves diagnostic precision and that the information it provides is often useful when counseling women about the cause of adverse pregnancy outcomes or other obstetric events and for providing guidance on future pregnancies, particularly on the risk of known recurrent placental disorders (8). Furthermore, pathological data on causes of stillbirth, fetal growth restriction, and other undesirable outcomes are necessary to develop appropriate public health initiatives at all levels, plan for clinical services including primary and referral needs, that ultimately will help achieve maternal and child health goals (10, 11).
Unfortunately, though targeted investigation can determine the cause of perinatal death in most cases, stillbirths and early neonatal deaths are rarely investigated using fetal or neonatal autopsy or placental pathology in resource-limited settings (12). Lack of pathology capacity is the main reason placental and fetal examination is rarely performed in sSA. Lack of capacity is multifactorial, including a scarcity of institutions capable of providing training in pathology techniques and research methods and an alarming scarcity of trained pathologists (10, 11). A 2012 survey of pathology resources in sSA demonstrated that all countries except South Africa and Botswana had fewer than one pathologist for every 500,000 people, less than 10% of the pathologist availability in the United States of America (11). Since the 1990s, we have been working in resource-limited settings to build capacity for perinatal pathology and carry out research studies to determine causes of maternal, fetal, and child morbidity and mortality (13–16). Through our individual and collective experience, we have witnessed the dire need to further improve the capacity for placental pathology in sSA.
Here, we report on our program’s experience building perinatal pathology capacity at Mbarara University of Science and Technology (MUST) and its affiliated Mbarara Regional Referral Hospital in southwestern Uganda. Recognizing that human resources for pathology training and research are limited in this setting, as elsewhere in sSA, we leveraged a 20-year multilateral research and teaching partnership involving MUST, Massachusetts General Hospital (MGH), and, more recently, Seed Global Health, to train a cadre of health workers and laboratory technicians to perform gross and histologic placental examination. We detail the structure of the training program and its outcome including quality of prepared slides and identified pathologic placental diagnoses.
In 2016, our collaborative intercontinental research group comprised of investigators and educators affiliated with MUST and MGH began a research program investigating infectious causes of stillbirth, early neonatal death, and adverse child health outcomes in Uganda. One team member (FAM), a perinatal pathologist from the United States with specialized training and extensive perinatal pathology experience, was stationed full-time on site at MUST for over 2 years, a volunteer position facilitated by the Seed Global Health program. Seed is a not-for-profit organization focused on human resource for health capacity-building in sSA through sustained collaborative engagement.1 Another team member (LMB), an infectious diseases physician and intensivist trained in the United States, lived on-site for 3 years coordinating the program’s development and carrying out research projects with a local obstetrician collaborator (JN). A third team member (DJR, an experienced perinatal pathologist) traveled to the site several times per year to provide the on-site team with support from the MGH Pathology Department and assist with building program capacity, especially training of a local team member (AB) in immunohistochemistry techniques.
Before initiating this program, placentas in Mbarara were rarely examined at birth for either pathologic gross or histologic changes. We began our initiative by developing a training program in gross placental examination and sampling for histologic review, starting with a 4-day combined didactic/practical curriculum to train nurses, midwives, and junior physicians in placental collection and gross assessment to identify lesions that would need sampling for histology. An example training schedule is provided in Table 1, which consisted of combined didactic presentations on placental function and structure and hands-on training in gross placental examination, umbilical cord blood sampling, placental dissection, and sampling of placental disc, membranes, and umbilical cord, and formalin-fixation of samples and sample trimming for histopathology block creation. Often, this training program was embedded in a larger training curriculum on research ethics and principles of informed consent along with additional procedures and data collection relevant to placental and maternal-child research. We employed a train-the-trainer model, with two experienced perinatal pathologists (DJR, FAM) who trained junior pathologists (including RA) and one junior physician scientist (LMB), who further trained local health workers. After carrying out placental gross examination and sampling procedures for at least 2 months, local health workers were then capable of training peers. The training program was modified during the COVID-19 pandemic to include remote live video didactic sessions (conducted by LMB) paired with in-person peer training (conducted by prior trainees with > 2 months of hands-on experience). One author (DJR) also separately facilitated training a histology technician (AB) to perform immunohistochemistry. This focused training program consisted of methods to prepare tissue for immunohistochemistry, battery testing, quality control and assurance practices, antigen retrieval, direct and indirect methods of manual immunohistochemistry staining, and development of a standardized operating protocol for immunohistochemistry.
Research grant funding (to LMB) and a volunteer stipend (to FAM) paid for time spent training health workers in gross placental examination and sampling. Histopathologic and histopathology supplies were funded by research grant funding (to LMB) as well as departmental partnership funds at MGH (to DJR and others). Gross placental examination findings were recorded on a standardized case report form developed by DJR in accordance with her practice and training at Harvard Medical School teaching hospitals. Histopathology findings were recorded on standardized case report forms using the categories defined in the Amsterdam consensus diagnostic nosology (9). Case report forms were then abstracted into a Research Electronic Capture (REDCap) database (17) for analysis.
All research participants provided written informed consent to participate in each research study. The first research study was approved by MUST (12/11-15), Mbale Regional Referral Hospital Research Ethics Committee (082/2016), Partners Healthcare (2016P000806), and Pennsylvania State University College of Medicine (STUDY0004199). The second research study was approved by MUST (11/03-17) and Partners Healthcare (2017P001319). The third research study was approved by MUST (10/06-19) and Partners Healthcare (2019P003248).
Training was conducted for three distinct studies over a 5-year time span from 2016 to 2021 (Table 2). One training program per study period was deemed sufficient to train all staff, with the exception of Study 3, when training was carried out in stages to accommodate newly hired staff. Refresher training was also provided due to interruptions in the research program from the COVID-19 pandemic. Overall, we trained 12 health workers, including six midwives, four nurses, and two junior medical doctors. All trainees are now capable of independently performing gross placental examination and collecting samples for histopathologic examination for research and/or clinical purposes (Figure 1). Altogether, the Mbarara trainees have collected, and performed gross placental assessment, on over 1,000 placentas to date (Table 2). Approximately 80% of all histologic slides prepared from these placentas were of adequate quality for diagnostic interpretation. Median placental weights ranged from a mean of 425 grams in Study 1 to a median of 456 (IQR 382–529) grams in Study 2 and 443 (IQR 375–511) grams in Study 3 (Table 2). Overall, 33.3% of placentas were in the < 10th percentile in weight, corrected for gestational age using a standard weight chart (18).
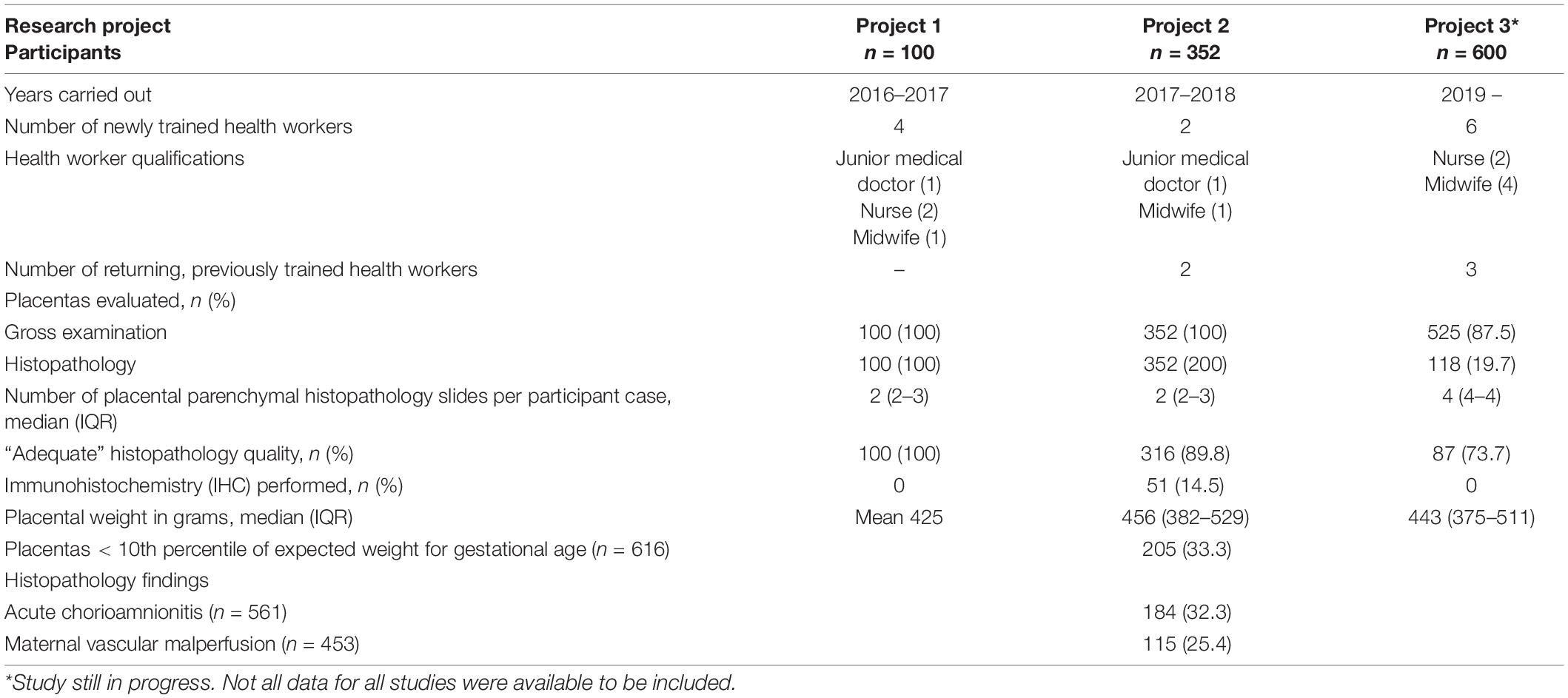
Table 2. Health workers trained, number of placentas processed and evaluated, and gross and histologic pathology findings in three separate research studies that constitute the foundation of perinatal pathology research at a Ugandan regional referral hospital.

Figure 1. Trained health workers independently dissecting and sampling placenta after gross examination in Mbarara, Uganda.
Trainees fixed and trimmed formalin-fixed samples of umbilical cord, membranes, placental parenchyma, and any focal disc or umbilical cord lesions to create formalin-preserved tissue specimens for paraffin embedding. In total, 5,408 tissue samples were processed and used for research diagnostics through the three studies. Grant funding supported local histology technicians (AB and four others) to create paraffin-embedded blocks, hematoxylin and eosin (H&E)-stain slides and generate slides for routine histopathology. Due to human resource constraints on local pathologists, slides were interpreted by perinatal pathologists based in the United States (DJR, FAM, and CKC) according to the Amsterdam consensus diagnostic nosology (9). Common histologic diagnoses included acute chorioamnionitis (present in 32.3% of cases), and features of maternal vascular malperfusion (MVM, present in 25.4% of cases).
To date, we have published three manuscripts reporting the results of this work (14, 15, 19), with one more currently under review and another three manuscripts in preparation. The local technician (AB) trained to perform immunohistochemistry staining on placental tissue for research (14) and clinical purposes has leveraged these skills to perform immunohistochemistry staining to assist in diagnosis of breast and other cancers.
Through a structured and targeted training program, we built capacity at a university-affiliated hospital in sSA to independently perform placental collection, gross pathologic examination and placental tissue processing for histology and special stains for placentas of diagnostic interest, adequate for research purposes. The health workers trained came from a variety of medical specialties including general nursing, midwifery, pediatrics, obstetrics and gynecology, and pathology. The training program demonstrated that health workers with diverse backgrounds can learn and teach essential placental gross examination and sampling techniques for histopathology. During the social disruption of the early COVID-19 pandemic, we hybridized our training model to include a virtual component that can be applied to other collaborative research endeavors in different resource-limited settings. We advocate for the train-the-trainer model to ensure ongoing local development of trainee skills to carry out research, improve on-site clinical diagnostic capacity and patient care, and allow trainees subsequently assume trainer roles to train others going forward.
We found a high proportion (33.3%) of placentas that weighed < 10th percentile of expected weight for gestational age. However, gestational age correction was performed using a standard weight chart based on women in the northeastern United States of America (18) since Ugandan or sSA-specific placental weight charts are not currently available. We believe that placental weights in Uganda appear smaller for gestational age when high-income reference standards are used, likely due to the high percentage of small placentas by weight. Furthermore, measurements aggregated from seven studies in high-resource settings reported a median weight of 520 (10–90% range 408–642) g (20). Thus, gestational age correction using non-local standards may lead to overdiagnosis of low placental weight. However, low placental weight could also indicate placental hypoplasia, a feature of MVM. Combined with small placental size, a relatively high prevalence MVM (25.4%) may point to a relatively high incidence of other features of MVM, including placental infarcts, increased syncytial knots, distal villous hypoplasia, increased perivillous fibrin, etc. Together, these findings are concerning indicators of possible intrauterine fetal growth restriction, which is a leading global cause of stillbirth, neonatal mortality, and morbidity (8). Furthermore, maternal HIV infection and antiretroviral treatment are associated with MVM, with prevalence as high as 30–40% (21). Large-scale perinatal pathology studies carried out in sSA have also demonstrated associations between utero-placental vascular pathology, acute chorioamnionitis, and stillbirth (22, 23). These results, reflecting a miniscule proportion of all deliveries in sSA, highlight the need for histopathological examination of placentas of unexplained stillbirth deliveries, fetal growth restriction, and maternal HIV infection, and the need to use standardized criteria for reporting diagnoses in order to compile and compare results across various settings (22). Alongside developing local placental weight charts, the high prevalence of low placental weight should be further investigated and addressed to improve mortality in children under age five.
Though largely successful, our initiative has several limitations. Only approximately 80% of the slides produced by our trainees were adequate for histopathologic interpretation. The 20% that were inadequate often had issues with processing and fixation but did not have issues with trimming/cutting of specimen tissue or paraffin blocks, or staining. The large proportion of inadequate slides emphasizes the continuing challenge of consistent slide production in resource-constrained settings. Furthermore, although local pathologists based at MUST and its affiliated regional referral hospital are capable of interpreting H&E-stained placental slides, unfortunately, human resource limitations currently limit their capacity to add placental cases to their current practice and prioritize these cases over other important diagnostic applications (e.g., new tumor diagnosis). In our future work, we aim to help define priority clinical cases for placental analysis and build capacity to perform on-site fetal autopsy. In addition, although we trained one histopathology technician to perform immunohistochemistry on placental specimens and other tissues, there remains limited local capacity to routinely perform special stains due to a lack of supplies and funding. However, the acquired immunohistochemistry skills can be readily useful for future research endeavors and clinical projects when supplies are available. Additionally, valuable skills may be passed on to other trainees thereby augmenting the pool of skilled individuals able to perform such tasks.
In the future, we hope that increasing research capacity will also translate into greater use of perinatal pathology for clinical diagnosis and management of stillbirth, neonatal death, and child health outcomes in sSA. Information gained from autopsies of stillbirths and neonatal deaths is essential to designing interventions to improve outcomes. Minimally invasive approaches should be considered as alternatives to conventional autopsy, which are proven acceptable alternatives to conventional pediatric autopsy in resource-limited settings (24). Toward this end, building perinatal pathology research capacity also contributes skills and resources that can have a positive collateral impact on patient care. Some strategies to address the shortage of pathology services include concentrating specialists in tertiary care centers and establishing structured referral systems to improve access. Digital telepathology has also been proposed as a stopgap measure to address the pathologist shortage in sSA, with some success, largely when embedded within long-term international collaborations (25). Multinational partnerships can also help with human resource limitations by providing training and diagnostic consultation, including virtual training, which has proven successful in some settings (26). A first step would be to survey current capacity and develop a tool for assessing needs. Although we report our experience of limited capacity to carry out perinatal pathology service in sSA, the true capacity is currently unclear. Recent efforts to assess anatomic pathology capacity have largely been focused on cancer diagnosis. Thus, regional surveys of perinatal pathology capacity should also be implemented.
Collaborative international research programs can, and should, contribute to clinical and research pathology capacity development in sSA (27). There are several good examples of successful programs in addition to ours, including a pathology program at Anokye Teaching Hospital that became self-sustaining after an 11-year partnership with University Hospital of North Norway and could serve as a model for others. The Anokye Pathology Department now provides surgical pathology, cytology, immunohistochemistry, frozen section services, and residency training, fully independent from international assistance (28). A similar partnership between the Fred Hutchinson Cancer Research Center at the University of Washington and Makerere University and its associated Mulago Hospital led to the establishment of a clinical pathology laboratory at the Uganda Cancer Institute that handled 5,700 tissue diagnoses in 2019 and routinely offers immunohistochemistry services (29). Though these examples are in the cancer field, similar programs could, and should, be established for clinical placental pathology and fetal autopsy. In settings where human resources are especially limited, the ability to conduct a thoughtful autopsy and having a keen eye for identifying gross placental lesions are key elements of perinatal pathology, and training health workers to develop these skills should be prioritized. In settings where histopathology is not available, even weights, measurements, and a thorough gross examination can be critically important, and on occasions sufficient to identify the potential cause of fetal or neonatal death (10).
In conclusion, we provide one example of a successful perinatal pathology research program in sSA that could serve as a model for others, increasing perinatal pathology capacity for both clinical and research applications. Furthermore, we will strive to continue our collaborative partnership for many years to come, building further capacity in clinical diagnostics to improve pregnancy and child health outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Mbarara University of Science and Technology Institutional Review Committee, Mbale Regional Referral Hospital Research Ethics Committee, Partners Healthcare, and Pennsylvania State University College of Medicine Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
LB: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, writing—original draft, and writing—review and editing. JN: conceptualization, data curation, investigation, methodology, project administration, resources, supervision, validation, and writing—review and editing. FM: conceptualization, data curation, histopathology slide interpretation, formal analysis, investigation, methodology, project administration, supervision, and writing—review and editing. CKC: conceptualization, data curation, histopathology slide interpretation, supervision, and writing—review and editing. AB and RA: data curation, methodology, project administration, supervision, validation, and writing—review and editing. VK: conceptualization, supervision, and writing—review and editing. DR: conceptualization, data curation, histopathology slide interpretation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, and writing—review and editing. All authors agreed to be accountable for the content of the work.
This work was supported by the Harvard University Center for AIDS Research National Institutes of Health/National Institute of Allergy and Infectious Diseases (grant no. P30AI060354 to LB), a KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst | The Harvard Clinical and Translational Science Center (grant no. KL2TR002542 to LB), the Charles H. Hood Foundation (to LB), a career development award from the National Institute of Allergy and Infectious Diseases (grant no. K23AI138856 to LB), the American Society of Tropical Medicine and Hygiene Burroughs Wellcome Postdoctoral Fellowship in Tropical Infectious Diseases (to LB), and a Seed Global Health volunteer stipend (to FM). The sponsors had no role in study design, data collection, analysis or interpretation, writing the report, or decision to submit the article for publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, the National Institutes of Health, or other funders.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to the study participants and to the Mbarara Regional Referral Hospital (MRRH) Maternity Staff, Mbarara University of Science and Technology, MUST Pathology Laboratory, and MRRH ISS Clinic for their partnership in this research. We are also grateful to Seed Global Health for building training capacity, Massachusetts General Hospital Global Health, and all other individuals who donated supplies, contributed to training, and assisted with capacity-building efforts.
1. Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet. (2014) 384:189–205. doi: 10.1016/s0140-6736(14)60496-7
2. Tiruneh D, Assefa N, Mengiste B. Perinatal mortality and its determinants in sub Saharan African countries: systematic review and meta-analysis. Matern Health Neonatol Perinatol. (2021) 7:1. doi: 10.1186/s40748-020-00120-4
3. Ismail MR, Noormahomed EV, Lawicki S, Eichbaum Q. Survey of clinical and anatomic pathology laboratory infrastructure in mozambique. Am J Clin Pathol. (2021) 156:810–7. doi: 10.1093/ajcp/aqab026
4. Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. (2000) 92:35–43. doi: 10.1016/s0301-2115(00)00423-1
5. Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front Physiol. (2018) 9:1091. doi: 10.3389/fphys.2018.01091
6. Gaccioli F, Lager S. Placental nutrient transport and intrauterine growth restriction. Front Physiol. (2016) 7:40. doi: 10.3389/fphys.2016.00040
7. Konkel L. Lasting impact of an ephemeral organ: the role of the placenta in fetal programming. Environ Health Perspect. (2016) 124:A124–9. doi: 10.1289/ehp.124-A124
8. Melamed N, Baschat A, Yinon Y, Athanasiadis A, Mecacci F, Figueras F, et al. FIGO (international federation of gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet. (2021) 152(Suppl. 1):3–57. doi: 10.1002/ijgo.13522
9. Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. (2016) 140:698–713. doi: 10.5858/arpa.2015-0225-CC
10. Roberts DJ. Perinatal pathology: practice suggestions for limited-resource settings. Arch Pathol Lab Med. (2013) 137:775–81. doi: 10.5858/arpa.2011-0560-SA
11. Adesina A, Chumba D, Nelson AM, Orem J, Roberts DJ, Wabinga H, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. (2013) 14:e152–7. doi: 10.1016/S1470-2045(12)70598-3
12. Madhi SA, Briner C, Maswime S, Mose S, Mlandu P, Chawana R, et al. Causes of stillbirths among women from South Africa: a prospective, observational study. Lancet Glob Health. (2019) 7:e503–12. doi: 10.1016/S2214-109X(18)30541-2
13. Roberts DJ. Perinatal pathology: practice suggestions for limited-resource settings. Arch Pathol Lab Med. 2013; 137(6):775–81. doi: 10.5858/arpa.2011-0560-SA
14. Bebell LM, Parks K, Le MH, Ngonzi J, Adong J, Boatin AA, et al. Placental decidual arteriopathy and vascular endothelial growth factor A (VEGF-A) expression among women with and without HIV. J Infect Dis. (2021) 224(12 Suppl. 2):S694–700. doi: 10.1093/infdis/jiab201
15. Bebell LM, Siedner MJ, Ngonzi J, Le MH, Adong J, Boatin AA, et al. Brief report: chronic placental inflammation among women living with HIV in Uganda. J Acquir Immune Defic Syndr. (2020) 85:320–4. doi: 10.1097/qai.0000000000002446
16. Wylie B, Matechi E, Kishashu Y, Fawzi W, Premji Z, Coull B, et al. Placental pathology associated with household air pollution in a cohort of pregnant women from Dar es Salaam, Tanzania. Environ Health Perspect. (2016) 125:134–40.
17. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
18. Pinar H, Sung CJ, Oyer CE, Singer DB. Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med. (1996) 16:901–7. doi: 10.1080/15513819609168713
19. Dolatshahi S, Butler AL, Siedner MJ, Ngonzi J, Edlow AG, Adong J, et al. Altered maternal antibody profiles in women with HIV drive changes in transplacental antibody transfer. Clin Infect Dis. (2022) ciac156. doi: 10.1093/cid/ciac156
20. Hayward CE, Lean S, Sibley CP, Jones RL, Wareing M, Greenwood SL, et al. Placental adaptation: what can we learn from birthweight:placental weight ratio? Front Physiol. (2016) 7:28. doi: 10.3389/fphys.2016.00028
21. Ikumi NM, Matjila M, Gray CM, Anumba D, Pillay K. Placental pathology in women with HIV. Placenta. (2021) 115:27–36. doi: 10.1016/j.placenta.2021.09.006
22. Lema G, Mremi A, Amsi P, Pyuza JJ, Alloyce JP, Mchome B, et al. Placental pathology and maternal factors associated with stillbirth: an institutional based case-control study in Northern Tanzania. PLoS One. (2020) 15:e0243455. doi: 10.1371/journal.pone.0243455
23. Salafia CM, Pezzullo JC, López-Zeno JA, Simmens S, Minior VK, Vintzileos AM. Placental pathologic features of preterm preeclampsia. Am J Obstet Gynecol. (1995) 173:1097–105. doi: 10.1016/0002-9378(95)91333-5
24. Roberts DJ, Njuguna HN, Fields B, Fligner CL, Zaki SR, Keating MK, et al. Comparison of minimally invasive tissue sampling with conventional autopsy to detect pulmonary pathology among respiratory deaths in a resource-limited setting. Am J Clin Pathol. (2019) 152:36–49. doi: 10.1093/ajcp/aqz016
25. Montgomery ND, Tomoka T, Krysiak R, Powers E, Mulenga M, Kampani C, et al. Practical successes in telepathology experiences in Africa. Clin Lab Med. (2018) 38:141–50. doi: 10.1016/j.cll.2017.10.011
26. Seymour DJL, Graef KM, Iliyasu Y, Diomande MIJM, Jaquet S, Kelly M, et al. Pathology training for cancer diagnosis in Africa. Am J Clin Pathol. (2022) 157:279–85. doi: 10.1093/ajcp/aqab131
27. Elliott A, Nerima B, Bagaya B, Kambugu A, Joloba M, Cose S, et al. Capacity for science in sub-Saharan Africa. Lancet. (2015) 385:2435–7. doi: 10.1016/S0140-6736(15)61111-4
28. Stalsberg H, Adjei EK, Owusu-Afriyie O, Isaksen V. Sustainable development of pathology in sub-Saharan Africa: an example from Ghana. Arch Pathol Lab Med. (2017) 141:1533–9. doi: 10.5858/arpa.2016-0498-OA
Keywords: Uganda, placenta, fetus, histopathology, histology, outcomes, pregnancy, stillbirth
Citation: Bebell LM, Ngonzi J, Meier FA, Carreon CK, Birungi A, Kerry VB, Atwine R and Roberts DJ (2022) Building Perinatal Pathology Research Capacity in Sub-Saharan Africa. Front. Med. 9:958840. doi: 10.3389/fmed.2022.958840
Received: 01 June 2022; Accepted: 24 June 2022;
Published: 08 July 2022.
Edited by:
Maria Contaldo, University of Campania L. Vanvitelli, ItalyReviewed by:
Robert Lukande, Makerere University, UgandaCopyright © 2022 Bebell, Ngonzi, Meier, Carreon, Birungi, Kerry, Atwine and Roberts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa M. Bebell, bGJlYmVsbEBtZ2guaGFydmFyZC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.