- 1Division of Nephrology, University Hospital of the Federal University of Maranhão, São Luís, Brazil
- 2Division of Nephrology, Hospital das Clínicas of the Federal University of Pernambuco, Recife, Brazil
- 3Ribeirão Preto Medical School, University of São Paulo, São Paulo, Brazil
- 4Division of Nephrology, University of São Paulo, São Paulo, Brazil
- 5Nephrology and Dialysis Center, Oswaldo Cruz German Hospítal, São Paulo, Brazil
The respiratory tract is the main infection site for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in many admissions to intensive care centers in several countries. However, in addition to lung involvement, kidney injury caused by the novel coronavirus has proven to be a significant factor related to high morbidity and mortality, alarming experts worldwide. The number of deaths has drastically reduced with the advent of large-scale immunization, highlighting the importance of vaccination as the best way to combat the pandemic. Despite the undeniable efficacy of the vaccine, the renal side effects associated with its use deserve to be highlighted, especially the emergence or reactivation of glomerulopathies mentioned in some case reports. This study aimed to identify the main renal morphological findings correlated with COVID-19 infection and its vaccination, seeking to understand the pathophysiological mechanisms, main clinical features, and outcomes.
Introduction
Since the description of the first report of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019, the upper respiratory tract has been well established to be the main infection site; however, much evidence has demonstrated that other organs, including the heart, liver, and kidneys in addition to the respiratory tract, can be severely affected (1). Acute kidney injury (AKI) caused by the new coronavirus is associated with the severe clinical status of patients and, consequently, a worse prognosis (2). Although the mechanism of injury is not completely understood, it is currently known to go beyond acute tubular necrosis secondary to hemodynamic instability in critically ill patients (2).
The binding of the viral S protein to the angiotensin-converting enzyme 2 (ACE2) receptor on the surface of host cells triggers SARS-CoV-2 infection. These receptors are in large quantities in type II pneumocytes in the lungs, heart, and kidneys. The virus incorporation into the cell occurs when proteins present on the surface of the virus, called “spikes,” bind to ACE2 and are endocytosed by activating transmembrane serine protease type 2 (TMPRSS 2), which starts the intracellular viral replication (3).
Studies from autopsies have shown that the kidneys are a special target organ of the virus, even in patients without a history of kidney disease. This is probably due to the high expression of proteins, such as ACE2, TMPRSS 2, and cathepsin L that enable viral infection (4). Detection of viral fragments in urine by polymerase chain reaction (PCR) was present in 21–50% of infected patients in the second or third week after infection, suggesting the possibility of renal tropism by the virus (5).
The infection course, morbidity, and mortality have changed favorably since the advent of vaccines against the new coronavirus in the last year, drastically reducing the number of deaths. However, with the spread of vaccination, adverse effects of vaccines, including kidney injury, have generated concerns globally (6). Since the beginning of large-scale immunization, the publication of case series of renal diseases with the emergence of new glomerulopathies or reactivation of previous glomerulopathies has increased; however, the related mechanisms, risk factors, and long-term consequences are not yet well established (7).
Search strategy
This review aimed to highlight and describe the main morphological and pathophysiological aspects of kidney injury described in the most recent publications related to SARS-CoV-2 infection and after the administration of vaccines against the new coronavirus. This narrative review was based on a comprehensive literature search on PUBMED/MEDLINE, PUBCOVID19, and GOOGLE SCHOLAR databases. The keywords related to “Glomerular,” “Glomerulopathy,” “Kidney,” “Tubular,” “Proteinuria,” and “COVID-19,” “SARS-CoV-2,” and “SARS-CoV vaccine” were used with Boolean combinations.
Morphological kidney findings associated with COVID-19 infection
Acute kidney injury in patients infected with COVID-19 has proven to be one of the main risk factors for worse prognosis in intensive care units (ICU). Despite studies showing a variation of 0.5–56% in AKI incidence in patients infected with SARS-CoV-2, this high frequency and association with unfavorable outcomes have been consistently reported in studies (8, 9).
Renal involvement ranges from mild proteinuria (44%) and microscopic hematuria (27%) to AKI requiring renal replacement therapy (RRT) (10). Approximately 20% of patients admitted to the ICU required RRT within 15 days after the onset of the disease, but the mechanisms leading to AKI are still not well established (11). Numerous studies have proposed multifactorial etiologies for renal involvement, highlighting hemodynamic instability caused by severe viral infections as the main factor. Other mechanisms, such as the renin-angiotensin-aldosterone system imbalance, dysregulation of the complement system cascade, pro-coagulant status, and release of pro-inflammatory mediators (“cytokine storm”), were also associated (9).
However, some studies have also proposed other etiologies of kidney injury, highlighting the direct viral action on the tubular epithelium and podocyte cells through the ACE2 receptor, causing mitochondrial dysfunction, acute tubular necrosis, and glomerulopathy (11).
Glomerular injury
Regardless of the presence of AKI, glomerular injury is an important cause of renal injury during COVID-19 infection (12). Many studies have been published showing that the most common glomerular injury type is podocytopathies, with collapsing glomerulopathy (CG) being the main cause of nephrotic syndrome associated with virus infection (13). However, it became clear after evaluating a series of biopsied cases in which glomerular involvement comprised a wide spectrum of lesions (Figure 1), including membranous glomerulopathy, minimal lesion disease, immunoglobulin A (IgA) nephropathy, and non-collapsing focal and segmental glomerulopathy (14).
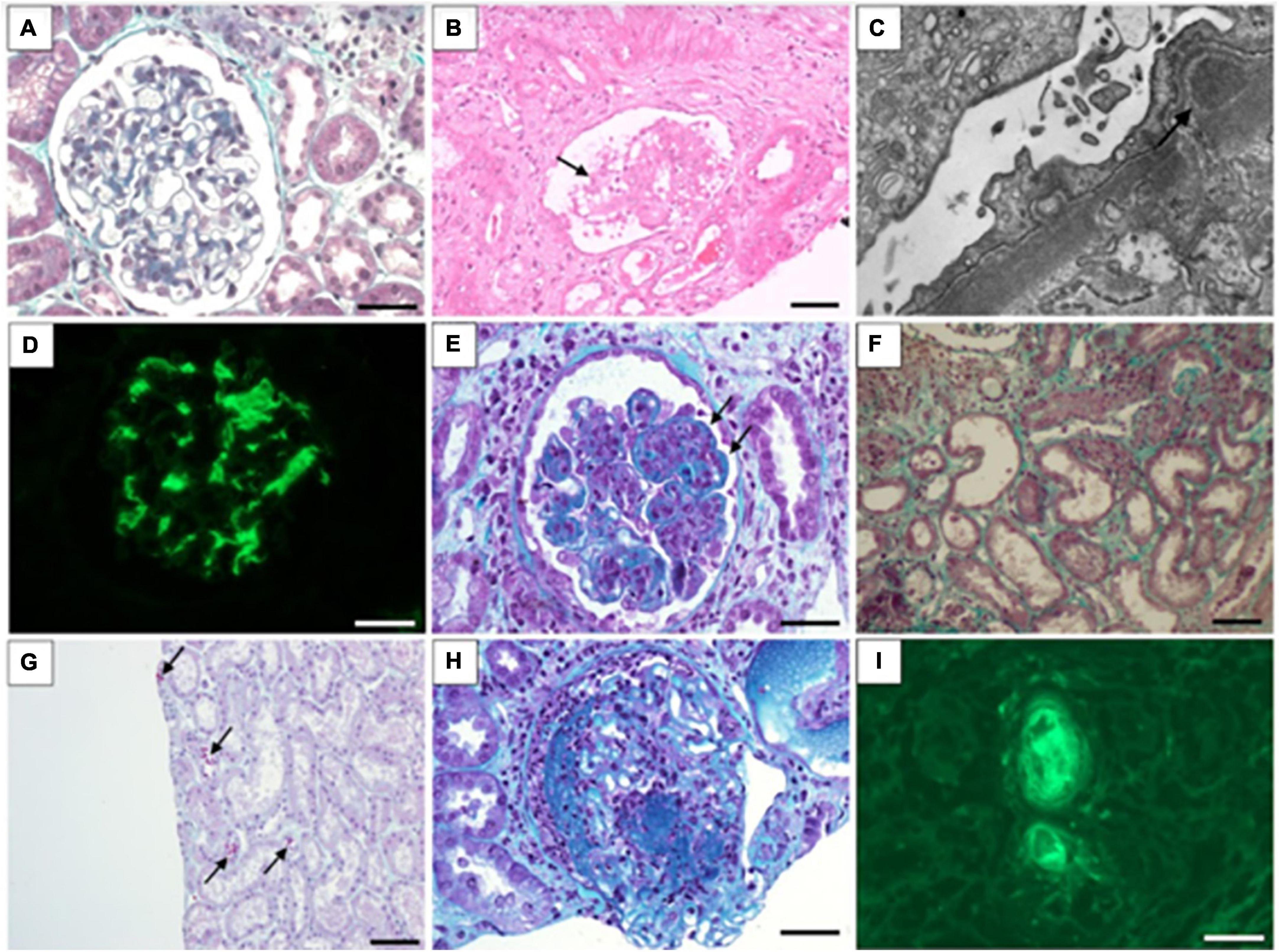
Figure 1. Morphological findings associated with COVID-19 infection in kidney biopsy. (A) Normal glomerulus in minimal change disease (Masson’s trichrome stain); (B) glomerular collapse and podocytes hyperplasia (arrow) in collapsing glomerulopathy (hematoxylin and eosin stain); (C) Subepithelial deposits in membranous nephropathy (electron microscopy); (D) Intense mesangial IgA deposits in IgA nephropathy (immunofluorescence); (E) Endocapilar proliferation and wire-loop hyaline deposits in class IV lupus nephritis (Masson’s trichrome stain); (F) Flattened tubular epithelial cells in dilated tubules in in acute tubular injury (Masson’s trichrome stain); (G) Ectatic tubules and ducts contain red-orange granular casts caused by rhabdomyolysis (Masson’s trichrome stain); (H) Glomerular necrosis and crescent formation in cases of immune complex-mediated crescentic glomerulonephritis (Masson’s trichrome stain); (I) Fluorescence shows intravascular thrombi in thrombotic microangiopathy (detected with antibody to fibrinogen). (A,D,H,I): Barr = 20 μm; (B,G,F): Barr = 50 μm.
In this sense, a renal biopsy is an essential tool in the context of SARS-CoV-2 infection since it identifies the histological diagnosis of glomerular disease and well as highlights the wide variety of other possible histopathological diagnoses (15).
COVID-associated nephropathy
Collapsing glomerulopathy was first described in the context of human immunodeficiency virus (HIV) infection and was later recognized as “HIV-associated nephropathy” (HIVAN). Subsequent studies have shown that the presence of the high-risk genotype for APOL-1 (HRG-APOL1) in African American individuals significantly increased the risk of developing HIVAN by 30–90% (16). It is currently known that other types of viral infections, including parvovirus B19, cytomegalovirus, and Epstein–Barr virus, also increase the risk of developing CG. Some studies have also pointed out that the common factor in CG cases may be the activation of interferon, given the presence of endothelial tubuloreticular inclusions characterized as “interferon footprints” (16).
In the context of COVID-19, many patients are diagnosed with COVID-associated nephropathy (COVAN), particularly those with HRG-APOL1. The presence of this genotype increases the risk of interferon-mediated podocyte injury in the presence of viral infections (8). In a study of 23 patients who presented with CG after SARS-CoV-2 infection, 91% of the patients were black. Furthermore, of the 17 patients who underwent genotyping, 16 (94%) presented HRG-APOL1. In the follow-up, seven patients with COVAN who required RRT managed to stay off dialysis; however, the prognosis regarding proteinuria and chronic kidney disease remained reserved (17).
In a study with six patients of African–American descent infected with the new coronavirus who had AKI and nephrotic proteinuria, the most prevalent diagnosis found in kidney biopsies was CG with extensive effacement of podocyte processes and focal/diffuse acute tubular injury. It should be noted that three of the six patients had HRG-APOL1, and none of them had evidence of viral particles on the biopsy. Thus, validating the hypothesis of the “two hits” combination mechanism–genetic predisposition and cytokine-mediated host response to COVID-19, as an important etiological factor (12).
A multicenter study evaluated 284 renal biopsies (240 native kidneys and 44 grafts) from patients infected with COVID-19 (240 native kidneys and 44 grafts) from March 2020 to March 2021 in the USA, India, and Switzerland. Statistical analyses showed that COVAN was the most prevalent finding, present in 62 (25.8%) patients, among which 91.7% were associated with HRG-APOL1 (18).
Table 1 show native kidney biopsy findings in adult and pediatric patients with COVID-19 from a literature review of case series reports. This review involved multicentric and unicentric studies from the USA, India, Switzerland, Italy, and France. Case reports and small series (< 5 cases) were not included.
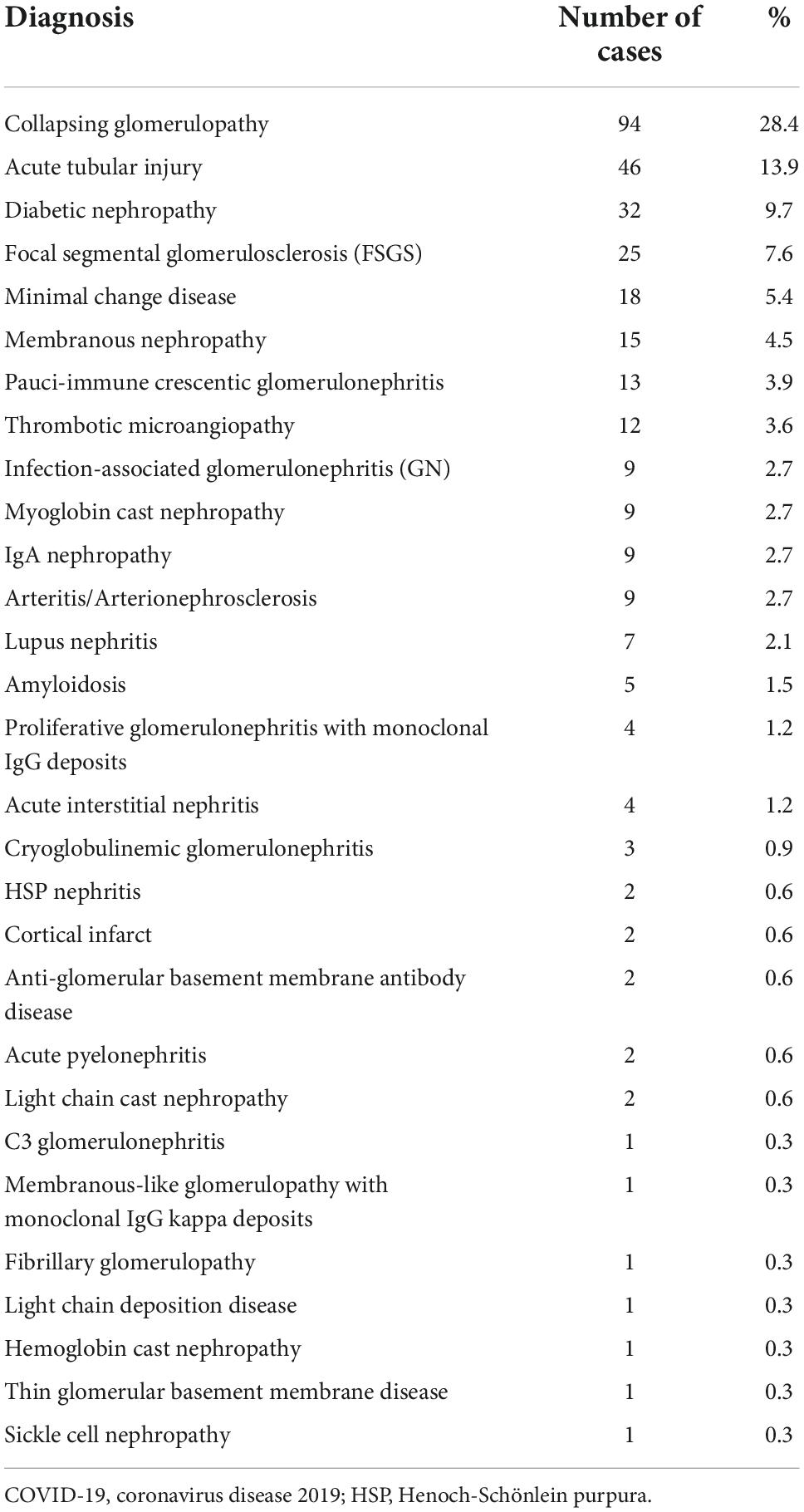
Table 1. Biopsy findings of native kidney in patients with COVID-19: Review of case-series reports (n = 331) (14, 18, 62–67).
Membranous nephropathy
Few cases of membranous nephropathy (MN) in the context of the COVID-19 pandemic have been described to date. In a multicenter study carried out in three countries, only 11 (4.6%) of 240 native kidney biopsies showed MN, four of them with positive phospholipase A2 receptor (PLA2R) (18). Kudose et al. detected MN in five (6.6%) of 76 infected patients, and PLA2R was positive in two of them (17). In one reported case, a patient presented MN PLA2R positive 4 weeks after diagnosis of mild COVID-19, with nephrotic syndrome, AKI and partial response to immunosuppressive treatment (19).
However, PLA2R was negative in most MN cases associated with COVID-19, strengthening the hypothesis that glomerular involvement is secondary to the infectious condition (10, 17, 18). The pathophysiological mechanisms are still unclear; however, MN in the context of SARS-CoV-2 infection may be secondary to an exacerbated immune response against the virus (10). Most experts propose postponing immunosuppression in cases of MN without changes in renal function or other complications; nevertheless, there is still little data on the clinical course and outcomes related to MN in the context of COVID-19 (19).
Immunoglobulin A nephropathy
IgAN is the most common glomerular disease worldwide (20). Some cases of COVID-19-associated IgAN have been reported in the literature since the pandemic began. Huang et al. reported a case of a 65-year-old woman with dark urine, renal dysfunction, and proteinuria after COVID-19 infection, with biopsy-proven IgAN and complete recovery after 3 days of glucocorticoids and angiotensin II receptor blockers therapy (21). Another study reported the case of a patient with AKI, nephrotic proteinuria and hematuria 3 weeks after COVID-19 infection. Renal biopsy was compatible with acute IgA-dominant infection-associated glomerulonephritis, and virus was detected in renal tissue with immunohistochemistry assay (22). In two large series evaluating kidney biopsies from patients with COVID-19, the frequency of IgAN was similar, being diagnosed in 2.6 and 2.9% of cases (17, 18). Apparently, the inflammatory environment and the “cytokine storm” provided by SARS-CoV-2 infection work as triggers in predisposed individuals (22).
Minimal change disease
In a multicenter study by May et al. only 11 patients were diagnosed with minimal change disease (MCD), corresponding to 4.6% of the evaluated cases (18). Yamada et al. (23) reported a case of MCD-like podocytopathy in a 49-year-old patient who had undergone a kidney transplant 25 years earlier. The patient developed nephrotic syndrome and worsened renal function after the COVID-19 infection. Intense effacement of podocyte processes with microvillous transformation was revealed by electron microscopy. After treatment with glucocorticoid and an angiotensin II receptor inhibitor, clinical improvement was observed, but with persistently elevated proteinuria for up to 6 weeks (23). Although the exact mechanism of MCD is not known, it is possible that the pathogenesis is related to T-lymphocyte activation and cytokine release triggered by viral infection. In a study that evaluated glomerulopathies during COVID-19, MCD was present in one case of 17 patients (14); this was the first case described in the literature of MCD associated with HRG-APOL-1 in a COVID-19 patient and revealed the presence of “interferon footprints,” demonstrating the importance of the role of cytokine-mediated podocyte injury in predisposed individuals.
Lupus nephritis
Previous studies have demonstrated a strong association between viral infections with mimicry, such as Epstein–Barr virus, cytomegalovirus, parvovirus B19, and HIV, and the emergence or reactivation of systemic lupus erythematosus (SLE) (24). The mechanism of SARS-CoV-2 infection inducing lupus nephritis may be related to the triggering of an intense immune response with the massive release of inflammatory cytokines such as interferon-gamma, tumor necrosis factor-alpha, interleukin-2 (IL-2), IL-6, IL-7, and IL-10, associated with the production of autoantibodies such as anti-cyclic citrullinated peptide antibody and antinuclear factor antibodies (25).
In the case series reported by Kudose et al. one case (7.14%) case of lupus nephritis (class IV + V) was detected (17). In contrast, May et al. detected six cases of lupus nephritis, corresponding to 2.5% of the native renal biopsy results (18); among these, three, two, and one were characterized as sclerotic lupus nephritis, membranous lupus nephritis, and minimal mesangial lupus nephritis (lupus podocytopathy), respectively. In a study published by Zamani et al. a patient diagnosed with SLE and lupus nephritis class I after COVID-19 underwent pulse therapy with glucocorticoids for 3 days plus monthly infusions of cyclophosphamide and daily oral prednisone (25). The patient was discharged with improvement in symptoms, proteinuria, and normalization of anti-DNA levels after 6 months.
Pauci-immune crescentic glomerulonephritis
Crescentic glomerulonephritis was not described among the 14 biopsies from patients with COVID-19 reported in a study published by Kudose et al. (17). However, in a multicentric publication by May et al. (18) 11 crescentic glomerulonephritis cases were found, eight of which were positive for antineutrophilic cytoplasmic antibody (ANCA) (18). Crescentic glomerulonephritis results from a wide range of disease associated with immune dysregulation (26). In a study published by Uppal et al. (27), two patients were diagnosed with pauci-immune crescentic glomerulonephritis (PICGN) a few days to 2 weeks after COVID-19 infection. Both patients received a glucocorticoid pulse followed by an infusion of rituximab after a negative reverse transcription-PCR for SARS-CoV-2 and showed improved renal function and symptoms after the first month of rituximab doses (27).
One of the mechanisms proposed to explain the emergence of this subtype of glomerular injury would be related to the uremic state associated “cytokine storm,” which could lead to an inadequate response in the face of an infectious condition, culminating in an ANCA-associated vasculitis (26, 27). Another related mechanism would be that the host’s factors predispose to certain types of renal pathologies due to a “second hit” (assuming SARS-CoV-2 infection) (27).
Injuries of tubulo-interstitial compartment
Acute tubular injury was the principal diagnosis in six of 17 patients with COVID-19 who underwent biopsy in a series of cases including native kidneys and allografts. Four patients had exposure to potentially nephrotoxic drugs and one patient had rhabdomyolysis with pigmented cast (14). In these cases, the etiology of kidney injury is multifactorial and complex, including sepsis, hypoxia, hemodynamic instability, nephrotoxin exposure, and multiorgan complications (14).
The kidney tissue is rich in ACE2 receptors and is characterized as one of the main targets of infection by the new coronavirus. The main site of these receptors in the kidneys is the apical membrane brush border of the proximal tubules and, to a lesser extent, in podocytes (9).
Direct cytotoxic action of the virus in the tubules has already been described as the main etiology among the probable mechanisms of tubular injury, resulting in mitochondrial dysfunction, acute tubular necrosis, tubular proteinuria, and hematuria (14). Another mechanism presented as a cause of tubular dysfunction in COVID-19 infection is acute interstitial nephritis, which, despite not being a rare cause of AKI, remains poorly recognized and diagnosed in the context of viralus infection. The main proposed mechanisms are be the direct action of the virus on the tubules and indirect action secondary to medications or other factors associated with the virus (5). In contrast, despite the most recent findings, viral particles were not directly detected in the renal tissue in a large multicenter study of 284 patients who underwent renal biopsy, supporting the hypothesis that the lesion has a multifactorial etiology (18).
Rhabdomyolysis has also been described as a possible cause of AKI in patients infected with COVID-19, associated with the need for RRT and high mortality (28, 29). Despite the multifactorial etiology, systemic muscle damage caused by the direct action of the virus, “cytokine storm,” and the hypoxemia environment seem to be the main causes (18, 28–30). Muscle injury triggers the release of large amounts of myoglobin-containing heme pigment, which can obstruct the tubular lumen, culminating in acute tubular necrosis (30).
Although the main hypothesis for the etiology of tubular damage is ischemia secondary to shock, e studies have demonstrated the presence of acute tubular necrosis in the absence of hemodynamic compromise or severe pulmonary alterations. Therefore, the hypothesis that the tubular lesion is predominantly ischemic does not seem to cover all cases with acute tubular necrosis, presenting the possibility of direct viral cytotoxic action as the main mechanism in some cases (31).
Vascular injury
Numerous cases have shown that the infection with the new coronavirus can lead to a prothrombotic inflammatory state, culminating in arterial or venous thrombosis with diverse clinical manifestations and outcomes (32, 33). Reports of stroke, AKI, and systemic and coronary vasculitis in COVID-19 have increased (34). The coagulopathy scenario generated by COVID-19 is usually evidenced by changes in tests, such as prothrombin time and D-dimer and fibrinogen serum levels.
The mechanism associated with a prothrombotic inflammatory state is possibly related to endothelial injury secondary to the activation of macrophages and monocytes and the release of inflammatory mediators, culminating in platelet activation, thrombin generation, and fibrin clot formation (9, 33). Another proposed mechanism to justify the procoagulant state is the activation of the complement system, causing significant damage to the microvasculature. There are also reports of strong evidence of systemic thrombophilia and microvascular injury associated with elevated plasma levels of C5d and endothelial deposits of C5b-9 in patients with COVID-19 (34).
The presence of endothelial dysfunction, activation of the coagulation cascade, and microcirculation thrombosis in the kidneys may be risk factors for AKI (9). The prevalence of thrombotic alterations in renal tissue was also described in a multicenter study, in which five cases of thrombotic microangiopathy were described among 240 native kidney biopsies analyzed (18). There is a correlation between COVID-19 infection and systemic vasculitis with different patterns; however, the mechanisms have not yet been well established. Thrombosis cases in the pulmonary vascular vessels, distal to the alveolar capillary bed, which work with a “clot filter,” may not be secondary to the systemic microembolism scenario but to a similar scenario as the vasculitis related to COVID-19, with repercussions on various organs, including the kidneys (32–34).
In a series of seven autopsies of patients who died from COVID-19 found cases of fibrin-rich microthrombi in scattered peritubular capillaries, thrombotic microangiopathy with large platelet-rich microthrombi, and microhemorrhage in the interstitium, as well as virions in proximal convoluted tubules and podocytes (35).
Comorbidities and severity of SARS-CoV-2 infection at the time of kidney biopsy
Most patients (70%) were symptomatic and had a moderate-to-severe disease at the time of kidney biopsy, and comorbid diseases were very common (85%). Therefore, these conditions can be considered risk factors for developing kidney injuries described above. Moderate-to-severe infections refer to infections requiring hospitalization, supplemental oxygen, intensive care, mechanical ventilation, and dialysis. The most frequent comorbidities were hypertension, diabetes mellitus, and obesity.
Morphological findings in renal involvement post COVID-19 vaccine
More than 8.2 billion COVID-19 vaccine doses have been administered globally to contain the contamination and mortality curve of the new coronavirus, resulting in a substantial reduction in the number of cases and deaths in several countries (36). Although adverse renal effects are rare in the context of COVID-19 vaccination, some cases of adverse effects in various organs, including the kidneys, have been reported and have been of concern to nephrologists (37).
It is well established that the immune response generated by the most diverse vaccines, such as vaccines against meningococcus C and B virus, influenza, and diphtheria-tetanus-pertussis (DTP), is a potential trigger for developing or reactivating nephrotic syndrome (36–40). However, in the most recently published studies related to post-vaccination conditions against COVID-19, in addition to nephrotic conditions, other forms of renal involvement have been reported, such as acute tubular necrosis and thrombotic microangiopathies (Table 1). In this sense, the nephrological community and other specializations remain vigilant regarding the evolution of cases of patients with post-vaccine involvement to better understand the mechanisms and associated outcomes (7, 39).
Glomerular injury
Although all vaccines against the new coronavirus are related to glomerular conditions, most studies have indicated that vaccines based on messenger ribonucleic acid (RNA) (Pfizer-BioNTech BNT162b2 and Moderna mRNA1273) are the most prevalent (38). The characteristics of post-vaccination COVID-19 kidney injury, based on the literature review, are summarized in Table 2.
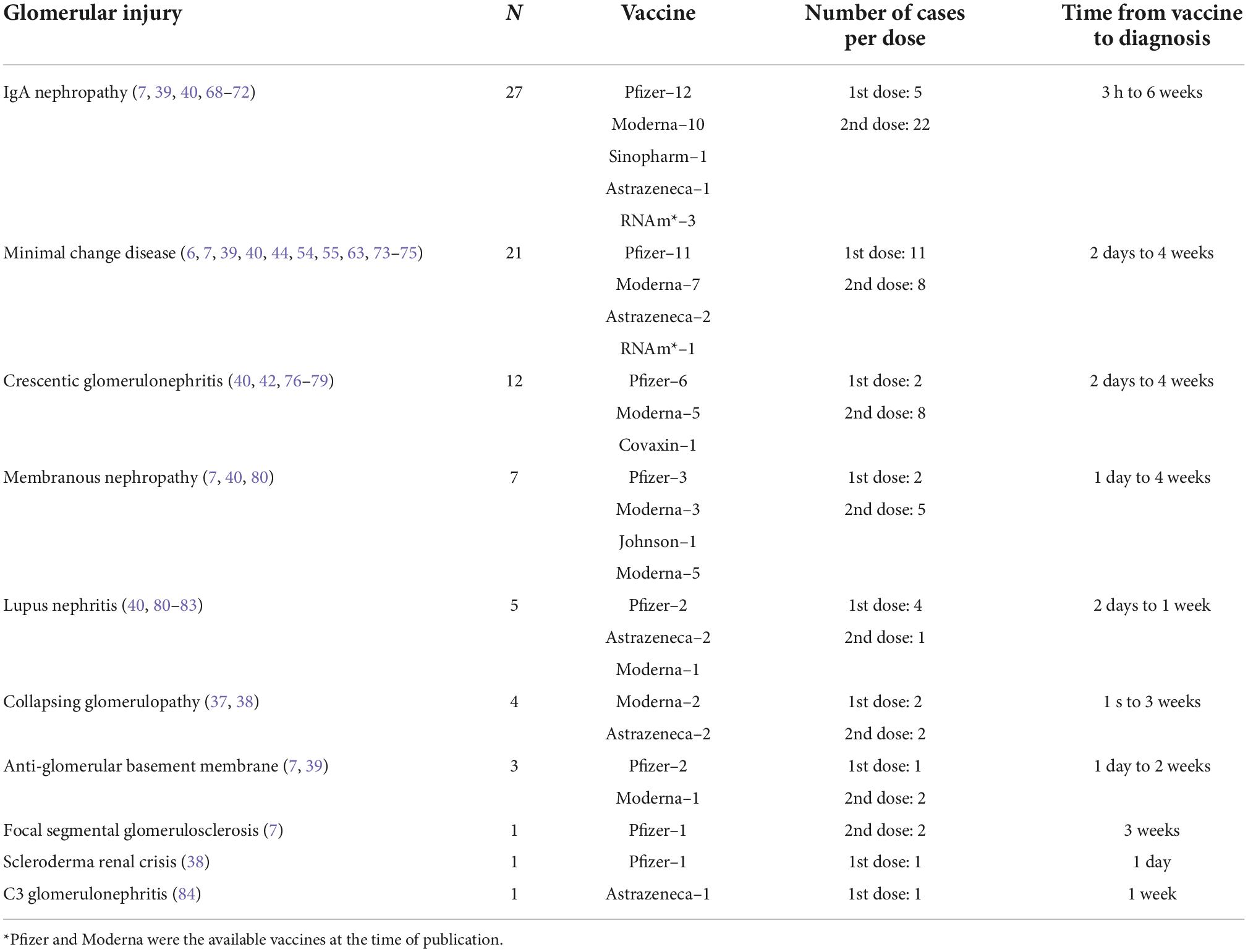
Table 2. Characteristics of post-vaccination COVID-19 kidney injury based on literature review (n = 82).
In a series of published cases with 13 patients who developed kidney damage after using a messenger-RNA-based vaccine, eight (62%) patients presented with newly diagnosed glomerulopathy, and five (38%) presented with reactivation of previous conditions. In this study, the most common glomerulopathy was IgAN (38%), followed by MN (23%) and podocytopathies (23%) (7). In another case series, patients who received messenger RNA-related (27) and adenovirus (2) vaccines showed changes in renal function and glomerular syndrome after 1 month. The main findings in the biopsies were IgAN (10), MCD (7), CG (2), crescentic glomerulopathy (6), MN (3), and lupus nephritis (1) (38). The most frequent presentation of glomerular injury post-COVID-19 vaccine was AKI with nephrotic or nephritic syndrome and gross hematuria, followed by nephritic syndrome and nephrotic syndrome with preserved renal function (7, 38).
The onset of glomerulonephritis usually occurs after 3 weeks of immunization, most of which occur within the first week (37). The activation time of IgAN was 1–2 days after receiving the 2nd dose of Pfizer-BioNTech BNT162b2 and Moderna mRNA1273, while MCD usually appeared on the 7th day after the 1st dose, suggesting a direct effect of immunization on the emergence of these two diseases (38). Furthermore, the work published by D’Agati et al. corroborates the hypothesis of the direct action of vaccines on the glomerulus since there is a strong temporal association between receipt of the vaccine and the onset of symptoms, suggesting a rapid response mediated by T-cells as a trigger for podocyte lesions (41).
Most patients with post-vaccine glomerulopathy were treated with immunosuppression according to histopathological diagnosis. In one case series, twenty-seven patients were treated with immunosuppression and followed up. Eight of them recovered renal function completely, five showed partial recovery, and fourteen did not improve, and five of them required hemodialysis (38). In another study, ten of thirteen patients were followed, nine of whom received immunosuppression. Eight patients responded to treatment (six with immunosuppression and two with conservative treatment) (7).
Vasculitis
Vaccine involvement in ANCA-related vasculitis may be systemic or limited to the renal system (39). In a review of 29 cases of ANCA-related vasculitis after COVID-19 vaccination, 24 cases were diagnosed after the vaccine, and the remaining cases had a recurrence or worsening of pre-existing vasculitis (40). Cases occurred 2–37 days after immunization, with the majority being associated with the RNA vaccine. The most frequent antibody was myeloperoxidase-ANCA (15), but proteinase 3-ANCA (3), double positivity (3), and anti-glomerular basement membrane were also found (40); approximately 50% occurred after the first dose and 50% after the second dose, and it could even occur after both doses (39, 40).
Kidney injury was present in 93% of the vaccine-associated vasculitis cases. The most common histopathologic findings were crescentic glomerulonephritis and fibrinoid necrosis without endocapillary proliferation and deposits on immunofluorescence (40). There was also a reported case of myeloperoxidase-vasculitis and rhabdomyolysis after Pfizer-BioNTech COVID-19 mRNA vaccination, whose biopsy demonstrated PICGN in addition to a severe acute tubular lesion with myoglobin cast and interstitial inflammation (42).
The exact mechanism of COVID vaccine-associated vasculitis is not fully understood. In addition to molecular mimicry mechanisms, RNA vaccines can lead to aberrant activation of the innate and acquired immune systems that, especially in genetically predisposed individuals, can serve as a basis for triggering autoimmune diseases (41–43). Regarding the inactivated vaccines, there may also be an induction of autoimmunity associated with the immune response to SARS-CoV-2 proteins (44). Most cases of COVID-19 vaccine-associated vasculitis tended to respond to immunosuppression as per the usual vasculitis treatment (42–45).
Rhabdomyolysis
Myalgia is one of the most common side effects associated with different types of COVID-19 vaccines and is often mild and self-limiting (46). More severe cases requiring hospitalization, including myositis and rhabdomyolysis, were less frequent (47, 48). This observation may lead to the belief that reported cases of vaccine-related rhabdomyolysis are underestimated.
A few cases of rhabdomyolysis have been reported, most of which are associated with mRNA vaccines (47–49). However, other vaccines, such as ChAdOx1nCoV-19 (AstraZeneca) and Ad26.COV2.S (Johnson & Johnson) have also been associated with this complication (50, 51). The clinical presentation can vary from mild manifestations without renal dysfunction (48) to severe manifestations with AKI, RRT, compartment syndrome, and death (49, 52, 53). The time between vaccination and symptom onset can vary from 1 to 14 days (50, 51, 53, 54).
Rhabdomyolysis secondary to vaccination has been reported previously, mainly following influenza vaccination (55). The mechanisms associated with this type of adverse effect of the COVID vaccine are not well understood, with some reported cases having potentially confounding factors such as statin use and previous neuromuscular disease (47, 53). However, this may arise from an exaggerated immune response to adjuvants, possibly potentiated by prior exposure to the COVID-19 virus (46, 54). Clinicians should be aware of the possibility of rhabdomyolysis as a complication of COVID-19 vaccination because, in such cases, early diagnosis and intervention, including vigorous hydration and elimination of factors that potentiate AKI, may be crucial for a better prognosis (56).
Vaccination recommendations in patients with immune-mediated renal diseases
Despite the adverse effects related to vaccination against the new coronavirus, immunization remains the main tool to control the number of new cases and mortality. Patients with glomerulopathies and proteinuria may be at a higher risk of severe infections, mainly because of the loss of immunoglobulins in the urine; it is important to use available prevention measures (57). However, more studies specific to immune-mediated kidney disease populations are needed.
Consideration should be given to patients’ current disease status and the use of immunosuppression. It is known that patients on immunosuppressants, such as B cell–depleting agents, mycophenolate mofetil, and glucocorticoids, may have reduced humoral response to the vaccine (57–60). For example, if rituximab is used, it may be necessary to delay vaccination for up to 6 months after stopping this medication to allow B-cell reconstitution and maximize vaccine response (59, 60).
Despite the greater number of activated or reactivated glomerular diseases being related to vaccines based on messenger RNA, conclusive data are still lacking for the non-recommendation of these vaccines to the detriment of others (38). For the pediatric population, an mRNA vaccine is recommended considering age restrictions for adenovirus-vectored vaccines and immunosuppression (61). It is also recommended that signs of relapse be monitored after vaccination and that treatment should follow the usual recommendations for the underlying disease (61).
However, available recommendations and data on the relationship between COVID-19 vaccines and kidney lesions are still scarce. Since this is a new disease whose vaccines were developed and applied only in late 2020, the current information is based on case reports and case series. Although case reports are useful for pharmacovigilance and are the first source of evidence for detecting adverse events related to drugs and vaccines, this type of scientific information alone is insufficient to establish a definitive causal relationship between the vaccine and kidney lesions. When analyzing these cases according to Bradford Hill’s causality criteria, temporality, coherence, plausibility, and analogy can be observed. Consistency can also be considered due to repeated events observed in different locations and circumstances. However, not all criteria have been met to date and cannot be used to establish causal relationship.
Conclusion
Renal involvement caused by COVID-19 has a strong impact on the evolutionary course of the disease, resulting in higher morbidity and mortality rates. This study aimed to elucidate the main forms of renal involvement in the context of SARS-CoV-2 infection, as well as the morphological findings and probable pathophysiological mechanisms involved. The main renal changes were listed in patients who received doses of the most diverse vaccinations against COVID-19. However, despite the aforementioned findings, mass vaccination has proven to be safe in the most diverse studies, constituting the main means of controlling new cases and reducing hospitalization and deaths, especially in the population with chronic kidney diseases.
Author contributions
IP, NF, GS, and PN: conceptualization. IP, DC, and GS: methodology. IP, GS, and PN: data curation and writing–original draft preparation. IP, DC, DS, NF, GS, and PN: writing–review and editing. DC, GS, and PN: supervision. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Rede EBSERH and CAPES.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Drain PK. Rapid diagnostic testing for SARS-CoV-2. N Engl J Med. (2022) 386:264–72. doi: 10.1056/NEJMcp2117115
2. Musah S. Uncovering SARS-CoV-2 kidney tropism. Nat Rev Mol Cell Biol. (2021) 22:509. doi: 10.1038/s41580-021-00370-w
3. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. (2020) 382:1653–9. doi: 10.1056/NEJMsr2005760
4. Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. (2020) 383:590–2. doi: 10.1056/NEJMc2011400
5. Ng JH, Zaidan M, Jhaveri KD, Izzedine H. Acute tubulointerstitial nephritis and COVID-19. ClinKidney J. (2021) 14:2151–7. doi: 10.1093/ckj/sfab107
6. Lebedev L, Sapojnikov M, Wechsler A, Varadi-Levi R, Zamir D, Tobar A, et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. (2021) 78:142–5. doi: 10.1053/j.ajkd.2021.03.010
7. Klomjit N, Alexander MP, Fervenza FC, Zoghby Z, Garg A, Hogan MC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep. (2021) 6:2969–78. doi: 10.1016/j.ekir.2021.09.008
8. Kant S, Menez SP, Hanouneh M, Fine DM, Crews DC, Brennan DC, et al. The COVID-19 nephrology compendium: AKI, CKD, ESKD and transplantation. BMC Nephrol. (2020) 21:449. doi: 10.1186/s12882-020-02112-0
9. Hassanein M, Radhakrishnan Y, Sedor J, Vachharajani T, Vachharajani VT, Augustine J, et al. COVID-19 and the kidney. Cleve Clin J Med. (2020) 87:619–31. doi: 10.3949/ccjm.87a.20072
10. Miao J, Fidler ME, Nasr SH, Larsen CP, Zoghby ZM. Membranous nephropathy in a patient with coronavirus disease 2019 (COVID-19): a case report. Clin Nephrol Case Stud. (2021) 9:11–8. doi: 10.5414/CNCS110379
11. Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. (2020) 8:738–42. doi: 10.1016/S2213-2600(20)30229-0
12. Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 High-risk genotype. J Am Soc Nephrol. (2020) 31:1688–95. doi: 10.1681/ASN.2020050558
13. Sethi S, D’Costa MR, Hermann SM, Nasr SH, Fervenza FC. Immune-complex glomerulonephritis after COVID-19 infection. Kidney Int Rep. (2021) 6:1170–3.
14. Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. (2020) 31:1959–68. doi: 10.1681/ASN.2020060802
15. Moeinzadeh F, Dezfouli M, Naimi A, Shahidi S, Moradi H. Newly diagnosed glomerulonephritis during COVID-19 infection undergoing immunosuppression therapy, a case report. Iran J Kidney Dis. (2020) 14:239–42.
16. Velez JCQ, Caza T, Larsen CP. COVAN is the new HIVAN: the re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol. (2020) 16:565–7. doi: 10.1038/s41581-020-0332-3
17. Kudose S, Santoriello D, Bomback AS, Sekulic M, Batal I, Stokes MB, et al. Longitudinal outcomes of COVID-19-associated collapsing glomerulopathy and other podocytopathies. J Am Soc Nephrol. (2021) 32:2958–69. doi: 10.1681/ASN.2021070931
18. May RM, Cassol C, Hannoudi A, Larsen CP, Lerma EV, Haun RS, et al. A multi-center retrospective cohort study defines the spectrum of kidney pathology in Coronavirus 2019 Disease (COVID-19). Kidney Int. (2021) 100:1303–15. doi: 10.1016/j.kint.2021.07.015
19. Guo W, Tan PH, Baikunje S. Membranous nephropathy in a patient with COVID-19 infection. J Nephrol. (2022) 35:351–5. doi: 10.1007/s40620-021-01165-0
21. Huang Y, Li XJ, Li YQ, Dai W, Shao T, Liu WY, et al. Clinical and pathological findings of SARS-CoV-2 infection and concurrent IgA nephropathy: a case report. BMC Nephrol. (2020) 21:504. doi: 10.1186/s12882-020-02163-3
22. Pérez A, Torregrosa I, D’Marco L, Juan I, Terradez L, Solís MA, et al. IgA-dominant infection-associated glomerulonephritis following SARS-CoV-2 infection. Viruses. (2021) 13:587. doi: 10.3390/v13040587
23. Yamada M, Rastogi P, Ince D, Thayyil A, Adela Mansilla M, Smith RJH, et al. Minimal change disease with nephrotic syndrome associated with coronavirus disease 2019 after apolipoprotein L1 risk variant kidney transplant: a case report. Transplant Proc. (2020) 52:2693–7. doi: 10.1016/j.transproceed.2020.08.012
24. Lythgoe H, Lj M, Hedrich CM, Aringer M. Classification of systemic lupus erythematosus in children and adults. Clin Immunol. (2022) 234:108898. doi: 10.1016/j.clim.2021.108898
25. Zamani B, MoeiniTaba SM, Shayestehpour M. Systemic lupus erythematosus manifestation following COVID-19: a case report. J Med Case Rep. (2021) 15:29. doi: 10.1186/s13256-020-02582-8
26. Antonelou M, Evans RDR, Henderson SR, Salama AD. Neutrophils are key mediators in crescentic glomerulonephritis and targets for new therapeutic approaches. Nephrol Dial Transplant. (2022) 37:230–8. doi: 10.1093/ndt/gfaa206
27. Uppal NN, Kello N, Shah HH, Khanin Y, De Oleo IR, Epstein E, et al. De novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. (2020) 5:2079–83.
28. Haroun MW, Dieiev V, Kang J, Barbi M, Marashi Nia SF, Gabr M, et al. Rhabdomyolysis in COVID-19 patients: a retrospective observational study. Cureus. (2021) 13:e12552. doi: 10.7759/cureus.12552
29. Han X, Ye Q. Kidney involvement in COVID-19 and its treatments. J Med Virol. (2021) 93:1387–95. doi: 10.1002/jmv.26653
30. de Oliveira P, Cunha K, Neves P, Muniz M, Gatto G, Salgado Filho N, et al. Renal morphology in coronavirus disease: a literature review. Medicina (Kaunas). (2021) 57:258. doi: 10.3390/medicina57030258
31. Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, et al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. (2020) 98: 228–31.
32. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. (2020) 194:101–15.
33. Song WC, FitzGerald GA. COVID-19, microangiopathy, hemostatic activation, and complement. J Clin Invest. (2020) 130:3950–3. doi: 10.1172/JCI140183
34. McGonagle D, Bridgewood C, Ramanan AV, Meaney JFM, Watad A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. (2021) 3:e224–33. doi: 10.1016/S2665-9913(20)30420-3
35. Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. (2020) 25:100434. doi: 10.1016/j.eclinm.2020.100434
36. Chan ATP, Tang SCW. De novo and relapsing glomerulonephritis after COVID-19 vaccination: how much do we know? Nephrology (Carlton). (2022) 27:5–6. doi: 10.1111/nep.14013
37. Neves PD, Caires RA, Guimarães MP, Costalonga EC, Cavalcante LB, Costa E Silva VT, et al. Collapsing glomerulopathy following SARS-CoV-2 adenovirus-vector-based vaccine: report of 2 cases. Kidney Int. (2022) 101:637–9. doi: 10.1016/j.kint.2021.12.016
38. Li NL, Coates PT, Rovin BH. COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation? Kidney Int. (2021) 100:959–65. doi: 10.1016/j.kint.2021.09.002
39. Izzedine H, Bonilla M, Jhaveri KD. Nephrotic syndrome and vasculitis following SARS-CoV-2 vaccine: true association or circumstantial? Nephrol Dial Transplant. (2021) 36:1565–9. doi: 10.1093/ndt/gfab215
40. Caza TN, Cassol CA, Messias N, Hannoudi A, Haun RS, Walker PD, et al. Glomerular disease in temporal association to SARS-CoV-2 vaccination-a series of 29 cases. Kidney360. (2021) 2:1770–80. doi: 10.34067/KID.0005372021
41. D’Agati VD, Kudose S, Bomback AS, Adamidis A, Tartini A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. (2021) 100:461–3.
42. Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. (2021) 78:611–3. doi: 10.1053/j.ajkd.2021.06.016
43. Prabhahar A, Naidu G, Chauhan P, Sekar A, Sharma A, Sharma A, et al. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol Int. (2022) 42:749–58. doi: 10.1007/s00296-021-05069-x
44. Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol. (2021) 224:108665. doi: 10.1016/j.clim.2021.108665
45. Hakroush S, Tampe B. Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and pauci-immune crescentic glomerulonephritis after Pfizer-BioNTech COVID-19 mRNA vaccination. Front Immunol. (2021) 12:762006. doi: 10.3389/fimmu.2021.762006
46. Patel R, Kaki M, Potluri VS, Kahar P, Khanna D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum Vaccin Immunother. (2022) 18:2002083.
47. Theodorou DJ, Theodorou SJ, Axiotis A, Gianniki M, Tsifetaki N. COVID-19 vaccine-related myositis. QJM. (2021) 114:424–5. doi: 10.1093/qjmed/hcab043
48. Nassar M, Chung H, Dhayaparan Y, Nyein A, Acevedo BJ, Chicos C, et al. COVID-19 vaccine induced rhabdomyolysis: case report with literature review. Diabetes Metab Syndr. (2021) 15:102170. doi: 10.1016/j.dsx.2021.06.007
49. Mack M, Nichols L, Guerrero DM. Rhabdomyolysis secondary to COVID-19 vaccination. Cureus. (2021) 13:e15004.
50. Gelbenegger G, Cacioppo F, Firbas C, Jilma B. Rhabdomyolysis following Ad26.COV2.S COVID-19 vaccination. Vaccines (Basel). (2021) 9:956. doi: 10.3390/vaccines9090956
51. Huang ST, Lee TJ, Chen KH, Sun HY, Chen WT, Hsieh SC, et al. Fatal myositis, rhabdomyolysis and compartment syndrome after ChAdOx1 nCoV-19 vaccination. J Microbiol Immunol Infect. (2022):S1684–1182(22)00057–3. doi: 10.1016/j.jmii.2022.04.003 [Epub ahead of print].
52. Tan A, Stepien KM, Narayana STK. Carnitine palmitoyltransferase II deficiency and post-COVID vaccination rhabdomyolysis. QJM. (2021) 114:596–7. doi: 10.1093/qjmed/hcab077
53. Banamah TA, Bogari AA, Neyazi A, Kotbi E, Almaghraby H, Atwah F. Severe rhabdomyolysis complicated with acute kidney injury required renal replacement therapy after Pfizer COVID-19 vaccine. Cureus. (2022) 14:e25199. doi: 10.7759/cureus.25199
54. Al-Rasbi S, Al-Maqbali JS, Al-Farsi R, Al Shukaili MA, Al-Riyami MH, Al Falahi Z, et al. Myocarditis, pulmonary hemorrhage, and extensive myositis with rhabdomyolysis 12 days after first dose of Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine: a case report. Am J Case Rep. (2022) 23:e934399. doi: 10.12659/AJCR.934399
55. Callado RB, Carneiro TG, Parahyba CC, Lima Nde A, da Silva Junior GB, Daher Ede F. Rhabdomyolysis secondary to influenza A H1N1 vaccine resulting in acute kidney injury. Travel Med Infect Dis. (2013) 11:130–3. doi: 10.1016/j.tmaid.2012.11.004
56. Unger K, Ponte CD, Anderson D. A possible case of COVID-19 booster vaccine-associated rhabdomyolysis and acute kidney injury. J Pharm Technol. (2022) 38:247–50. doi: 10.1177/87551225221093944
57. Shoenfeld Y, Agmon-Levin N. ‘ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. (2011) 36:4–8. doi: 10.1016/j.jaut.2010.07.003
58. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis – an overview for clinicians. Crit Care. (2005) 9:158–69. doi: 10.1186/cc2978
59. Ogi M, Yokoyama H, Tomosugi N, Hisada Y, Ohta S, Takaeda M, et al. Risk factors for infection and immunoglobulin replacement therapy in adult nephrotic syndrome. Am J Kidney Dis. (1994) 24:427–36. doi: 10.1016/s0272-6386(12)80899-7
60. Stevens KI, Frangou E, Shin JIL, Anders HJ, Bruchfeld A, Schönermarck U, et al. Perspective on COVID-19 vaccination in patients with immune-mediated kidney diseases: consensus statements from the ERA-IWG and EUVAS. Nephrol Dial Transplant. (2022) 37:1400–10. doi: 10.1093/ndt/gfac052
61. Morello W, Vianello FA, Proverbio E, Peruzzi L, Pasini A, Montini G. COVID-19 and idiopathic nephrotic syndrome in children: systematic review of the literature and recommendations from a highly affected area. Pediatr Nephrol. (2022) 37:757–64. doi: 10.1007/s00467-021-05330-2
62. Ferlicot S, Jamme M, Gaillard F, Oniszczuk J, Couturier A, May O, et al. The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria. Nephrol Dial Transplant. (2021) 12:gfab042.
63. Gambella A, Barreca A, Biancone L, Roccatello D, Peruzzi L, Besso L, et al. Spectrum of kidney injury following COVID-19 disease: renal biopsy findings in a single Italian pathology service. Biomolecules. (2022) 12:298. doi: 10.3390/biom12020298
64. Nasr SH, Alexander MP, Cornell LD, Herrera LH, Fidler ME, Said SM, et al. Kidney biopsy findings in patients with COVID-19, kidney injury, and proteinuria. Am J Kidney Dis. (2021) 77:465–8.
65. Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, et al. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. (2020) 31:1948–58. doi: 10.1681/ASN.2020050699
66. Nomura E, Finn LS, Bauer A, Rozansky D, Iragorri S, Jenkins R, et al. Pathology findings in pediatric patients with COVID-19 and kidney dysfunction. Pediatr Nephrol. (2022) 15:1–7. doi: 10.1007/s00467-022-05457-w
67. Akilesh S, Nast CC, Yamashita M, Henriksen K, Charu V, Troxell ML, et al. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis. (2021) 77:82–93.e1.
68. Fernández P, Alaye ML, Chiple MEG, Arteaga J, Douthat W, Fuente J, et al. Glomerulopathies after vaccination against COVID-19. Four cases with three different vaccines in Argentina. Nefrologia. (2021): doi: 10.1016/j.nefro.2021.09.003 [Epub ahead of print].
69. Nakatani S, Mori K, Morioka F, Hirata C, Tsuda A, Uedono H, et al. New-onset kidney biopsy-proven IgA vasculitis after receiving mRNA-1273 COVID-19 vaccine: case report. CEN Case Rep. (2022) 25:1–5. doi: 10.1007/s13730-021-00677-9
70. Hanna C, Herrera Hernandez LP, Bu L, Kizilbash S, Najera L, Rheault MN, et al. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. (2021) 100:705–6. doi: 10.1016/j.kint.2021.06.032
71. Kudose S, Friedmann P, Albajrami O, D’Agati VD. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int. (2021) 100:468–9. doi: 10.1016/j.kint.2021.06.011
72. Lo WK, Chan KW. Gross haematuria after mRNA COVID-19 vaccination in two patients with histological and clinical diagnosis of IgA nephropathy. Nephrology (Carlton). (2022) 27:110–1. doi: 10.1111/nep.13992
73. Pran K, Cohen AWS, Weerasinghe N, Vilayur E. Report of two cases of minimal change disease following vaccination for COVID-19. Nephrology (Carlton). (2022) 27:111–2.
74. Psyllaki A, Stavrakaki I, Androvitsanea A, Gakiopoulou H, Petrakis I, Stylianou K. Two cases of glomerular involvement after vaccination against COVID-19: epiphenomenon or causality? Clin Kidney J. (2021) 15:574–5.
75. Park HJ, An WS, Rha SH, Kim SE, Lee SM. Minimal change glomerulonephritis following the second dose of the Moderna COVID-19 vaccine. QJM. (2022) 115:490–1. doi: 10.1093/qjmed/hcac094
76. Sekar A, Campbell R, Tabbara J, Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. (2021) 100:473–4.
77. Dube GK, Benvenuto LJ, Batal I. Antineutrophil cytoplasmic autoantibody-associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int Rep. (2021) 6:3087–9. doi: 10.1016/j.ekir.2021.08.012
78. Kim S, Jung J, Cho H, Lee J, Go H, Lee JH. A child with crescentic glomerulonephritis following SARS-CoV-2 mRNA (Pfizer-BioNTech) vaccination. Pediatr Nephrol. (2022):1–4. doi: 10.1007/s00467-022-05681-4 [Epub ahead of print].
79. Bansal SB, Rana AS, Manhas N, Rana A. Post COVID vaccination (COVAXIN™ -BB152 V) pauci-immune crescentic glomerulonephritis. Indian J Nephrol. (2022) 32:495–7.
80. Tuschen K, Bräsen JH, Schmitz J, Vischedyk M, Weidemann A. Relapse of class V lupus nephritis after vaccination with COVID-19 mRNA vaccine. Kidney Int. (2021) 100:941–4. doi: 10.1016/j.kint.2021.07.019
81. Sekar A. Lupus nephritis flare post Moderna mRNA-1273 coronavirus vaccine. QJM. (2022) 114:882–3. doi: 10.1093/qjmed/hcab284
82. Zavala-Miranda MF, González-Ibarra SG, Pérez-Arias AA, Uribe-Uribe NO, Mejia-Vilet JM. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. (2021) 100:1340–1. doi: 10.1016/j.kint.2021.09.009
83. Kim HJ, Jung M, Lim BJ, Han SH. New-onset class III lupus nephritis with multi-organ involvement after COVID-19 vaccination. Kidney Int. (2022) 101:826–8. doi: 10.1016/j.kint.2022.01.013
Keywords: COVID-19, SARS-CoV-2, acute kidney injury, COVAN, kidney biopsy, vaccination, kidney morphology
Citation: Pacheco ICR, Costa DMN, Sousa DS, Salgado Filho N, Silva GEB and Neves PDMM (2022) Kidney injury associated with COVID-19 infection and vaccine: A narrative review. Front. Med. 9:956158. doi: 10.3389/fmed.2022.956158
Received: 29 May 2022; Accepted: 11 November 2022;
Published: 05 December 2022.
Edited by:
Chan Kam Wa, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Leila Mostafavi, Massachusetts General Hospital and Harvard Medical School, United StatesWilliam Morello, Policlinico di Milano, Italy
Copyright © 2022 Pacheco, Costa, Sousa, Salgado Filho, Silva and Neves. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gyl Eanes Barros Silva, Z3lsZWFuZXNAYWx1bW5pLnVzcC5icg==
 Iago Carvalho Rezende Pacheco
Iago Carvalho Rezende Pacheco Denise Maria do Nascimento Costa
Denise Maria do Nascimento Costa Deborah Serra Sousa
Deborah Serra Sousa Natalino Salgado Filho
Natalino Salgado Filho Gyl Eanes Barros Silva
Gyl Eanes Barros Silva Precil Diego Miranda de Menezes Neves
Precil Diego Miranda de Menezes Neves