
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Med. , 02 September 2022
Sec. Pulmonary Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.950883
 Cristiano Caruso1*
Cristiano Caruso1* Paolo Cameli2
Paolo Cameli2 Elena Altieri3
Elena Altieri3 Maria Aliani4
Maria Aliani4 Pietro Bracciale5
Pietro Bracciale5 Luisa Brussino6
Luisa Brussino6 Maria Filomena Caiaffa7
Maria Filomena Caiaffa7 Giorgio Walter Canonica8,9
Giorgio Walter Canonica8,9 Stefano Centanni10
Stefano Centanni10 Maria D’Amato11
Maria D’Amato11 Stefano Del Giacco12
Stefano Del Giacco12 Fausto De Michele13
Fausto De Michele13 Elide Anna Pastorello14
Elide Anna Pastorello14 Girolamo Pelaia15
Girolamo Pelaia15 Paola Rogliani16,17
Paola Rogliani16,17 Micaela Romagnoli18
Micaela Romagnoli18 Pietro Schino19
Pietro Schino19 Marco Caminati20,21
Marco Caminati20,21 Alessandra Vultaggio22
Alessandra Vultaggio22 Alessandro Zullo23
Alessandro Zullo23 Sara Rizzoli23
Sara Rizzoli23 Silvia Boarino24
Silvia Boarino24 Gianfranco Vitiello25
Gianfranco Vitiello25 Francesco Menzella26†
Francesco Menzella26† Fabiano Di Marco27†
Fabiano Di Marco27†Background: Severe asthma is a heterogeneous inflammatory disease driven by eosinophilic inflammation in the majority of cases. Despite biologic therapy patients may still be sub-optimally controlled, and the choice of the best biologic is a matter of debate. Indeed, switching between biologics is common, but no official guidelines are available and real-world data are limited.
Materials and methods: In this post hoc analysis of the Italian, multi-center, observational, retrospective study, ANANKE. Patients with severe eosinophilic asthma treated with benralizumab were divided in two groups based on history of previous biologic therapy (biologic-experienced [suboptimal response] vs naïve). Baseline clinical and laboratory characteristics were collected in the 12 months prior to benralizumab treatment. Change over time in blood eosinophils, annualized exacerbation rate (AER), asthma control (ACT), lung function and oral corticosteroid (OCS) use following benralizumab initiation were collected in the two groups.
Results: A total of 147 biologic-naïve and 58 biologic-experienced (34 omalizumab, 19 mepolizumab, and 5 omalizumab-mepolizumab) patients were enrolled. Biologic-experienced patients were more likely to be atopic and have a higher AER despite more frequent OCS use. Similar reductions in AER (>90% in both groups), OCS use (≥49% reduction in dosage and ≥41% able to eliminate OCS), ACT improvement (≥7 points gained in 48 weeks) and lung function (≥300 mL of FEV1 improvement in 48 weeks) were observed after benralizumab introduction within the two groups. There were no registered discontinuations of benralizumab for safety reasons.
Conclusion: In this post hoc analysis, patients who were switched to benralizumab because of suboptimal control with a previous biologic therapy were more likely to be atopic and more often treated with omalizumab. Benralizumab is effective in both naïve patients and those previously treated with a biologic.
Severe asthma is a complex and heterogeneous disease that affects 5–10% of patients with asthma (1), and is characterized by the presence of severe exacerbations, systemic corticosteroid use and costs related to healthcare resource utilization (2).
To date, five monoclonal antibodies have been approved for the treatment of asthma, namely omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab. Omalizumab was the first biologic drug approved for severe allergic asthma, defined by elevation of total serum IgE and perennial allergen sensitization (3). Mepolizumab, reslizumab, benralizumab, and dupilumab have since been approved for severe eosinophilic asthma (SEA), one of the most frequent, severe and difficult-to-treat asthma subtypes (4, 5).
Patients are often eligible for multiple biological treatments because of overlapping characteristics (e.g., eosinophilia and sensitization to a perennial allergen). There is a lack of clear guidelines on how to prioritize the best biologics in patients meeting multiple prescribing criteria (6), direct head-to-head comparison trials of all biologics are not available, and indirect meta-analysis has shown discordant non-conclusive results (7). Presence of specific clinical characteristics such as CRSwNP or allergic rhinitis may help clarify patient phenotype and predict clinical response to biologics (8–10). In line with these evidences, a recent ANANKE post hoc analysis confirmed clinical trial data which indicated CRSwNP as the cardinal predictor of benralizumab response (11).
For all of these reasons, the choice of which biologic to prescribe should be individualized for each patient on the basis of many factors, including asthma severity, phenotype, endotype, safety, costs, and expected treatment goals (12).
Once initiated, the effectiveness of a biologic should be evaluated after 4–6 months of treatment in terms of asthma control, exacerbation history, lung function, and other metrics (13). Suboptimal response should ideally be defined as a composite outcome as the reduction of exacerbation rates may not be apparent for more than 1 year after the introduction of a biologic (14). A recent US study using claims data from patients with severe asthma treated with biologics (N = 3,262) showed that roughly 60% of patients were uncontrolled or suboptimally controlled despite biologic treatment (15). In this subgroup of patients, switching from one biologic to another should be carefully evaluated according to characteristics related to disease- (e.g., presence of autoimmunity, elevated eosinophils, and fractional exhaled nitric oxide [FeNO]) and patient-related factors (13, 16).
This post hoc analysis from the ANANKE (chAracterization of ItaliaN severe uncontrolled Asthmatic patieNts Key features when receiving benralizumab in a real-life setting: the observational rEtrospective) study aimed to describe the clinical characteristics and efficacy of benralizumab in terms of asthma exacerbation, asthma control, lung function and oral corticosteroid (OCS) use in patients with severe eosinophilic asthma who were biologic treatment-naïve compared to patients switched from other biologics (omalizumab and mepolizumab) because of suboptimal response (biologic experienced).
The design of the ANANKE study has previously been described (17). In brief, ANANKE (ClinicalTrials.gov Identifier: NCT04272463) is an Italian multi-center, observational, retrospective, cohort study including patients with SEA who started benralizumab therapy as per clinical practice or within the Italian Sampling Program that was activated following benralizumab approval in January 2018 and before reimbursement. Patients were consecutively enrolled between December 2019 and July 2020 at 21 Italian sites. As per the protocol, data collection covered a period of >15 months, i.e., 12 months prior to the index date (initiation of the treatment with benralizumab within the sampling program or per clinical practice) to retrieve a restricted set of clinical data plus at least 3 months between the index date and the enrollment visit. ANANKE was performed in accordance with the principles of the Declaration of Helsinki and the regulations and guidelines governing medical practice and ethics in Italy. Ethical approval was provided by the ethics committees/institutional review boards at each participating site. Each patient signed the informed consent and privacy form. Data were collected from each hospital medical charts according to clinical practice and were entered into the electronic case report form.
The study included patients aged ≥ 18 years at the index date with SEA requiring stable treatment with high doses of inhaled corticosteroids and a long-acting β2-agonist ± additional asthma controller (e.g., LAMA, LTRA, OCS, according to clinicians’ judgment); and who had started benralizumab, receiving at least one injection ≥3 months before enrollment, with hospital medical charts available from the index date. Patients were excluded if during the observation period they received benralizumab in a clinical trial, or participated in studies imposing a specific patient management strategy which did not correspond to the site’s normal clinical practice.
Patients were stratified into two groups: the first group, defined as “naïve,” included patients who previously had not received an asthma biologic treatment; the second group, “biologic-experienced,” comprised patients who had switched from one or more previous biologics because of uncontrolled disease.
The primary objective was to describe the clinical features of naïve and biologic-experienced patients as recorded at the index date and during the 12 months prior to benralizumab treatment. Demographics (age, sex, body mass index [BMI], comorbidities, and smoking status), asthma features (age at diagnosis and duration), laboratory features (blood eosinophil count [BEC] and total serum immunoglobulin E [IgE]), and atopic status (defined as the presence of a perennial allergen sensitization demonstrated by skin prick test) were recorded. Lung function parameters, asthma control [defined by Asthma Control Test [ACT] (18)], and OCS use and dosage were also measured. Exacerbations were analyzed according to annualized exacerbation rates for any exacerbation (defined as a physician diagnosed clinically relevant asthma exacerbation) and severe exacerbations [defined as worsening of asthma that lead to one of the following: (a) use of systemic corticosteroids for 3 days or more or a temporary increase in a stable, background dosage of oral corticosteroids; (b) an emergency department or urgent care visit (<24 h) due to asthma that required systemic corticosteroids; or (c) an inpatient admission to hospital (≥24 h) due to asthma].
The secondary objective was to describe clinical outcomes assessed during benralizumab treatment between the index date and end of observation; when available, data at 16, 24, and 48 weeks after the index date were described. Outcomes included: (1) change over time of BEC; (2) annualized rate of any exacerbation and severe exacerbations during benralizumab treatment; (3) change over time of asthma control; (4) change over time of FEV1; (5) change over time of OCS use and dosage; (6) benralizumab discontinuation and reasons for discontinuation during the observation period. These outcomes were collected and compared in naïve and biologic-experienced patients.
The statistical analysis has previously been described (17). In brief, the analyses were descriptive and carried out using mean, standard deviation (SD), median, interquartile range (IQR), range, and absolute and relative frequencies. No formal hypotheses were pre-specified. The analyses were performed using SAS software v9.4 (SAS Institute, Cary, NC, United States).
Between December 2019 and July 2020, a total of 205 patients were recruited and met the eligibility criteria for the ANANKE study. A total of 147 (71.7%) naïve and 58 (28.3%) biologic-experienced patients were considered evaluable for this post hoc analysis (Table 1). Thirty-four of these patients had been previously treated with omalizumab (58.6%), 19 patients (32.8%) with mepolizumab and 5 patients (8.6%) with omalizumab followed by mepolizumab.
The proportion of male and female patients was well balanced in the biologic-experienced group but females were more common in the naïve group (50 vs. 66%, respectively). Duration of asthma from time of diagnosis was longer in biologic-experienced patients. As expected from a higher number of patients treated with omalizumab before benralizumab, biologic-experienced patients were more likely to be atopic (58.6 vs. 34.7% in the naïve group) with numerically higher serum IgE levels at baseline. As expected, BEC at baseline was lower in the biologic-experienced group (median 500 cells/mm3, IQR = 300–719) when compared to naïve patients (618 cells/mm3, IQR = 440–915). The two groups were otherwise comparable in terms of age, age at diagnosis of asthma, BMI and smoking status.
Lung function based on FEV1, FVC, and FEV1/FVC as well as asthma control (ACT) were impaired (under the normal range) in both groups. A numerically higher rate of any exacerbation (4.34 vs. 3.91 in the naïve patients) and severe exacerbations (1.79 vs. 0.83 in the naïve patients) were present in the biologic-experienced group. A slightly higher percentage of patients in the biologic-experienced group were treated with OCS before benralizumab start (34.5 vs. 22.4% in the naïve group), with a higher prednisone-equivalent daily dosage (mean of 18 vs. 11.3 mg/day in the naïve group). Ten patients in the naïve group (7.1%) and four patients in the biologic-experienced group (7.2%) did not show any exacerbation during the 12 months before benralizumab prescription.
Comorbidities considered to be possibly related to OCS use, such as hypertension, osteoporosis, cataract, anxiety/depression, type 2 diabetes mellitus and cardiovascular disease were numerically more prevalent in the biologic-experienced group when compared to the naïve group (Table 2).
Both groups had a mean ± SD exposure to benralizumab therapy of 10.3 ± 5.0 months and a median [IQR] exposure of 9.8 [6.1–13.9] months after index date.
A near complete depletion of peripheral eosinophils was seen in both groups at the first timepoint (16 weeks), and BEC remained low thereafter, in accordance with the known mechanism of action of benralizumab (Supplementary Figure 1).
The annualized exacerbation rate was consistently reduced after the introduction of benralizumab in both groups (−93.6% reduction in naïve patients and −91.9% reduction in biologic-experienced patients) (Figure 1A). In particular, during the observation period, the percentage of patients without any exacerbations increased from 7.1 to 82.4% in the naïve group and from 7.2 to 80% in the biologic-experienced group of patients. Benralizumab reduced the severe annualized exacerbation rate in naïve and biologic-experienced patients by −94 and −95%, respectively (Figure 1B), and there were no differences between the two groups. At index date biologics-experienced patients seemed more severe than naïve patients as they showed trend toward higher AER, OCS use and lower FEV1.
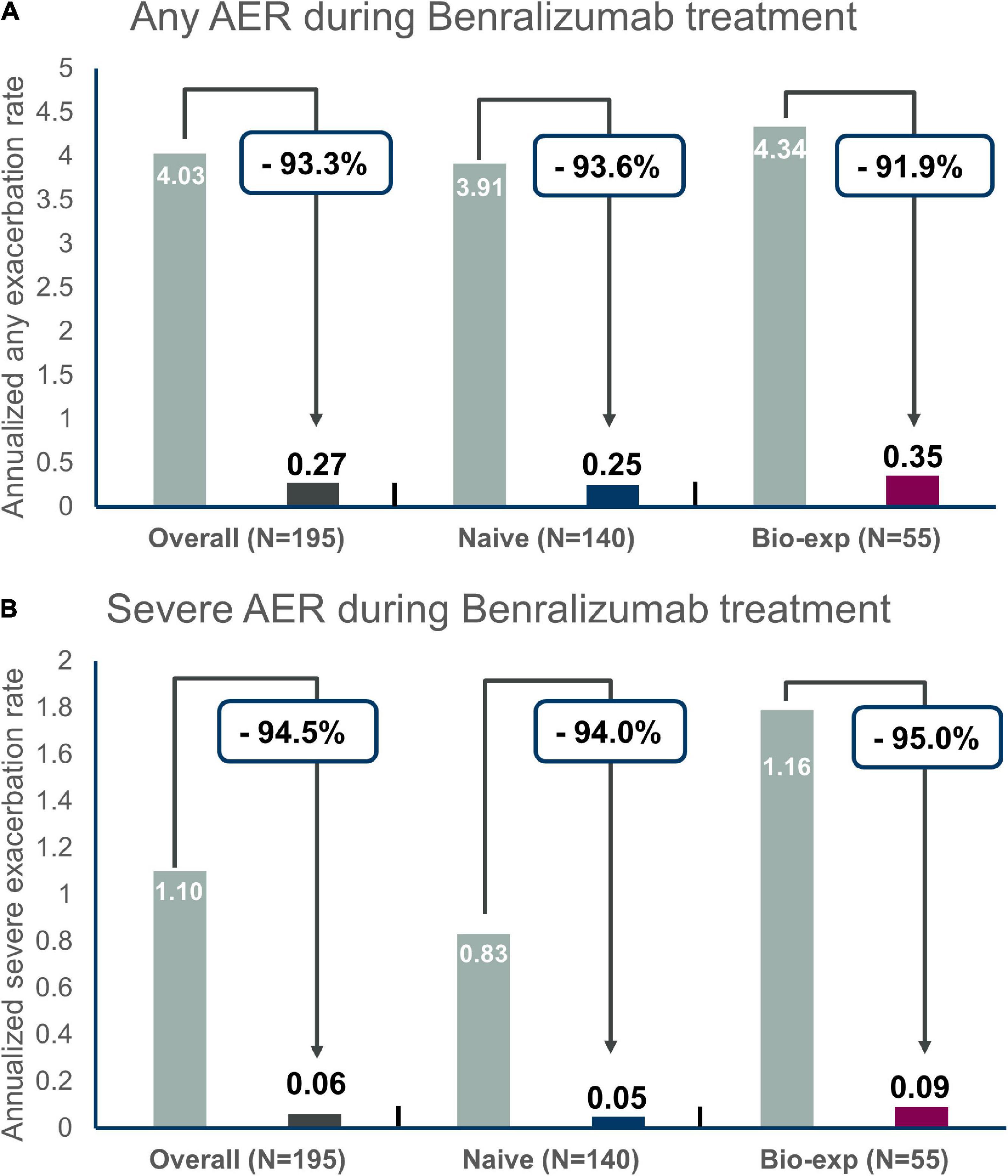
Figure 1. Annualized exacerbation rates (AER) of any severity (A) and for severe exacerbations (B) during benralizumab treatment in the entire population and in naïve severe eosinophilic asthma patients versus biologic-experienced patients.
Improvements in ACT were seen as early as at the first timepoint (16 weeks), reaching a median of 21 points in both groups. The improvement was sustained throughout the other timepoints (24 and 48 weeks) both in naïve (22 points, IQR = 20–24.5) and biologic-experienced patients (21 points, IQR = 20–24 points) (Figure 2). A total of 82% of the naïve patients scored at least 20 points and 77.8% achieved the minimal important difference (MID) of ACT at 48 weeks. Similarly, 79.2% of the biologic-experienced patients presented an ACT of at least 20 points and 73.7% achieved an MID of ACT at Week 48.
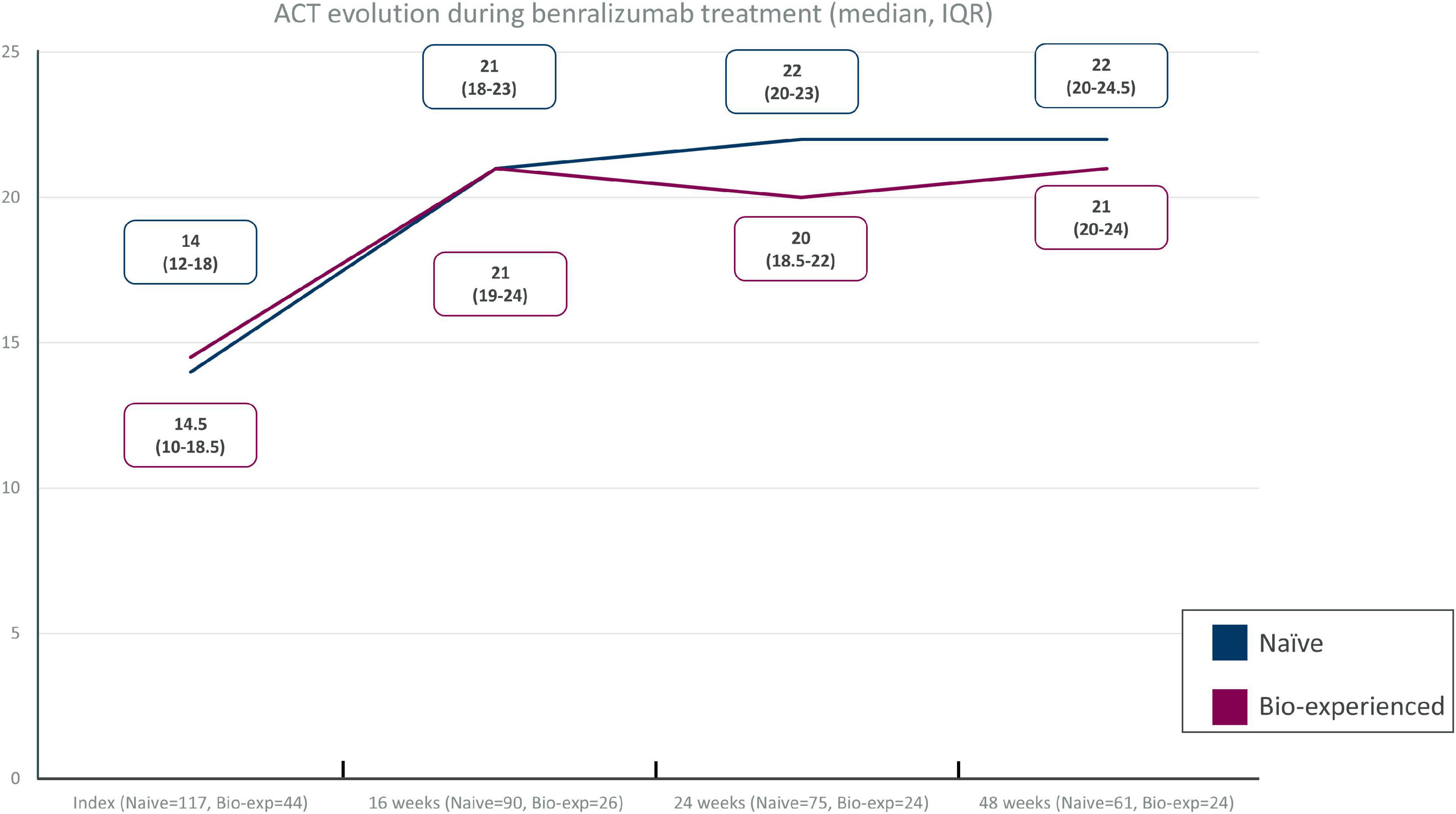
Figure 2. Asthma control test (ACT) improvement in different timepoints in severe eosinophilic asthma (SEA) patients without (naïve) and with (biologic-experienced) previous use of a biologic drug during benralizumab treatment.
Sufficient data for the evaluation of lung function were only available for pre-bronchodilator FEV1 at 16, 24, and 48 weeks during benralizumab treatment in the two groups. Improvement of FEV1 was evident in both groups, with +200 mL gain after 16 weeks of treatment and +400 and +300 mL gain at 48 weeks in the naïve and biologic-experienced group, respectively (Figure 3). Data are not shown for the other lung function parameters described at baseline.
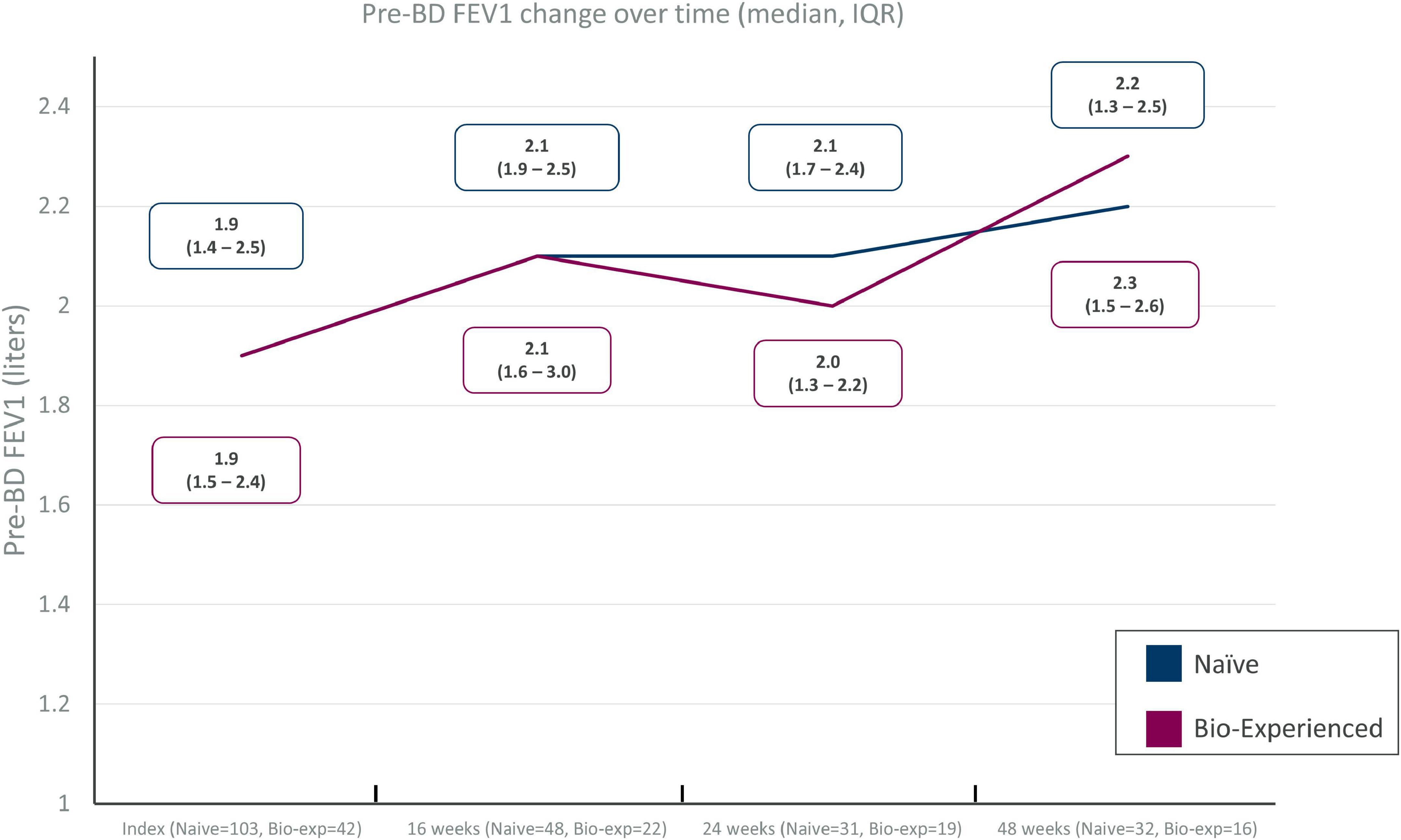
Figure 3. Pre-BD FEV1 change over time in severe eosinophilic asthma (SEA) patients with (biologic-experienced) and without (naïve) previous exposure to a biologic drug during benralizumab treatment.
Data on OCS reduction and elimination during benralizumab treatment was available for 27 out of 33 naïve patients and 17 out of 20 biologic-experienced patients with OCS use at index date (Figure 4 and Table 3).
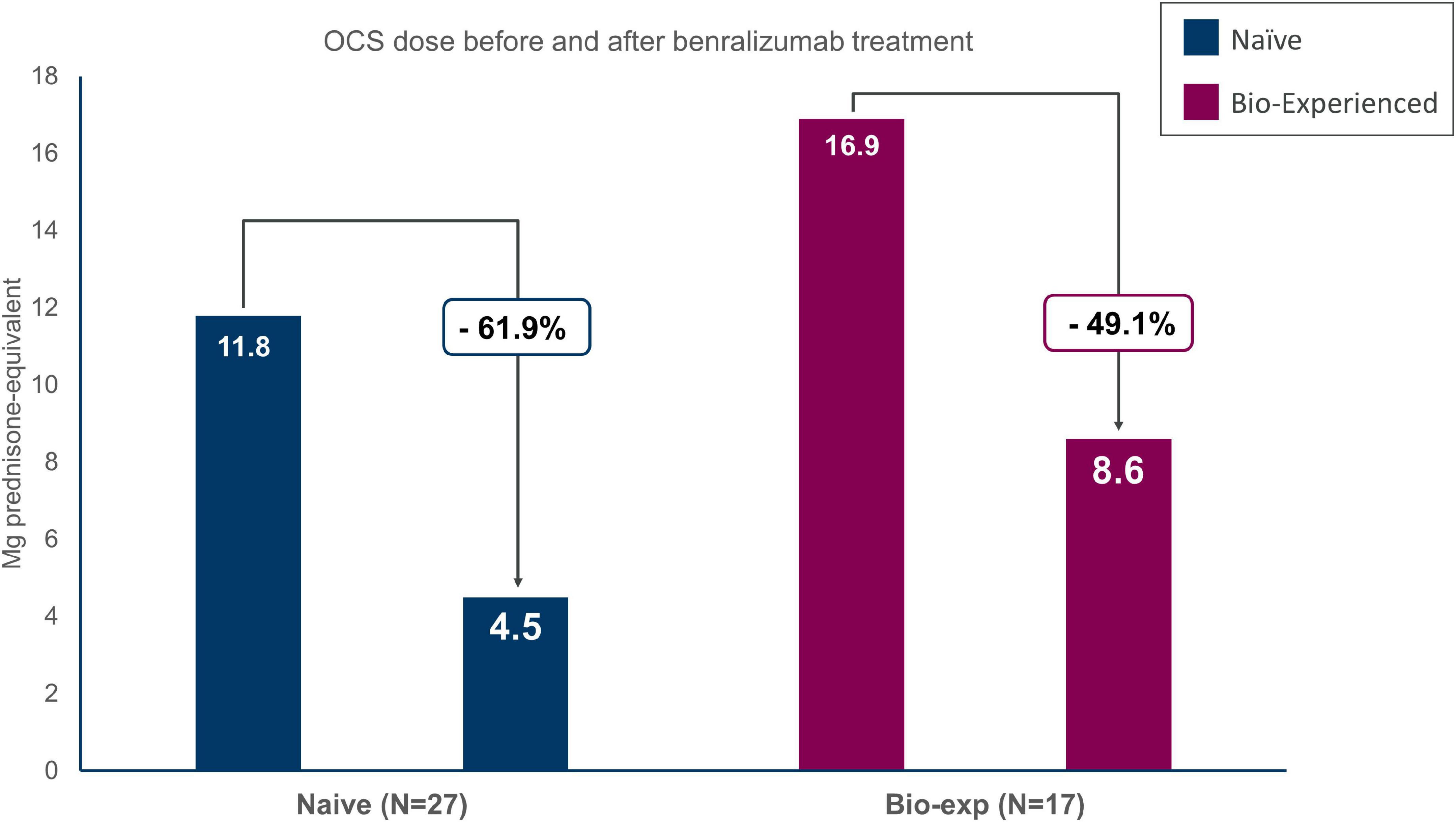
Figure 4. Oral corticosteroids (OCS) sparing effect of benralizumab in severe eosinophilic asthma (SEA) patients with (biologic-experienced) and without (naïve) previous use of a biologic drug. Dose is reported in milligrams of prednisone equivalent.
Naïve patients reduced OCS dosage by 61.9%, decreasing from 11.8 ± 8.5 to 4.5 ± 5.6 mg/day of prednisone-equivalent at the end of the observation period. Forty-one percent of the patients were able to eliminate OCS and 48.1% obtained any reduction of the OCS dosage. Similarly, biologic-experienced patients reduced OCS dose from 16.9 ± 9.1 to 8.6 ± 10.3 mg/day of prednisone-equivalent (−49.1%); 47.1% of the patients being able to completely eliminate OCS at EOB.
Three patients in the naïve group and one patient in the biologic-experienced group discontinued benralizumab during the observation period. Lack of efficacy, physician, or patient decision were the reasons recorded for discontinuation. No discontinuation for safety reasons were registered after index date.
In this post hoc analysis of the real-world ANANKE study (17) we evaluated the clinical characteristics of 205 SEA patients who were either biologic-naïve or switched from another biologic therapy because of suboptimal response. We confirmed the efficacy of benralizumab in all the outcomes evaluated, even in patients who failed a previous biologic therapy (omalizumab or mepolizumab). Benralizumab showed a reduction of over 90% in asthma exacerbations (even severe exacerbations), a reduction in concomitant OCS use (with almost 50% of patients having elimination of OCS), as well as improvements in asthma control amelioration and lung function independent of previous biologic therapy failure.
In this study, nearly 30% of the patients were biologic-experienced at the time of benralizumab prescription, a percentage similar to that recently described in the literature (19–21). More than half of the patients in this study switched from omalizumab to benralizumab, and 8.6% were eligible for omalizumab before switching to mepolizumab and then to benralizumab because of lack of efficacy. Despite previous treatment with omalizumab or mepolizumab, the subgroup of biologic-experienced patients seemed more severe than biologics naïve as they presented higher AER, more OCS dependence and lower FEV1. As expected, the presence of atopy, defined as the presence of sensitization to a perennial allergen along with serum IgE levels (17), was numerically more frequent in the biologic-experienced population. To date, no specific biomarker has been proven to be useful for predicting response to omalizumab in severe allergic asthmatic patients (22). In contrast, eosinophil counts of ≥300 cells/mm3, the presence of nasal polyps, basal corticosteroid use and a late onset of asthma have been found to predict an enhanced response to benralizumab (23).
Switching from omalizumab to an anti-interleukin (IL)-5 biologic therapy has been proven efficacious in ameliorating exacerbation rates and OCS use, as well as improving asthma control and lung function in severe allergic asthma patients with an eosinophilic phenotype (6, 24–28). To date, only one real-world study has been published on the efficacy of benralizumab in severe allergic asthma patients with a BEC of ≥300 cells/mm3 and who had previously received omalizumab (29). In particular, benralizumab was shown to significantly reduce asthma exacerbation rates and OCS use, with parallel improvement in asthma symptom control and lung function, not only versus baseline, but also with respect to omalizumab treatment. These results are most likely related to the specific mechanism of action of benralizumab – antibody-dependent cellular cytotoxicity (ADCC) – which allows almost complete elimination of peripheral blood and tissue eosinophils (30). A direct correlation between eosinophil levels and risk of exacerbations has been observed in allergic patients who had eosinophils levels of 300 cells/mm3 or higher (31). In contrast, no association between serum IgE levels and risk of exacerbation or other asthma outcomes has been evidenced so far (31).
Nineteen patients in this study switched to benralizumab from mepolizumab due to suboptimal control. To date, limited information is available regarding the effectiveness of switching between agents targeting the anti-IL-5/anti-IL-5R pathway (32). A retrospective study by Jackson et al. (33) demonstrated that benralizumab was able to reduce exacerbation rates and OCS use while improving asthma control and lung function in patients sub-optimally controlled with mepolizumab therapy. One-third of the patients did not experience any exacerbations after 1 year of benralizumab treatment, and more than half of the patients completely eliminated OCS use. The limited number of patients in our study precludes us from making specific conclusions about these 19 patients. However, the reduction of exacerbation rate by over 90% is commensurate with the efficacy of benralizumab even in this subgroup of patients.
To date, no clinical differences between responders and non-responders to mepolizumab have been identified that predict subsequent response to benralizumab (33). Three main reasons can potentially explain the efficacy of benralizumab in patients who have failed an anti-IL-5 drug such as mepolizumab. Firstly, benralizumab is the only drug that is able to achieve a near complete depletion of eosinophils both in peripheral blood and tissues, including the airways (30, 34, 35). The phenotyping Mepolizumab EXacerbations in severe eosinophilic asthma (MEX) study revealed that approximately 50% of the exacerbations in patients treated with mepolizumab were eosinophilic in nature, showing persistence of sputum eosinophil levels ≥2% after treatment (36). In these sub-optimally controlled patients, identified by increasing FeNO levels during exacerbations (36), benralizumab should be considered, even during the phenotyping process in biologic-naïve patients (37). Alternatively, switching to anti-IL-4/IL-13 could be considered in this subgroup of patients, but careful monitoring of BEC and clinical parameters are recommended to assess the risk of symptomatic hypereosinophilia and/or evolution toward eosinophilic granulomatosis with polyangiitis (EGPA, former known as Churg-Strauss Syndrome) (38, 39). Secondly, autoimmune features may be present in severe asthma patients (40). In particular, IL-5-anti-IL-5 complement activating immune-complexes and anti-eosinophils peroxidase have been reported to reduce the effectiveness of mepolizumab fixed dose (100 mg every 4 months), but not intravenous mepolizumab 750 mg, reslizumab 3 mg/kg or benralizumab 30 mg every 8 weeks (12, 13, 16, 40, 41). Finally, switching to benralizumab from another biologic of the anti-IL-5 pathway can be adequate when the presence of anti-drug autoantibodies (ADA) has been suspected as the cause of failure (13). No clear factors have been associated with higher ADA risk and, to date, intermittent biologic therapy and re-exposure after a long treatment-free interval may be associated with higher ADA risk (42). The presence of ADA has not been assessed in our study, as a limited number of Centers offer this possibility in real-life.
Few reports of double switching from omalizumab to mepolizumab and then benralizumab are reported in the literature (43). In our study, five patients switched from omalizumab to mepolizumab and then to benralizumab for suboptimal response to the first two biologics. Further analyses are planned for this special subgroup of patients and specific data are not shown in this study because of limited long-term information (e.g., ACT score and FEV1 at 48 weeks).
The use of OCS in SEA patients deserves specific commentary (44). In this study, half of the patients who switched from another biologic eliminated OCS use and an overall reduction of 50% in OCS from the baseline dose was achieved in less than 1 year of follow-up. The particular pharmacodynamic characteristics of benralizumab, such as the unique mechanism of action (near complete eosinophil depletion through ADCC), the action on precursors of eosinophils (45) and the more prominent suppression of the IL-5 axis (46) may account for these effects on patients who failed treatment with omalizumab and mepolizumab.
This study does have several limitations, some of which have already been discussed above. We acknowledge the retrospective nature of the study and the absence of a comparator arm may be limiting, as well as the absence of baseline clinical and laboratory data for switched patients before the introduction of the first biologic.
Moreover, the observational nature of the study did not allow to make any formal statistical hypotheses and the results are descriptive only. However, we think that this study represents the real-world experience on the use of benralizumab in both naïve and biologic-experienced patients, and we believe it could be informative for clinicians in daily clinical practice (47).
This study described the clinical features of patients switching from other biologics to benralizumab, with a numerically higher prevalence of atopy related to the frequency of omalizumab use in our population. Benralizumab has been shown to be effective in naïve patients and biologic-experienced patients in reducing asthma exacerbation and OCS use and improving lung function and asthma control. Previous use of mepolizumab should not be a deterrent for the use of benralizumab in suboptimal responders, likely due to differences in the mechanism of action between the two drugs. Further studies characterizing the clinical profile of patients benefiting the most from biologics in severe asthma are warranted in order to avoid multiple switches between biologics.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The ethical approval was provided by the Ethics Committees/Institutional Review Boards at each participating site. The patients/participants provided their written informed consent to participate in this study.
FM, PC, MA, PB, LB, MFC, CC, SC, MD’A, SD, FDi, FDe, EP, GP, PR, MR, PS, MC, AV, and GC: investigation, resources, and writing – review and editing. AZ and SR: methodology, software, validation, formal analysis, resources, data curation, and writing – review and editing. SB: conceptualization, writing – original draft, writing – review and editing, and project administration. GV: conceptualization, writing – original draft, writing – review and editing, and visualization. All authors contributed to the article and approved the submitted version.
This work was supported by AstraZeneca SpA Italy, which had a role in the study design and in the collection and analysis of data.
We thank all the patients and physicians who participated in this study. We are grateful to Fabio Ferri, Claudio Marchese, Barbara Roncari, and the entire MediNeos team for the support during the design, management, and statistical analysis of the data. Additional medical writing support was provided by the Vicky Hinstridge for InScience Communications, Springer Healthcare Ltd., United Kingdom, and funded by the AstraZeneca.
FM declares research fundings as Principal Investigator by AstraZeneca, Chiesi Farmaceutici, Novartis, Sanofi; fees as speaker/lecturer by AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, Novartis, Sanofi. SC declares grants and/or personal fees from AstraZeneca, Boheringer Ingelheim, Chiesi, Glaxo Smith Kline, Guidotti, Menarini, Novartis, Valeas. SD received grants and/or personal fees from AstraZeneca, Chiesi, Glaxo Smith Kline, Menarini, Novartis. FDi has received lectures fees at national and international meetings and consultancy fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Dompe, Guidotti/Malesci, GlaxoSmithKline, Menarini, Novartis, and Zambon. GP has received lecture fees and consultancy fees from Alfasigma, AstraZeneca, Chiesi, GlaxoSmithKline, Guidotti-Malesci, Menarini, Mundipharma, Novartis, Sanofi, and Zambon. PR has participated as a lecturer, speaker, and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis, her department has received funding from Almirall, Boehringer Ingelheim, Chiesi, Novartis, and Zambon. MR declares grants and personal fees from Boehringer Ingelheim, Roche, AstraZeneca, Novartis, Chiesi, GSK, Menarini, Guidotti, AlfaSigma, and Zambon. AV received payment for lectures and consultant arrangements from Novartis, GlaxoSmithKline, Teva, and AstraZeneca. GC has received grant/research support from Boehringer Ingelheim, ALK, and Stallergenes, and honoraria or consultation fees from Menarini, GSK, Sanofi, Teva, Hal, AstraZeneca, and Novartis. AZ and SR were employed by MediNeos Observational Research – an IQVIA company. SB and GV were employed by AstraZeneca SpA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.950883/full#supplementary-material
1. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. (2014) 43:343–73.
2. Canonica GW, Colombo GL, Bruno GM, Di Matteo S, Martinotti C, Blasi F, et al. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the severe asthma network in Italy (SANI) registry. World Allergy Organ J. (2019) 12:100007. doi: 10.1016/j.waojou.2018.12.001
3. Pelaia G, Vatrella A, Busceti M, Gallelli L, Terracciano R, Maselli R. Anti-IgE therapy with omalizumab for severe asthma: current concepts and potential developments. Curr Drug Targets. (2015) 16:171–8. doi: 10.2174/1389450116666141219122157
4. de Groot JC, ten Brinke A, Bel EHD. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. (2015) 1:00024-2015.
5. Heaney LG, Perez de Llano L, Al-Ahmad M, Backer V, Busby J, Canonica GW, et al. Eosinophilic and noneosinophilic asthma. Chest. (2021) 160:814–30.
6. Carpagnano GE, Pelaia C, D’Amato M, Crimi N, Scichilone N, Scioscia G, et al. Switching from omalizumab to mepolizumab: real-life experience from Southern Italy. Ther Adv Respir Dis. (2020) 14:175346662092923. doi: 10.1177/1753466620929231
7. Bourdin A, Husereau D, Molinari N, Golam S, Siddiqui MK, Lindner L, et al. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: a systematic review. Eur Respir J. (2018) 52:1801393. doi: 10.1183/13993003.01393-2018
8. Nolasco S, Crimi C, Pelaia C, Benfante A, Caiaffa MF, Calabrese C, et al. Benralizumab effectiveness in severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: a real-world multicenter study. J Allergy Clin Immunol Pract. (2021) 9:4371–80.e4. doi: 10.1016/j.jaip.2021.08.004
9. Bagnasco D, Brussino L, Bonavia M, Calzolari E, Caminati M, Caruso C, et al. Efficacy of benralizumab in severe asthma in real life and focus on nasal polyposis. Respir Med. (2020) 171:106080. doi: 10.1016/j.rmed.2020.106080
10. Bakakos A, Loukides S, Usmani OS, Bakakos P. Biologics in severe asthma: the overlap endotype – opportunities and challenges. Expert Opin Biol Ther. (2020) 12:1427–34.
11. D’Amato M, Menzella F, Altieri E, Bargagli E, Bracciale P, Brussino L, et al. Benralizumab in patients with severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: an ANANKE study post-hoc analysis. Front Allergy. (2022) 3:881218. doi: 10.3389/falgy.2022.881218
12. Agache I, Akdis CA, Akdis M, Canonica GW, Casale T, Chivato T, et al. EAACI biologicals guidelines—recommendations for severe asthma. Allergy. (2021) 76:14–44.
13. Pepper AN, Hanania NA, Humbert M, Casale TB. How to assess effectiveness of biologics for asthma and what steps to take when there is not benefit. J Allergy Clin Immunol Pract. (2021) 9:1081–8. doi: 10.1016/j.jaip.2020.10.048
14. Global Initiative for Asthma [GINA]. Difficult-to-treat & severe asthma in adolescent and adult patients: diagnosis and management. (2019). Available online at: https://ginasthma.org/wp-content/uploads/2021/08/SA-Pocket-guide-v3.0-SCREEN-WMS.pdf (accessed May 2022).
15. Reibman J, Tan L, Ambrose C, Chung Y, Desai P, Llanos J-P, et al. Clinical and economic burden of severe asthma among US patients treated with biologic therapies. Ann Allergy Asthma Immunol. (2021) 127:318–25.e2.
16. Mukherjee M, Forero DF, Tran S, Boulay M-E, Bertrand M, Bhalla A, et al. Suboptimal treatment response to anti-IL-5 monoclonal antibodies in severe eosinophilic asthmatics with airway autoimmune phenomena. Eur Respir J. (2020) 56:2000117. doi: 10.1183/13993003.00117-2020
17. Menzella F, Bargagli E, Aliani M, Bracciale P, Brussino L, Caiaffa MF, et al. ChAracterization of ItaliaN severe uncontrolled asthmatic patieNts key features when receiving benralizumab in a real-life setting: the observational rEtrospective ANANKE study. Respir Res. (2022) 23:36. doi: 10.1186/s12931-022-01952-8
18. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test. A survey for assessing asthma control. J Allergy Clin Immunol. (2004) 113:59–65. doi: 10.1016/j.jaci.2003.09.008
19. Numata T, Araya J, Miyagawa H, Okuda K, Fujita Y, Utsumi H, et al. Effectiveness of switching biologics for severe asthma patients in Japan: a single-center retrospective study. J Asthma Allergy. (2021) 14:609–18. doi: 10.2147/JAA.S311975
20. Silver J, Bogart M, Molfino NA, Siddall J, Small M, Hanson M, et al. Factors leading to discontinuation of biologic therapy in patients with severe asthma. J Asthma. (2022) 59:1839–49. doi: 10.1080/02770903.2021.1971700
21. Jackson DJ, Burhan H, Menzies-Gow A, Pfeffer P, Nanzer A, Gil EG, et al. Benralizumab effectiveness in severe asthma is independent of previous biologic use. J Allergy Clin Immunol Pract. (2022) 10:1534–44.e4. doi: 10.1016/j.jaip.2022.02.014
22. Casale TB, Luskin AT, Busse W, Zeiger RS, Trzaskoma B, Yang M, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract. (2019) 7:156–64.e1. doi: 10.1016/j.jaip.2018.04.043
23. Bleecker ER, Wechsler ME, FitzGerald JM, Menzies-Gow A, Wu Y, Hirsch I, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. (2018) 52:1800936. doi: 10.1183/13993003.00936-2018
24. Carpagnano GE, Resta E, Povero M, Pelaia C, D’Amato M, Crimi N, et al. Clinical and economic consequences of switching from omalizumab to mepolizumab in uncontrolled severe eosinophilic asthma. Sci Rep. (2021) 11:5453. doi: 10.1038/s41598-021-84895-2
25. Chapman KR, Albers FC, Chipps B, Muñoz X, Devouassoux G, Bergna M, et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy. (2019) 74:1716–26. doi: 10.1111/all.13850
26. Bagnasco D, Menzella F, Caminati M, Caruso C, Guida G, Bonavia M, et al. Efficacy of mepolizumab in patients with previous omalizumab treatment failure: real-life observation. Allergy. (2019) 74:2539–41. doi: 10.1111/all.13937
27. Liu MC, Chipps B, Munoz X, Devouassoux G, Bergna M, Smith SG, et al. Benefit of switching to mepolizumab from omalizumab in severe eosinophilic asthma based on patient characteristics. Respir Res. (2021) 22:144. doi: 10.1186/s12931-021-01733-9
28. Albers FC, Hozawa S, Bratton DJ, Yancey SW, Prazma CM, Humbert M, et al. Update: mepolizumab treatment in patients with severe eosinophilic asthma and prior omalizumab use. Allergy. (2020) 75:942–6. doi: 10.1111/all.14048
29. Pelaia C, Crimi C, Nolasco S, Carpagnano GE, Brancaccio R, Buonamico E, et al. Switch from omalizumab to benralizumab in allergic patients with severe eosinophilic asthma: a real-life experience from southern Italy. Biomedicines. (2021) 9:1822. doi: 10.3390/biomedicines9121822
30. Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. (2013) 132:1086–96.e5.
31. Jackson DJ, Humbert M, Hirsch I, Newbold P, Garcia Gil E. Ability of serum IgE concentration to predict exacerbation risk and benralizumab efficacy for patients with severe eosinophilic asthma. Adv Ther. (2020) 37:718–29. doi: 10.1007/s12325-019-01191-2
32. Papaioannou AI, Fouka E, Papakosta D, Papiris S, Loukides S. Switching between biologics in severe asthma patients. When the first choice is not proven to be the best. Clin Exp Allergy. (2021) 51:221–7.
33. Kavanagh JE, Hearn AP, D’Ancona G, Dhariwal J, Roxas C, Green L, et al. Benralizumab after sub-optimal response to mepolizumab in severe eosinophilic asthma. Allergy. (2021) 76:1890–3. doi: 10.1111/all.14693
34. Kavanagh JE, Hearn AP, Dhariwal J, D’Ancona G, Douiri A, Roxas C, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest. (2021) 159:496–506.
35. Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti–IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. (2010) 125:1344–53.e2. doi: 10.1016/j.jaci.2010.04.004
36. McDowell PJ, Diver S, Yang F, Borg C, Busby J, Brown V, et al. The inflammatory profile of exacerbations in patients with severe refractory eosinophilic asthma receiving mepolizumab (the MEX study): a prospective observational study. Lancet Respir Med. (2021) 9:1174–84. doi: 10.1016/S2213-2600(21)00004-7
37. Hearn AP, Kavanagh J, D’Ancona G, Roxas C, Green L, Thomson L, et al. The relationship between Feno and effectiveness of mepolizumab and benralizumab in severe eosinophilic asthma. J Allergy Clin Immunol Pract. (2021) 9:2093–6.e1.
38. Eger K, Pet L, Weersink EJM, Bel EH. Complications of switching from anti–IL-5 or anti–IL-5R to dupilumab in corticosteroid-dependent severe asthma. J Allergy Clin Immunol Pract. (2021) 9:2913–5.
39. Spadaro G, Lagnese G, Punziano A, Poto R, Varricchi G, Detoraki A. The immunology of switching biologics in severe eosinophilic asthma patients. J Allergy Clin Immunol Pract. (2021) 9:3528–9.
40. Mukherjee M, Nair P. Autoimmune responses in severe asthma. Allergy Asthma Immunol Res. (2018) 10:428.
41. Bormioli S, Vultaggio A, Nencini F, Comin C, Bercich L, Bezzi M, et al. Benralizumab: resolution of eosinophilic pulmonary vasculitis in a patient with EGPA. J Investig Allergy Clin Immunol. (2021) 31:519–21. doi: 10.18176/jiaci.0689
42. Vultaggio A, Perlato M, Nencini F, Vivarelli E, Maggi E, Matucci A. How to prevent and mitigate hypersensitivity reactions to biologicals induced by anti-drug antibodies? Front Immunol. (2021) 12:765747. doi: 10.3389/fimmu.2021.765747
43. Davison J, Doe S. A patient case demonstrating the efficacy of benralizumab in uncontrolled severe eosinophilic asthma refractory to omalizumab and mepolizumab treatment. Respir Med Case Rep. (2021) 34:101557. doi: 10.1016/j.rmcr.2021.101557
44. Suehs CM, Menzies-Gow A, Price D, Bleecker ER, Canonica GW, Gurnell M, et al. Expert consensus on the tapering of oral corticosteroids for the treatment of asthma. A Delphi study. Am J Respir Crit Care Med. (2021) 203:871–81. doi: 10.1164/rccm.202007-2721OC
45. Menzella F, Biava M, Bagnasco D, Galeone C, Simonazzi A, Ruggiero P, et al. Efficacy and steroid-sparing effect of benralizumab: has it an advantage over its competitors? Drugs Context. (2019) 8:1–11. doi: 10.7573/dic.212580
46. Caruso C, Colantuono S, Tolusso B, Di Mario C, Pentassuglia A, Rumi G, et al. Basophil activation and serum IL-5 levels as possible monitor biomarkers in severe eosinophilic asthma patients treated with anti-IL-5 drugs. Allergy. (2021) 76:1569–71. doi: 10.1111/all.14643
Keywords: severe eosinophilic asthma, switch, benralizumab, observational, biologics
Citation: Caruso C, Cameli P, Altieri E, Aliani M, Bracciale P, Brussino L, Caiaffa MF, Canonica GW, Centanni S, D’Amato M, Del Giacco S, De Michele F, Pastorello EA, Pelaia G, Rogliani P, Romagnoli M, Schino P, Caminati M, Vultaggio A, Zullo A, Rizzoli S, Boarino S, Vitiello G, Menzella F and Di Marco F (2022) Switching from one biologic to benralizumab in patients with severe eosinophilic asthma: An ANANKE study post hoc analysis. Front. Med. 9:950883. doi: 10.3389/fmed.2022.950883
Received: 23 May 2022; Accepted: 10 August 2022;
Published: 02 September 2022.
Edited by:
Amy Klion, National Institutes of Health (NIH), United StatesReviewed by:
Petros Bakakos, Athens Chest Hospital Sotiria, GreeceCopyright © 2022 Caruso, Cameli, Altieri, Aliani, Bracciale, Brussino, Caiaffa, Canonica, Centanni, D’Amato, Del Giacco, De Michele, Pastorello, Pelaia, Rogliani, Romagnoli, Schino, Caminati, Vultaggio, Zullo, Rizzoli, Boarino, Vitiello, Menzella and Di Marco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristiano Caruso, Y2FydXNvY3Jpc3RpYW5vMUBnbWFpbC5jb20=
†These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.