- 1Savaid Stomatology School, Hangzhou Medical College, Hangzhou, China
- 2Department of Prosthodontics, Xi’an Savaid Stomatology Hospital, Xi’an, China
- 3Department of Plastic Surgery, Xijing Hospital, Fourth Military Medical University, Xi’an, China
- 4Clinic of Dental Experts, Xi’an Savaid Stomatology Hospital, Xi’an, China
- 5Department of Oral and Maxillofacial Surgery, Xi’an Daxing Hospital, Xi’an, China
Increasing attention to skin regeneration has rapidly broadened research on the topic. However, no bibliometric analysis of the field’s research trends has yet been conducted. In response to this research gap, this study analyzed the publication patterns and progress of skin regeneration research worldwide using a bibliometric analysis of 1,471 papers comprising 1,227 (83.4%) original articles and 244 (16.6%) reviews sourced from a Web of Science search. Publication distribution was analyzed by country/region, institution, journal, and author. The frequency of keywords was assessed to prepare a bibliometric map of the development trends in skin regeneration research. China and the United States were the most productive countries in the field: China had the greatest number of publications at 433 (29.4%) and the United States had the highest H-index ranking (59 with 15,373 citations or 31.9%). Author keywords were classified into four clusters: stem cell, biomaterial, tissue engineering, and wound dressing. “Stem cells,” “chitosan,” “tissue engineering,” and “wound dressings” were the most frequent keywords in each cluster; therefore, they reflected the field’s current focus areas. “Immunomodulation,” “aloe vera,” “extracellular vesicles,” “injectable hydrogel,” and “three-dimensional (3D) bioprinting” were relatively new keywords, indicating that biomaterials for skin regeneration and 3D bioprinting are promising research hotspots in the field. Moreover, clinical studies on new dressings and techniques to accelerate skin regeneration deserve more attention. By uncovering current and future research hotspots, this analysis offers insights that may be useful for both new and experienced scholars striving to expand research and innovation in the field of skin regeneration.
Introduction
The skin is the largest organ and an essential part of the human body (1). By functioning harmoniously with other organs, the skin is the first body’s barrier against the damage from the external environment (2). “Skin regeneration” refers to the complete replacement of damaged skin with new skin (3). Impaired skin regeneration is a common outcome in patients with diabetes, pressure ulcers, and burns, making skin regeneration treatments necessary (4). However, global population growth has increased the demand for and costs of skin regeneration treatments (5). In light of this, skin regeneration has become an extensively researched subject (6).
Bibliometrics is a statistical method that involves quantitatively analyzing research papers on special topics via mathematical methods (7). Unlike traditional citation counts, a bibliometric approach considers the connections within the literature; notably, it identifies intellectual structures and emerging trends (8). Within the field of tissue regeneration, bibliometric analyses have been employed to estimate its research trends, including cardiac (9), neural (10), periodontal (11), cartilage (12), and bone regeneration (13). However, no bibliometric reports assessing the relevant scientific outputs and research trends of studies on skin regeneration have yet been published.
To fill this gap in the scholarly archive, this study sought to investigate the publication pattern and progress of skin regeneration research worldwide. Data were obtained from the Web of Science Core Collection (WoSCC). The publication distribution was systematically assessed by geography, institution, journal, and author. Furthermore, we assessed the frequency of keywords and conducted bibliometric mapping to uncover the development of skin regeneration research.
Materials and methods
Data sources and search strategies
WoSCC is a credible database for conducting bibliometric analyses across many publications (11, 12, 14). Therefore, we conducted a comprehensive online search of skin regeneration research from 1900 when WoSCC was launched, on 29 April 2022. To analyze the data, such as the number of publications annually and the number of articles published by country, institution, journal, and author, we downloaded plaintext versions of the articles. As this review used data obtained from a public database and did not involve human subjects, no ethical consent was required.
The search was only conducted on one day (29 April 2022) to prevent inconsistency caused by rapid database renewal. The search strategies were as follows: Topic = (“skin regeneration”) AND language = English. Original articles and reviews that had undergone a standard peer review and which appeared in Science Citation Index Expanded were eligible for inclusion. The two researchers (JZ and CD) performed a preliminary screening by reading the titles and abstracts of the skin regeneration literature presented by the search to exclude irrelevant papers. In the case of a disagreement, a third person (GM) assisted in resolving the discrepancy by reading the full text.
Data collection
Two authors (JZ and CD) independently extracted data, including titles, keywords, publication dates, origin countries or regions, authors, institutions, journals, sums of citations, and H-indices, from correlative publications. The data from WoSCC were inputted into Microsoft Excel 2016 (Redmond, WA, United States), GraphPad Prism 7 (GraphPad Prism Software Inc., San Diego, CA, United States), and VOSviewer version 1.6.18 (Leiden University, Leiden, Netherlands) and were subsequently analyzed quantitatively and qualitatively. Meanwhile, the World Bank website was used to collect the latest information regarding the gross domestic product (GDP) of the countries/regions.
Bibliometric analyses
Bibliometric analyses were conducted on the following aspects: growth trend of publications, publication countries/regions, journals, institutions, authors, keywords, and citations. We performed the calculations in the following order: (1) the contributions of countries/regions to global publications in terms of the number of publications produced, number of citations, H-index, GDP of the country/region, and country/region-wise co-authorship; (2) the publication distribution of different journals; (3) the frequency of different institutions and institution-wise co-authorship; (4) the authors with the most publications and the most cited papers, both in the research scope of skin regeneration and author-wise co-authorship; (5) clusters and emergence time of keywords; (6) and research progress in skin regeneration based on a constructed network of direct and co-citations of published papers.
We applied the web statistical tool in the Web of Science to analyze the characteristics of the included publications and then used VOSviewer to create a collaborative map based on countries/regions, institutions, and authors. The size of an item’s circle on the map was proportional to the number of its publications and the width of a line between two items was proportional to the magnitude of their collaboration. Items of the same color belonged to the same cluster, indicating that they cooperated closely in this field (9).
The main concepts in bibliometric analyses are briefly outlined below (15).
• Relative research interest (RRI) is determined by the total number of publications in all fields divided by the annual number of publications in one specific field; this eliminates bias from an increase in total publications.
• Average publication year (APY) is used to quantify the relative novelty of a keyword.
• Co-citation analysis involves tracking pairs of papers that are cited together in the source articles. When the same pairs of papers are co-cited by many authors, clusters of research begin to form. The co-cited papers in these clusters tend to share common themes.
• The total link strength of an item reflects the degree of cooperation with other items. The higher its value, the higher the level of cooperation (9).
Results
Growth trend of publications
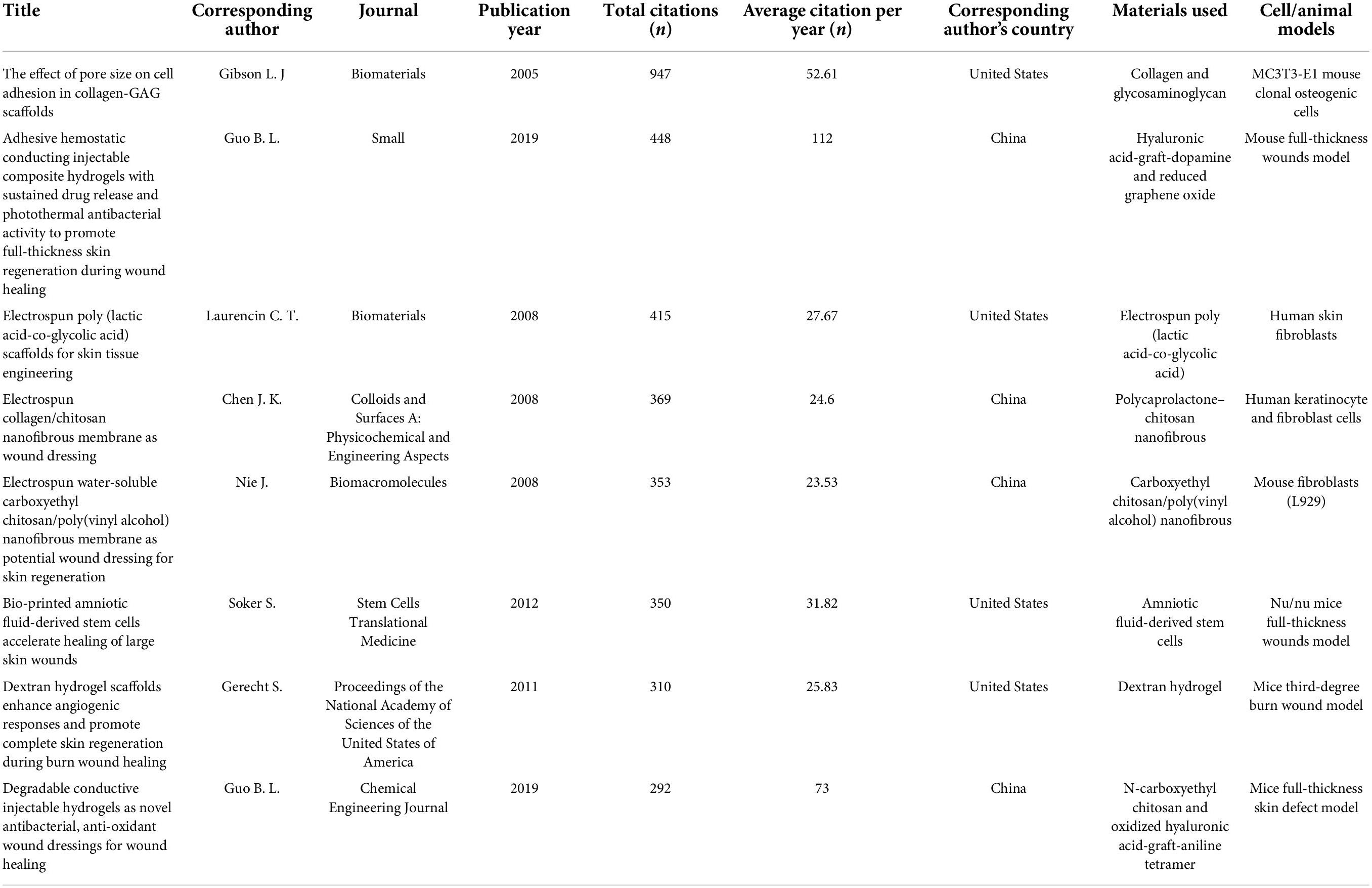
A total of 1,471 publications, including 1,227 (83.4%) articles and 244 (16.6%) reviews, met our screening criteria. The number of articles on skin regeneration worldwide increased significantly over time, from 2 in 2000 to 242 in 2021 (Figure 1A). When considering the number of all-field publications, global interest in skin regeneration was measured in terms of the RRI, which was approximately 0.0003% before 2000 and 0.009% in 2021. In addition to the above publications, only one retracted publication was searched for incorrect data interpretation and inaccurate citation.
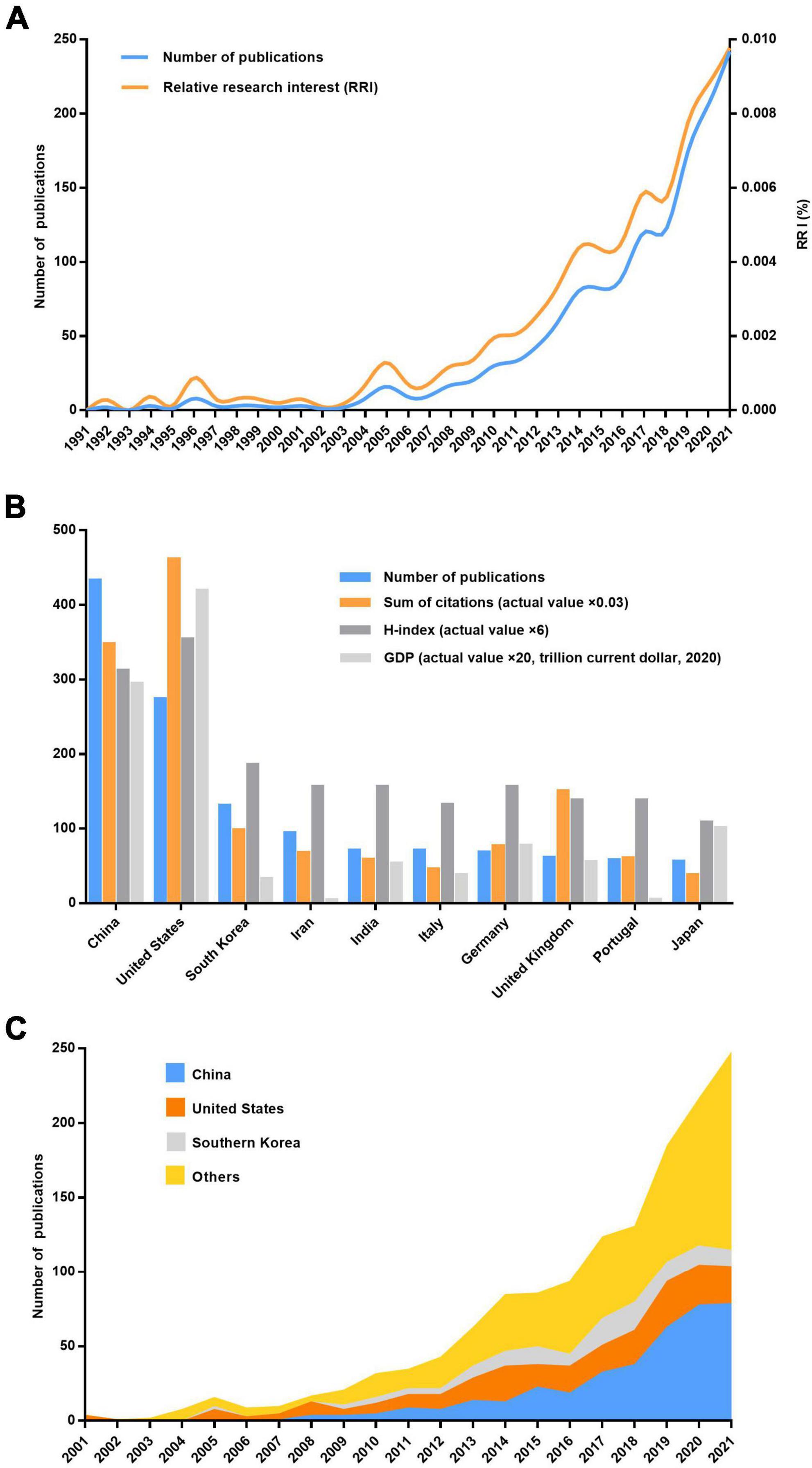
Figure 1. Contributions of different countries/regions to the field of skin regeneration. (A) Number of publications worldwide and the time course of relative research interest in skin regeneration; (B) number of publications, citation frequency (×0.03), H-index (×6), and GDP (×20, per trillion US dollars, 2020) of countries/regions (no less than five publications regarding skin regeneration); (C) number of publications from the top three and other countries per year (RRI, relative research interest).
Bibliometric analysis of countries/regions
China ranked first for the number of publications at 433 (29.4%), followed by the United States at 274 (18.6%) and South Korea at 131 (8.9%; Figures 1B,C). However, regarding the H-index, the United States ranked first (59 with 15,373 citations or 31.9%), followed by China (52 with 11,575 citations or 24.0%) and South Korea (31 with 3,272 citations or 6.8%); these results are consistent with these countries’ 2020 GDP rankings. Moreover, four clusters were mapped by coauthors of countries/regions (Figure 2). Cluster 1 mainly included Western European countries/regions, such as England, France, and Netherlands. China, Iran, the United States, and Italy had the highest number of published papers in Clusters 1–4.
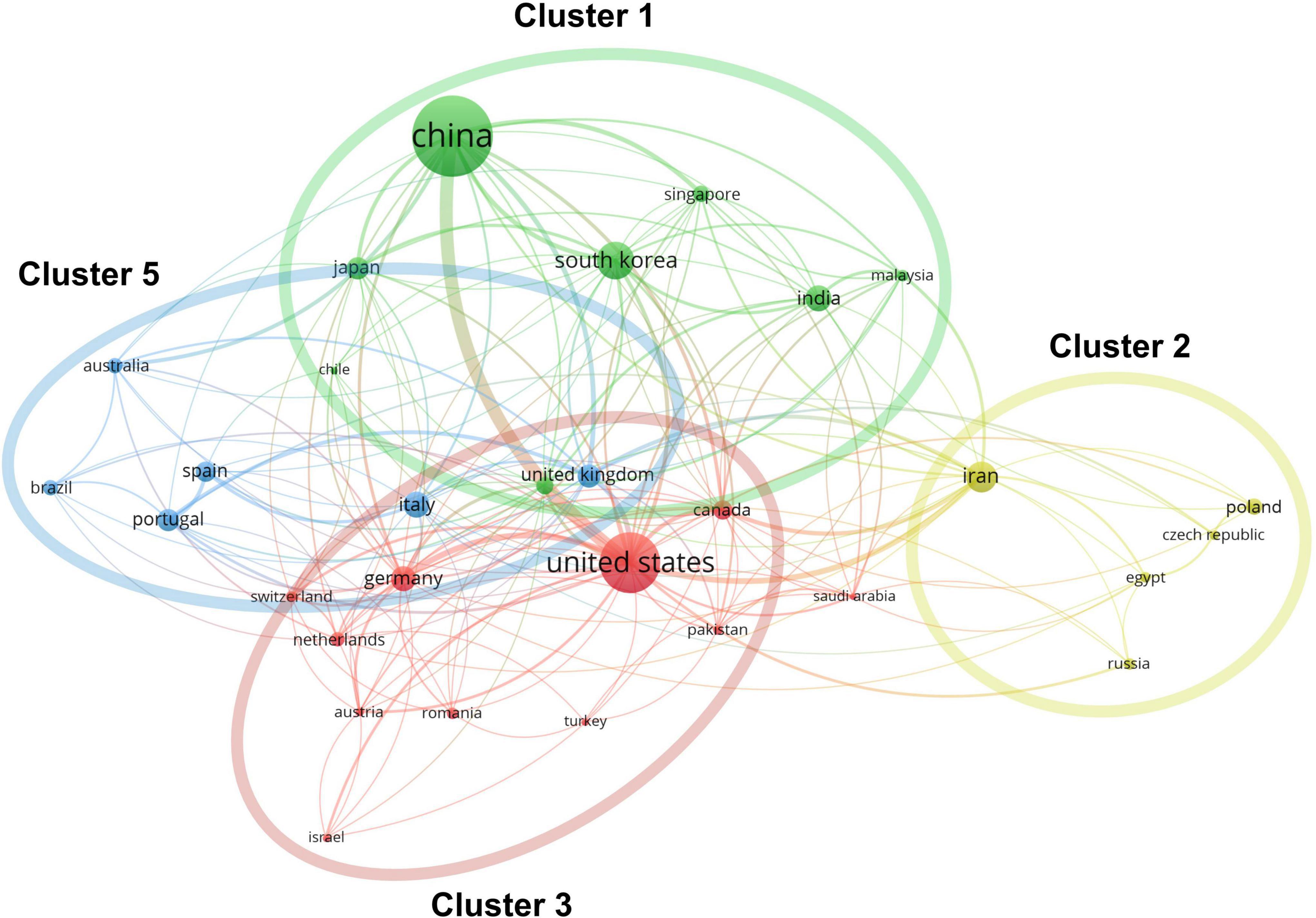
Figure 2. Co-authorship analysis of countries/regions divided into five clusters indicated with different colors. The large icon indicates countries/regions with high frequencies. Maximum number of countries per paper: 25; minimum number of countries per paper: 10; and minimum number of citations from one country: 10.
Bibliometric analysis of journals, institutions, and department
Approximately one-sixth of the skin regeneration papers (240, 16.3%) were published in the top 10 journals ranked by number of publications. Specifically, the International Journal of Molecular Sciences (33 publications; 2.2%), the International Journal of Biological Macromolecules (28 publications; 1.9%), and Acta Biomaterialia (26 publications; 1.8%) were ranked first, second, and third, respectively. The top 10 journals with the most publications are listed in Figure 3A. With 53 papers (3.6%) each, the League of European Research Universities (LERU) and Shanghai Jiao Tong University have published more than any other institution in the world. The top 10 institutions with the most publications are listed in Figure 3B. An analysis of co-authorship between institutions showed five clusters (Figure 4). The institutions with the highest number of publications in Clusters 1–4 were Shanghai Jiao Tong University (50 publications), the Chinese Academy of Sciences (29 publications), Seoul National University (19 publications), and Tehran University of Medical Sciences (23 publications). Besides, in the top 100 most frequently cited publications, the department of biomedical engineering ranked first in the number of publications at 20, followed by materials science at 16 and biology at 10.
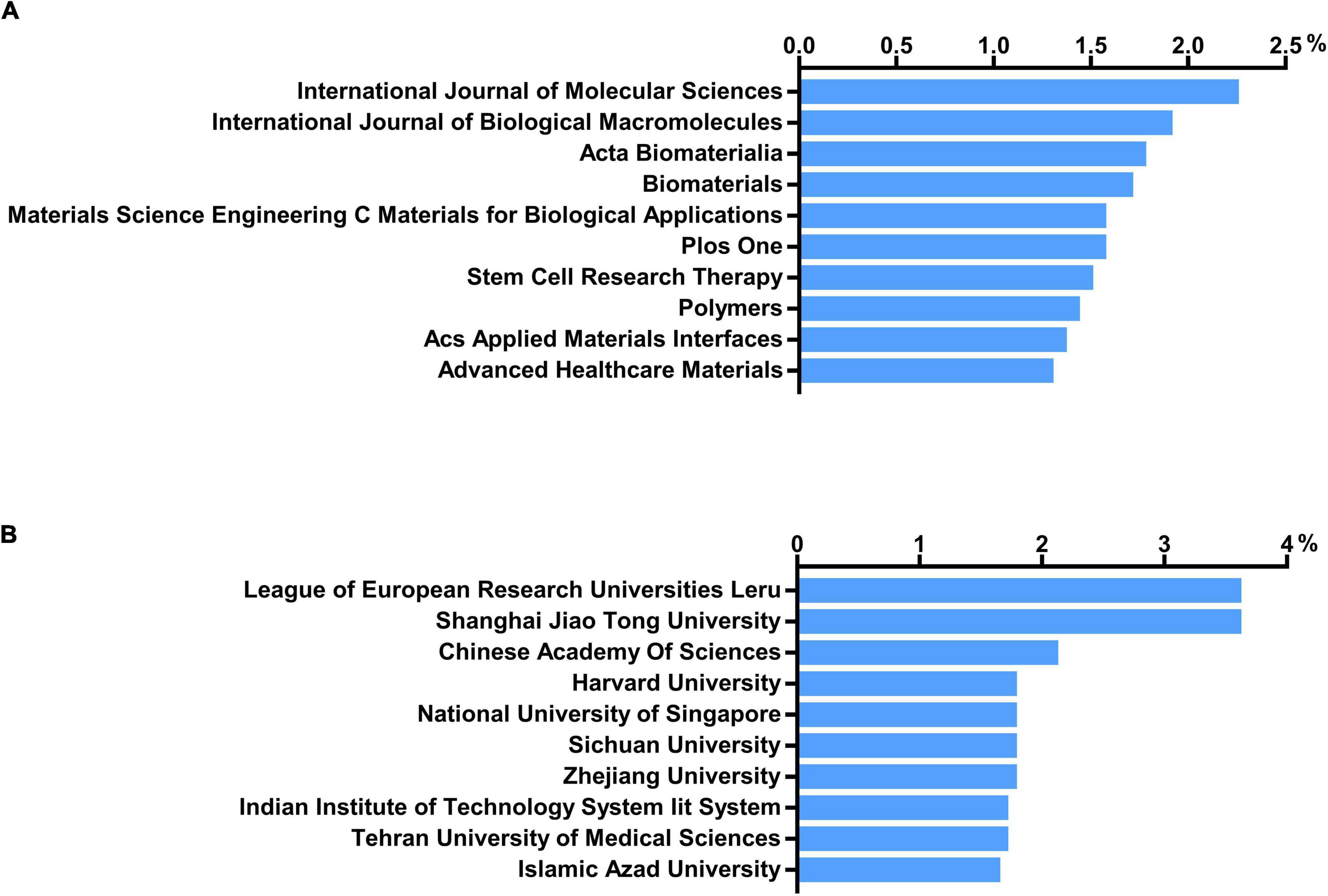
Figure 3. Number of institutions and journals focusing on skin regeneration. (A) Top 10 journals publishing research on skin regeneration; (B) top 10 institutes publishing research on skin regeneration.
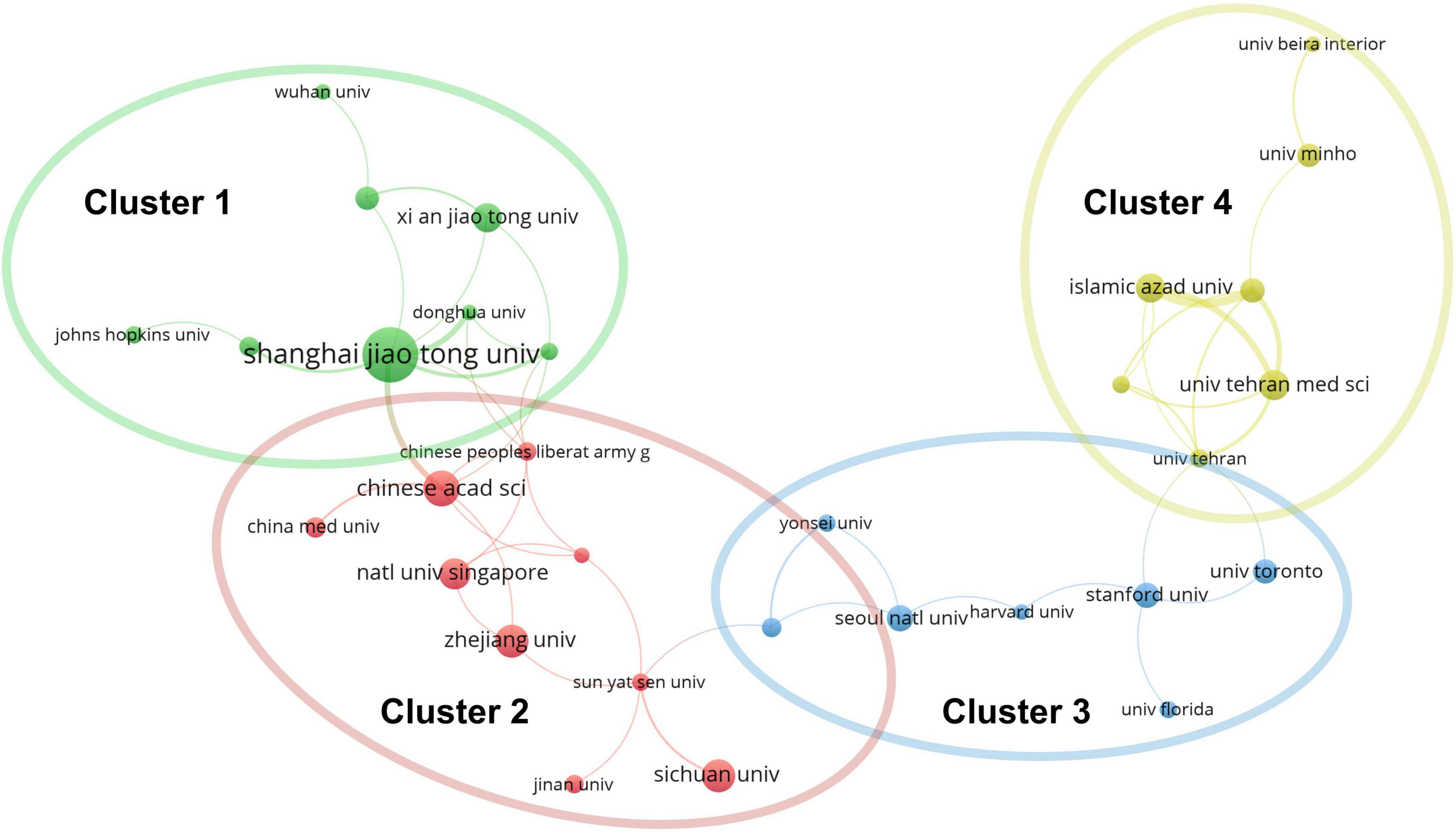
Figure 4. Co-authorship analysis of organizations divided into five clusters, indicated with different colors. The large icon indicates organizations with high frequencies. Maximum number of countries per paper: 25; minimum number of countries per paper: 10; and minimum number of citations from one country: 10.
Bibliometric analysis of authors
The top 10 authors wrote a total of 135 papers, accounting for 9.2% of all studies on skin regeneration. Li Qingfeng from Shanghai Jiao Tong University published 17 papers related to skin regeneration, thus ranking first in terms of the number of publications. Ramakrishna Seeram from the National University of Singapore and Fu Xiaobing from the Chinese People’s Liberation Army General Hospital ranked second and third with 15 and 14 papers, respectively. Furthermore, Lorna J. Gibson from the Massachusetts Institute of Technology had the highest citation frequency (Table 1 and Supplementary Table 1) (16–23). These highly cited articles are all basic science studies. The eight clusters of co-authorship among authors are illustrated in Figure 5; most show that Chinese authors cooperate relatively frequently.

Figure 5. Co-authorship analysis of authors. The authors were divided into eight clusters, indicated with different colors. The large icon indicates authors with high frequencies. Maximum number of authors per paper: 25; minimum number authors per paper: 4; and minimum number of citations of one author: 4.
Bibliometric analysis of keywords
A total of 3,054 author keywords were extracted from the publications using VOSviewer. As presented in Figure 6A, 69 keywords that occurred more than seven times were identified and classified into four clusters: stem cell (Cluster 1), biomaterial (Cluster 2), tissue engineering (Cluster 3), and wound dressing (Cluster 4). In Cluster 1, stem cells (106 times), skin (82 times), and keratinocytes (47 times) were the top three search terms. In Cluster 2, skin regeneration (329 times), chitosan (60 times), and collagen (38 times) were the most frequently searched terms. In Cluster 3, the top three keywords were wound healing (405 times), tissue engineering (86 times), and biomaterials (53 times). In Cluster 4, wound dressing (72 times), electrospinning (67 times), and hydrogels (60 times) appeared the most frequently.
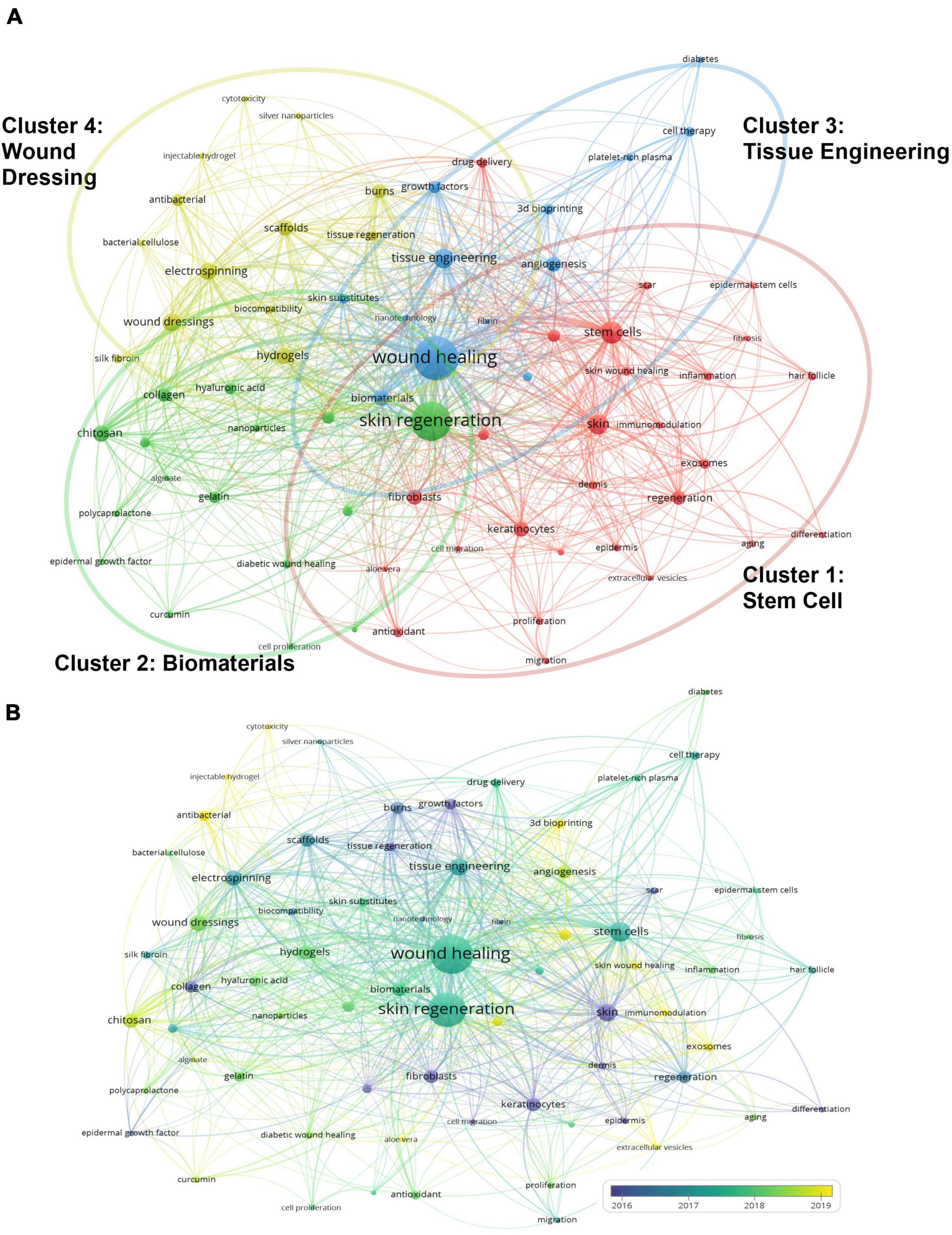
Figure 6. Analysis of keywords in publications on skin regeneration. (A) Map of the keywords concerning skin regeneration. The keywords were divided into three clusters, indicated with different colors. The large icon indicates keywords with high frequencies. (B) The keyword distribution is presented according to the average date of appearance, with blue representing an early appearance and yellow indicating a recent appearance. The smaller the distance between two keywords, the greater the frequency of their co-occurrence. Minimum number of occurrences of one keyword: 7.
As presented in Figure 6B, VOSviewer colored the keywords according to the date on which each word was published. Specifically, blue indicates that the word was published a relatively long time ago and yellow indicates that it was published recently (15). For example, early in the history of research on skin regeneration, the APY for “epidermis” (Cluster 1) was 2012.6. Meanwhile, “extracellular vesicles” (Cluster 1) is a relatively new keyword with an APY of 2020.3. “Immunomodulation,” “aloe vera,” and “extracellular vesicles”; “chitosan”, “alginate,” and “curcumin”; “three-dimensional (3D) bioprinting,” “angiogenesis,” and “diabetes”; and “injectable hydrogel,” “antibacterial,” and “wound dressing” were the top three most recent keywords in Clusters 1–4, respectively. The detailed results of the co-occurrence analysis of all keywords are presented in Supplementary Table 2.
Bibliometric analysis of citations and co-citations
All the articles related to skin regeneration considered in this study have been cited 48,171 times since 1990, with an average citation frequency of 32.7 times per paper and 1,459.7 times per year. To reveal whether these publications on skin regeneration were interlinked (i.e., whether they cited each other), VOSviewer was used to create a direct citation map of publications with over 50 citations (Figure 7A). In this map, Martin et al.’s (24) paper was most frequently cited (3,359 times) and Tottoli et al.’s (25) paper was the most cited (121 times) among recent publications. The bibliometric analysis results of co-citations (cited references) are shown in Figure 7B. Cluster 1, which had a similar keyword co-occurrence to Clusters 2–4, was named “applied study” while Cluster 2, which had a similar keyword co-occurrence to Cluster 1, was named “basic study.”
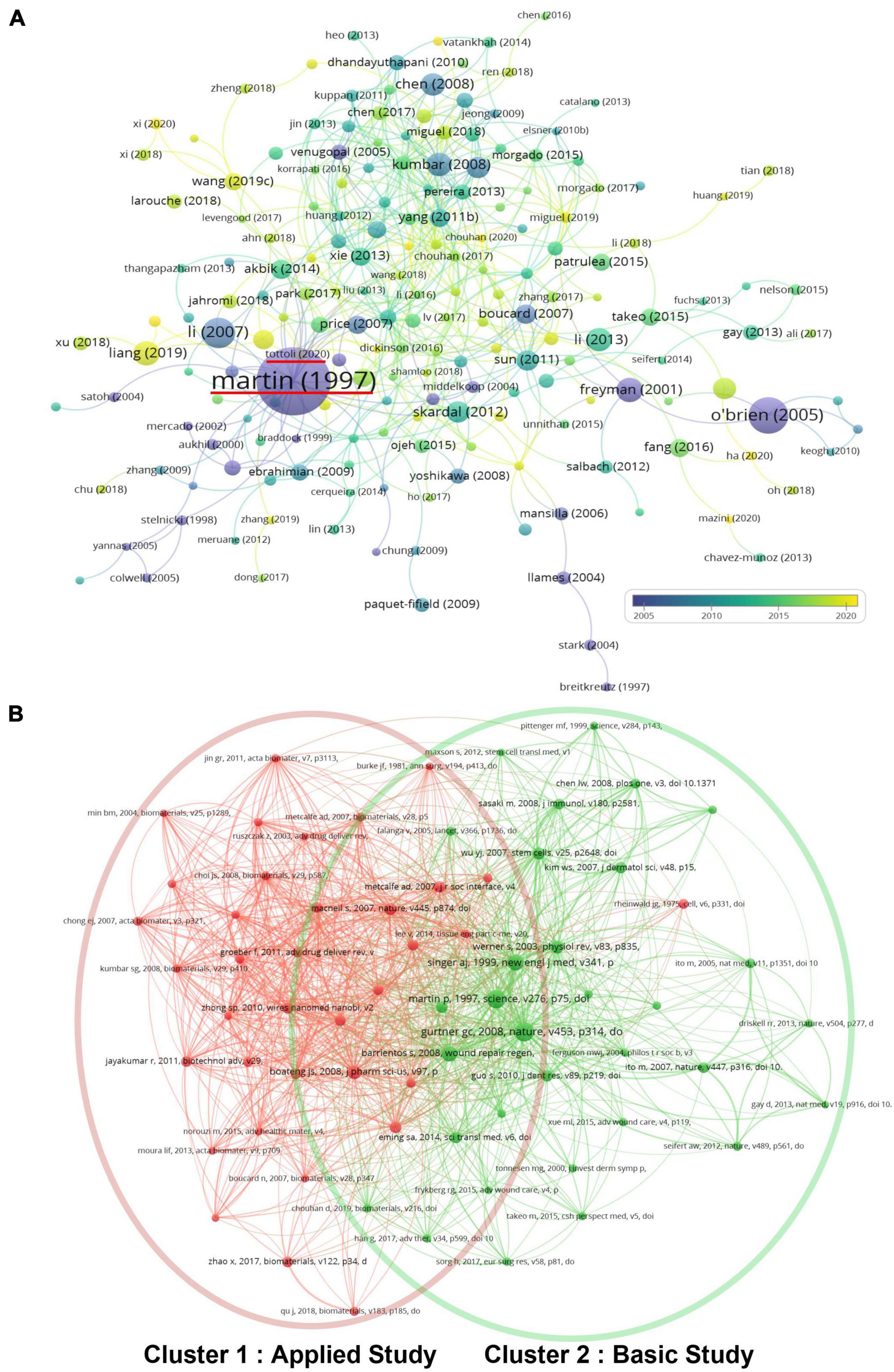
Figure 7. Citation and co-citation analysis of papers on skin regeneration. (A) Citation map; the distribution of publications is presented according to the average date of appearance, with blue representing an early appearance and yellow indicating a recent appearance; the red horizontal line indicates the most cited articles and the most cited articles among the latest articles; minimum number of times a paper was cited: 50. (B) Co-citation map; the publications were divided into three clusters in accordance with different colors. The large icon indicates publications with high frequencies; minimum number of citations of one document: 25.
Discussion
Young scholars and trained experts researching skin regeneration must find relevant scientific literature, clarify its past and current development, uncover important information, and identify active research frontiers and development trends in the field. This paper contributes to this work by summarizing these key concerns. More specifically, this study identified fundamental details of the skin regeneration field by conducting co-author analyses across journals, institutions, and authors.
The number of research publications on skin regeneration has increased rapidly over the last 30 years, indicating that skin regeneration is a research hotspot. Surveying this landscape, this study found that China is the most active country in skin regeneration research, particularly in terms of its total number of published articles. Accordingly, Chinese authors, such as Li Qingfeng and Fu Xiaobing, have been the most productive and active researchers on the topic. Notably, Li Qingfeng, the most productive author in the field, studies mechanical induced skin regeneration. Recently, his team identified EZH2 and CDH1 as therapeutic targets for skin regeneration after mechanical loading (26, 27). However, in terms of the number of total citations and H-index, the United States is the most influential country in the field of skin regeneration—this may be related to the United States’ early start and significant financial investment in the field. Notably, China and the United States frequently cooperate in skin regeneration research, as illustrated by the co-occurrence network of countries/regions. Generally, this study found that European and Asian authors are increasingly contributing to research on skin regeneration and that the influence of authors from the United States is extremely significant. Meanwhile, the International Journal of Molecular Sciences, an international, peer-reviewed, open access journal providing an advanced forum for all aspects of molecular research, published the largest number of studies on skin regeneration. Regarding institutions, the LERU has published the largest number of articles in the field.
Research focuses
The co-occurrence analysis of author keywords yielded the following four clusters (Clusters 1–4) of keywords based on the bibliographic map. The more frequently the keywords occur, the more noteworthy the related topics. In addition to keywords representing skin regeneration itself, such as “skin regeneration” and “wound healing,” the keywords revealed the following focus areas.
“Stem cells” was the most frequent keyword in Cluster 1. When the skin barrier is breached during wounding, re-establishing tissue integrity and function requires considerable coordination between various cell types, signaling factors, and matrix interactions (28). In this process, tissue-resident stem cells play an important role in self-renewing and maintaining their population during homeostasis (28). The stem cells must also cooperate with other cell types, including fibroblasts and immune cells, to ensure efficient and harmonious skin regeneration during wound healing (28). An exogenous addition of stem cells to skin injury lesions increases cell proliferation and neovascularization while reducing inflammation (29). Moreover, stem cell-based therapeutic strategies have shown considerable potential for improving the rate and quality of wound healing and skin regeneration (30).
“Chitosan” was the most frequently seen in Cluster 2. Chitosan is one of the most frequently studied biomaterials. It is formed by N-acetyl-D-glucosamine monomers with β-1,4-glycosidic bonds and is acquired by deacetylating the chitin extracted from crustacean shells. Studies have reported many benefits of chitosan and its derivatives in skin regeneration, including its desirable pharmacological value (due to its antibacterial, anti-inflammatory, and hemostatic properties) (31, 32); superior biocompatibility and biodegradability (33); good water absorption and retention properties; and the amino (−NH2) and hydroxyl (−OH) groups in its molecular chains, which enable it to graft to other groups and chemical components to enhance particular biological functions (34). When combined with other materials—namely, biological macromolecules or bioactive factors—chitosan is more effective in promoting skin regeneration, as seen with chitosan microneedle array patches and chitosan-based hydrogels with nanotechnologically modified curcumin and epidermal growth factors (35, 36). Moreover, the treatment effect of a chitosan dressing has been tested in many clinical trials, which reveal that chitosan acts as an effective antimicrobial and procoagulant agent, provides beneficial microbiota, facilitates wound re-epithelialization, and reduces patients’ pain levels (37–39).
“Tissue engineering” was the most frequent keyword in Cluster 3. Over the past few years, tissue engineering has enormously contributed to the progress of skin regeneration and wound healing (40). Through the use of biomaterials, bioactive molecules, cells, and their combinations, skin tissue engineering develops engineered scaffolds that can assist skin reconstruction (41). Skin can be replaced or modeled with tissue-engineered constructs that mimic native physiological characteristics (42). In clinical applications, engineered skin substitutes are effective in accelerating wound healing in cases of extensive burns, venous leg ulcers, and diabetic foot ulcers (43–45). Nevertheless, there is a need to redesign the currently available alternatives to make them more user-friendly, affordable, and viable (30). Other trending topics in tissue engineering include functional artificial skin grafts for nerve reconstruction, pigmentation, and skin appendages (e.g., hair follicles and sweat glands) (46).
“Wound dressings” was the most frequent keyword that appeared in Cluster 4. For several millennia, primary wound dressings, such as plasters, have served as physical barriers to protect wounds from the external environment (47). Over time, newer wound dressing materials have been developed to provide appropriate care for wounds and ensure optimal healing (48). Apart from chitosan, as previously described, collagen (49), alginate (50), cellulose (51), gelatin (52), hyaluronic acid (53), silk (54), and foam dressings (55) have been extensively studied in the field of skin wound dressings. In recent years, novel wound dressings have served as not only physical and chemical barriers, but also real-time monitors of the wound environment by allowing the growth factor and cellular delivery to be monitored and act as antimicrobial barriers (56–58).
Research trends
This study found that “immunomodulation” (Cluster 1), “aloe vera” (Cluster 1), “extracellular vesicles” (Cluster 1), “injectable hydrogel” (Cluster 4), and “3D bioprinting” (Cluster 3) are the newest keywords representing trends in skin regeneration research. These keywords suggest that emerging research trends are concerned with biomaterials that can facilitate skin regeneration. Below, the insights on biomaterials and skin regeneration uncovered by this study are presented.
First, bioactive components extracted from aloe vera have complex constituents and various pharmacological properties (59), such as antioxidant, anti-inflammatory, immunomodulatory, antimicrobial, antiviral, antidiabetic, hepatoprotective, anticancer, skin-protective, and wound-healing properties. These properties have been attributed to the presence of many active compounds, including anthraquinones, anthrones, chromones, flavonoids, amino acids, lipids, carbohydrates, vitamins, and minerals (60). When aloe vera is applied externally, it accelerates the regeneration of damaged skin (61, 62). Its healing property stems from a compound called glucomannan, which affects the fibroblast growth factor and stimulates the activity and proliferation of these cells, consequently improving collagen production and secretion (63). Thus, recent functional wound dressings contain aloe vera extracts as bioactive agents in combination with other scaffold materials (64–66).
Second, extracellular vesicles or exosomes have recently gained tremendous attention in the field of skin regeneration. These nanosized extracellular particles can break cellular boundaries and facilitate intracellular signal delivery during tissue regeneration (67). By conveying functional cargos (e.g., growth factor, cytokine, and miRNA) to target cells, extracellular vesicles not only participate in normal physiological processes, such as hemostasis, inflammation, proliferation, and remodeling, but also serve as a new style of wound treatment, particularly when derived from stem cells (68, 69).
Third, hydrogels were designed to gel at body temperature for injectable delivery for in situ forming (70). Compared with the traditional hydrogel formed outside of a patient or implanted using invasive surgical techniques, the injectable counterparts have several advantages, such as low cost, convenience, ability to deliver therapeutic payloads with minimal invasiveness, and ability to fill complex tissue defects (71, 72). Numerous chemical and material processing techniques have been utilized to produce injectable hydrogels, which are broadly categorized as covalent and non-covalent hydrogels (72). These materials can be injected as viscous liquids and subsequently solidified through variations in their local microenvironment (temperature, pH, and ion concentration), the application of an external stimulus (light), or affinity-based self-organization (in the case of peptides and other physically associating functional moieties) (73–78). The composite materials, formed by combining injectable hydrogels with other materials and bioactive components or active cells, exhibit great potential applications for skin regeneration (79–81).
Additionally, because immunomodulation plays a crucial role in skin regeneration (82, 83), specific cell-laden immunostimulating biomaterials may potentially be applicable in skin tissue restoration (84). Chen et al. (85) found that a combination of cryogel/hydrogel biomaterials and acupuncture can promote diabetic skin wound healing through immunomodulation. Meanwhile, Saleh et al. (86) used adhesive hydrogels loaded with miRNA-laden nanoparticles to promote wound healing caused by the polarization of macrophages to the M2 phenotype.
Moreover, 3D bioprinting can bridge the divergence between artificial tissue constructs and natural tissues; specifically, computer-aided design techniques can stack cell-laden materials layer by layer into 3D structures (87, 88). 3D bioprinting has several advantages for rapidly creating prototypes of customized structures, delivering cell-laden materials with high precision in space, and tissue engineering in a highly controllable microenvironment (89). Scholars have developed various bioprinting strategies on the basis of their fundamental working principles for fabricating functional tissue constructs, such as inkjet-based bioprinting, laser-assisted bioprinting, pressure-assisted (extrusion) bioprinting, acoustic bioprinting, stereolithography-based bioprinting, and magnetic bioprinting (90, 91). Through these strategies, 3D-printed skin possesses enormous potential as grafts for wound healing, burned skin replacement, and in vitro human skin modeling for product and drug testing (92–94). Notably, 3D bioprinting depends heavily on bioink for the development of functional organs or tissues. Recent studies have focused on bioinks used in 3D bioprinting, such as gelatin methacryloyl, collagen, and extracellular matrix collagen-based hydrogel (95).
Despite the obvious advantages of biomaterials and 3D bioprinting, most studies on these topics are experimental or preclinical. While no strict randomized controlled clinical trials are listed on WoSCC, ClinicalTrials.gov suggests that clinical trials in the field are currently underway. Moving forward, attention should be paid to clinical studies on how biomaterials and 3D bioprinting may be applied to accelerate skin regeneration.
This study has two main limitations. First, given the large number of relevant terms, it is difficult to guarantee the inclusion of all relevant articles and the exclusion of all articles that are largely irrelevant to the bibliometric analysis. Second, some of the latest publications were not emphasized in the study due to the common limitations of bibliometric analysis: if studies appear for only a short time and are insufficient in terms of number and frequency of cited literature, then a bibliometric analysis will not identify them. To be sure, this does not mean that recent literature is unimportant, but that more time is necessary for testing.
Conclusion
In conclusion, this study deciphered the progress of skin regeneration research using a bibliometric analysis. It found that the number of articles on skin regeneration worldwide has significantly increased over time. China and the United States have been the most productive in the field. Going forward, the application of biomaterials that facilitate skin regeneration and 3D bioprinting are promising research hotspots; moreover, clinical studies on new dressings and techniques to accelerate skin regeneration deserve attention.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JZ and CD conceptualized, designed, and conducted the study, acquired and analyzed the data, and wrote the manuscript. QS, YC, QW, and DW conducted the study and edited the manuscript. GM designed the study and wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.947649/full#supplementary-material
References
1. Martins AM, Ascenso A, Ribeiro HM, Marto J. The brain-skin connection and the pathogenesis of psoriasis: a review with a focus on the serotonergic system. Cells. (2020) 9:796. doi: 10.3390/cells9040796
2. Gonzales K, Fuchs E. Skin and its regenerative powers: an alliance between stem cells and their niche. Dev Cell. (2017) 43:387–401. doi: 10.1016/j.devcel.2017.10.001
3. Kamolz LP, Griffith M, Finnerty C, Kasper C. Skin regeneration, repair, and reconstruction. Biomed Res Int. (2015) 2015:892031. doi: 10.1155/2015/892031
4. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. (2009) 17:763–71. doi: 10.1111/j.1524-475X.2009.00543.x
5. Park U, Kim K. Multiple growth factor delivery for skin tissue engineering applications. Biotechnol Bioprocess Eng. (2017) 22:659–70. doi: 10.1007/s12257-017-0436-1
6. Nemeth K, Key S, Bottlik G, Masszi T, Mezey E, Karpati S. Analyses of donor-derived keratinocytes in hairy and nonhairy skin biopsies of female patients following allogeneic male bone marrow transplantation. Stem Cells Dev. (2012) 21:152–7. doi: 10.1089/scd.2010.0593
7. Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000 - 2014). Expert Opin Biol Ther. (2014) 14:1295–317. doi: 10.1517/14712598.2014.920813
8. Gutiérrez-Salcedo M, Martínez MÁ, Moral-Munoz JA, Herrera-Viedma E, Cobo MJ. Some bibliometric procedures for analyzing and evaluating research fields. Appl Intell. (2018) 48:1275–87. doi: 10.1007/s10489-017-1105-y
9. Ma S, Yan J, Chen L, Zhu Y, Chen K, Zheng C, et al. A bibliometric and visualized analysis of cardiac regeneration over a 20-year period. Front Cardiovasc Med. (2021) 8:789503. doi: 10.3389/fcvm.2021.789503
10. Pan Y, Zhang Y, Gao X, Jia J, Gao J, Ma Z. Scientific progress regarding neural regeneration in the Web of Science: a 10-year bibliometric analysis. Neural Regen Res. (2013) 8:3449–54. doi: 10.3969/j.issn.1673-5374.2013.36.011
11. Shaikh MS, Ullah R, Lone MA, Matabdin H, Khan F, Zafar MS. Periodontal regeneration: a bibliometric analysis of the most influential studies. Regen Med. (2019) 14:1121–36. doi: 10.2217/rme-2019-0019
12. Mc Donald CK, Moriarty P, Varzgalis M, Murphy C. The top 50 most cited articles in cartilage regeneration. Biores Open Access. (2017) 6:58–62. doi: 10.1089/biores.2017.0006
13. Hussin M, Mohd Serah A, Azlan KA, Abdullah HZ, Idris MI, Ghazali I, et al. A bibliometric analysis of the global trend of using alginate, gelatine, and hydroxyapatite for bone tissue regeneration applications. Polymers. (2021) 13:647. doi: 10.3390/polym13040647
14. Yu Y, Li Y, Zhang Z, Gu Z, Zhong H, Zha Q, et al. A bibliometric analysis using VOSviewer of publications on COVID-19. Ann Transl Med. (2020) 8:816. doi: 10.21037/atm-20-4235
15. Xia DM, Wang XR, Zhou PY, Ou TL, Su L, Xu SG. Research progress of heat stroke during 1989-2019: a bibliometric analysis. Mil Med Res. (2021) 8:5. doi: 10.1186/s40779-021-00300-z
16. O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. (2005) 26:433–41. doi: 10.1016/j.biomaterials.2004.02.052
17. Liang YP, Zhao X, Hu TL, Chen BJ, Yin ZH, Ma PX, et al. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small. (2019) 15:1990046. doi: 10.1002/smll.201900046
18. Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. (2008) 29:4100–7. doi: 10.1016/j.biomaterials.2008.06.028
19. Chen JP, Chang GY, Chen JK. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf Physicochem Eng Asop. (2008) 313:183–8. doi: 10.1016/j.colsurfa.2007.04.129
20. Zhou YS, Yang DZ, Chen XM, Xu Q, Lu FM, Nie J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules. (2008) 9:349–54. doi: 10.1021/bm7009015
21. Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med. (2012) 1:792–802. doi: 10.5966/sctm.2012-0088
22. Sun GM, Zhang XJ, Shen YI, Sebastian R, Dickinson LE, Fox-Talbot K, et al. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Aca Sci USA. (2011) 108:20976–81. doi: 10.1073/pnas.1115973108
23. Qu J, Zhao X, Liang YP, Xu YM, Ma PX, Guo BL. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem Eng J. (2019) 362:548–60. doi: 10.1016/j.cej.2019.01.028
24. Martin P. Wound healing–aiming for perfect skin regeneration. Science (1997) 276:75–81. doi: 10.1126/science.276.5309.75
25. Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics (2020) 12. doi: 10.3390/pharmaceutics12080735
26. Wang J, Zhang YF, Gao Y, Shan SZ, Li QF. EZH2 regulates the correlation between skin regeneration and the duration of mechanical stretch. J Invest Dermatol. (2021) 141:894–894. doi: 10.1016/j.jid.2020.09.007
27. Huang XL, Liang X, Zhou YW, Li HZ, Du HY, Suo YJ, et al. CDH1 is identified as a therapeutic target for skin regeneration after mechanical loading. Int J Bio Sci. (2021) 17:353–67. doi: 10.7150/ijbs.51309
28. Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. (2019) 21:18–24. doi: 10.1038/s41556-018-0237-6
29. Jo H, Brito S, Kwak BM, Park S, Lee MG, Bin BH. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int J Mol Sci. (2021) 22:2410. doi: 10.3390/ijms22052410
30. Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. (2019) 10:111. doi: 10.1186/s13287-019-1212-2
31. Mohan K, Ganesan AR, Muralisankar T, Jayakumar R, Sathishkumar P, Uthayakumar V, et al. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci Technol. (2020) 105:17–42. doi: 10.1016/j.tifs.2020.08.016
32. Khan MA, Mujahid M. A review on recent advances in chitosan based composite for hemostatic dressings. Int J Biol Macromol. (2019) 124:138–47. doi: 10.1016/j.ijbiomac.2018.11.045
33. Li M, Liang Y, He J, Zhang H, Guo B. Two-pronged strategy of biomechanically active and biochemically multifunctional hydrogel wound dressing to accelerate wound closure and wound healing. Chem Mater. (2020) 32:9937–53. doi: 10.1021/acs.chemmater.0c02823
34. Abd El-Hack ME, El-Saadony MT, Shafi ME, Zabermawi NM, Arif M, Batiha GE, et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int J Biol Macromol. (2020) 164:2726–44. doi: 10.1016/j.ijbiomac.2020.08.153
35. Chi J, Zhang X, Chen C, Shao C, Zhao Y, Wang Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact Mater. (2020) 5:253–9. doi: 10.1016/j.bioactmat.2020.02.004
36. Hu B, Gao M, Boakye-Yiadom KO, Ho W, Yu W, Xu X, et al. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact Mater. (2021) 6:4592–606. doi: 10.1016/j.bioactmat.2021.04.040
37. Wang CH, Cherng JH, Liu CC, Fang TJ, Hong ZJ, Chang SJ, et al. Procoagulant and antimicrobial effects of chitosan in wound healing. Int J Mol Sci. (2021) 22:7067. doi: 10.3390/ijms22137067
38. Totsuka Sutto SE, Rodríguez Roldan YI, Cardona Muñoz EG, Garcia Cobian TA, Pascoe Gonzalez S, Martínez Rizo A, et al. Efficacy and safety of the combination of isosorbide dinitrate spray and chitosan gel for the treatment of diabetic foot ulcers: a double-blind, randomized, clinical trial. Diab Vasc Dis Res. (2018) 15:348–51. doi: 10.1177/1479164118769528
39. Mo X, Cen J, Gibson E, Wang R, Percival SL. An open multicenter comparative randomized clinical study on chitosan. Wound Repair Regen. (2015) 23:518–24. doi: 10.1111/wrr.12298
40. Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci. (2017) 18:789. doi: 10.3390/ijms18040789
41. Amirsadeghi A, Jafari A, Eggermont LJ, Hashemi SS, Bencherif SA, Khorram M. Vascularization strategies for skin tissue engineering. Biomater Sci. (2020) 8:4073–94. doi: 10.1039/d0bm00266f
42. Yu JR, Navarro J, Coburn JC, Mahadik B, Molnar J, Holmes JH IV, et al. current and future perspectives on skin tissue engineering: key features of biomedical research, translational assessment, and clinical application. Adv Healthc Mater. (2019) 8:e1801471. doi: 10.1002/adhm.201801471
43. Momeni M, Fallah N, Bajouri A, Bagheri T, Orouji Z, Pahlevanpour P, et al. A randomized, double-blind, phase I clinical trial of fetal cell-based skin substitutes on healing of donor sites in burn patients. Burns. (2019) 45:914–22. doi: 10.1016/j.burns.2018.10.016
44. Stone RC, Stojadinovic O, Rosa AM, Ramirez HA, Badiavas E, Blumenberg M, et al. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci Transl Med. (2017) 9:aaf8611. doi: 10.1126/scitranslmed.aaf8611
45. Frykberg RG, Cazzell SM, Arroyo-Rivera J, Tallis A, Reyzelman AM, Saba F, et al. Evaluation of tissue engineering products for the management of neuropathic diabetic foot ulcers: an interim analysis. J Wound Care. (2016) 25:S18–18. doi: 10.12968/jowc.2016.25.Sup7.S18
46. Przekora A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: can we reconstruct functional skin tissue in vitro. Cells. (2020) 9:9071622. doi: 10.3390/cells9071622
47. Shah JB. The history of wound care. J Am Col Certif Wound Spec. (2011) 3:65–6. doi: 10.1016/j.jcws.2012.04.002
48. Aljghami ME, Saboor S, Amini-Nik S. Emerging innovative wound dressings. Ann Biomed Eng. (2019) 47:659–75. doi: 10.1007/s10439-018-02186-w
49. Chattopadhyay S, Raines RT. Review collagen-based biomaterials for wound healing. Biopolymers. (2014) 101:821–33. doi: 10.1002/bip.22486
50. Peng W, Li D, Dai K, Wang Y, Song P, Li H, et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int J Biol Macromol. (2022) 208:400–8. doi: 10.1016/j.ijbiomac.2022.03.002
51. Alven S, Aderibigbe BA. Chitosan and cellulose-based hydrogels for wound management. Int J Mol Sci. (2020) 21:21249656. doi: 10.3390/ijms21249656
52. Kang JI, Park KM. Advances in gelatin-based hydrogels for wound management. J Mater Chem B. (2021) 9:1503–20. doi: 10.1039/d0tb02582h
53. Graça M, Miguel SP, Cabral C, Correia IJ. Hyaluronic acid-based wound dressings: a review. Carbohydr Polym. (2020) 241:116364. doi: 10.1016/j.carbpol.2020.116364
54. Chouhan D, Mandal BB. Silk biomaterials in wound healing and skin regeneration therapeutics: from bench to bedside. Acta Biomater. (2020) 103:24–51. doi: 10.1016/j.actbio.2019.11.050
55. Walker RM, Gillespie BM, Thalib L, Higgins NS, Whitty JA. Foam dressings for treating pressure ulcers. Cochrane Database Syst Rev. (2017) 10:CD011332. doi: 10.1002/14651858.CD011332.pub2
56. Farahani M, Shafiee A. Wound healing: from passive to smart dressings. Adv Healthc Mater. (2021) 10:e2100477. doi: 10.1002/adhm.202100477
57. Pinto RV, Carvalho S, Antunes F, Pires J, Pinto ML. Emerging nitric oxide and hydrogen sulfide releasing carriers for skin wound healing therapy. ChemMedChem. (2022) 17:e202100429. doi: 10.1002/cmdc.202100429
58. Dumitru CD, Neacsu IA, Grumezescu AM, Andronescu E. Bee-derived products: chemical composition and applications in skin tissue engineering. Pharmaceutics. (2022) 14:14040750. doi: 10.3390/pharmaceutics14040750
59. Liang J, Cui L, Li J, Guan S, Zhang K, Li J. Aloe vera: a medicinal plant used in skin wound healing. Tissue Eng Part B Rev. (2021) 27:455–74. doi: 10.1089/ten.TEB.2020.0236
60. Kumar R, Singh AK, Gupta A, Bishayee A, Pandey AK. Therapeutic potential of Aloe vera-A miracle gift of nature. Phytomedicine. (2019) 60:152996. doi: 10.1016/j.phymed.2019.152996
61. Hêś M, Dziedzic K, Górecka D, Jêdrusek-Goliñska A, Gujska E. Aloe vera (L.) Webb.: natural sources of antioxidants - A review. Plant Foods Hum Nutr. (2019) 74:255–65. doi: 10.1007/s11130-019-00747-5
62. Burusapat C, Supawan M, Pruksapong C, Pitiseree A, Suwantemee C. Topical aloe vera gel for accelerated wound healing of split-thickness skin graft donor sites: a double-blind, randomized, controlled trial and systematic review. Plast Reconstr Surg. (2018) 142:217–26. doi: 10.1097/PRS.0000000000004515
63. Hashemi SA, Madani SA, Abediankenari S. The review on properties of aloe vera in healing of cutaneous wounds. Biomed Res Int. (2015) 2015:714216. doi: 10.1155/2015/714216
64. Ezhilarasu H, Ramalingam R, Dhand C, Lakshminarayanan R, Sadiq A, Gandhimathi C, et al. Biocompatible aloe vera and tetracycline hydrochloride loaded hybrid nanofibrous scaffolds for skin tissue engineering. Int J Mol Sci. (2019) 20:5174. doi: 10.3390/ijms20205174
65. Abdel-Mohsen AM, Frankova J, Abdel-Rahman RM, Salem AA, Sahffie NM, Kubena I, et al. Chitosan-glucan complex hollow fibers reinforced collagen wound dressing embedded with aloe vera. II. Multifunctional properties to promote cutaneous wound healing. Int J Pharm. (2020) 582:119349. doi: 10.1016/j.ijpharm.2020.119349
66. Kudłacik-Kramarczyk S, Głąb M, Drabczyk A, Kordyka A, Godzierz M, Wróbel PS, et al. Physicochemical characteristics of chitosan-based hydrogels containing albumin particles and aloe vera juice as transdermal systems functionalized in the viewpoint of potential biomedical applications. Materials. (2021) 14:5832. doi: 10.3390/ma14195832
67. Wan R, Hussain A, Behfar A, Moran SL, Zhao C. The therapeutic potential of exosomes in soft tissue repair and regeneration. Int J Mol Sci. (2022) 23:3869. doi: 10.3390/ijms23073869
68. Narauskaitë D, Vydmantaitë G, Rusteikaitë J, Sampath R, Rudaitytë A, Stašytë G, et al. Extracellular vesicles in skin wound healing. Pharmaceuticals. (2021) 14:811. doi: 10.3390/ph14080811
69. Bian D, Wu Y, Song G, Azizi R, Zamani A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. (2022) 13:24. doi: 10.1186/s13287-021-02697-9
70. Mathew AP, Uthaman S, Cho KH, Cho CS, Park IK. Injectable hydrogels for delivering biotherapeutic molecules. Int J Biol Macromol. (2018) 110:17–29. doi: 10.1016/j.ijbiomac.2017.11.113
71. Ghandforoushan P, Golafshan N, Babu Kadumudi F, Castilho M, Dolatshahi-Pirouz A, Orive G. Injectable and adhesive hydrogels for dealing with wounds. Expert Opin Biol Ther. (2022) 22:519–33. doi: 10.1080/14712598.2022.2008353
72. Dimatteo R, Darling NJ, Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv Drug Deliv Rev. (2018) 127:167–84. doi: 10.1016/j.addr.2018.03.007
73. Liow SS, Dou Q, Kai D, Karim AA, Zhang K, Xu F, et al. Thermogels: in situ gelling biomaterial. ACS Biomater Sci Eng. (2016) 2:295–316. doi: 10.1021/acsbiomaterials.5b00515
74. Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. (2008) 49:1993–2007. doi: 10.1016/j.polymer.2008.01.027
75. Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. (2007) 13:2369–85. doi: 10.1089/ten.2007.0093
76. Koutsopoulos S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: progress, design guidelines, and applications. J Biomed Mater Res A. (2016) 104:1002–16. doi: 10.1002/jbm.a.35638
77. Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. (2003) 21:1171–8. doi: 10.1038/nbt874
78. Webber MJ, Appel EA, Meijer EW, Langer R. Supramolecular biomaterials. Nat Mater. (2016) 15:13–26. doi: 10.1038/nmat4474
79. Yu Y, Yang B, Tian D, Liu J, Yu A, Wan Y. Thiolated hyaluronic acid/silk fibroin dual-network hydrogel incorporated with bioglass nanoparticles for wound healing. Carbohydr Polym. (2022) 288:119334. doi: 10.1016/j.carbpol.2022.119334
80. Gong X, Luo M, Wang M, Niu W, Wang Y, Lei B. Injectable self-healing ceria-based nanocomposite hydrogel with ROS-scavenging activity for skin wound repair. Regen Biomater. (2022) 9:rbab074. doi: 10.1093/rb/rbab074
81. Yang S, Jiang H, Qian M, Ji G, Wei Y, He J, et al. MSC-derived sEV-loaded hyaluronan hydrogel promotes scarless skin healing by immunomodulation in a large skin wound model. Biomed Mater. (2022) 17:ac68bc. doi: 10.1088/1748-605X/ac68bc
82. Heydari P, Kharaziha M, Varshosaz J, Javanmard SH. Current knowledge of immunomodulation strategies for chronic skin wound repair. J Biomed Mater Res B Appl Biomater. (2022) 110:265–88. doi: 10.1002/jbm.b.34921
83. Ding J, Lei L, Liu S, Zhang Y, Yu Z, Su Y, et al. Macrophages are necessary for skin regeneration during tissue expansion. J Transl Med. (2019) 17:36. doi: 10.1186/s12967-019-1780-z
84. Tavakoli S, Kisiel MA, Biedermann T, Klar AS. Immunomodulation of skin repair: cell-based therapeutic strategies for skin replacement (a comprehensive review). Biomedicines. (2022) 10:118. doi: 10.3390/biomedicines10010118
85. Chen TY, Wen TK, Dai NT, Hsu SH. Cryogel/hydrogel biomaterials and acupuncture combined to promote diabetic skin wound healing through immunomodulation. Biomaterials. (2021) 269:120608. doi: 10.1016/j.biomaterials.2020.120608
86. Saleh B, Dhaliwal HK, Portillo-Lara R, Shirzaei Sani E, Abdi R, Amiji MM, et al. Local immunomodulation using an adhesive hydrogel loaded with mirna-laden nanoparticles promotes wound healing. Small. (2019) 15:e1902232. doi: 10.1002/smll.201902232
87. Levato R, Jungst T, Scheuring RG, Blunk T, Groll J, Malda J. From shape to function: the next step in bioprinting. Adv Mater. (2020) 32:e1906423. doi: 10.1002/adma.201906423
88. Chimene D, Kaunas R, Gaharwar AK. Hydrogel bioink reinforcement for additive manufacturing: a focused review of emerging strategies. Adv Mater. (2020) 32:e1902026. doi: 10.1002/adma.201902026
89. Tan B, Gan S, Wang X, Liu W, Li X. Applications of 3D bioprinting in tissue engineering: advantages, deficiencies, improvements, and future perspectives. J Mater Chem B. (2021) 9:5385–413. doi: 10.1039/d1tb00172h
90. Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med. (2016) 14:271. doi: 10.1186/s12967-016-1028-0
91. Leberfinger AN, Ravnic DJ, Dhawan A, Ozbolat IT. Concise review: bioprinting of stem cells for transplantable tissue fabrication. Stem Cells Transl Med. (2017) 6:1940–8. doi: 10.1002/sctm.17-0148
92. Cubo N, Garcia M, Del Cañizo JF, Velasco D, Jorcano JL. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication. (2016) 9:015006. doi: 10.1088/1758-5090/9/1/015006
93. Vijayavenkataraman S, Lu WF, Fuh JY. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication. (2016) 8:032001. doi: 10.1088/1758-5090/8/3/032001
94. Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. (2020) 226:119536. doi: 10.1016/j.biomaterials.2019.119536
Keywords: bibliometric analysis, skin regeneration research, tissue engineering, wound dressing, 3D bioprinting
Citation: Zhou J, Dong C, Shu Q, Chen Y, Wang Q, Wang D and Ma G (2022) Deciphering the focuses and trends in skin regeneration research through bibliometric analyses. Front. Med. 9:947649. doi: 10.3389/fmed.2022.947649
Received: 19 May 2022; Accepted: 07 July 2022;
Published: 22 July 2022.
Edited by:
Teng Su, Duke University, United StatesReviewed by:
Shiqi Hu, North Carolina State University, United StatesXiao Long, Peking Union Medical College Hospital (CAMS), China
Hanlin Zhang, Peking Union Medical College Hospital (CAMS), China
Copyright © 2022 Zhou, Dong, Shu, Chen, Wang, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ge Ma, MzYwOTUyNjAyQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jian Zhou1,2†
Jian Zhou1,2† Chen Dong
Chen Dong