
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 03 August 2022
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.945913
This article is part of the Research Topic Role of Complement Activation in Kidney Diseases View all 6 articles
 Wei-yi Guo
Wei-yi Guo Xiu-ping An
Xiu-ping An Li-jun Sun
Li-jun Sun Hong-rui Dong
Hong-rui Dong Wen-rong Cheng
Wen-rong Cheng Nan Ye
Nan Ye Guo-qin Wang
Guo-qin Wang Xiao-yi Xu
Xiao-yi Xu Zhi-rui Zhao
Zhi-rui Zhao Hong Cheng*
Hong Cheng*Introduction: IgA nephropathy (IgAN) encompasses a wide range of clinical and histology features. Some patients present without hematuria, with or without hypertension, still rapidly progress in renal function. Renal pathology of this part of patients were predominant intrarenal arteriolar lesions, rarely presented glomerular proliferative lesions. We aim to investigate the clinical and pathological characteristics and prognosis of these IgAN patients and initially explore whether the abnormal activation of complement is involved in the intrarenal arteriolar lesions of IgAN.
Methods: A total of 866 patients with renal biopsy-proven IgAN diagnosed at Beijing Anzhen Hospital were recruited. IgAN patients without intrarenal arteriolar lesions and proliferative lesions were excluded (n = 115), the rest were divided into arteriolar lesions group (n = 202) and proliferative lesions group (n = 549). Among them, 255 patients were regularly followed up for at least 1 year. Renal biopsy tissues of 104 IgAN patients were stained for complement components by immunohistochemistry and immunofluorescence.
Results: Compared with proliferative lesions group, the arteriolar lesions group experienced high percentage of hypertension (p = 0.004), low percentage of gross hematuria (p = 0.001), microscopic hematuria (p < 0.001) and less initial proteinuria (p = 0.033). Renal survival between the two groups was not significantly different (p = 0.133). MBL, C4d, FH and FHR5, C3c, and MAC deposited on intrarenal arteriole in arteriolar lesions group. Compare with the proliferative lesion group, the arteriolar lesions group exhibited a higher intensity of C3c deposition on the intrarenal arterioles (p = 0.048). C3c and CD31 co-deposited on intrarenal arterioles area in patients with intrarenal arteriolar lesions.
Conclusion: Renal survival of the IgAN patients in arteriolar lesions group was not better than those in proliferative lesions group. Abnormal activation of complement may be involved in the pathogenesis of arteriolar damage through the injury of endothelial cells in this clinical phenotype of IgAN.
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis worldwide (1). It is a leading cause of chronic kidney disease and progresses to end-stage kidney disease approximately 20 years after diagnosis in up to 20–40% of patients (2). Patients with IgAN display considerable heterogeneity in clinical manifestations and pathological phenotypes (3). In addition to the typical clinical manifestations, such as recurrent episodes of gross hematuria during or immediately following mucosal infections (4), some patients also present without hematuria, with or without hypertension, and show a rapid decline in renal function. The renal pathology of this group of patients shows predominantly intrarenal arteriolar lesions, and seldom presents as glomerular proliferative lesions. At present, there is no unified understanding of the clinical manifestations, prognosis or pathogenesis of patients with IgAN with predominant intrarenal arteriolar lesions.
The role of intrarenal arteriolar lesions in the prognosis of IgAN remains under debate owing to inconsistent study results. For the treatment of this clinical phenotype dominated by intrarenal arteriolar lesions, clinical practice is to control blood pressure to reach the target. There remains a lack of guidance and recommendations for immunosuppressive therapy for these patients (5). Previous studies have shown that blood pressure is an independent factor that affects intrarenal arteriolar disease (6). However, we noticed that some IgAN patients with normal blood pressure also presented with severe intrarenal arteriolar damage (7–9), suggesting that in addition to blood pressure, other factors may be involved in the pathogenesis of intrarenal arteriolar lesions.
Increasing evidence has implied the importance of alternative pathway- and/or lectin pathway-induced complement activation in IgAN (10). Complement activation can generally occur through three different pathways (Figure 1). In patients with IgAN, the components of complement activation have been commonly detected in the renal biopsy specimens, circulatory immune complexes, blood and urine (10–17). In recent years, several studies have showed that complement C4d is related to vascular lesions, especially thrombotic microvascular disease-like lesions (18). Arteriolar C4d is a potential biomarker for disease progression in IgAN (19, 20). Studies of atherosclerosis have revealed that sublytic C5b-9 assembly leads to the activation and proliferation of smooth muscle and endothelial cells (21). Previous studies on complement in vascular injury have focused mainly on thrombotic microangiopathy and atherosclerotic damage to the large and middle arteries. The role of complement system activation in intrarenal arteriosclerosis of IgAN is rarely discussed.
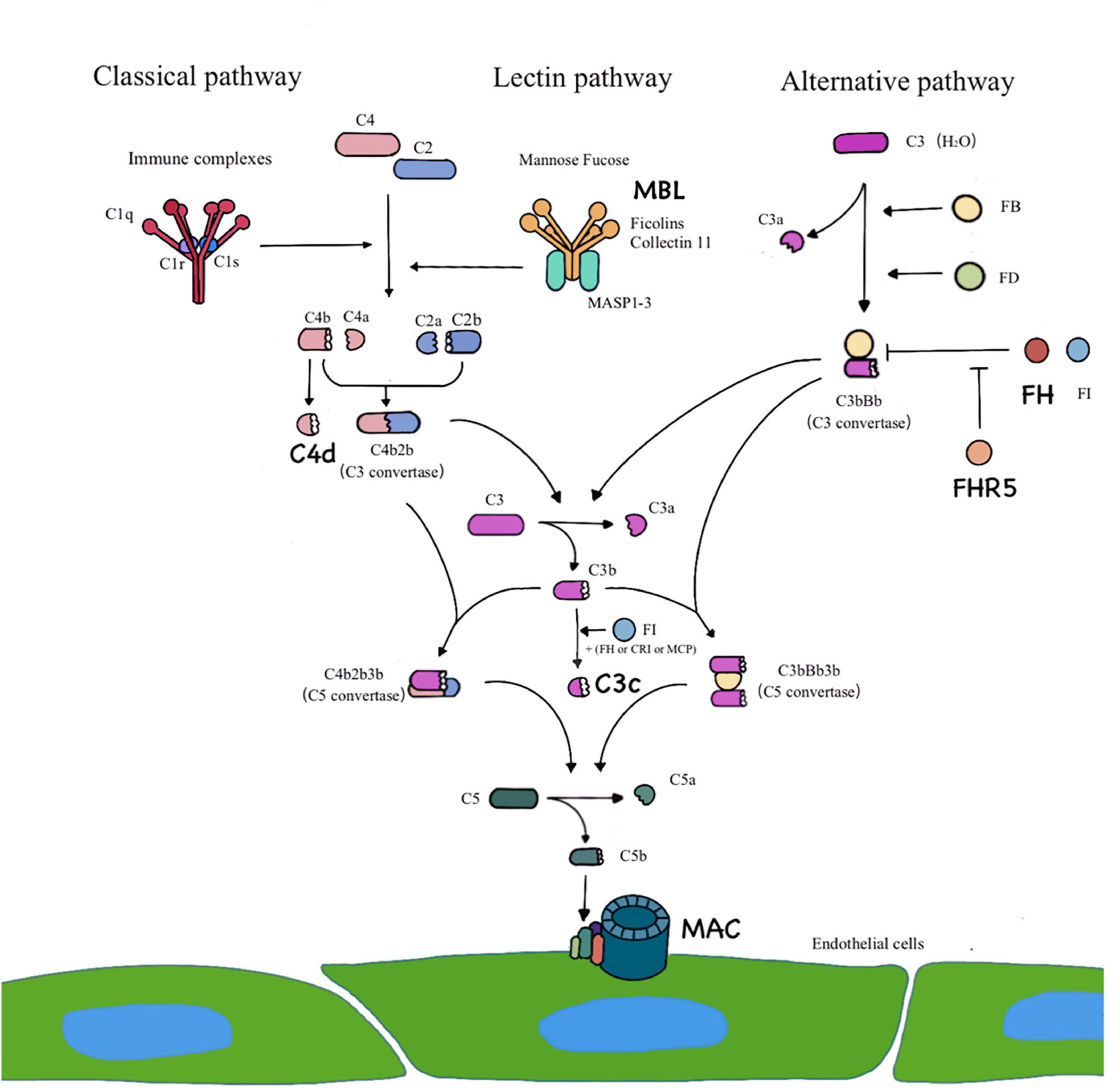
Figure 1. Three pathways of complement activation. The classical pathway is activated by IgG– and/or IgM–containing immune complexes. The lectin pathway requires a particular sugar moiety pattern to be recognized and bound by MBL/Ficolins/Collectin 11, leading to a classical pathway–like activation cascade. C4d represents the activation of classical pathway or/and lectin pathway. The alternative pathway is constantly initiated by spontaneous hydrolysis of C3 [C3b (H2O)] that is efficiently powered by the covalent attachment of C3b on an activating surface. Complement factor H (FH) and factor H related protein 5 (FHR5) are regulator proteins of alternative pathway. Three pathways lead to formation of C3 convertase and converge at the C3 level. The addition of C3b to the C3 convertase creates C5 convertase, which triggers the formation of the terminal pathway complete complex (C5b-9), which is also known as membrane attack complex.
This study aims to investigate the clinical and pathological characteristics and prognosis of patients with IgAN with predominant arteriolar lesions and to initially explore whether the abnormal activation of complement is involved in the intrarenal arteriolar lesions of IgAN.
From January 2010 to March 2021, the baseline data of 866 patients with renal biopsy-proven IgAN diagnosed at Beijing Anzhen Hospital were included in this study. The diagnosis of IgAN was based upon the presence of dominant IgA deposition in the mesangial area as detected by immunofluorescence and electron-dense material deposition in the mesangial area as observed with electron microscopy. Patients with IgA vasculitis, liver cirrhosis, systemic lupus and other secondary etiologies of IgAN identified by detailed clinical and laboratory examinations were excluded. IgAN patients without intrarenal arteriolar lesion and proliferative lesion were excluded (n = 115). Among the enrolled patients (n = 751), frozen renal biopsies of 104 patients from January 2019 to March 2021 were enrolled for immunofluorescence of C3c. A flow diagram is shown in Figure 2.
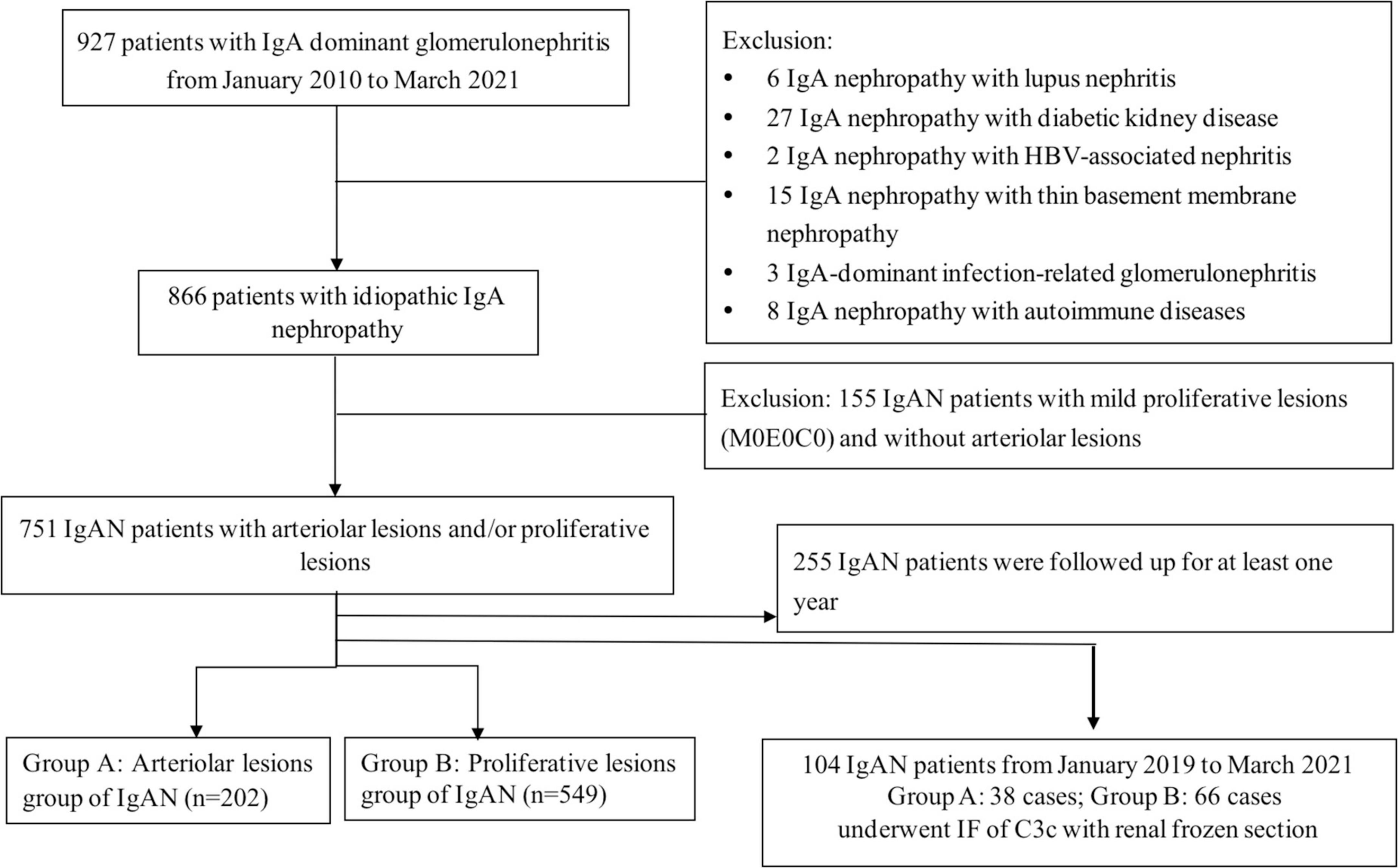
Figure 2. Flow diagram of patients of IgA nephropathy enrolled in the study. A total of 866 patients with renal biopsy-proven IgAN diagnosed from January 2010 to April 2021. IgAN patients without intrarenal arteriolar lesions and proliferative lesions were excluded (n = 115), the rest were divided into arteriolar lesions group (group A: n = 202) and proliferative lesions group (group B: n = 549). Among them, 255 patients were regularly followed up for at least 1 year. Among the enrolled patients (n = 751, group A and group B), frozen renal biopsies of 104 patients from January 2019 to March 2021 were enrolled for immunofluorescence of C3c.
The research was conducted in compliance with the principles of the Declaration of Helsinki and approved by the ethics committees of Beijing Anzhen Hospital. Informed consent was obtained from all enrolled individuals.
Clinical manifestations at the time of renal biopsy were collected from the medical records. High blood pressure (HBP) was defined as a systolic blood pressure (SBP) of 130 mmHg or more, a diastolic blood pressure (DBP) of 80 mmHg or more, or the use of antihypertensive medications to prevent hypertension. Hematuria was defined as gross hematuria or microscopic hematuria (RBC > 3/HP, with microscopic examination of sediment after centrifugation). The glomerular filtration rate (GFR) of IgAN patients was calculated using the Modified Glomerular Filtration Rate Estimating Equation (22).
Renal biopsy specimens were processed routinely for light, immunofluorescence and electron microscopy. Processing for light microscopy examination included hematoxylin and eosin, periodic acid-Schiff, periodic acid-silver methenamine, and Masson’s trichrome (PAM-Masson) staining. Immunofluorescence staining for IgG, IgA, IgM, C3, C1q, and FRA was routinely performed. Intrarenal arterial lesions include intrarenal arterial arteriolar wall thickening, arterial occlusion, arterial wall sclerosis and hyaline change. Arterial arteriolar wall thickening was defined when the cross-sectional ratio of the luminal diameter to the outer diameter was less than approximately 0.48, according to our previous study (23). Hyaline change was defined as a glassy, pink or red eosinophilic homogenous appearance of the arteriolar wall without cell proliferation.
Wall thickening was scored based on over 50% of intrarenal arteries and arterioles being affected relative to the total number of all arterial cross-sections, and the ratio of the luminal diameter to the outer diameter was as follows: 0 (> 0.48) (Figure 3A), 1 (0.45∼0.48) (Figure 3B), 2 (< 0.45) (Figure 3C), and 3 (0, arterial occlusion) (Figure 3D). Hyaline changes (Figure 3E) were scored based on the percentage of affected intrarenal arteries and arterioles relative to the total number of all arterial cross-sections: 0, no hyaline; 1, intrarenal arterial lesion involvement < 25%; 2, intrarenal arterial lesion involvement > 25%. The sum score of all changes was used to grade intrarenal arterial lesions as mild (1), moderate (2–3), or severe (4–5) based on the total of the score of the ratio of the luminal diameter to the outer diameter and Hyaline changes.
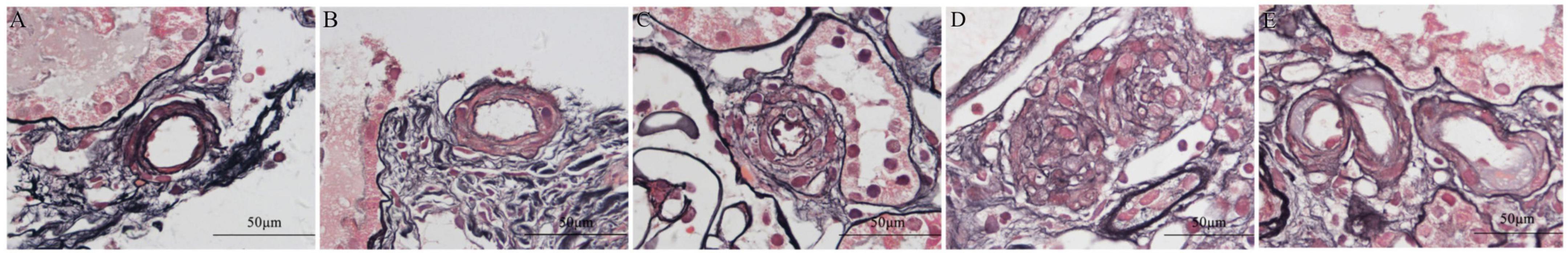
Figure 3. Periodic acid-silver methenamine and Masson’s trichrome (PAM-Masson) staining of intrarenal arterioles in patients with immunoglobulin A nephropathy (IgAN). Wall thickening was scored based on over 50% of intrarenal arteries and arterioles being affected relative to the total number of all arterial cross-sections, and the ratio of the luminal diameter to the outer diameter was as follows: 0 (> 0.48) [0.575, (A)], 1 (0.45∼0.48) [0.479, (B)], 2 (<0.45) [0.253, (C)], and 3 (0, arterial occlusion) [0, (D)]. Hyaline changes (E) (Original magnification × 1,000).
The Oxford classification and the intrarenal arterial lesions score was used to evaluate pathological lesions for individuals with more than eight glomeruli and more than eight intrarenal arteries and arterioles in biopsy specimens (24). Kidney biopsies from all patients were reviewed independently by Wei-yi Guo and Li-jun Sun who were blinded to clinical data.
Patients without intrarenal arteriolar lesions and proliferative lesions were excluded (n = 115), and the rest were divided into two groups based on the main histological lesion: the arteriolar lesions group (group A) (n = 202), including patients with predominant intrarenal arterial lesions with mild Oxford mesangial hypercellularity, and without endocapillary hypercellularity or crescents (M0E0C0); the proliferative lesions group (group B) (n = 549), which consisted of patients with glomerular predominant proliferative lesions including Oxford mesangial hypercellularity and/or endocapillary hypercellularity and/or crescents (M1 and/or E1 and/or C1/2) with or without intrarenal arterial lesions. Among these patients, 68 patients in group A and 187 patients in group B were regularly followed up for at least 1 year, or reaching end-stage renal disease (ESRD) within 12 months.
The composite outcome was defined as a 30% decline in eGFR, ESRD (eGFR < 15 ml/min/1.73 m2, the requirement for maintenance dialysis for at least 6 months, or kidney transplantation) or death. Patients who did not reach the event of interest during follow-up or those who were lost to follow-up were defined as censored. A 30% decline in eGFR or ESRD, whichever occurred first, was used as the definition of the primary endpoint.
Frozen renal biopsies from 104 patients with IgAN (from June 2019 to March 2021) were stained for C3c and galactose deficient IgA1 (Gd-IgA1) by immunofluorescence. Paraffin-embedded renal tissues from 10 patients with IgAN in group A were stained for FH, FHR-5, MBL, C4d, C3c, and membrane attack complex (MAC) by immunohistochemistry and immunofluorescence. Detail staining protocols were optimized for frozen renal biopsy tissue and formalin-fixed paraffin-embedded renal biopsy tissue with the following antibodies: mouse monoclonal anti-human FH antibody (Santa Cruz, United States), and rabbit polyclonal anti-human FHR-5 antibody (GeneTex, California, United States), mouse monoclonal anti-human MBL antibody (clone 3E7) (Hycult Biotech, United States), rabbit polyclonal anti-human C4d antibody (Bio-Rad, United States), mouse monoclonal anti-human C5b-9 antibody (Abcam, United States), rabbit polyclonal anti-human C3c (Dako, Glostrup, Denmark), rat monoclonal anti-human Gd-IgA1 antibody (KM55) (Immuno-Biological Laboratories, Japan) (11, 15). The method to detect colocalization of C3c and CD31 (biomarker of endothelial cells, Abcam, United States) were the same as previously described (15).
Immunofluorescence was scored using a fluorescence microscope (Nikon 80i, Japan). Two observers (Wei-yi Guo and Li-jun Sun), who were blinded to the clinical data, graded the staining intensity from anonymized sections as 0 (absent), 1+, 2+, or 3+. Sections that contained fewer than 2 glomeruli and 2 arterioles were excluded.
Plasma samples of 60 patients with IgAN (from June 2019 to March 2021) were collected on the day of biopsy and stored at −80°C. Levels of Gd-IgA1 was measured using a commercially available enzyme-linked immunosorbent assay test kit with KM55 (Immuno-Biological Laboratories, Japan) according to the manufacture’s protocol.
Quantitative variables are presented as the means ± standard deviation (for normally distributed data) or medians with interquartile range (IQR) (for non-normally distributed data). Categorical data are summarized as absolute frequencies and percentages. For continuous variables, an independent-samples t-test was used when the data were normally distributed; for non-normally distributed data, the Mann-Whitney or Kruskal-Wallis test was performed. Categorical variables were compared using the χ2-test. The prognostic factors were identified by multivariate analysis with Cox regression. The results are expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). SPSS 11.0 statistical software (SPSS, Chicago, IL, United States) was used for mean/median/percentage comparison and survival analysis.
At the time of biopsy, there were 403/751 (53.7%) male patients, and the mean age was 38.17 ± 13.02 years. Prodromic infection and gross hematuria were found in 114 (15.2%) and 136 (18.2%) patients, respectively. At the time of renal biopsy, the median proteinuria value of the patients was 1.26 (0.60, 2.50) g/24 h, while the average estimated glomerular filtration rate (eGFR) was 82.31 (50.48, 107.67) ml/min/1.73 m2. Of our patients, 557/751 (74.2%) presented with hypertension. Histologically, mesangial hypercellularity (M1), endocapillary hypercellularity (E1), and segmental glomerulosclerosis (S1) were found in 300/751 (39.9%), 465/751 (61.9%) and 404 (53.8%) patients, respectively. For tubular atrophy and interstitial fibrosis (Oxford-T), 342/751 (45.5%), 252/751 (33.6%), and 157/751 (20.9%) of patients were scored as T0/T1/T2, respectively. For crescent lesions, 487/751 (64.8%), 203/751 (27.0%), and 61/751 (8.1%) of patients were scored as C0/C1/C2, respectively (Table 1).
There were significantly more IgAN patients in group A than in group B who experienced hypertension [165/202 (81.7%) vs. 392/549 (71.4%), p = 0.004, Table 1] and non-microscopic hematuria [54/202 (26.7%) vs. 47/549 (8.6%), p < 0.001, Table 1]. Compared with those in group B, fewer patients in group A experienced prodromic infection [21/202 (10.4%) vs. 93/549 (16.9%), p = 0.027, Table 1] and gross hematuria [21/202 (10.4%) vs. 115/549 (21.0%), p = 0.001, Table 1]. In addition, compared with those in group B, patients in group A presented with less initial proteinuria [1.00 (0.50, 2.40) g/day vs. 1.31 (0.62, 2.53) g/day, p = 0.033, Table 1], and percentages of segmental glomerulosclerosis/adhesion [S1: 65/202 (32.2%) vs. 339/549 (61.7%), p < 0.001, Table 1]. In terms of treatment, more patients in group A were treated with a renin-angiotensin system inhibitor (RASI) [155 (79.1%) vs. 364 (69.7%), p = 0.013, Table 1], and fewer patients in group A were treated with steroids or immunosuppressive agents [31 (15.8%) vs. 175 (33.5%), p < 0.001, Table 1] than patients in group B.
According to the severity of intrarenal arterial lesions, we divided the arteriolar lesions group into mild, moderate and severe subgroups. The severe subgroup presented with more severe left ventricular mass index (LVMI) [severe/moderate/mild subgroup: 105.50 (88.99, 129.26) g/m2 vs. 91.80 (70.67, 109.68) g/m2 vs. 80.33 (68.13, 101.03) g/m2, p = 0.002, Table 2], urine osmotic pressure (severe/moderate/mild subgroup: 536.28 ± 190.88 mOsm/kg⋅H2O vs. 642.20 ± 176.19 mOsm/kg⋅H2O vs. 697.60 ± 175.11 mOsm/kg⋅H2O, p < 0.001, Table 2), initial proteinuria [severe/moderate/mild subgroup: 2.59 (0.98, 4.05) g/day vs. 0.75 (0.44, 2.24) g/day vs. 0.98 (0.49, 2.36) g/day, p = 0.021, Table 2], and eGFR [severe/moderate/mild group: 47.11 (23.14, 72.59) ml/min/1.73 m2 vs. 68.28 (42.51, 87.38) ml/min/1.73 m2 vs. 91.42 (63.52, 111.49) ml/min/1.73 m2, p < 0.001, Table 2] and a high percentage of Oxford T lesions [severe/moderate/mild subgroup: T1/T2, 7 (38.9%)/10 (55.6%) vs. 19 (41.3%)/11 (23.9%) vs. 38 (27.5%)/18 (13.0%), p < 0.001, Table 2].
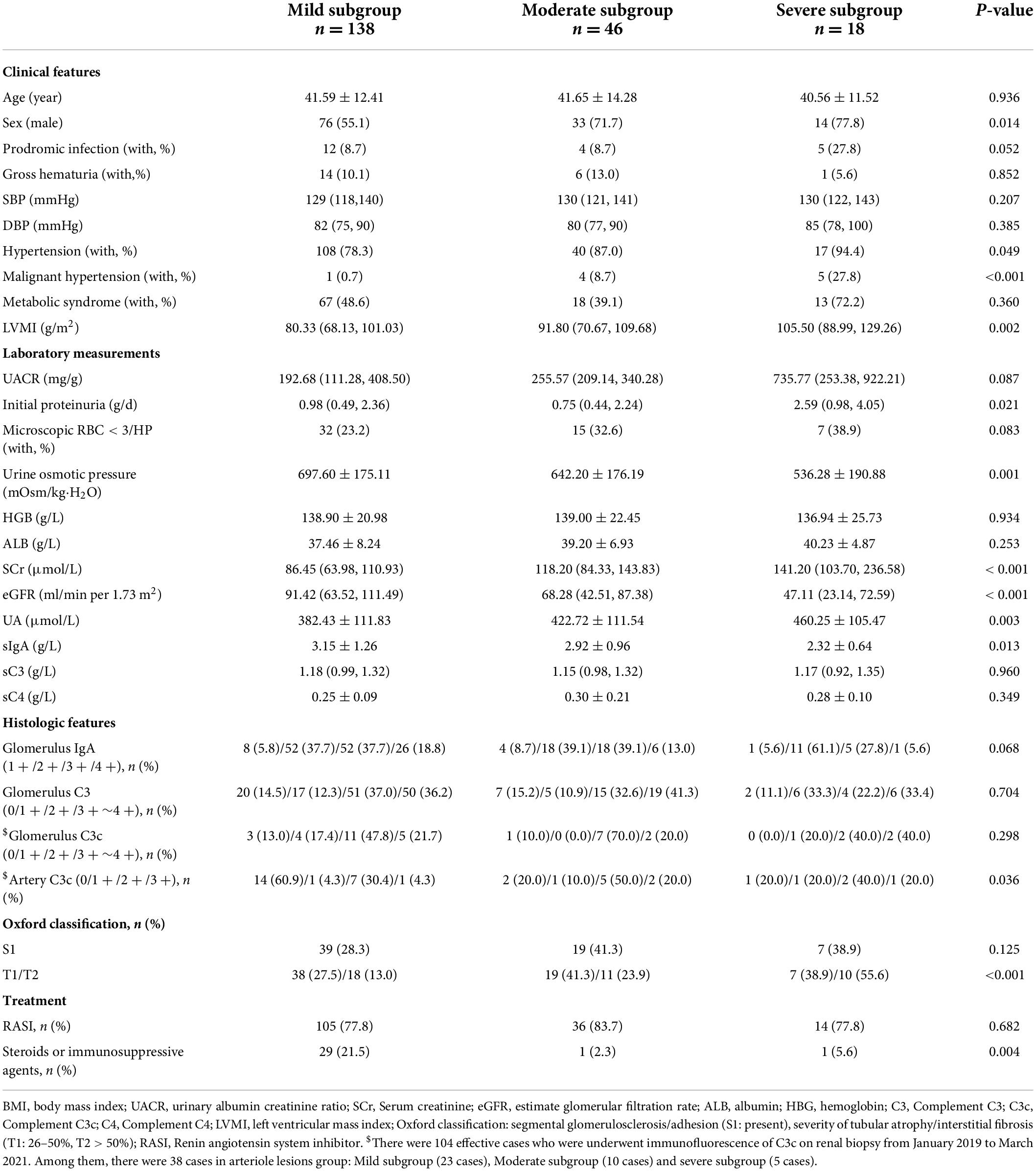
Table 2. Demographic, clinical, and histological characteristics of the subgroup of the arteriolar lesions group with IgAN based on the severity of arterial lesions. (n = 202).
In the arteriolar lesions group, patients without hypertension had a higher eGFR [97.38 (75.81, 120.22) ml/min/1.73 m2 vs. 78.10 (49.38, 102.87) ml/min/1.73 m2, p < 0.001] and fewer arteriolar lesions [mild/moderated/severe lesions: 30 (81.1%)/6 (16.2%)/1 (2.7%) vs. 108 (65.5%)/40 (24.2%)/17 (10.3%), p = 0.049, Table 3]. Interestingly, the hypertension and non-hypertension subgroups of IgAN patients in the arteriolar lesion group histological exhibited no differences in the presence of lesions such as Oxford S lesions and T lesions and in treatment with a RASI, steroids or immunosuppressive agents.
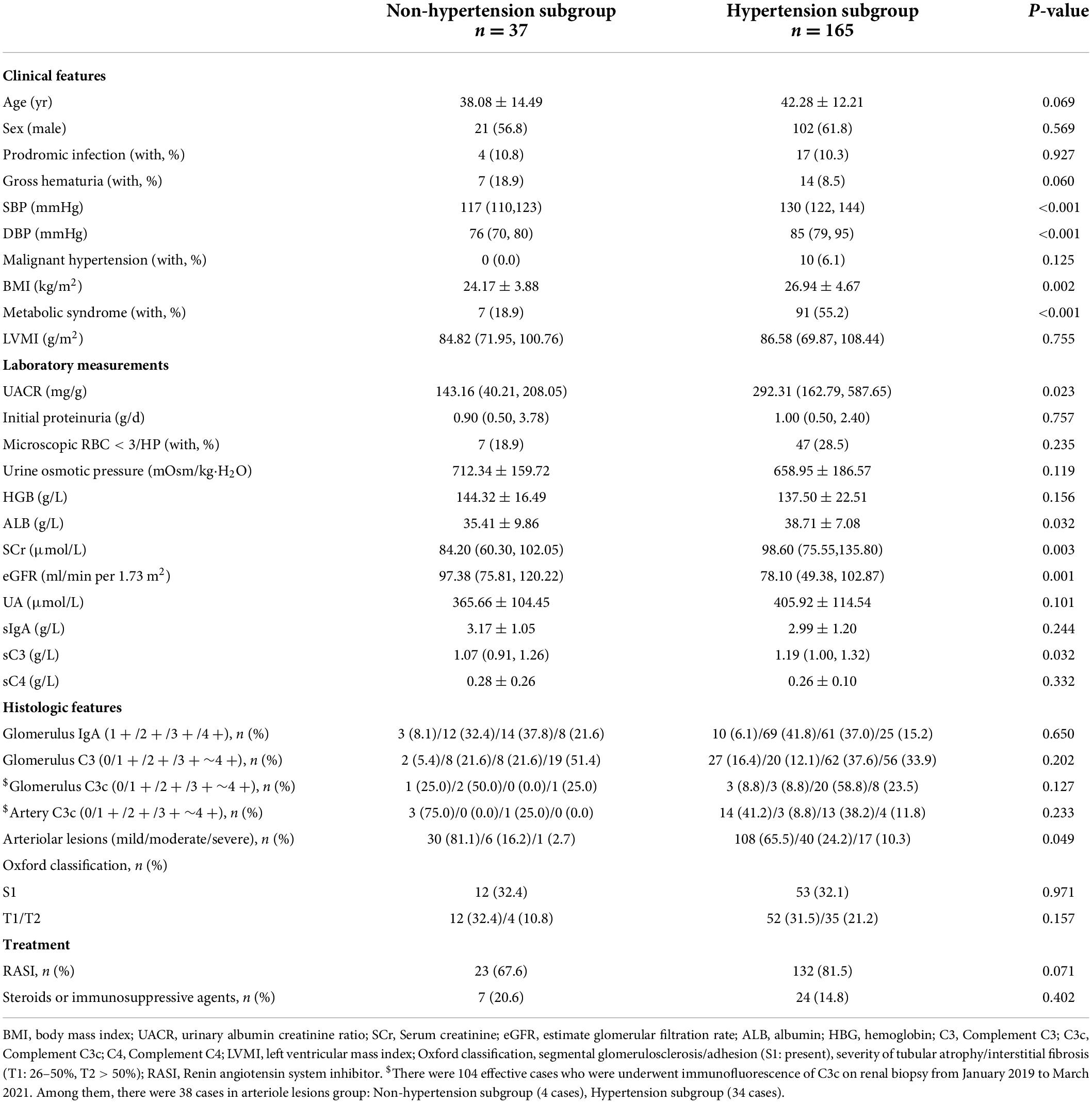
Table 3. Demographic, clinical, and histologic characteristics of hypertension and non-hypertension subgroup in arteriolar lesions group of IgAN patients.
The clinical, laboratory and pathologic parameters between group A and group B with regular follow-up were showed in Table 4. During follow-up, 18 patients (24.0%) in group A and 67 patients (32.2%) in group B reached the composite endpoint (p = 0.184). Among these patients, 11 patients (16.2%) in group A and 46 patients (24.6%) in group B showed a 30% decline in eGFR (p = 0.153). Moreover, end-stage renal disease occurred in 5 patients (5.2%) in group A and 33 patients (11.4%) in group B (p = 0.075).
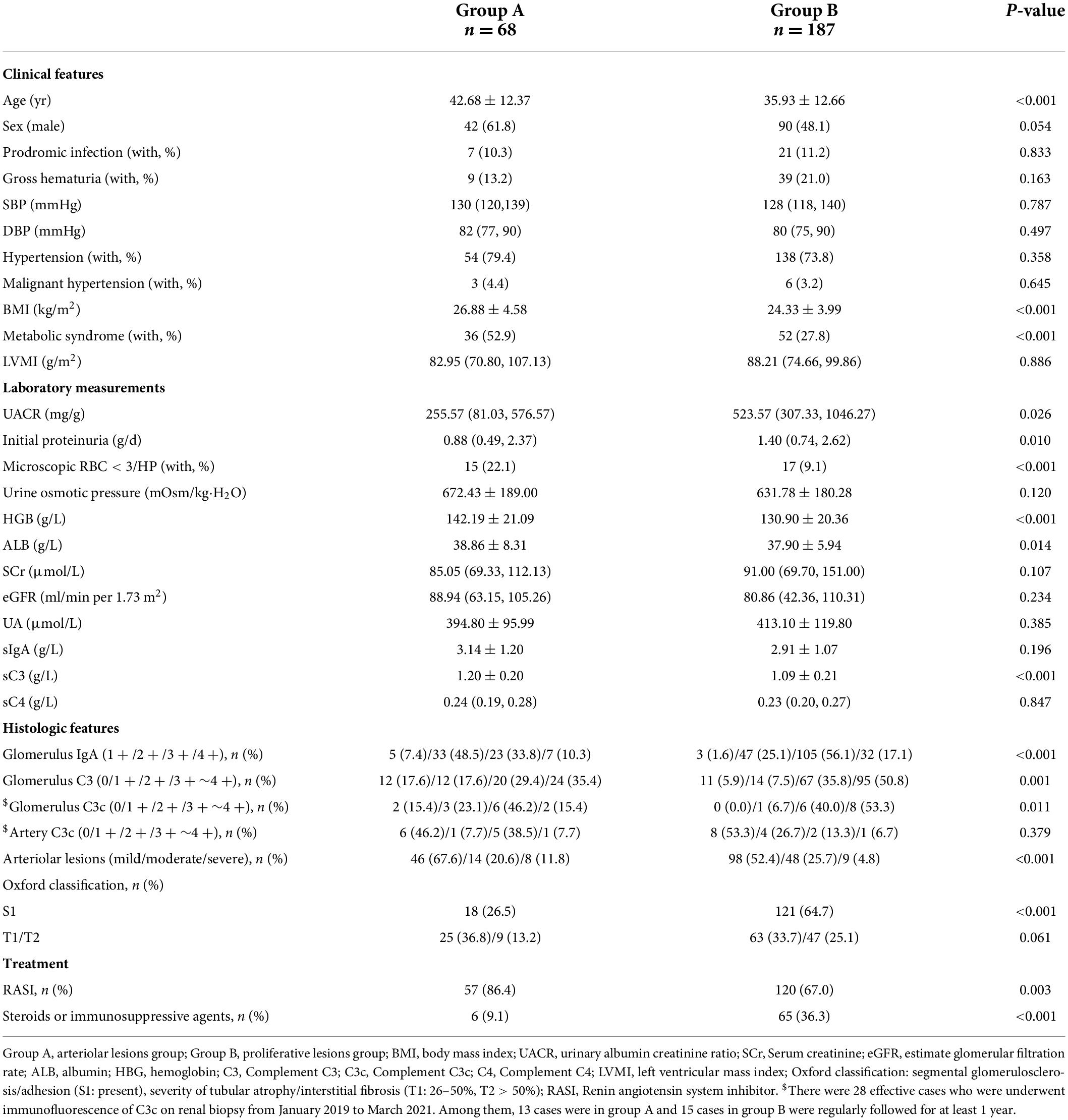
Table 4. Demographic, clinical, and histologic characteristics of patients with IgAN with follow-up. (n = 255).
We used the Cox proportional hazards model to compare prognosis between group A and group B. After adjustment for multiple clinical and histological risk factors, as well as steroids/immunosuppressive therapy, patients with group A showed no significantly risks of reaching the composite outcome compared with group B (HR = 2.253, 95% CI: 0.693–7.330, p = 0.194, Table 5 and Figure 4), which revealed that renal survival in group A was not better than that in group B.
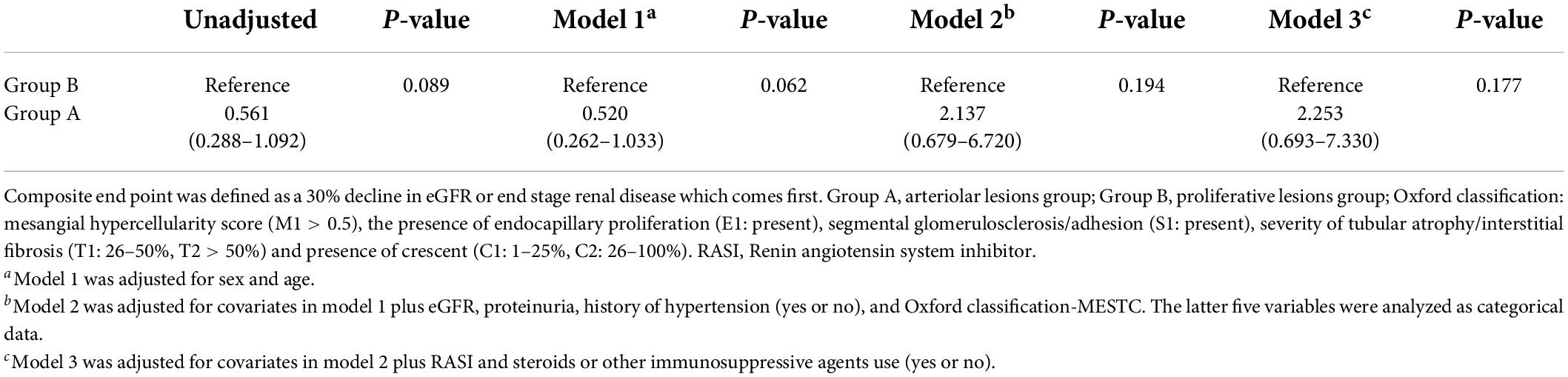
Table 5. Cox regression to compare the prognosis between the arteriolar lesion group and proliferative lesion group.
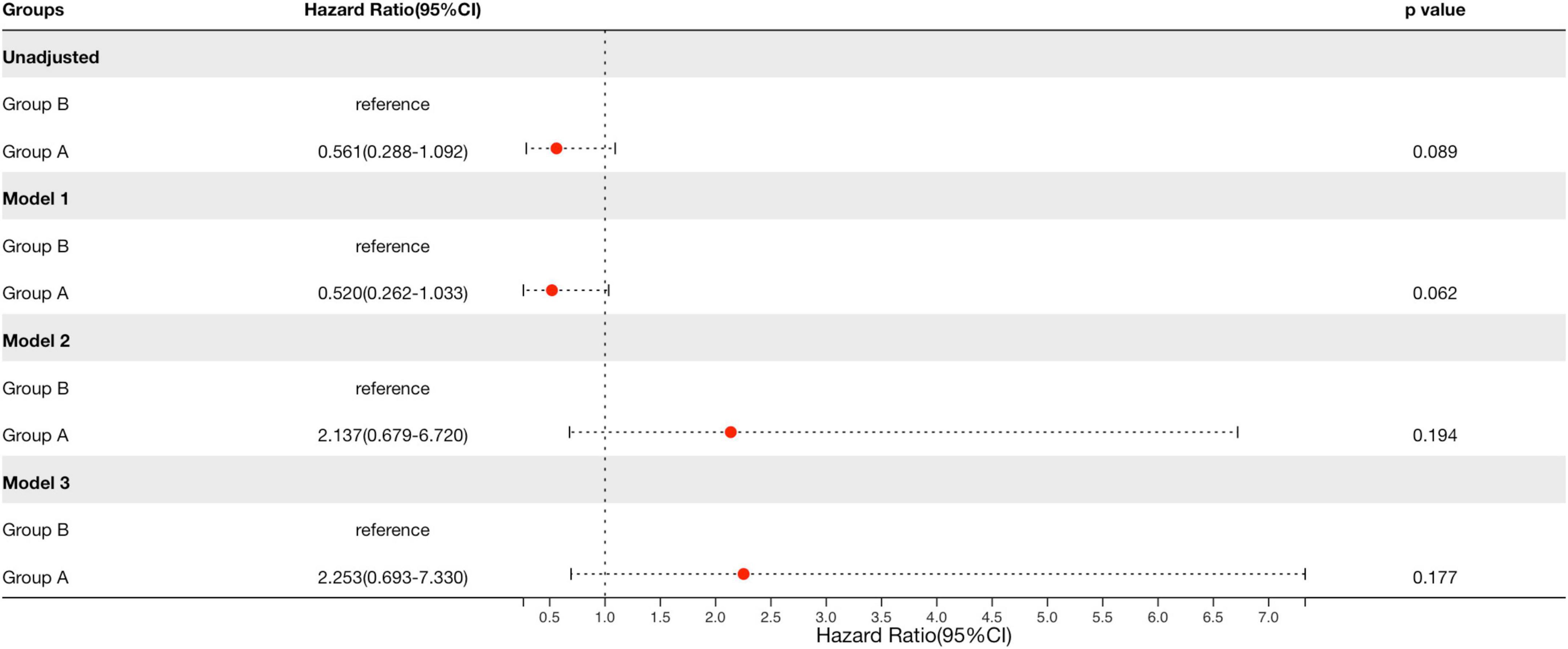
Figure 4. Cox regression to compare the prognosis between the arteriolar lesions group and proliferative lesions group in patients with IgAN. Group A, arteriolar lesion group; Group B, proliferative lesion group. Model 1 was adjusted for sex and age. Model 2 was adjusted for covariates in model 1 plus eGFR, proteinuria, history of hypertension (yes or no), and Oxford classification-MESTC. The latter five variables were analyzed as categorical data. Model 3 was adjusted for covariates in model 2 plus RASI and steroids or other immunosuppressive agents use (yes or no). Renal survival in group A was not better than that in group B after adjustment for multiple clinical and histological risk factors, as well as therapy.
Interestingly, patients in group A exhibited a lower intensity of C3 [0/1 + /2 + /3 + ∼4 + : 17 (44.7%)/3 (7.9%)/14 (36.8%)/4 (10.5%) vs. 38 (57.6%)/14 (21.2%)/9 (13.6%)/5 (7.6%), p = 0.027, Table 1 and Figure 5A] deposition in the glomerular mesangial and capillary areas than those in group B. In addition, the serum C3 level was higher in group A than in group B [1.18 (0.98, 1.32) mmol/L vs. 1.07 (0.94, 1.22) mmol/L, p < 0.001, Table 1], indicating that complement activation in both the circulation and glomeruli was more severe in group B than in group A. Moreover, patients in group A exhibited a higher intensity of C3c deposition [0/1 + /2 + /3 + ∼4 + : 17 (44.7%)/3 (7.9%)/14 (36.8%)/4 (10.5%) vs. 38 (57.6%)/14 (21.2%)/9 (13.6%)/5 (7.6%), p = 0.048, Table 1 and Figure 5B] in intrarenal arterioles than those in group B, indicating that complement activation was more severe in intrarenal arterioles in group A than in group B. In group A, as the severity of intrarenal arteriolar lesions worsened, the intensity of C3c deposition in intrarenal arterioles increased [0/1 + /2 + /3 + ∼4 + : mild subgroup: 14 (60.9%)/1 (4.3%)/7 (30.4%)/1 (4.3%) vs. moderate subgroup: 2 (20.0%)/1 (10.0%)/5 (50.0%)/2 (20.0%) vs. severe subgroup: 1 (20.0%)/1 (20.0%)/2 (40.0%)/1 (20.0%), p = 0.036, Table 2], and patients with or without hypertension showed no differ in the intensity of C3c deposition in intrarenal arterioles [0/1 + /2 + /3 + ∼4 + : hypertension subgroup: 14 (41.2%)/3 (8.8%)/13 (38.2%)/4 (11.8%) vs. non-hypertension subgroup: 3 (75%)/0 (0.0%)/1 (25.0%)/0 (0.0%), p = 0.233, Table 3]. Figures 6A–D shows representative images of immunofluorescence staining for C3c deposition in intrarenal arterioles (grades 0–3 +) in patients with IgAN. C3c and CD31 (biomarker of endothelial cells) co-deposited in intrarenal arterioles area in patients with IgAN with intrarenal arteriolar lesions. 2-dimensional (2D) fluorograms were used to analyze colocalization by Image-Pro Plus software (Media Cybernetics, Rockville, MD) (Pearson correlation = 0.928389, overlap coefficient = 0.928594) (Figures 6E–H).
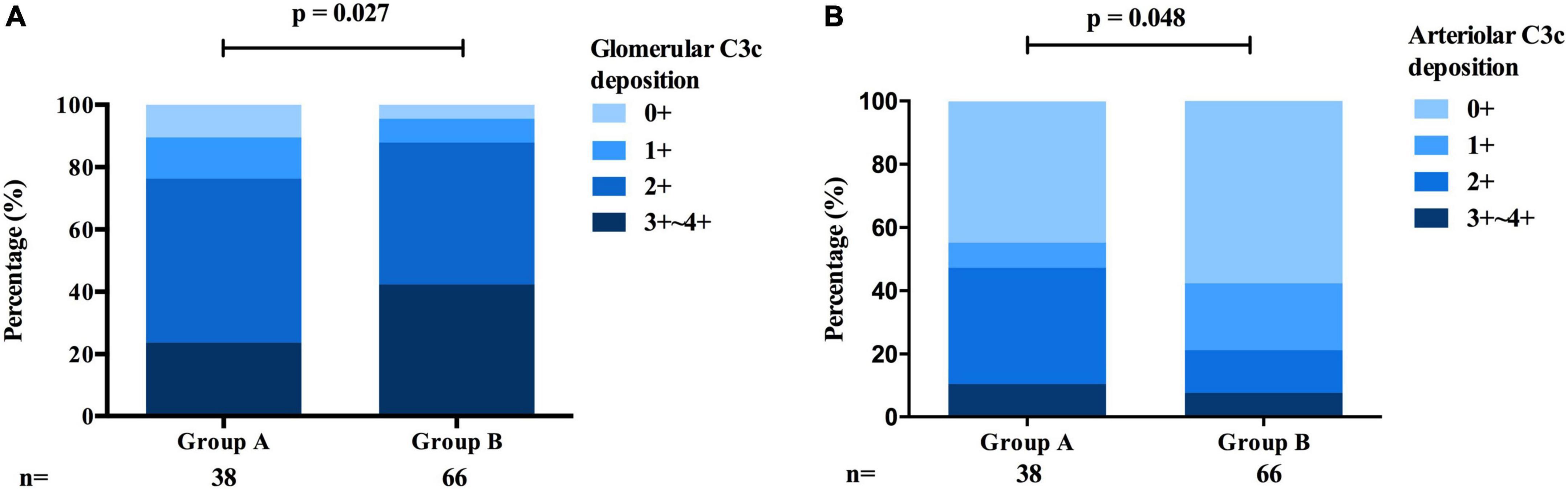
Figure 5. Comparison of the deposition of C3c between the arteriolar lesions group and the proliferative lesions group of patients with IgAN. (A) The intensity of C3c deposition in the glomerular mesangial and capillary areas was more severe in the proliferative lesions group than in the arteriolar lesions group. (B) The intensity of C3c deposition in intrarenal arterioles was more severe in the arteriolar lesions group than in the proliferative lesions group.
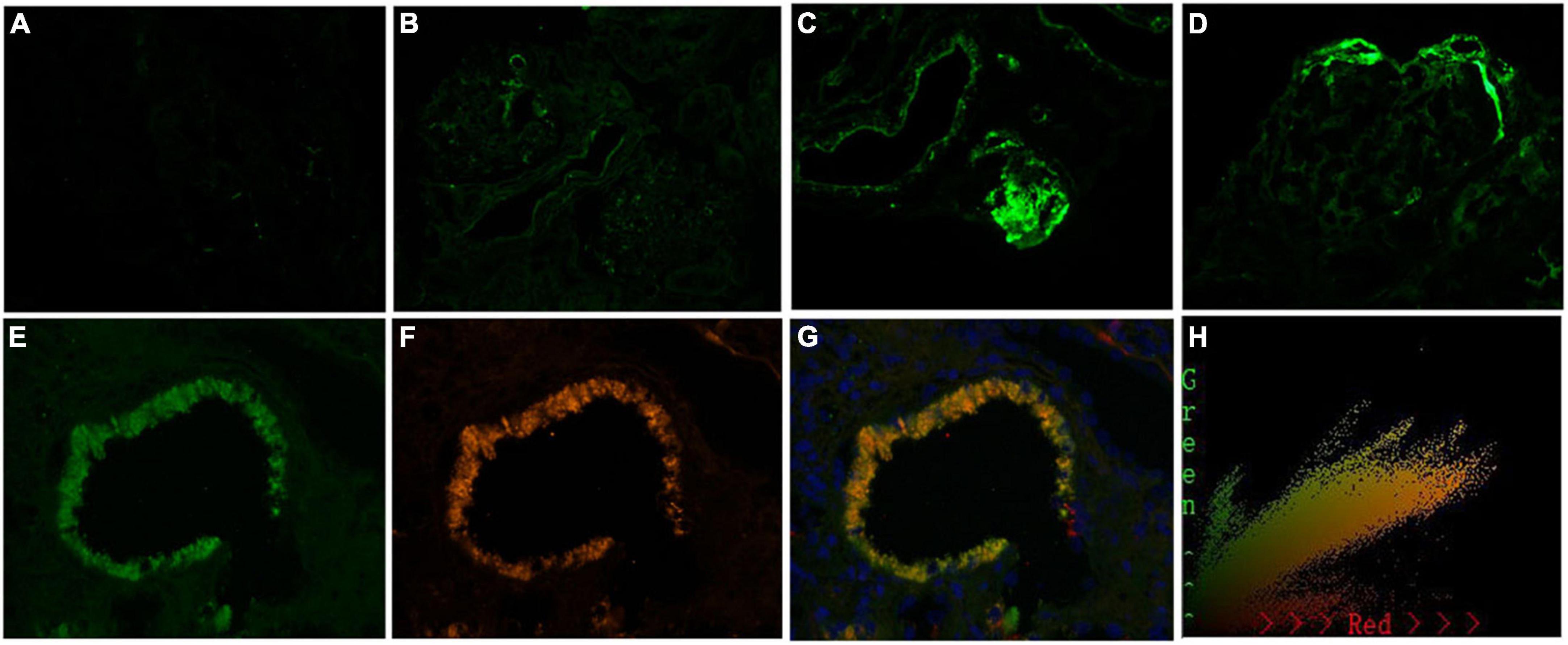
Figure 6. Representative images of immunofluorescence staining for C3c in arteries grade 0–3 +, and the colocalization of C3c and CD31 in patients with immunoglobulin A nephropathy (IgAN). Granular positive staining for C3c by immunofluorescence along intrarenal arterioles in patients with IgAN. (A) Negative. (B) 1 + intensity. (C) 2 + intensity. (D) 3 + intensity. (Original magnification × 200). (E) Granular positive staining for C3c, and (F) Granular positive staining for CD31 (biomarker of endothelial cells) by immunofluorescence along intrarenal arterioles. (G) C3c and CD31 colocalized along the intrarenal arterioles. (H) The corresponding 2-dimensional fluorograms have been included to confirm the degree of colocalization (Pearson correlation = 0.928389, overlap coefficient = 0.928594). (Original magnification ×400).
Furthermore, renal biopsies were stained with immunohistochemistry and immunofluorescence for complement components such as MBL, C4d, FH, and FHR5 and MAC. MBL (Figures 7A,G), C4d (Figures 7B,H), FH (Figures 7C,I), FHR5 (Figures 7D,J), C3c (Figures 7E,K), and MAC (Figures 7F,L) were found to be deposited in the intrarenal arterioles in group A of IgAN.

Figure 7. Positive staining for MBL (A,G), C4d (B,H), FH (C,I), FHR5 (D,J), C3c (E,K), and MAC (F,L) by immunohistochemistry and immunofluorescence, respectively, along intrarenal arterioles in the arteriolar lesions group of IgAN. (Original magnification ×200).
Gd-IgA1 mainly deposited in glomerulus and rarely deposited in the renal intrarenal arterioles (Figure 8A). Compared with IgAN patients in group B, patients in group A. In histological features, patients in group A exhibited a lower intensity of IgA [1 + /2 + /3 + /4 + : 13 (6.4%)/81 (40.1%)/75 (37.1%)/33 (16.3%) vs. 9 (1.6%)/137 (25.0%)/308 (56.1%)/95 (17.3%), p < 0.001, Table 1] and glomerular Gd-IgA1 [0/1 + /2 + /3∼4 + : 18 (47.4%)/7 (18.4%)/9 (23.7%)/4 (10.5%) vs. 7 (10.6%)/23 (34.8%)/28 (42.4%)/8 (12.1%), p = 0.003, Table 1 and Figure 8C] deposition in the glomerular mesangial and capillary areas than those in group B. There is no significant difference in serum IgA (group A: 3.02 ± 1.18 g/L vs. group B: 2.97 ± 1.13 g/L, p = 0.653, Table 1) and plasma Gd-IgA1 [group A: 6.24 (3.53, 12.94) g/L vs. group B: 7.27 (4.88, 9.25) g/L, p = 0.687, Table 1 and Figure 8C] between group A and group B.
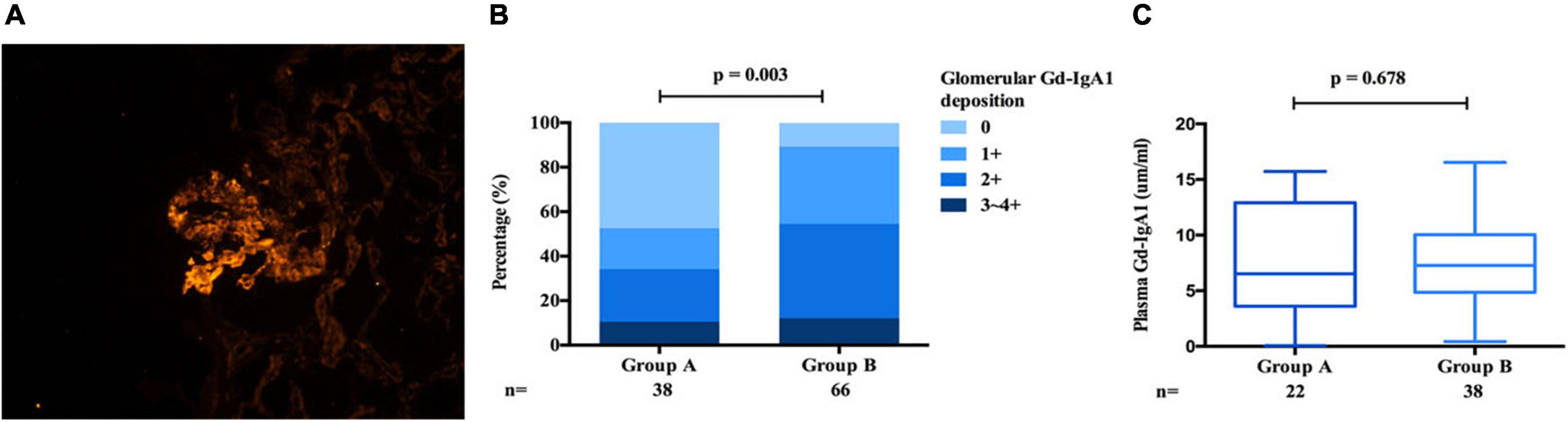
Figure 8. Gd-IgA1 in IgAN. Granular Gd-IgA1 (KM55) by immunofluorescence along the glomerular mesangial and capillary area in patients with IgAN (A). Gd-IgA1 mainly deposited in glomerulus and the intensity of Gd-IgA1 deposition in group B was significantly stronger than that of group A (B). Plasma Gd-IgA1 shows no differ in group A and group B (C).
Some patients with IgAN present atypical clinical manifestations, that is, present without protruding hematuria, with or without hypertension. The renal biopsy pathology of these patients shows predominant intrarenal arteriolar lesions. The renal function of patients with IgAN with this phenotype can deteriorate rapidly. This study focused on studying the clinical and pathological characteristics and prognosis of patients with this phenotype of IgAN and preliminarily explored whether complement is overactivated in this vascular disease.
In the present study, we found that compared with those in the proliferative lesions group, more IgAN patients in the arteriolar lesions group experienced hypertension, and fewer patients in the arteriolar lesions group experienced gross hematuria and microscopic hematuria. In addition, compared with those in the proliferative lesions group, patients in the arteriolar lesions group presented with less initial proteinuria. We also found that the renal prognosis of IgAN with only arteriolar lesions was not better than that of IgAN with proliferative lesions. A retrospective study found that the prevalence of intrarenal arterial lesions was up to 72.2% in patients with IgAN and that the presence of vascular lesions was associated with a reduced eGFR and poorer renal outcomes (6). Wu et al. (8) analyzed the intrarenal arterial lesions in 1005 Chinese patients with IgAN and found that the prevalence of intrarenal small artery and arteriolar lesions was 54.6%. Moreover, the severity of small arterial-arteriolar lesions was linked to adverse renal outcomes. Similarly, Nasri and Mubarak (25) found that 47.8% of IgAN patients presented with arteriosclerosis and 41.9% with arterial intimal fibrosis, and these arteriolar lesions were related to elevated levels of serum creatinine and proteinuria. However, the Oxford multicenter cohort study indicated that arteriolar lesions are not an independent risk factor that affects the prognosis of IgAN. They found that 40% of patients presented with arteriolar lesions, which was strongly correlated with the level of blood pressure and eGFR but not with proteinuria severity, and there was no significant correlation between arterial lesion severity and renal prognosis (26). Previous studies on vascular lesions in IgAN mostly focused on those patients with arteriolar lesions accompanied by proliferative lesions. However, our study mainly investigated IgAN with intrarenal arteriolar sclerosis lesions and rarely with glomerular proliferative lesions. We found that the renal prognosis of the arteriolar lesions group was not better than that of the proliferative lesions group.
Previous studies have shown that blood pressure is an independent factor that affects arteriolar injury in IgAN (6). However, our research and previous studies have shown that patients with IgAN, even those with normal blood pressure present with renal arteriolar sclerosis (8). Thus, we speculate that, apart from hypertension, other factors are involved in the pathogenesis and induce a poor renal prognosis in this phenotype of IgAN. In this study, we found that the positive rate of complement C3c deposition on the intrarenal arteriolar wall was 50/116 (43.1%). In the arteriolar lesions group, the intensity of C3c deposition on the walls of intrarenal arterioles was significantly stronger than that in the proliferative lesions group. Complement activation can generally occur through three different pathways (Figure 1). In this study, we found that complement components such as MBL (representing lectin pathway), C4d (the classical and lectin pathways), FH, and FHR5 (the alternative pathway), and MAC (the terminal pathway) are deposited on the walls of injured intrarenal arterioles, which suggests that the abnormal deposition of the above complement components on the intrarenal arteriolar wall may be involved in the pathogenesis of arteriole damage. Faria et al. (19) found that arteriolar C4d deposition was identified in 21/126 (16.7%) IgAN patients and that arteriolar C4d was a potential biomarker for disease progression in IgAN. Importantly, we also found that both hypertensive and non-hypertensive patients in the arteriolar lesions group showed no difference in the intensity of complement C3c deposition on the arterial wall. We speculated that the abnormal activation of the complement system may be involved in the pathogenesis of arteriolar damage, which might affect the prognosis of this clinical phenotype of IgAN.
Our study showed that, in addition to the deposition of complement MBL, C4d, C3c, and MAC on the damaged arteriolar wall, complement FH and FHR5 are also deposited on the wall of injured arterioles. FH is a negative regulatory protein in the alternative pathway that prevents overactivation of complement (27). FHR5 is a natural antagonist of FH and deregulates FH to overactivate the complement system (28, 29). Our previous research found that glomerular staining for FHR5 was observed in 57% of predominantly mesangial pattern with IgAN in biopsy specimens (11). The glomerular intensity of FHR5 deposition could indicate the severity of histologic lesions in IgAN (11, 30). Previous studies have also shown that FHR5 is deposited on the surface of vascular endothelial cells (31). We also speculate that FHR5 and FH are deposited in the endothelial cells of renal intrarenal arterioles and that then FHR5 competitively antagonizes the negative regulation of complement activation by FH, thus overactivating the complement system (32). Then, MACs form and damage endothelial cells, leading to arteriolar damage. The specific mechanism needs further exploration.
Gd-IgA1 is a pivotal molecule in the pathogenesis of IgAN (33–37). We found that plasma Gd-IgA1 shows no differ in proliferative lesions group and the arteriolar lesion group. Gd-IgA1 mainly deposited in glomerulus and the intensity of Gd-IgA1 deposition in the proliferative lesions group was significantly stronger than that of the arteriolar lesions group. However, Gd-IgA1 barely deposited in the renal intrarenal arterioles. We speculate that the damage of Gd-IgA1 to kidney might focus on glomerulus rather than intrarenal arterioles. We also found that complement C3c colocalized with CD31, a biomarker of endothelial cells, in intrarenal arterioles. We speculate that complement overactivation may cause the damage to intrarenal arterioles by injuring endothelial cells. In previous studies on atherosclerosis of the large and medium vasculature, the terminal complement complex C5b-9 affects atherogenesis by propelling vascular smooth muscle cell proliferation and migration, stimulating endothelial proliferation, and promoting vascular lesions formation (38). However, the specific mechanism of the complement system involved in intrarenal arteriole injury in this clinical phenotype of IgAN, which deserves further exploration.
We found that abnormal activation of complement may be involved in the pathogenesis of arteriolar damage in patients with IgAN, leading to a poor renal prognosis. We hope that our research might provide a theoretical basis for the intervention of complement activation in this clinical phenotype of IgAN in the future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committees of Beijing Anzhen Hospital. The patients/participants provided their written informed consent to participate in this study.
W-YG and HC made substantial contributions to the study concept and design. W-YG drafted the manuscript. HC critically revised the manuscript, supervised the entire study, and gave final approval to the article. W-YG, X-PA, and H-RD performed the experiments. W-YG and L-JS reviewed kidney biopsies from all patients and graded the staining intensity from anonymized sections. W-YG, X-PA, and NY conducted statistical analyses. NY, X-YX, G-QW, Z-RZ, and W-RC treated the patients and collected the primary data. All authors read and approved the final manuscript.
This work was supported by grants from the National Natural Science Foundation of China (81900653), the Natural Science Foundation of Beijing Municipality (7194258 and 7192050), and the Capital’s Funds for Health Improvement and Research (2022-2-2066).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. (1987) 64:709–27.
2. Berthoux FC, Mohey H, Afiani A. Natural history of primary IgA nephropathy. Semin Nephrol. (2008) 28:4–9. doi: 10.1016/j.semnephrol.2007.10.001
3. Rodrigues JC, Haas M, Reich HN. IgA nephropathy. Clin J Am Soc Nephrol. (2017) 12:677–86. doi: 10.2215/CJN.07420716
4. Pattrapornpisut P, Avila-Casado C, Reich HN. IgA nephropathy: Core curriculum 2021. Am J Kidney Dis. (2021) 78:429–41. doi: 10.1053/j.ajkd.2021.01.024
5. Kidney Disease: Improving Global Outcome (KDIGO) Glomerular Disease Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100: S1–276.
6. Zhang Y, Sun L, Zhou S, Xu Q, Xu Q, Liu D, et al. Intrarenal arterial lesions are associated with higher blood pressure, reduced renal function and poorer renal outcomes in patients with IgA nephropathy. Kidney Blood Press Res. (2018) 43:639–50. doi: 10.1159/000489290
7. Karoui KE, Hill GS, Karras A, Jacquot C, Mouloguet L, Kourilsky O, et al. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol. (2012) 23:137–48. doi: 10.1681/ASN.2010111130
8. Wu J, Chen X, Xie Y, Yamanaka N, Shi S, Wu D, et al. Characteristics and risk factors of intrarenal arterial lesions in patients with IgA nephropathy. Nephrol Dial Transplant. (2005) 20:719–27. doi: 10.1093/ndt/gfh716
9. Cai Q, Shi S, Wang S, Ren Y, Hou W, Liu L, et al. Microangiopathic lesions in IgA nephropathy: A cohort study. Am J Kidney Dis. (2019) 74:629–39. doi: 10.1053/j.ajkd.2019.03.416
10. Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, et al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. (2015) 26:1503–12. doi: 10.1681/ASN.2014101000
11. Guo WY, Sun LJ, Dong HR, Wang GQ, Xu XY, Zhao ZR, et al. Glomerular complement factor H-Related protein 5 is associated with histologic injury in immunoglobulin a nephropathy. Kidney Int Rep. (2021) 6:404–13. doi: 10.1016/j.ekir.2020.11.019
12. Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. (2006) 17:1724–34. doi: 10.1681/ASN.2005090923
13. Guo WY, Zhu L, Meng SJ, Shi SF, Liu LJ, Lv JC, et al. Mannose-binding lectin levels could predict prognosis in IgA nephropathy. J Am Soc Nephrol. (2017) 28:3175–81. doi: 10.1681/ASN.2017010076
14. Zhu L, Zhai YL, Wang FM, Hou P, Lv JC, Xu DM, et al. Variants in complement factor H and complement Factor H-related protein genes, CFHR3 and CFHR1, affect complement activation in IgA nephropathy. J Am Soc Nephrol. (2015) 26:1195–204. doi: 10.1681/ASN.2014010096
15. Wang Z, Xie X, Li J, Zhang X, He J, Wang M, et al. Complement activation is associated with crescents in IgA nephropathy. Front Immunol. (2021) 12:676919. doi: 10.3389/fimmu.2021.676919
16. Medjeral-Thomas NR, Lomax-Browne HJ, Beckwith H, Willicombe M, McLean AG, Brookes P, et al. Circulating complement factor H-related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int. (2017) 92:942–52. doi: 10.1016/j.kint.2017.03.043
17. Zhu L, Guo WY, Shi SF, Liu LJ, Lv JC, Medjeral-Thomas NR, et al. Circulating complement factor H-related protein 5 levels contribute to development and progresson of IgA nephropathy. Kidney Int. (2018) 94:150–8. doi: 10.1016/j.kint.2018.02.023
18. Trimarchi H, Coppo R. Glomerular endothelial activation, C4d deposits and microangiopathy in immunoglobulin A nephropathy. Nephrol Dial Transplant. (2021) 36:581–6. doi: 10.1093/ndt/gfz241
19. Faria B, Canão P, Cai Q, Henriques C, Matos AC, Poppelaars F, et al. Arteriolar C4d in IgA nephropathy: A cohort study. Am J Kidney Dis. (2020) 76:669–78. doi: 10.1053/j.ajkd.2020.03.017
20. Espinosa M, Ortega R, Sánchez M, Segarra A, Salcedo MT, González F, et al. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin J Am Soc Nephrol. (2014) 9:897–904. doi: 10.2215/CJN.09710913
21. Vlaicu SI, Tatomir A, Rus V, Mekala AP, Mircea PA, Niculescu F, et al. The role of complement activation in atherogenesis: The first 40 years. Immunol Res. (2016) 64:1–13. doi: 10.1007/s12026-015-8669-6
22. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Internal Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
23. Chen TY, Chen YP, Dong HR, Wang GQ, Cheng H. Clinical and pathological significance of intrarenal arteriolar lesions in the patients with IgA nephropathy with or without hypertension. Chin J Pract Internal Med. (2014) 34:391–4.
24. Working Group of the International IgA Nephropathy Network and the Renal Pathology Sociatty Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, et al. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. (2009) 76:546–56.
25. Nasri H, Mubarak M. Significance of vasculopathy in IgA nephropathy patients with regard to Oxford classification and immunostaining findings: A single center experience. J Renal Inj Prev. (2013) 2:41–5. doi: 10.12861/jrip.2013.16
26. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. (2009) 76:534–45.
27. Rodríguez de Córdoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sánchez-Corral P. The human complement factor H: Functional roles, genetic variations and disease associations. Mol Immunol. (2004) 41:355–67.
28. Skerka C, Chen Q, Fremeaux-Bacchi V, Roumenina LT. Complement factor H related proteins (CFHRs). Mol Immunol. (2013) 56:170–80. doi: 10.1016/j.molimm.2013.06.001
29. Goicoechea de Jorge E, Caesar JJE, Malik TH, Patel M, Colledge M, Johnson S, et al. Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci U S A. (2013) 110:4685–90. doi: 10.1073/pnas.1219260110
30. Medjeral-Thomas NR, Troldborg A, Constantinou N, Lomax-Browne HJ, Hansen AG, Willicombe M, et al. Progressive IgA nephropathy is associated with low circulating mannan-binding lectin-associated serine Protease-3 (MASP-3) and increased glomerular factor H-related Protein-5 (FHR5) deposition. Kidney Int Rep. (2018) 3:426–38. doi: 10.1016/j.ekir.2017.11.015
31. Murphy B, Georgiou T, Machet D, Hill P, McRae J. Factor H-related protein-5: A novel component of human glomerular immune deposits. Am J Kidney Dis. (2002) 39:24–7. doi: 10.1053/ajkd.2002.29873
32. Csincsi AI, Kopp A, Zöldi M, Bánlaki Z, Uzonyi B, Hebecker M, et al. Factor H-related protein 5 interacts with pentraxin 3 and the extracellular matrix and modulates complement activation. J Immunol. (2015) 194:4963–73. doi: 10.4049/jimmunol.1403121
33. Wyatt RJ, Julian BA. IgA nephropathy. N Engl JMed. (2013) 368:2402–14. doi: 10.1056/NEJMra1206793
34. Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. (1995) 100:470–4. doi: 10.1111/j.1365-2249.1995.tb03724.x
35. Coppo R, Amore A. Aberrant glycosylation in IgA nephropathy (IgAN). Kidney Int. (2004) 65:1544–7. doi: 10.1111/j.1523-1755.2004.05407.x
36. Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. (2007) 71:1148–54. doi: 10.1038/sj.ki.5002185
37. Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, et al. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. (2012) 82:790–6. doi: 10.1038/ki.2012.197
Keywords: IgA nephrology, intrarenal arteriolar lesion, complement system, C3c, renal survival
Citation: Guo W-y, An X-p, Sun L-j, Dong H-r, Cheng W-r, Ye N, Wang G-q, Xu X-y, Zhao Z-r and Cheng H (2022) Overactivation of the complement system may be involved in intrarenal arteriolar lesions in IgA nephropathy. Front. Med. 9:945913. doi: 10.3389/fmed.2022.945913
Received: 17 May 2022; Accepted: 18 July 2022;
Published: 03 August 2022.
Edited by:
Toshihiro Sawai, Shiga University of Medical Science, JapanReviewed by:
Laureline Berthelot, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceCopyright © 2022 Guo, An, Sun, Dong, Cheng, Ye, Wang, Xu, Zhao and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Cheng, ZHJjaGVuZ2hAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.