- 1First Military Hospital, Lublin, Poland
- 2Clinical Research Development Centre, Medical Research Agency, Warsaw, Poland
- 3Department of Clinical Immunology, Medical University of Warsaw, Warsaw, Poland
- 4Centre for Studies on Research Integrity, Institute of Law Studies, Polish Academy of Sciences, Warsaw, Poland
- 5Department of Medical Ethics and Palliative Medicine, Medical University of Warsaw, Warsaw, Poland
Pain is one of the most common symptoms in cancer patients including older adults. The objective of this study was to evaluate the enrollment criteria that can limit the inclusion of older adults in clinical trials concerning cancer-related pain (CRP). The study included 356 trials registered with ClinicalTrials.gov. Our primary outcome measures were the proportion of trials that excluded patients based on upper age limits (80 years of age or less), strict organ-specific exclusion criteria, broad and imprecise criteria, and inadequate performance score. One hundred and twenty-six trials (35.4%) had upper age limits. Strict exclusion criteria were used in 95 (26.7%) trials. Broad and imprecise exclusion criteria were listed in 57 (16.2%) trials. Low performance score was used as an exclusion criterion in 4 trials (1.1%). Overall, in 241 trials (67.7%) there was either an upper age limit or at least one strict or broad and imprecise exclusion criterion, or a criterion involving the performance status. The odds of excluding older adults were significantly higher in certain neoplasm types, study objectives, intervention types, and center locations. In conclusion, considerable proportion of recent clinical trials concerning CRP either explicitly exclude older adults or create high risk of such exclusion which substantially limits the evidence base for the treatment of such patients in clinical practice. Sponsors and investigators should consider careful modification of the enrollment criteria to improve the inclusion of older individuals who make up the major proportion of cancer patients population.
Introduction
Pain is one of the most common symptoms in cancer patients (1). It frequently occurs both in patients with solid tumors (2) and those with hematological malignancies (3). A recent meta-analysis showed that the prevalence of pain in patients receiving anticancer treatment, after the treatment, and those with advanced, metastatic, or terminal disease is 55, 39.3, and 66.4%, respectively (4). Pain is one of the most significant factors reducing the quality of life of cancer patients (5).
Treatment of cancer-related pain (CRP) is a major clinical challenge; a recent systematic review showed that ~30% of cancer patients do not receive analgesic treatment adequate to the pain intensity (6). Inadequate control of CRP can have a number of serious consequences including substantial disturbance of patient daily activities (7), reduced compliance with anticancer treatments (8), higher medical costs (9), and higher level of depression and anxiety of family caregivers of patients (10).
Pain is even the greater clinical problem in older adults with cancer (11–13). In view of a number of factors such as renal or hepatic impairment, other co-morbidities, and polypharmacy, older cancer patients can respond to various treatments differently to younger ones (14). Therefore, to ensure optimal care of older patients, doctors need the data on the benefits and harms of analgesic treatments coming from clinical trials involving such individuals. However, it is known that older patients have been underrepresented in clinical trials concerning cancer (15). One of the main barriers which can limit the enrollment of older cancer patients in clinical studies are stringent eligibility criteria (16–20).
However, to our knowledge, no studies have yet been performed to evaluate the enrollment criteria in clinical trials concerning CRP. We hypothesized that in many clinical trials concerning CRP these criteria also can limit the enrollment of older individuals. To verify our hypothesis, we assessed the enrollment criteria in clinical trials related to CRP that have been registered with ClinicalTrials.gov (CT.gov), the most comprehensive register of clinical studies in the world (https://www.clinicaltrials.gov/). We examined both the age limits used in the trials and the criteria that may indirectly limit the inclusion of older adults.
Methods
Selection of clinical trials
Pain in cancer patients can have a wide range of causes. In this study we classified as CRP both pain caused by the neoplasm itself (including the metastases) and that resulting from anticancer treatment including pharmacotherapy, radiotherapy, and surgery. However, it is estimated that ~9% of cancer patients experience pain that is unrelated to either the cancer itself or anticancer treatment (21). Therefore, to select eligible trials, in each case we checked whether a trial record contains information that a given study concerns CRP, and not pain due to other etiologies (e.g., pain due to osteoarthritis).
Clinical trials concerning CRP were searched for in CT.gov. In order to identify eligible trials we used the search term “Cancer pain” (field “Condition or disease”) which results in the selection of trials not only based on this specific term, but also its synonyms used by the CT.gov search engine including “Cancer related pain,” “Tumor related pain,” “Oncological pains,” “Oncology pains,” “Cancer associated pain,” and “Neoplasm related pain'. We used the following inclusion criteria: (1) Interventional studies; (2) Study start date on 01/01/2014 or later; (3) Primary purpose “Prevention,” “Treatment,” or “Supportive Care”. We excluded studies with the recruitment status “Suspended” or “Withdrawn”, trials performed on healthy volunteers, trials concerning pain other than CRP as defined above and pediatric clinical trials (however, if a trial enabled the enrollment of both adolescents and adults, it was included).
Data extraction and analysis
From record of each eligible study we extracted the following data: CT.gov identifier, recruitment status, sponsor(s), cancer type, intervention type, phase, enrollment, allocation, primary purpose, study start date. We also extracted the data on basic pain characteristics and relevant enrollment criteria, especially the age limits and the criteria related to bone marrow, liver, kidney, the cardiovascular system, and the pulmonary system. Moreover, we recorded psychiatric diseases and prior or concurrent malignancies listed as the exclusion criteria.
In the analysis of the enrollment criteria we used a classification system developed by Lewis et al. (22) and used in other studies on the exclusion of the elderly from clinical trials (19). In brief, the exclusion criteria related to any of the above-mentioned organs, systems, and diseases have been divided into two main categories—moderate and strict. Strict criteria required normal or nearly normal organ functions and/or laboratory parameters, while moderate criteria permitted the inclusion of patients with mild abnormalities. If a trial had both moderate and strict exclusion criteria related to the same organ or system, it was classified as having strict criteria. Of note, the classification includes “Mental illness making informed consent impossible” as a criterion related to psychiatry. Therefore, we considered cognitive impairment as one of such criteria (the classification does not include a separate category related to neurological diseases). Full list of the moderate and strict exclusion criteria related to different organs and systems is available at (https://theoncologist.onlinelibrary.wiley.com/doi/suppl/10.1634/theoncologist.2014-0093).
Apart from the exclusion criteria concerning function of specific organs and systems listed in the above-mentioned classification, we also recorded broad and imprecise criteria open to investigators' interpretation (e.g., “the presence of any significant disease”). Moreover, we extracted the data about the patient performance score listed as an exclusion criterion. When analyzing the data on the performance score, we assumed that grade 0 in the Eastern Cooperative Oncology Group (ECOG) scale corresponds to score 100 in Karnofsky scale (KS), ECOG grade 1 is equal to score 80–90 in KS, ECOG grade 2 corresponds to score 60–70 in KS, ECOG grade 3 is equal to score 40–50 in KS, and ECOG grade 4 corresponds to score ≤30 in KS (19).
Our primary outcome measures included the proportion of trials that excluded patients based on strict organ/system-specific exclusion criteria, broad and imprecise criteria, inadequate performance score (grade 2 or more in the ECOG scale), and arbitrary upper age limits (80 years of age or less).
Statistical analysis
Discrete variables were presented as absolute numbers and percentages, whereas continuous variables as medians with interquartile ranges. Statistical calculations were performed using R package (23). “Tidyverse” was used to process all the data (24). Multivariate logistic regression was employed to determine the relationship between the primary outcome measures and other characteristics of clinical trials. Temporal trends regarding the primary outcome measures were evaluated using univariate logistic regression. “Jtools” R package was used to export the created models to.xlsx format (25). Chi-Square test was employed to assess whether the presence of strict or broad exclusion criteria depended on the presence of pre-defined age limits of 80 years old or lower. In all analyses, p < 0.05 was considered a statistically significant threshold.
Results
Characteristics of the included clinical trials
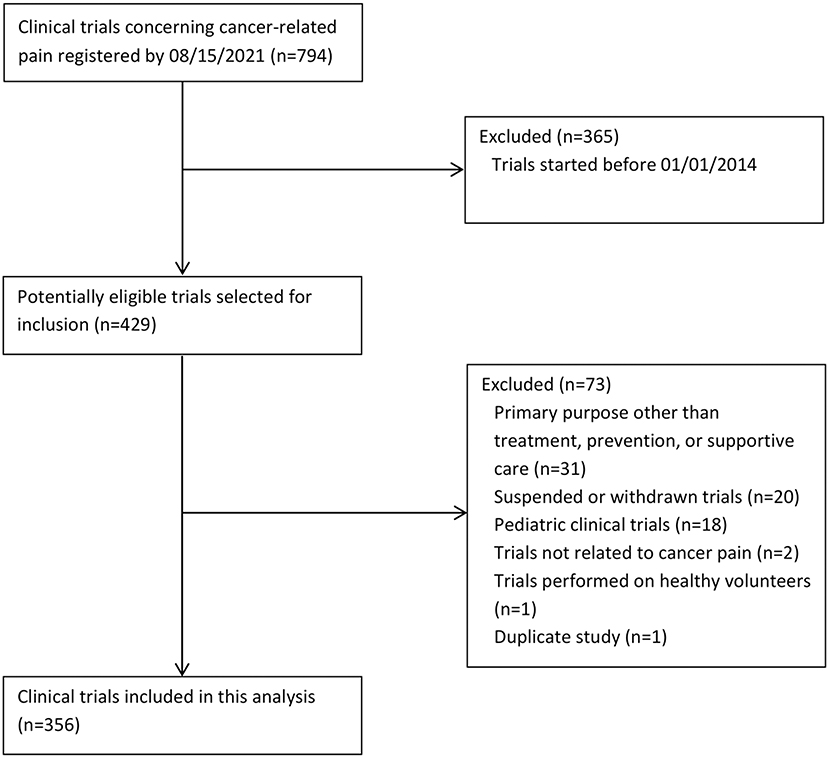
Clinical trials of interventions being evaluated in the prevention, treatment, or supportive care of patients with CRP were searched for in CT.gov (search date 08/15/2021). The selection of eligible trials is shown in Figure 1. Our initial search yielded 794 trials. Eventually we included 356 trials.
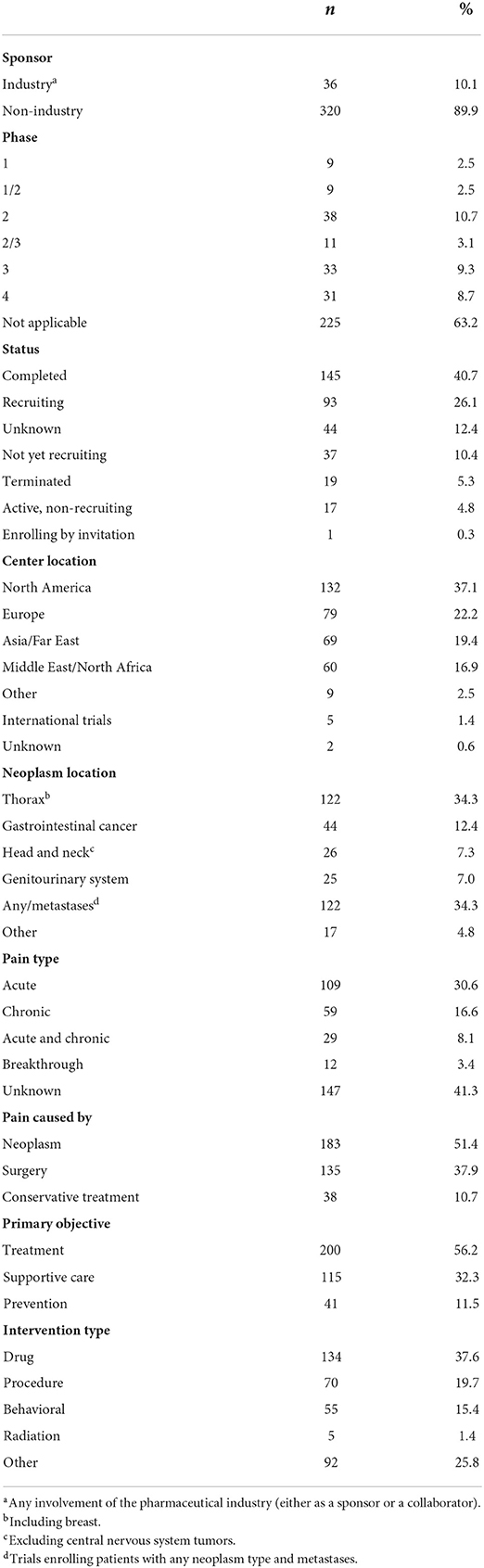
The characteristics of the included trials are shown in Table 1. Therapeutic intervention that was used most often were drugs (n = 134; 37.6%). Many trials enabled the enrollment of patients with any neoplasm type (n = 122; 34.3%). When a specific neoplasm type or neoplasm location was listed as the targeted condition, it was most often breast cancer (n = 104; 29.2%) followed by gastrointestinal cancer (n = 44; 12.4%), and head and neck cancer (HNC; n = 26; 7.3%). The median number of participants was 68 (IR range 37.5–120). The most common center locations included the USA (n = 111; 31.2%), Egypt (n = 38; 10.7%), and China (n = 36; 10.1%). Most of the trials were entirely funded by non-industrial sources (n = 320; 89.9%).
Assessment of the enrollment criteria
Regarding basic pain characteristics, 59 trials (16.6%) enabled the inclusion of patients with chronic pain and 109 (30.6%) recruited participants with acute pain. In addition, 29 trials (8.1%) allowed for the enrollment of individuals with both acute and chronic pain. In 12 trials (3.4%) patients with breakthrough pain could be included. However, in as many as 156 trials (41.3%) there was no information regarding pain type. We also found that 173 trials (48.6%) recruited patients with pain due to anticancer treatments; these included surgery (n = 135; 37.9%) and conservative treatments (n = 38; 10.7%).
Ninety-three trials (26.1%) listed as an inclusion criterion the minimal intensity of pain, as determined by numeric rating scale (NRS; median value, 4; IR range, 4–5). We also found that only 9 trials (2.5%) specified the maximal acceptable pain intensity (median value, 3; IR range 3–7). Nine (2.5%) and 1 (0.3%) trials specified the minimal and maximal intensity of pain, respectively, as determined by Brief Pain Inventory (BPI) scale.
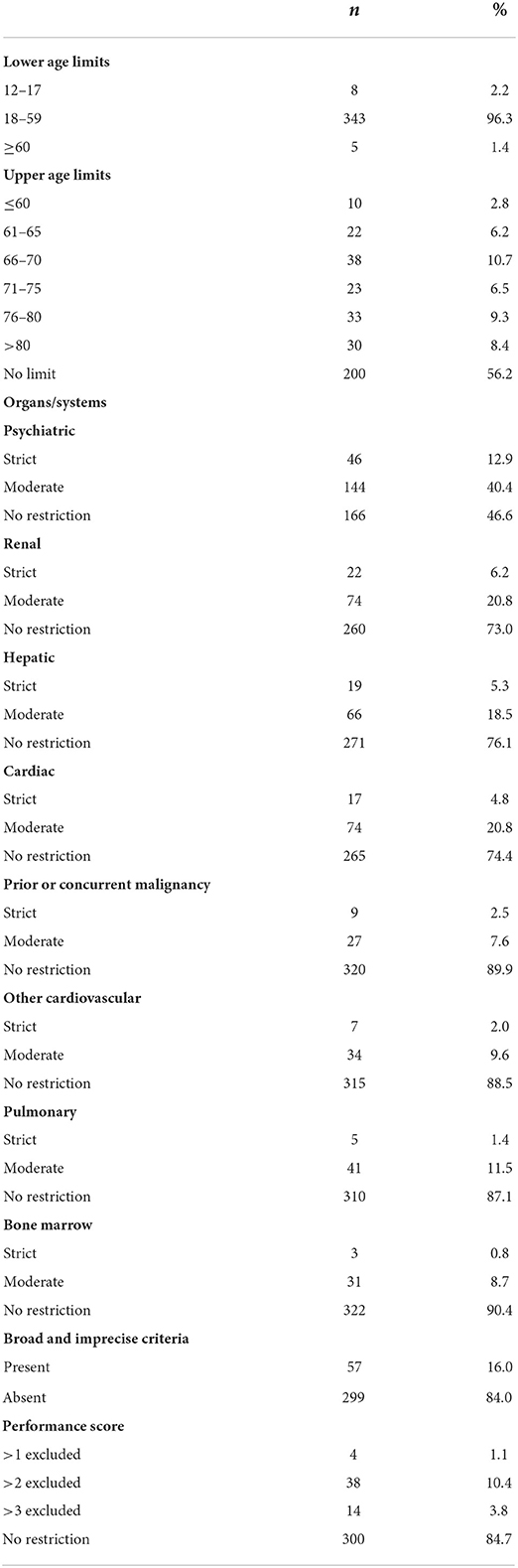
Detailed data on the age limits used in clinical trials concerning CRP are shown in Table 2. In most trials (n = 343; 96.6%) the minimal age of participants was between 18 and 59 years. Only five trials (1.4%) enrolled solely participants aged 60 or older. We also found that 156 trials (43.8%) excluded participants based on upper age limits. In many trials, upper age limits were within range 66–70, followed by 76–80 years of age (Table 2). The number of trials with the limits of 80 years of age or less (a primary outcome measure) was 126 (35.4%).
We also employed multivariate logistic regression to identify the factors significantly affecting the odds of excluding older adults based on the upper age limits, strict organ-specific criteria, and broad and imprecise criteria. The following factors were included in each logistic regression analysis: primary objective of the trial, neoplasm location, intervention type, phase, sponsor type, center location, number of patients, and whether trial concerned pain following cancer surgery. Detailed results of logistic regression are shown in Supplementary Tables 1–3. On multivariate analysis, higher odds of the limits of 80 years of age or less was found for trials in which the primary objective was the treatment [adjusted odds ratio (aOR), 4.05; confidence interval (CI), 1.86–8.8; p < 0.001], trials enrolling patients with certain neoplasm types, especially head and neck cancer (aOR, 4.9; CI, 1.59–15.11; p = 0.005) and cancer of the genitourinary system (aOR, 5.52; CI, 1.61–18.92; p = 0.006) as well as those conducted in Middle East and North African countries (aOR, 8.64; CI, 1.89–39.56; p = 0.005; Supplementary Table 1). On the other hand, the odds of excluding older adults based on the upper age limits was lower in trials in which a drug was used (aOR, 0.24; CI, 0.09–0.64; p < 0.01; Supplementary Table 1). However, we found no significant temporal trend toward decreasing the frequency of the upper age limits between 2014 and 2021 (p > 0.05).
We also assessed moderate and strict exclusion criteria pertaining to function of different organs and systems. Detailed data on the frequency of different criteria are presented in Table 2. These mostly concerned psychiatry (n = 190; 53.4%), specifically different psychiatric disorders (139 trials; 39%), cognitive impairment (83 trials; 23.3%), and substance abuse (65 trials; 18.3%); many trials listed a combination of these criteria. In most of these trials, the exclusion criteria related to psychiatry were fairly broad. Among the trials that listed psychiatric disorders as an exclusion criterion, only 33 (23.7%) provided concrete examples (mostly psychoses and depression) and 39 (28.1%) specified the severity of the disease, while 75 (54%) did not provide either any examples or details about the severity; rather, these referred mostly to “psychiatric diseases,” “psychiatric disorders” or other similar general terms. Among the trials in which cognitive impairment was listed as an exclusion criterion, only 29 (34.9%) provided a specific threshold value of the impairment beyond which the patient was ineligible.
Remarkably, in 46 trials (12.9%) the exclusion criteria pertaining to psychiatric disorders were strict (i.e., a history of a psychiatric disease and/or substance abuse). Other strict exclusion criteria involved impaired renal function (n = 22; 6.2%) and impaired hepatic function (n = 19; 5.3%). Few trials excluded patients based on the criteria concerning bone marrow function, malignancies, and the pulmonary and cardiovascular systems (Table 2). Overall, strict exclusion criteria (a primary outcome measure) were listed in 95 (26.7%) trials.
Multivariate logistic regression showed that the odds of strict exclusion criteria concerning the function of any organ/system was higher in trials in which a drug was used (aOR, 3.4; CI, 1.42–8.16; p = 0.006; Supplementary Table 2). We found no significant temporal trend toward decreasing the frequency of the strict exclusion criteria between 2014 and 2021 (p > 0.05).
Apart from the criteria pertaining to function of specific organs and systems, we also assessed broad and imprecise exclusion criteria. These generally did not involve any specific organs or diseases and were open to investigators' interpretation. We found that such criteria were applied in 57 trials (16.0%; Table 2). On multivariate analysis, the odds of broad and imprecise criteria was lower in trials recruiting patients with pain following surgical treatment of cancer (aOR, 0.35; CI, 0.13–0.97; p = 0.04) and those conducted in Middle East and North African countries (aOR, 0.08; CI, 0.01–087; p = 0.03; Supplementary Table 3). We found no significant trend toward decreasing the frequency of these criteria between 2014 and 2021 (p > 0.05).
Moreover, in 59 trials (16.5%) participants were excluded based on low performance status. The scales that were most commonly used included the ECOG scale (n = 40; 11.2%), followed by the KS (n = 18; 5%). However, only 4 trials (1.1%) excluded participants with the performance score of 2 or more in the ECOG scale or its equivalent in KS (a primary outcome measure; Table 2).
Overall, 241 trials (67.7%) excluded patients based on an upper age limit of 80 years of age or less, or at least one strict or broad and imprecise exclusion criterion, or inadequate performance score. We also noted that among 230 trials without an upper age limit, as many as 118 (51.3%) listed the criteria indirectly increasing the odds of the exclusion of older patients.
We also performed a sub-group analysis for the trials in which a drug was used. This analysis showed that among these trials the most common reason for a patient exclusion were strict organ/system specific criteria (n = 54; 40.3%) followed by the upper age limits (n = 48; 35.8%), and broad and imprecise criteria (n = 27; 20.1%). Very few trials excluded patients with inadequate performance score (n = 4; 3%). Overall, 103 trials (76.9%) either explicitly excluded older adults or had high risk of such exclusion.
Discussion
CRP can occur before the start of anticancer therapy, but it can also be a long-lasting and serious consequence of anticancer treatment including chemotherapy (26), radiotherapy (27), and surgery (28). To cover different causes of pain in cancer patients, we included to our study clinical trials concerning both pain due to the development of the cancer process itself, and pain being a result of chemotherapy, radiotherapy, and surgical treatment of cancer.
The most frequent intervention type in our study were drugs. However, the evidence is mounting that treatment of pain involving solely pharmacotherapy can be ineffective and unsafe (29). Therefore, recently there has been a clear tendency in pain medicine toward comprehensive pain management involving also non-pharmacological options (29, 30). Importantly, as shown by a number of recent systematic reviews and meta-analyses, some non-pharmacological treatments can be effective also in patients with cancer pain (31–35). Therefore, we included to our study clinical trials of non-pharmacological interventions alongside those evaluating the effects of analgesic drugs. Overall, the sample of the included clinical trials reflects the diversity of different causes of CRP and relevant investigational interventions.
Older adults can be excluded from clinical trials in a number of ways. The first and most apparent one involves the use of arbitrary upper age limits. However, older individuals can also be excluded in an indirect way—based on stringent criteria pertaining to function of different organs as well as the performance score (19). An analysis of 495 cancer clinical trials involving 59,300 participants showed that the relaxation of the criteria concerning function of different organs and the performance score would have increased the participation of the elderly by ~50% (22). Moreover, broad and imprecise exclusion criteria are considered to increase risk of excluding older adults (36). Each of these potential barriers was examined in our study.
Overall, we showed that many clinical trials concerning CRP either explicitly exclude older individuals based on the upper age limits or pose high risk of such exclusion due to stringent enrollment criteria. This substantially limits the evidence base for treating older adults with CRP. For instance, more than one third of the analyzed trials had upper age limits. However, the use of arbitrary age limits in clinical trials does not seem to be well-justified in view of the substantial heterogeneity of the aging process. Generally, the chronological age alone does not seem to be a good parameter reflecting an individual's state of health (37, 38). Rather than to exclude patients on the basis of the chronological age, investigators should consider performing geriatric assessment to identify older individuals who may be more susceptible to harms associated with investigational treatments (39, 40).
Remarkably, over a half of the trials that did not have upper age limits, did pose high risk of excluding older patients based on other criteria. This leads to a serious problem—even if older adults are enrolled to clinical trials, they are generally healthier and fitter compared with the average patient encountered in clinical practice. This problem was already reported for other cancer clinical trials (16).
Of note, only 1.4% of the analyzed trials were designed solely for participants aged 60 years or older. For comparison, 5% of the trials concerning the treatment of hematological malignancies enrolled solely participants aged 60 years and older (19). Another study showed that 5% of phase III trials of anticancer treatments published between 2011 and 2014 were dedicated to patients aged 60 years or older (41). Therefore, we believe that investigators should consider the design of more trials enrolling solely the elderly; otherwise, the data on the effects of drugs in these patients can be obtained from subgroup analyses which provide only preliminary evidence of the efficacy and safety of new therapeutic interventions (41).
Regarding the exclusion criteria that may indirectly limit the enrollment of older patients, most of these concerned psychiatry. In fact, 51% of the trials included to this analysis contained such criteria, of which 13.2% were strict. This fairly broad category included psychiatric diseases, substance abuse/addiction, and/or cognitive impairment.
Generally, there are several reasons for which patients with such disorders have been excluded from clinical trials. One of these are problems with obtaining informed consent which is one of the fundamental ethical and legal requirements for a participant's inclusion to a clinical trial. In clinical trials concerning pain an important problem is also a fact that psychiatric disorders may increase risk of abuse of at least some investigational analgesic drugs, especially opioids. The scale of this problem is very serious; in fact it is estimated that three million individuals in the USA and 16 million individuals worldwide have been affected by opioid use disorder—“opioid epidemic” (42). Importantly, psychiatric diseases and other substance abuse are risk factors for opioid misuse and addiction (43). Some other painkillers such as gabapentinoids also have some potential for addiction (44).
Another important reason for frequent exclusion of patients with psychiatric diseases and/or cognitive impairment is associated with the nature of pain which is a subjective symptom whose intensity cannot be assessed by any objective measurement method. Therefore, of primary importance is a clinical trial participant's ability to self-report the effect of an investigational treatment on the pain intensity. However, the ability to self-report pain symptoms is compromised in patients with cognitive impairment (45–47). Furthermore, some psychiatric diseases including schizophrenia and depression can substantially affect pain perception (48, 49).
Thus, on the one hand, the exclusion of patients with psychiatric diseases and/or cognitive impairment has solid justification. However, the prevalence of these disorders in older adults is fairly high. In fact, older age is a risk factor for the development of some neurodegenerative diseases, especially Alzheimer's disease and Parkinson's disease which are associated with serious cognitive impairment (50, 51). It is also known that cognitive impairment is a serious clinical problem in older patients with cancer (52, 53). For instance, one study revealed that its prevalence in individuals with cancer aged 65 years or more at the initiation of the treatment was as high as 46% (54). In patients with hematological malignancies the prevalence of cognitive impairment was even higher—up to 70% (55). Furthermore, some psychiatric diseases are known to occur frequently in older adults. An example of such a disease is depression; a recent systematic review revealed that the global prevalence of major depression in older individuals was 13.3% (56). Importantly, a substantial proportion of patients with depression also has cognitive impairment (57).
Overall, the frequent use of broad exclusion criteria concerning psychiatric disorders and/or cognitive impairment substantially limits the generalizability of the results of clinical trials concerning CRP. In our view, rather than to use broad exclusion criteria (such as “psychiatric disorder,” “mental illness,” or “cognitive impairment”), investigators should consider at least the inclusion of participants with disorders with relatively mild course, for instance mild cognitive impairment. There are several tools that can be used for cognitive assessment including examination of a patient with suspected cognitive impairment. Recent systematic review showed that 14 such tools can be used in clinical research settings, and six of these were evaluated in older patients (58).
A specific group of studies in our sample were trials evaluating the effects of perioperative analgesia in patients undergoing cancer surgery (mostly mastectomy). We consider this group of trials relevant to our study because they are very important for cancer survivors. This results from a fact that acute post-operative pain is a known to be a significant risk factor for developing persistent post-mastectomy pain (PPMP), a syndrome known to negatively affect mood, sleep, cognition, activities of daily living, social interactions, and overall quality of life of breast cancer survivors. Thus, by alleviating acute pain, perioperative analgesia can reduce risk of developing PPMP thereby substantially improving the quality of life of breast cancer survivors (59). This problem is important because PPMP is known to affect up to 50% of women following mastectomy (29). Logistic regression showed that trials concerning the treatment of pain following cancer surgery do not have higher risk of excluding older adults either based on the upper age limits or other criteria.
The main limitation to our study is that we analyzed only trials registered with CT.gov. There are several other registries of clinical trials which make up the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; https://www.who.int/clinical-trials-registry-platform). CT.gov is one of the data providers for the ICTRP. Thus, some clinical trials concerning CRP may have been registered with other registries and are missing from our analysis. However, CT.gov is the most comprehensive register of clinical studies in the world. Of note, a number of studies on the exclusion of older adults from clinical trials have been performed based on trials registered with CT.gov (17–19, 60).
In conclusion, many recent clinical trials concerning CRP either explicitly or implicitly exclude older participants. Given that it is older adults that make up the majority of cancer patients (14), overly restrictive enrollment criteria substantially limit the generalizability of trial results. Sponsors and investigators should consider careful modification of some of the exclusion criteria to improve the enrollment of older participants. In addition, separate trials with less stringent exclusion criteria may be designed to recruit solely older patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JB and TP contributed to conception and design of the study. KK and EK analyzed clinical trials concerning cancer-related pain. JB wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by funds from the research subvention obtained by the Medical University of Warsaw.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.945481/full#supplementary-material
References
1. Neufeld NJ, Elnahal SM, Alvarez RH. Cancer pain: a review of epidemiology, clinical quality and value impact. Future Oncol. (2017) 13:833–41. doi: 10.2217/fon-2016-0423
2. Nadler MB, Desnoyers A, Langelier DM, Amir E. The effect of exercise on quality of life, fatigue, physical function, and safety in advanced solid tumor cancers: a meta-analysis of randomized control trials. J Pain Symptom Manage. (2019) 58:899–908.e7. doi: 10.1016/j.jpainsymman.2019.07.005
3. Niscola P, Tendas A, Scaramucci L, Giovaninni M, Cupelli L, De Sanctis V, et al. Pain in malignant hematology. Expert Rev Hematol. (2011) 4:81–93. doi: 10.1586/ehm.10.79
4. van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. (2016) 51:1070–90.e9. doi: 10.1016/j.jpainsymman.2015.12.340
5. Kahan B. Cancer pain and current theory for pain control. Phys Med Rehabil Clin N Am. (2014) 25:439–56. doi: 10.1016/j.pmr.2014.01.013
6. Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. (2014) 32:4149–54. doi: 10.1200/JCO.2014.56.0383
7. Te Boveldt N, Vernooij-Dassen M, Burger N, Ijsseldijk M, Vissers K, Engels Y. Pain and its interference with daily activities in medical oncology outpatients. Pain Phys. (2013) 16:379–89. doi: 10.36076/ppj.2013/16/379
8. Richardson JL, Marks G, Levine A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol. (1988) 6:1746–52. doi: 10.1200/JCO.1988.6.11.1746
9. Fortner BV, Okon TA, Portenoy RK. A survey of pain-related hospitalizations, emergency department visits, and physician office visits reported by cancer patients with and without history of breakthrough pain. J Pain. (2002) 3:38–44. doi: 10.1054/jpai.2002.27136
10. Miaskowski C, Kragness L, Dibble S, Wallhagen M. Differences in mood states, health status, and caregiver strain between family caregivers of oncology outpatients with and without cancer-related pain. J Pain Symptom Manage. (1997) 13:138–47. doi: 10.1016/S0885-3924(96)00297-7
11. Brant JM. Assessment and management of cancer pain in older adults: strategies for success. Asia Pac J Oncol Nurs. (2018) 5:248–53. doi: 10.4103/apjon.apjon_11_18
12. Finnerty D, O'Gara Á, Buggy DJ. Managing pain in the older cancer patient. Curr Oncol Rep. (2019) 21:100. doi: 10.1007/s11912-019-0854-7
13. Hachem GE, Rocha FO, Pepersack T, Jounblat Y, Drowart A, Lago LD. Advances in pain management for older patients with cancer. Ecancermedicalscience. (2019) 13:980. doi: 10.3332/ecancer.2019.980
14. Marosi C, Köller M. Challenge of cancer in the elderly. ESMO Open. (2016) 1:e000020. doi: 10.1136/esmoopen-2015-000020
15. Hurria A, Levit LA, Dale W, Mohile SG, Muss HB, Fehrenbacher L, et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology Statement. J Clin Oncol. (2015) 33:3826–33. doi: 10.1200/JCO.2015.63.0319
16. Sedrak MS, Freedman RA, Cohen HJ, Muss HB, Jatoi A, Klepin HD, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. (2021) 71:78–92. doi: 10.3322/caac.21638
17. Duma N, Kothadia SM, Azam TU, Yadav S, Paludo J, Vera Aguilera J, et al. Characterization of comorbidities limiting the recruitment of patients in early phase clinical trials. Oncologist. (2019) 24:96–102. doi: 10.1634/theoncologist.2017-0687
18. Lockett J, Sauma S, Radziszewska B, Bernard MA. Adequacy of inclusion of older adults in NIH-funded phase III clinical trials. J Am Geriatr Soc. (2019) 67:218–22. doi: 10.1111/jgs.15786
19. Hamaker ME, Stauder R, van Munster BC. Exclusion of older patients from ongoing clinical trials for hematological malignancies: an evaluation of the National Institutes of Health Clinical Trial Registry. Oncologist. (2014) 19:1069–75. doi: 10.1634/theoncologist.2014-0093
20. Bellera C, Praud D, Petit-Monéger A, McKelvie-Sebileau P, Soubeyran P, Mathoulin-Pélissier S. Barriers to inclusion of older adults in randomised controlled clinical trials on Non-Hodgkin's lymphoma: a systematic review. Cancer Treat Rev. (2013) 39:812–7. doi: 10.1016/j.ctrv.2013.01.007
21. Grond S, Zech D, Diefenbach C, Radbruch L, Lehmann KA. Assessment of cancer pain: a prospective evaluation in 2266 cancer patients referred to a pain service. Pain. (1996) 64:107–14. doi: 10.1016/0304-3959(95)00076-3
22. Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. (2003) 21:1383–9. doi: 10.1200/JCO.2003.08.010
23. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/ (accessed May 10, 2022).
24. Wickham H, Averick M, Bryan J, Chang W. Welcome to the tidyverse. J Open Source Soft. (2019) 4:1686. doi: 10.21105/joss.01686
25. Long J. Jtools: Analysis and Presentation of Social Scientific Data (2020). Available online at: https://jtools.jacob-long.com/ (accessed May 10, 2022).
26. Quintão NLM, Santin JR, Stoeberl LC, Corrêa TP, Melato J, Costa R. Pharmacological treatment of chemotherapy-induced neuropathic pain: PPARγ agonists as a promising tool. Front Neurosci. (2019) 13:907. doi: 10.3389/fnins.2019.00907
27. Liu S, Zhao Q, Zheng Z, Liu Z, Meng L, Dong L, et al. Status of treatment and prophylaxis for radiation-induced oral mucositis in patients with head and neck cancer. Front Oncol. (2021) 11:642575. doi: 10.3389/fonc.2021.642575
28. Tait RC, Zoberi K, Ferguson M, Levenhagen K, Luebbert RA, Rowland K, et al. Persistent post-mastectomy pain: risk factors and current approaches to treatment. J Pain. (2018) 19:1367–83. doi: 10.1016/j.jpain.2018.06.002
29. Becker WC, Dorflinger L, Edmond SN, Islam L, Heapy AA, Fraenkel L. Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Fam Pract. (2017) 18:41. doi: 10.1186/s12875-017-0608-2
30. Tick H, Nielsen A, Pelletier KR, Bonakdar R, Simmons S, Glick R, et al. Evidence-based nonpharmacologic strategies for comprehensive pain care: the consortium pain task force white paper. Explore. (2018) 14:177–211. doi: 10.1016/j.explore.2018.02.001
31. Ruano A, García-Torres F, Gálvez-Lara M, Moriana JA. Psychological and non-pharmacologic treatments for pain in cancer patients: a systematic review and meta-analysis. J Pain Symptom Manage. (2022) 63:e505–20. doi: 10.1016/j.jpainsymman.2021.12.021
32. Sine H, Achbani A, Filali K. The effect of hypnosis on the intensity of pain and anxiety in cancer patients: a systematic review of controlled experimental trials. Cancer Invest. (2022) 40:235–53. doi: 10.1080/07357907.2021.1998520
33. Chiu HY, Hsieh YJ, Tsai PS. Systematic review and meta-analysis of acupuncture to reduce cancer-related pain. Eur J Cancer Care. (2017) 26:e12457. doi: 10.1111/ecc.12457
34. Lee SH, Kim JY, Yeo S, Kim SH, Lim S. Meta-analysis of massage therapy on cancer pain. Integr Cancer Ther. (2015) 14:297–304. doi: 10.1177/1534735415572885
35. Gallagher LM, Lagman R, Rybicki L. Outcomes of music therapy interventions on symptom management in palliative medicine patients. Am J Hosp Palliat Care. (2018) 35:250–7. doi: 10.1177/1049909117696723
36. Helfand BKI, Webb M, Gartaganis SL, Fuller L, Kwon CS, Inouye SK. The exclusion of older persons from vaccine and treatment trials for coronavirus disease 2019-missing the target. JAMA Intern Med. (2020) 180:1546–9. doi: 10.1001/jamainternmed.2020.5084
37. Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. (2014) 69:640–9. doi: 10.1093/gerona/glt162
38. Mitnitski A, Howlett SE, Rockwood K. Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci. (2017) 72:877–84. doi: 10.1093/gerona/glw089
39. Li D, Sun CL, Kim H, Soto-Perez-de-Celis E, Chung V, Koczywas M, et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. (2021) 7:e214158. doi: 10.1001/jamaoncol.2021.4158
40. Liu WY, Jin J, Tang Y, Li N, Tang Y, Wang J, et al. Safety and efficacy of preoperative chemoradiotherapy in fit older patients with intermediate or locally advanced rectal cancer evaluated by comprehensive geriatric assessment: a planned interim analysis of a multicenter, phase II trial. J Geriatr Oncol. (2021) 12:572–7. doi: 10.1016/j.jgo.2020.10.016
41. Le Saux O, Falandry C, Gan HK, You B, Freyer G, Péron J. Inclusion of elderly patients in oncology clinical trials. Ann Oncol. (2016) 27:1799–804. doi: 10.1093/annonc/mdw259
42. Azadfard M, Huecker MR, Leaming JM. Opioid addiction. In: StatPearls, eds Abai B, Abu-Ghosh A, and Acharya AB (Treasure Island, CA: StatPearls Publishing) (2021).
43. Webster LR. Risk factors for opioid-use disorder and overdose. Anesth Analg. (2017) 125:1741–8. doi: 10.1213/ANE.0000000000002496
44. Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. (2017) 27:1185–215. doi: 10.1016/j.euroneuro.2017.08.430
45. Kunz M, Mylius V, Scharmann S, Schepelman K, Lautenbacher S. Influence of dementia on multiple components of pain. Eur J Pain. (2009) 13:317–25. doi: 10.1016/j.ejpain.2008.05.001
46. Scherder EJ, Sergeant JA, Swaab DF. Pain processing in dementia and its relation to neuropathology. Lancet Neurol. (2003) 2:677–86. doi: 10.1016/S1474-4422(03)00556-8
47. Dubé CE, Mack DS, Hunnicutt JN, Lapane KL. Cognitive impairment and pain among nursing home residents with cancer. J Pain Symptom Manage. (2018) 55:1509–18. doi: 10.1016/j.jpainsymman.2018.02.012
48. Vaughan S, Failla MD, Poole HM, Forshaw MJ, McGlone F, Cascio CJ, et al. Pain processing in psychiatric conditions: a systematic review. Rev Gen Psychol. (2019) 23:336–58. doi: 10.1177/1089268019842771
49. Nitzan U, Hecht M, Braw Y, Maoz H, Levkovitz Y, Yarnitsky D, et al. Initial evaluation of pain intensity among depressed patients as a possible mediator between depression and pain complaints. Front Psychiatry. (2019) 10:48. doi: 10.3389/fpsyt.2019.00048
50. Atri A. The Alzheimer's disease clinical spectrum: diagnosis and management. Med Clin North Am. (2019) 103:263–93. doi: 10.1016/j.mcna.2018.10.009
51. Goldman JG, Sieg E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. (2020) 6:365–77. doi: 10.1016/j.cger.2020.01.001
52. Loh KP, Janelsins MC, Mohile SG, Holmes HM, Hsu T, Inouye SK, et al. Chemotherapy-related cognitive impairment in older patients with cancer. J Geriatr Oncol. (2016) 7:270–80. doi: 10.1016/j.jgo.2016.04.008
53. Edelstein A, Pergolizzi D, Alici Y. Cancer-related cognitive impairment in older adults. Curr Opin Support Palliat Care. (2017) 11:60–9. doi: 10.1097/SPC.0000000000000254
54. Dubruille S, Libert Y, Merckaert I, Reynaert C, Vandenbossche S, Roos M, et al. The prevalence and implications of elderly inpatients' desire for a formal psychological help at the start of cancer treatment. Psychooncology. (2015) 24:294–301. doi: 10.1002/pon.3636
55. Koll TT, Sheese AN, Semin J, Ernst W, High R, Wildes TM, et al. Screening for cognitive impairment in older adults with hematological malignancies using the Montreal Cognitive Assessment and neuropsychological testing. J Geriatr Oncol. (2020) 11:297–303. doi: 10.1016/j.jgo.2019.11.007
56. Abdoli N, Salari N, Darvishi N, Jafarpour S, Solaymani M, Mohammadi M, et al. The global prevalence of major depressive disorder (MDD) among the elderly: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2022) 132:1067–73. doi: 10.1016/j.neubiorev.2021.10.041
57. Varghese S, Frey BN, Schneider M, Kapczinski F, Cardoso TA. Functional and cognitive impairment in the first episode of depression: a systematic review. Acta Psychiatr Scand. (2022) 145:156–85. doi: 10.1111/acps.13385
58. Gilbert T, Bosquet A, Thomas-Antérion C, Bonnefoy M, Le Saux O. Assessing capacity to consent for research in cognitively impaired older patients. Clin Interv Aging. (2017) 12:1553–63. doi: 10.2147/CIA.S141905
59. Yuksel SS, Chappell AG, Jackson BT, Wescott AB, Ellis MF. Post mastectomy pain syndrome: a systematic review of prevention modalities. JPRAS Open. (2021) 31:32–49. doi: 10.1016/j.jpra.2021.10.009
Keywords: elderly, older adults, clinical trial, cancer pain, cancer-related pain, enrollment criteria
Citation: Krysa K, Kowalczyk E, Borysowski J, Lachota M and Pasierski T (2022) Exclusion of older adults from clinical trials in cancer-related pain. Front. Med. 9:945481. doi: 10.3389/fmed.2022.945481
Received: 16 May 2022; Accepted: 21 July 2022;
Published: 04 August 2022.
Edited by:
Simone Scarlata, Campus Bio-Medico University, ItalyReviewed by:
Marco Russano, Campus Bio-Medico University, ItalyTomasz Kostka, Medical University of Lodz, Poland
Copyright © 2022 Krysa, Kowalczyk, Borysowski, Lachota and Pasierski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Borysowski, amJvcnlzb3dza2lAaW50ZXJpYS5wbA==
 Krzysztof Krysa1
Krzysztof Krysa1 Jan Borysowski
Jan Borysowski Mieszko Lachota
Mieszko Lachota