
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 26 September 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.944208
 Klervi Golhen1†
Klervi Golhen1† Carolyn Winskill2†
Carolyn Winskill2† Martin Theiler3
Martin Theiler3 Michael Buettcher1,4
Michael Buettcher1,4 Yu-Hsin Yeh2
Yu-Hsin Yeh2 Nancy Zhang2
Nancy Zhang2 Tatjana Welzel1,5
Tatjana Welzel1,5 Marc Pfister1,2*
Marc Pfister1,2*Background: Psoriasis is a chronic immune-mediated inflammatory skin disease affecting both adults and children. To better understand the efficacy-safety profile of biologics in children with moderate-to-severe psoriasis, this study aimed to analyze efficacy and safety data of randomized controlled trials (RCTs) performed in pediatric psoriasis and to compare efficacy outcomes in children with those in adults.
Methods: RCTs investigating biologics in children with moderate-to-severe psoriasis were identified in a systematic literature review. PASI75/90 treatment responses at weeks 11/12 were analyzed comparing biologics with control arms. Serious adverse events (SAEs) were analyzed at the end of each study. Efficacy data from RCTs in adults with psoriasis were selected for the same biologics. Risk ratios (RR) of selected RCTs were pooled together in a statistical random effects model using the inverse variance method.
Results: For children, there were 1 etanercept, 2 secukinumab, 1 ixekizumab and 1 ustekinumab placebo-controlled RCTs and 1 adalimumab RCT using methotrexate as reference arm at weeks 11/12. For adults, out of 263 RCTs, 7 adalimumab and 15 etanercept (TNF inhibitors) and 4 ixekizumab and 12 ustekinumab (IL-17 and IL-12/23 inhibitors) RCTs reported PASI75/90 efficacy responses at weeks 11/12. Regarding efficacy, all biologics showed improved PASI responses over control arms. RRs ranges were 2.02–7.45 in PASI75 and 4.10–14.50 in PASI90. The highest PASI75 responses were seen for ustekinumab 0.375 mg/kg (RR = 7.25, 95% CI 2.83–18.58) and ustekinumab 0.75 mg/kg (RR = 7.45, 95% CI 2.91–19.06) in the CADMUS study. The highest PASI90 response was seen for ixekizumab (RR = 14.50, 95% CI 4.82–43.58) in the IXORA-PEDS study. SAE incidences in pediatric and adult arms with biologics were 0 to 3% except for a pediatric arm with adalimumab 0.40 mg/kg (8%). For adults, pooled RR also showed improved PASI responses over placebo for all biologics, with highest PASI75 response observed for ixekizumab (pooled RR = 16.18, 95% CI 11.83–22.14).
Conclusion: Both adults and children with psoriasis show superior efficacy with biologics compared to control arms after 3 months of treatment with SAE incidences in the low percentages. Additional longer-term clinical studies are warranted to fully understand the overall efficacy-safety profile of biologics in children with moderate-to-severe psoriasis.
Psoriasis is a chronic immune-mediated systemic disease with the skin as the major affected organ presenting with well-demarcated pink-to-erythematous plaques with overlying hyperkeratotic plaques. The onset may be during child- or adulthood. The worldwide prevalence is estimated at 0.51 to 11.3% in adults and 0 to 1.37% in children (1). Chronic plaque psoriasis is the most common form in children, typically involving the face and scalp. Psoriasis is often associated with serious comorbidities such as cardiovascular disease, metabolic syndrome, chronic kidney disease, arthritis, psychosocial effects, and inflammatory bowel disease (2, 3). Risk factors may include environmental (skin trauma, infections, medications, and psychological stress) and genetic [human leukocyte antigen (HLA) type Cw6 (PSORS1) or CARD14 mutation] factors (4). Psoriasis is driven by the innate and adaptive immune systems, partly characterized by the chronic activation of T-helper cells (Th17) and secretion of proinflammatory cytokines such as interleukin 17 (IL-17) and tumor necrosis factor-α (TNF-α) in response to IL-23 (5). Diagnosis is based on the clinical features and most patients do not need a skin biopsy for histopathological evaluation. The Physician’s Global Assessment of Disease Activity (PGA) is a 6-point scale used to measure the severity of disease at the time of the evaluation. Severity and extent of psoriasis disease activity can be measured in children and adults with the Psoriasis Area and Severity Index (PASI). Psoriasis is grouped into “mild-to-moderate” (less than 10% of body surface area involved) and “moderate-to-severe” (more than 10% of body surface area involved) plaque psoriasis. The “moderate-to-severe” group, approximately 10 to 20% of the pediatric age group, often requires phototherapy and/or systemic treatment. Without effective treatment, psoriasis results in decreased psychosocial health and reduced quality of life (6). Several indices exist to assess the impact of psoriasis on the health-related quality of life (HRQOL) in children. The Children’s Dermatology Life Quality Index (CDLQI) is a 10-item questionnaire for measuring HRQOL in children aged from 4 to 16 years (7).
In the past, established therapeutic management included topical agents, phototherapy, conventional systemic treatments (e.g., methotrexate, ciclosporin, and acitretin), and biologics such as TNF inhibitors (8). Recent breakthroughs in the understanding of psoriasis pathogenesis have resulted in the use of IL-17 and IL-12/23 targeted therapies. The approval of IL-17 and IL-12/23 inhibitors has revolutionized treatment of moderate-to-severe plaque psoriasis in adults and most compounds have recently been approved for use in children aged 6 years and older (9). These new treatment options have significantly facilitated treatment of children with moderate-to-severe psoriasis (10). Given the requirement for potentially lifelong treatment, an excellent safety profile is paramount, especially when treating children. Currently, there are only a limited number of studies comparing different biologics in the pediatric population, making decisions on what biologic to use difficult. Finding the preferred initial biologic for children is challenging and has to be based on factors of safety, patient comorbidities, frequency of dosing and drug availability and licensing in the respective country.
This study aimed to (i) summarize and analyze available efficacy and safety data of randomized controlled trials (RCTs) performed in pediatric psoriasis patients, (ii) compare published treatment outcomes with those in adults, and (iii) provide an overview of current psoriasis treatment strategies in children and adults to (iv) better understand the efficacy-safety balance of biologics with the goal of supporting clinicians in their therapeutic decisions in clinical practice, and those developing new treatment options for children with moderate-to-severe psoriasis.
This systematic review was conducted based on the Cochrane Handbook for Systematic Reviews of Interventions and reporting items in the PRISMA statement (11, 12) and focused on comparing risk ratios (RRs) of PASI75 or PASI90 (efficacy endpoints) and incidence of adverse events (AEs) of interest (safety endpoints) between RCTs in adults and children with psoriasis treated with biologics. It should be noted that the typical timepoint for efficacy and safety evaluation for pivotal RCTs in pediatric drug development and approval is 12 weeks. Therefore, a majority of reported pivotal clinical RCTs in children with moderate-to-severe psoriasis investigated efficacy and safety endpoints 3 months after treatment start. For this reason we focused our systematic review and meta-analysis on one key timepoint: drug-related effects on efficacy and safety at week 11/12 of treatment.
Randomized controlled trials for all pediatric rheumatology patients were identified in a systematic literature search initially conducted on July 26, 2020 in MEDLINE, ClinicalTrials.gov and the EU Clinical Trials Register with a sample size of ≥5 children with pediatric inflammatory rheumatic disease (PiRD), including moderate-to-severe psoriasis (PiRD) aged ≤20 years and treated with predefined biologics (13). On February 3, 2022 this literature search was updated in line with the previously described review protocol to ensure that the most recent publications on pediatric moderate-to-severe psoriasis are included (Figure 1) (13). An additional search was conducted in the SCOPUS database using the same search terms used for MEDLINE but this did not yield any additional publications of relevance. When multiple references for a single study (e.g., more than one journal article1, regulatory documents) were available, data from one study were pulled from all available sources. Identified RCTs fulfilling the following inclusion criteria were included in the analysis: (i) population: plaque psoriasis; (ii) treatment: abatacept, adalimumab, anakinra, baricitinib, belimumab, brodalumab, canakinumab, certolizumab, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rilonacept, risankizumab, rituximab, sarilumab, secukinumab, tildrakizumab, tocilizumab, tofacitinib, upadacitinib, or ustekinumab; (iii) control: placebo, no treatment, or conventional systemic therapies including methotrexate, ciclosporin, or acitretin; (iv) outcomes: PASI75 or PASI90 responses; (v) time point: 3 months after treatment start (at week 11/12). Funnel plots of all included studies were performed to assess publication bias (Supplementary Figure 2).
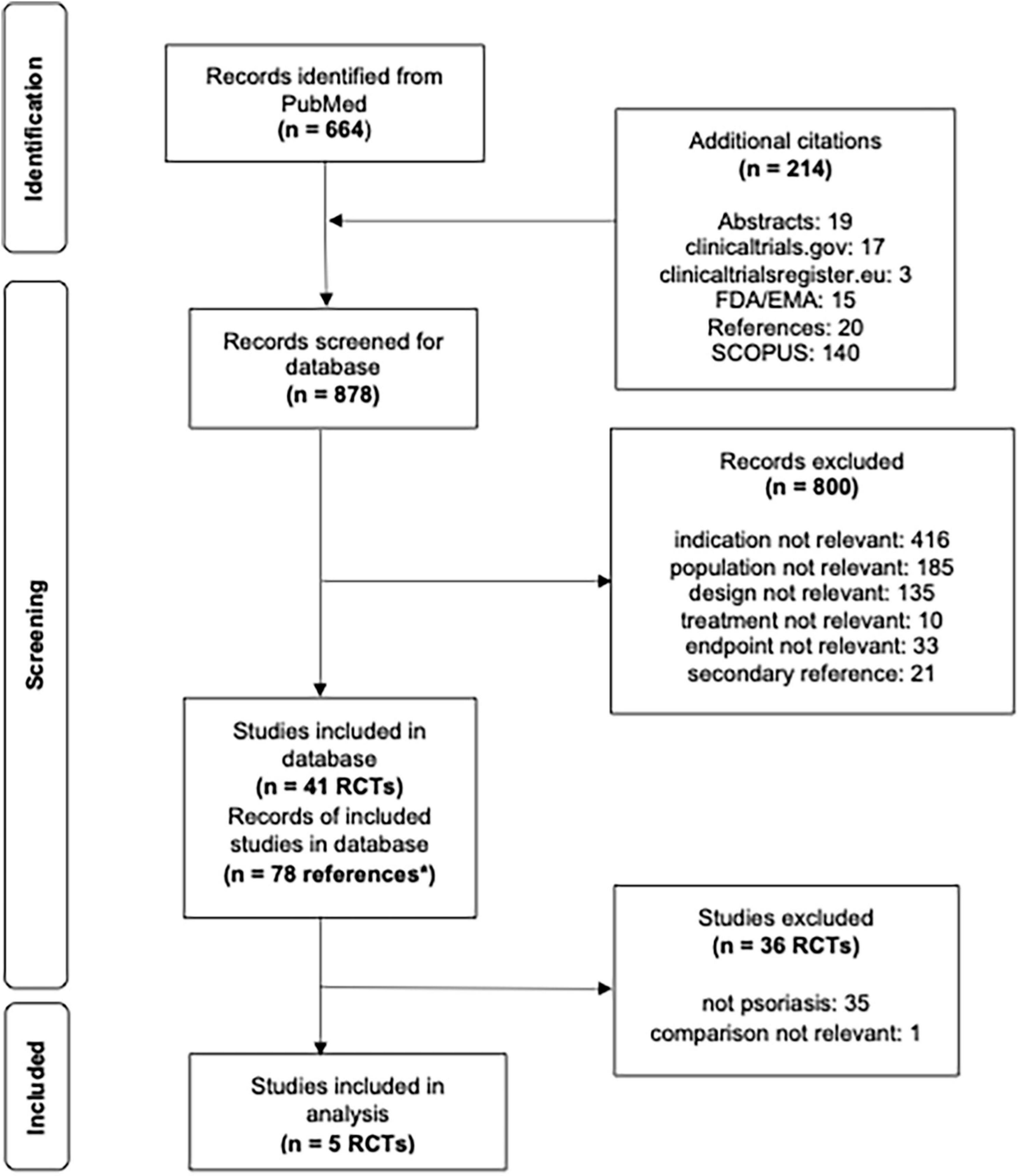
Figure 1. Flowsheet literature review and pediatric study selection for analysis. *n = 78 correspond to multiple references to a single study, i.e., more than one journal article, clinicaltrials.gov and/or regulatory documents.
Randomized controlled trials for adult plaque psoriasis patients that met similar criteria as the RCTs for pediatric psoriasis were similarly identified from an existing up-to-date clinical outcomes database (Certara Psoriasis CODEx database) as of October 11, 2021. The search for the adult psoriasis RCTs used the following search terms: (psoria*[TIAB]) AND (list of drugs) AND (randomized controlled trial[publication type] OR (randomized [TIAB] OR randomized [TIAB])) NOT case reports[publication type] NOT review[publication type] NOT comment[publication type] NOT letter[publication type] NOT rats[MeSH] AND humans[MeSH]. Studies strictly in psoriatic arthritis patients were excluded. To facilitate analysis, other exclusion criteria were applied that differed from the selection of pediatric studies: (i) control: placebo only; and (ii) sample size of ≥ 20 patients.
It should be noted that only data from RCTs were considered for systematic review and meta-analyses. Observational studies and review articles were excluded.
Aggregate (summary)-level data was extracted for each selected RCT. Study design, baseline demographic and clinical characteristics such as location, patient population, sample size, age criteria, and treatment, as well as efficacy and safety data were captured. In addition, detailed dosing information from published RCTs in children and adults with psoriasis were tabulated.
The Psoriasis Area and Severity Index responses PASI75 and PASI90 at weeks 11 or 12 (i.e., after 3 months of treatment) were used to describe efficacy for both adults and children. In cases of duplicate data within a study, data from the intent-to-treat (ITT) population was selected over per-protocol or completed populations and imputed data was selected over non-imputed data. PGA ≤ 1 and CDLQI ≤ 1 responses after 3 months of treatment were also extracted for exploratory analysis.
Each RCT for adult and pediatric psoriasis patients included in the systematic literature review was screened for safety data at the end of induction treatment (i.e., 12 or 16 weeks). Safety data was selected according to hierarchical inclusion criteria to ensure each study arm had only one data point: (1) overall population with all grades of AEs, (2) stratified population with all grades of AEs (if data was reported separately for different subgroups, like regions of an arm instead of the arm overall), and (3) overall population with non-serious AEs. Safety data of interest included (i) overall AEs, (ii) serious adverse events (SAEs), (iii) overall infections, (iv) serious infections, (v) upper respiratory tract infections (URTIs), (vi) gastroenteritis, (vii) autoimmune reactions, and (viii) dermatologic AEs. To maintain consistency across studies, only the proportion or number of patients with AEs was captured. Rate data (events/patient-year or total number of events) was not captured.
For the pediatric data, raw proportions (%) in each study arm for PASI75 and PASI90 responses 3 months after treatment initiation were recorded or calculated as the number of subjects with response divided by the total number of subjects evaluable for response. RR were calculated as percentage of response in biologics arm divided by percentage of response in control arm. Further, 95% confidence intervals (CIs) for RRs were computed utilizing the exact method. All CIs that did not include 1 indicated significant effects. RRs of PASI75 and PASI90 3 months after treatment initiation were visualized using forest plots. The pooled RR for each outcome was calculated utilizing the DerSimonian-Laird method with a random effects model. Heterogeneity between studies was assessed using I2 (the proportion of variability between studies due to heterogeneity). Heterogeneity was defined by the following I2 thresholds: no heterogeneity I2 = 0%, low I2 < 30%, moderate 30 ≤ I2 ≤ 59%, and high I2 ≥ 60%. Since the meta-analysis for each PASI outcome included fewer than 10 studies, statistical significance of heterogeneity was assessed as p < 0.10. Meta-regression was also not performed due to the number of studies. Raw proportions (%) of participants achieving a PGA score of 0 or 1 and a CDLQI score of 0 or 1 3 months after treatment initiation were also recorded for each study. For the adult data, RR for all four efficacy outcomes for each distinct biologic were grouped and pooled together in a statistical random effects model using the inverse variance method.
The incidence of each AE of interest was summarized descriptively for each arm at the end of each study in addition to calculating the risk difference (percentage of AEs in biologic arm - percentage of AEs in control arm). For the adult data, the number of patients with the AE and the number of patients evaluable for safety analysis were combined across arms evaluating the same treatment of interest (i.e., all adalimumab data were pooled together and all placebo data were pooled together for the same group of studies).
Dosing information from approved biologics in children and adults was summarized in Table 5.
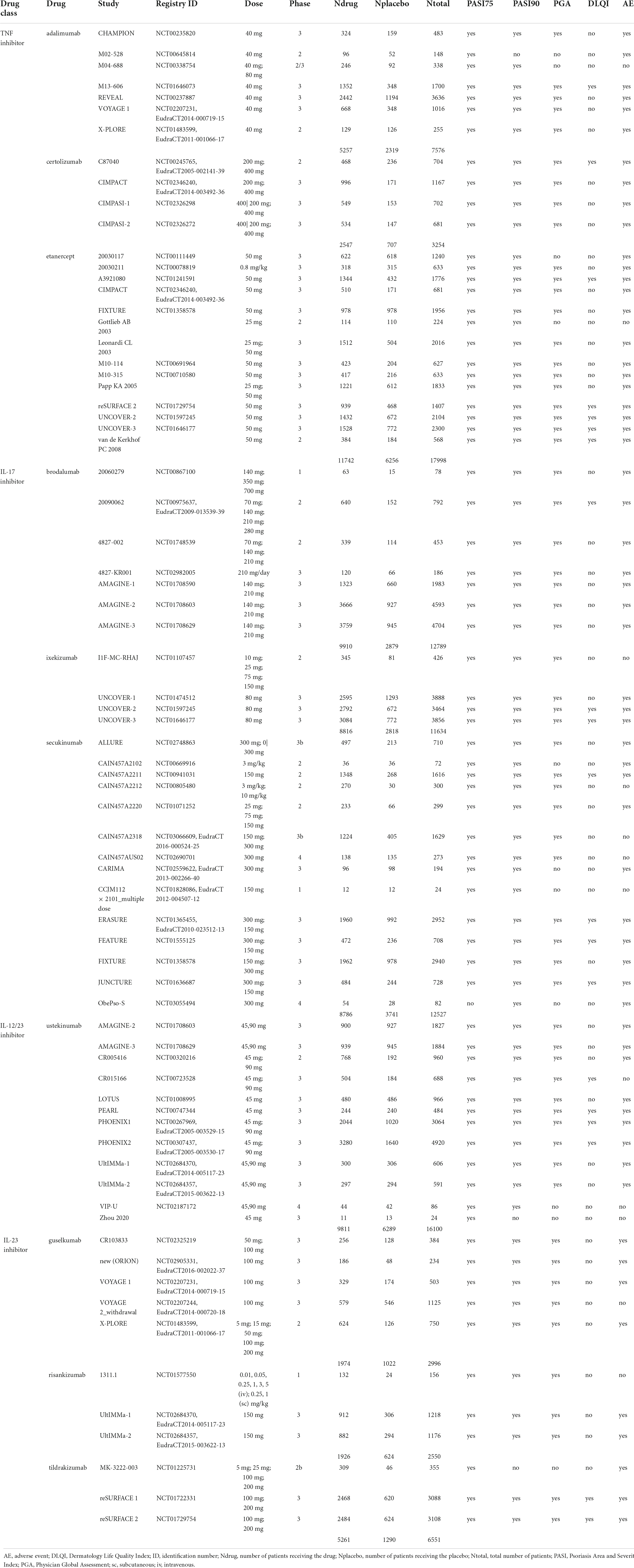
Table 1. Overview of adult psoriasis studies used for comparison against pediatric psoriasis studies.
Publication bias was assessed using visual inspection of the funnel plots; asymmetry of the funnel plots was assessed using Begg’s test (rank correlation method) and Egger’s test (linear regression method). Statistical significance for publication bias was assessed as p < 0.05. Meta-analyses were performed using the “meta” package in R (version 4.0.3). All forest plots and other graphs were generated using RStudio (version 1.2.5042).
A comprehensive overview of currently available dose information from approved biologics in children and adults is provided.
In this section we summarize results from the performed literature search, data collection, and systematic literature review for efficacy and safety outcomes in pediatric and adult RCTs. We also provide an overview of approved doses of biologics in pediatric psoriasis and compare those with dosing approaches in adults with psoriasis.
Six out of a total of 41 PiRD studies were conducted in pediatric psoriasis patients. Two studies investigated secukinumab [IL-17 inhibitor], CAIN457A2310 (NCT02471144) (14) and CAIN457A2311 (NCT03668613) (15, 16). The other four studies investigated other biologics (M04-717 (NCT01251614) [adalimumab, TNF inhibitor] (16), 20030211 (NCT00078819) [etanercept, TNF inhibitor] (17–19), IXORA-PEDS (NCT03073200) [ixekizumab, IL-17 inhibitor] (20), CADMUS (NCT01090427) [ustekinumab, IL-12/23 inhibitor]) (21). No studies were identified in pediatric patients treated with JAK inhibitors. All the inclusion criteria of the pediatric studies required the patients to be candidates for systemic therapy; to be poorly controlled by topical therapy; to have a history of psoriasis for at least 6 months; a PASI of at least 12 (with the exception of a PASI score of at least 20 for adalimumab, secukinumab, and ixekizumab in countries where etanercept was approved); a PGA of at least 3; body surface area (BSA) involvement at screening and baseline of at least 10% (except adalimumab with at least 20% BSA involved). Only the adalimumab trial required heliotherapy or phototherapy to have failed, be contraindicated or not tolerated; in no other trial previous treatment with a conventional systemic or phototherapy was required. All six studies reported PASI75 and PASI90 at weeks 11 or 12. The CAIN457A2311 secukinumab study was excluded from analysis as the comparison was out of scope (secukinumab high dose versus secukinumab low dose). The five remaining studies reported PGA ≤ 1 at week 12 and three studies reported CDLQI ≤ 1 at week 12. The comparator arm for the adalimumab study was methotrexate while the other four studies were placebo controlled (Figure 1).
Out of a total of 263 adult studies available in the Certara CODEx database, there were 7 adalimumab (22–28), 7 brodalumab (29–34), 4 certolizumab (35–37), 14 etanercept (17, 36, 38–49), 4 ixekizumab (46, 50, 51), 5 guselkumab (27, 28, 52, 53), 3 risankizumab (54, 55), 14 secukinumab (42, 56–63), 3 tildrakizumab (47, 64), and 12 ustekinumab (34, 55, 65–71) placebo-controlled RCTs that reported PASI75/PASI90 treatment responses at weeks 11 or 12 (Table 1). To be consistent with the pediatric studies, only monotherapy arms were selected for analysis. Inclusion criteria for pediatric studies compared to adult studies seem to be comparable or slightly more stringent regarding severity (PASI) and less stringent regarding pre-treatment with systemic treatments and phototherapy, given that these treatments are not licensed for the studied population. Overall, it is not possible to exclude that the adult population had slightly more severe disease at inclusion, given that patients had to be refractory to standard care including systemic treatment or phototherapy.
In this section we report the key efficacy endpoints PASI75 and PASI90, Physician’s Global Assessment of Disease Activity (PGA) ≤ 1 and Children’s Dermatology Life Quality Index (CDLQI) ≤ 1 in children with psoriasis, and PASI75, PASI90, PGA, and DLQI in adults with psoriasis.
All biologics arms in the pediatric psoriasis RCTs indicated significant treatment effects in PASI responses over control arms (Table 2). RRs ranged from 2.02–7.45 in PASI75 (Figure 2A) and 4.10–29.72 in PASI90 (Figure 2B). Disease severity was moderate to severe in all RCTs at inclusion with the exception of the CADMUS study (severe). The lowest PASI75 treatment response was seen for adalimumab and the lowest PASI90 treatment response in a study arm with etanercept (both TNF inhibitors). The highest PASI75 treatment responses were seen for ustekinumab 0.375 mg/kg (RR = 7.25, 95% CI 2.83–18.58) and ustekinumab 0.75 mg/kg (RR = 7.45, 95% CI 2.91–19.06) compared to placebo in the CADMUS study (Figure 2A). The highest PASI90 treatment responses were seen for ixekizumab (RR = 14.50, 95% CI 4.82–43.58) in the IXORA-PEDS study and for secukinumab (RR = 29.72 for low dose and RR = 27.68 for high dose) in the CAIN457A2310 study (Figure 2B). The pooled RR was 4.16 (95% CI 3.21–5.39) for PASI75 (Figure 2A) and 9.28 (95% CI 5.80–14.86) for PASI90 (Figure 2B), indicating that arms with biologics are significantly superior compared to control arms. There was low heterogeneity in the meta-analysis of both PASI outcomes (I2 = 21% and p = 0.26 for PASI75; I2 = 3% and p = 0.41 for PASI90) (data not shown).
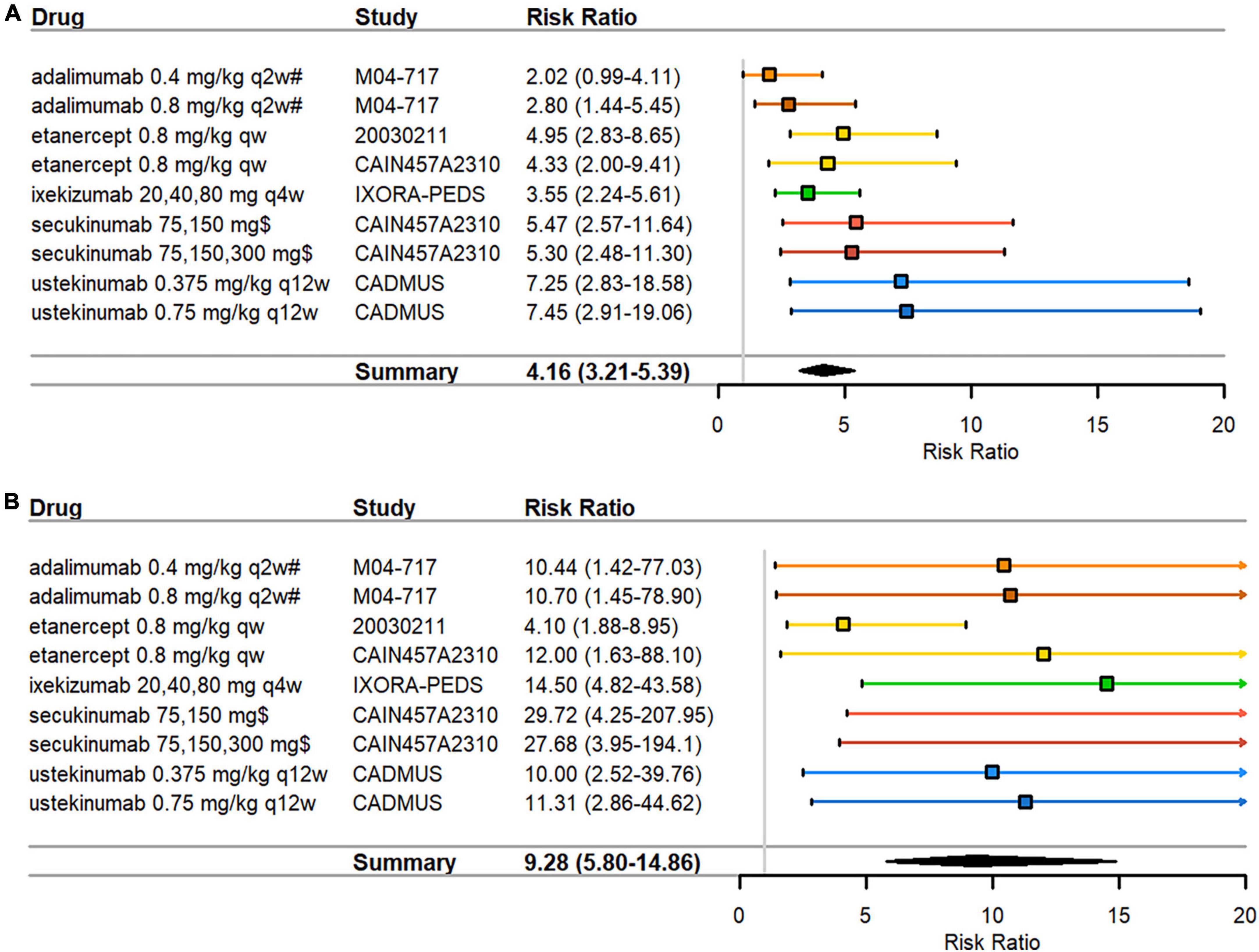
Figure 2. Pediatric psoriasis RCTs that reported efficacy data at 3 months. (A) PASI75 (B) PASI90 RRs—mean represented by the square—were calculated as percent response in biologics arm divided by percent response in control arm. Overall effect estimate is represented by the diamond, with the width showing the CIs for the overall estimated effect estimate. Further, 95% CIs for RRs—represented by the whiskers—were computed utilizing the exact method. All CIs that did not include 1 indicated significant effects. Experimental treatment is preferred when RR > 1. PASI, Psoriasis Area and Severity Index; qw, every week; q2w, every 2 weeks; q4w, every 4 weeks; q12w, every 12 weeks; RCT, randomized controlled trial; RR, risk ratio. $ dosed at weeks 1, 2, 3, 4, and every 4 weeks thereafter; # compared against methotrexate monotherapy instead of placebo.
Funnel plot asymmetry was significant by Egger’s test for PASI90 (p = 0.017) (Supplementary Figure 2B) but not significant for PASI75 (p = 0.243) (Supplementary Figure 2A). Funnel plot asymmetry was not significant by Begg’s test for PASI75 (p = 0.144) or PASI90 (p = 0.677). Due to the low number of studies, results must be interpreted with caution as tests for publication bias are underpowered when there are fewer than 10 studies in an analysis.
The pooled RR for the corresponding adult psoriasis data (Supplementary Table 1) also showed improved PASI responses over placebo for all biologics, with the highest PASI75 response observed for ixekizumab (pooled RR = 16.18, 95% CI 11.83–22.14) and secukinumab (pooled RR = 15.35, 95% CI 12.49–18.86). Ixekizumab and secukinumab also showed the highest PASI90 treatment effects compared to placebo (Supplementary Table 1). Of the biologics approved in adults but still undergoing investigation in children, guselkumab had the largest PASI75 RR compared to the other biologics (pooled RR = 23.02, 95% CI 7.70–68.83) (Supplementary Table 1).
The proportion of patients achieving a PGA of 0 or 1 after 3 months of treatment was higher in arms with biologics than in placebo or standard of care (SOC) arms. In the IXORA-PEDS study, 81% of patients achieved a PGA score of 0 or 1 at week 12 in the ixekizumab arm versus 11% in the placebo arm (RR = 7.56, 95% CI 3.53–16.20) (Table 3). Ustekinumab (68% for low dose and 69% for high dose) and secukinumab low dose (70%) reported the highest percentages of patients achieving a PGA score of 0 or 1 after 3 months of treatment.
Pooled RRs for PGA of 0 or 1 indicated significant treatment effects for biologics compared to placebo in adult studies (Supplementary Table 2). The highest PGA ≤ 1 response was observed for certolizumab (pooled RR = 30.47, 95% CI 11.48–80.86) and the lowest response observed for etanercept (pooled RR = 7.74, 95% CI 5.83–10.27). Pooled RRs were also high for the IL-17 inhibitors (brodalumab, ixekizumab, secukinumab).
Absolute change and percentage of improvement from baseline in CDLQI at week 12 was higher in biologic arms than in placebo or SOC arms. Ixekizumab (64%) and ustekinumab high dose (57%) reported the highest percentages of patients achieving CDLQI ≤ 1 after 3 months of treatment (Table 3). Compared to placebo responses, ustekinumab showed the highest treatment response (RR = 4.25, 95% CI 1.62–11.15). In the 20030211 study, CDLQI improved by 52% from baseline in the etanercept arm versus 18% in the placebo arm (data not shown). In the M04-717 study, CDLQI scores decreased by 4.9 points in the adalimumab low-dose arm, 6.6 points in the adalimumab high-dose arm, and 5 points in the methotrexate arm (data not shown).
Available data indicates that biologics in adult RCTs showed improved responses over placebo (Supplementary Table 2). No DLQI endpoints were reported for guselkumab or risankizumab studies, and only one study was only available for other biologics. Ustekinumab had the highest treatment response compared to placebo (pooled RR = 12.23, 95% CI 8.72–17.15). Pooled RRs were also high for all IL-17 inhibitors.
In this section we report SAEs, adverse events, and anti-drug antibodies in children and adults with psoriasis.
Serious adverse events incidences were low with the IL-17 inhibitor ixekizumab (1%), the IL-12/23 inhibitor ustekinumab (3%), and the TNF inhibitor etanercept (0%). With the TNF inhibitor adalimumab, compared to the 0.4 mg/kg study arm (8%; gastrointestinal infection, hand fracture, agitation), while it was low in the 0.8 mg/kg study arm (0%). No SAEs were reported in any of the pediatric control study arms. No serious infections were reported except for the study arm with adalimumab 0.4 mg/kg (3%) (Figure 3).
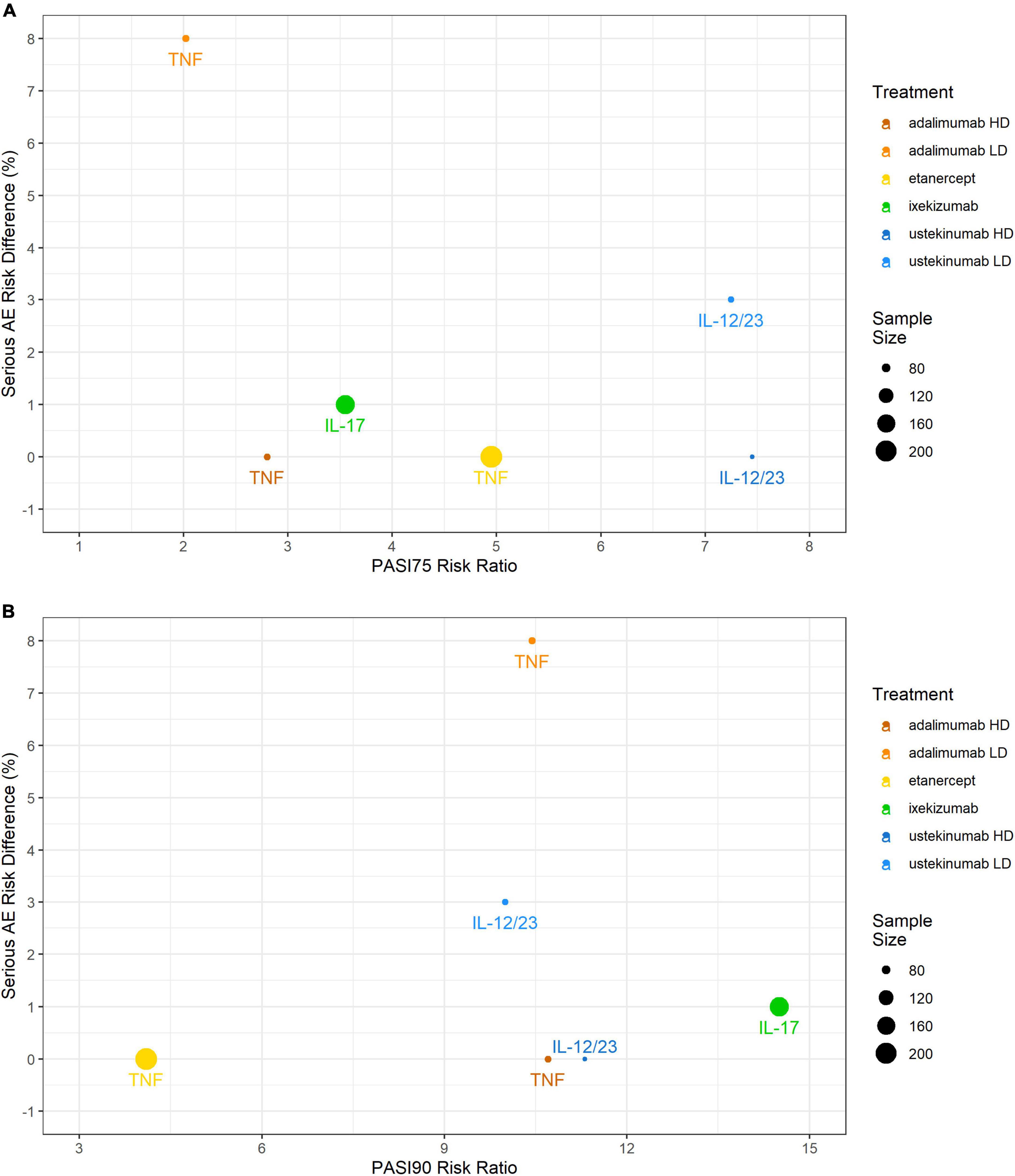
Figure 3. Comparing efficacy (PASI75 and PASI90 RRs) against safety (RD) in the pediatric psoriasis RCTs that reported both outcomes. (A) PASI75 RR versus SAEs RD; (B) PASI90 RR versus SAEs RD. PASI, Psoriasis Area and Severity Index; RCT, randomized controlled trial; RD, risk difference; SAE, serious adverse event.
Serious adverse event incidences in adults were low in all study arms (up to 3%) with an RR of 1.08 in IL-17 inhibitors brodalumab, ixekizumab and secukinumab, and an RR of 1.09 in the IL-12/23 inhibitor ustekinumab (Supplementary Table 3).
Supplementary Figure 1 and Figure 4 show an overview of the frequency of overall AEs, overall infections, URTIs and gastroenteritis in pediatric plaque psoriasis studies with the exception of the secukinumab studies. There was limited safety data for the induction phase of the CAIN457A2310 study, and hence this study was not included in this analysis. The incidence of overall AEs, overall infections, and URTIs appeared to be more frequent with TNF inhibitors etanercept (64, 47, and 17% respectively), adalimumab 0.4 mg/kg (77, 56, and 10%, respectively) and adalimumab 0.8 mg/kg (68, 45, and 5%, respectively) than with the IL-12/23 ustekinumab 0.375 mg/kg (51, 32, and 3%, respectively) or ustekinumab 0.75 mg/kg (44, 22, and 8%, respectively) (Figure 4). In the pediatric IL-17 studies, frequencies of Candida infections were 0–1.8%, for inflammatory bowel disease (IBD) 0–1.5%. The relative incidence of overall AEs and overall infections was higher with etanercept (64 versus 59% and 47 versus 31%, respectively) (Supplementary Figure 1 and Table 4) and ixekizumab (56 versus 45% and 32 versus 25%, respectively) compared to placebo arms. Of other AEs of interest, airway infections such as nasopharyngitis, pharyngitis, sinusitis and URTI were the most frequently reported (Table 4). Injection-site reactions were reported in 14 patients in the ixekinumab arm versus 1 patient in the control arm in the IXORA-PEDS trial. Overall, no unexpected safety outcomes were found.
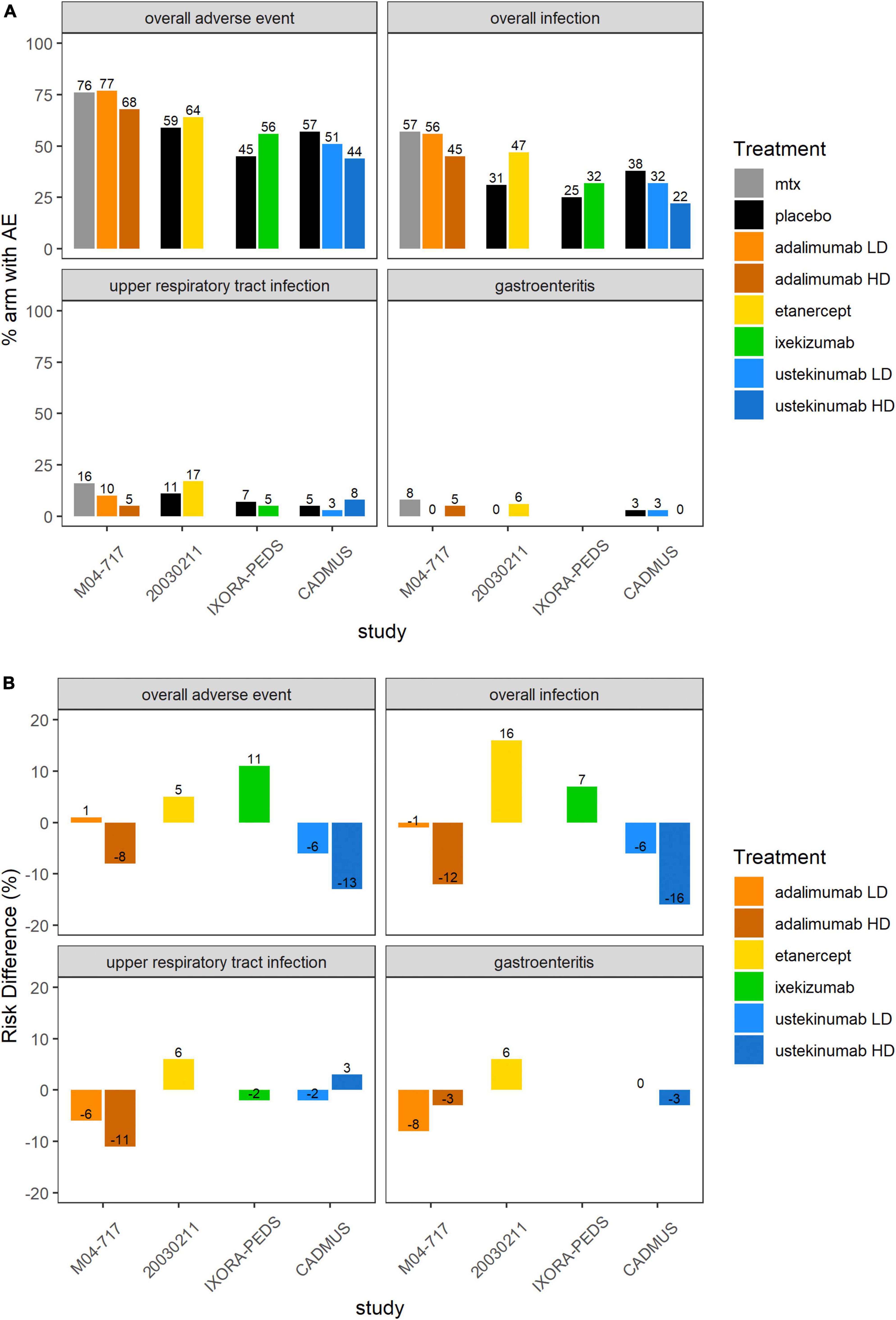
Figure 4. Overall AEs, overall infections, UTRIs or gastroenteritis in the 5 included pediatric psoriasis RCTs. (A) Proportion (%) of patients with AEs in each study arm. Higher proportion of AEs in the control arm favors experimental treatment. (B) RD (%) of patients with AEs in each treatment arm compared to placebo or SOC. Treatment effect < 0 favors experimental treatment over control arm. AE, adverse event; MTX, methotrexate; RCT, randomized controlled trial; RD, risk difference.
The incidence of overall AEs, SAEs, overall infections and URTIs in adult psoriasis studies irrespective of dose is shown in Supplementary Table 3. It should be noted that two ustekinumab studies did not report any safety data. Ten other studies did not report safety data at the end of induction (i.e., after 12 and 16 weeks of treatment) and thus were not included in the analysis. Overall AEs were higher in bordalumab, ixekizumab, and secukinumab (58, 59, and 61%, respectively) than in guselkumab, risankizumab, and tildrakizumab (51, 48, and 49%, respectively). Overall infections had higher incidences in certolizumab and secukinumab (37 and 30%, respectively) than in guselkumab and risankizumab (24 and 22%, respectively). Overall infections were not reported in tildrakizumab studies. Incidence of gastroenteritis was low (up to 3%) in all study arms. URTIs were more prevalent in adalimumab, etanercept, and guselkumab (7, 6, and 6%, respectively) than in ixekizumab, secukinumab, and tildrakizumab (4, 3, and 2%, respectively). In general, the relative incidence of overall AEs and overall infections was higher in ixekizumab (59 versus 47% and 27 versus 23%, respectively) and secukinumab (61 versus 53% and 30 versus 20%, respectively) than in placebo arms. The IL-17 drug class had higher risk (RR = 1.16) and the IL-23 drug class had lower risk (RR = 0.97) for overall AEs. In general, SAE incidences in investigated RCTs in adult psoriasis were low with low relative risk (RR = 1.08 for IL-17 drug class, and RR = 1.09 for IL-23 drug class).
There were no anti-drug antibodies reported in RCTs in children with psoriasis (Table 4).
There was limited information on anti-drug antibodies in RCTs in adults with psoriasis. Administration of adalimumab and ixekizumab (9% for both) was found to have higher incidences of anti-drug antibodies than other investigated biologics (Supplementary Table 3).
Table 5 provides an overview of adult and pediatric doses of biologics as approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) (February 3, 2022). All listed drugs are approved by both the FDA and EMA with the exception of adalimumab (approved for pediatric psoriasis by the EMA but not the FDA). Etanercept is approved for psoriasis in children ≥6 years by the EMA and ≥4 years by the FDA but all other biologics are approved for psoriasis in children ≥6 years.
Adalimumab (TNF inhibitor), etanercept (TNF inhibitor), and ustekinumab (IL-12/23 inhibitor) are approved for both adult and pediatric psoriasis. Certolizmab pegol and infliximab are approved for adults but not children. Of the IL-17 inhibitors, ixekizumab and secukinumab are approved for both adult and pediatric populations but brodalumab is only approved in adults (since 2017). IL-23 inhibitors guselkumab, risankizumab and tildakizumab are also only approved for adults (approved between 2017 and 2019). There are ongoing studies of certolizumab (NCT04123795), brodalumab (NCT04305327), guselkumab (NCT03451851), risankizumab (NCT04435600), and tildakizumab (NCT03997786) in pediatric psoriasis patients. On average, biologics in children are approved 4–8 years after approval in adults with plaque psoriasis (Table 5).
As compared to adult doses, children with psoriasis are dosed primarily by weight. Dosing for ixekizumab and secukinumab in children is based on a threshold of 50 kg. Secukinumab is approved for children under 25 kg by the FDA but not the EMA. The dosing algorithm for ustekinumab is similar for adults and children with the exception of children under 60 kg, where it is adjusted by weight (0.75 mg/kg). Besides ustekinumab, dosing of approved biologics in adults with plaque psoriasis is not dependent on weight (Table 5).
To the best of our knowledge, this is the first integrated study that analyzes and compares the efficacy and safety balance of biologics in children and adults with psoriasis. The study by Sun et al. performed a systematic review and meta-analysis of five RCTs assessing biologic therapy in pediatric patients with psoriasis and showed a high efficacy and safety profile (9). In the present work, we summarize and compare efficacy and safety outcomes in children and adults with moderate-to-severe psoriasis, and provide an overview of approved doses in pediatric and adult psoriasis to better understand the efficacy-safety balance of biologics, especially in pediatrics, and to be aware of what we know and do not know about the efficacy-safety biologics in children with moderate-to-severe psoriasis. In addition to PASI75 and PASI90 scores, we summarize Physician’s Global Assessment of Disease Activity (PGA) and Children’s Dermatology Life Quality Index (CDLQI), bringing additional insights into associations between disease progression and health-related quality of life and socioeconomic impacts on patients’ lives.
PASI75 indicates a 75% or greater reduction in PASI scores from baseline and is indicative of excellent disease improvement. Investigated TNF inhibitors (adalimumab and etanercept) and IL-17 and IL-12/23 inhibitors (ixekizumab, secukinumab, and ustekinumab) evaluated in pediatric plaque psoriasis achieve superior efficacy over control arms, measured by PASI75 and PASI90 after 3 months of treatment. For PASI75, secukinumab and ustekinumab had the highest RR, whereas the TNF inhibitor adalimumab had the lowest RR in studies with pediatric psoriasis. For PASI90, ixekizumab and secukinumab had the highest RR, whereas the TNF inhibitor etanercept had the lowest RR in studies with pediatric psoriasis. For the adult studies, ixekizumab and secukinumab had the highest pooled RR for PASI75 and PASI90. Achieving a PGA score of 0 (cleared) to 1 (minimal) also indicates extensive reduction in disease burden. Ustekinumab and ixekizumab led to the highest percentages of patients achieving a PGA score of 0 or 1 after 3 months of treatment. Treatment with ixekizumab, etanercept and secukinumab high-dose showed the most significant effect on percent improvement from baseline in CDLQI after 3 months of treatment, therefore accounting for a higher impact on psychosocial health.
Overall, no unexpected safety outcomes were found in this systematic literature review and psoriasis treatment with biologics appears to be safe. Incidences of overall AEs, overall infections, and URTIs were more frequent with the TNF inhibitors etanercept and adalimumab than with ustekinumab. The relative incidence of overall AEs and overall infection was higher in etanercept and ixekizumab compared to placebo arms. Literature suggests that children might be slightly less prone to Candida infection with IL-17 treatment (72). A clear association of IBD and IL-17 treatment could not be corroborated in adults and pediatric studies tend to support this conclusion (42). Overall, etanercept administration yielded more infections than in the control group. SAE incidences with biologics were low (up to 3%) in investigated pediatric and adult psoriasis RCTs, except for a pediatric study arm with the TNF inhibitor adalimumab (8%). This finding is also reflected in the literature of adult patients and seems to mirror a real effect of a higher risk with anti-TNF as compared to the newer classes of biologics (73). The aims of the present study were to compare and review efficacy and safety data at 12 weeks, which is the standard timepoint for the pivotal trials leading to approval. Long-term safety outcomes could therefore not be assessed beyond 2 years in this study as investigated RCTs reported safety data up to 2 years only (74). It should be noted that several prior registries have focused on long-term observations in children treated with biologics (75, 76).
Overall, the risk difference (RD) of overall infections, overall AEs, and URTIs was similar between adults and children with psoriasis. A recent meta-analysis on plaque psoriasis treated with biologic drugs performed by Cui et al. showed a similar relative risk of overall AEs and SAEs in comparison to our report (77). In general, study arms with biologics did not have considerably higher short-term safety concerns than control arms. Additional clinical trials in pediatric psoriasis patients are warranted to further characterize longer-term safety outcomes and bring additional evidence to the use of this new class of biologics in this particularly vulnerable patient population.
Besides ustekinumab and etanercept, dosing of approved biologics in adults with plaque psoriasis is not dependent on weight. In contrast, children with psoriasis are dosed primarily by weight. Dosing for ixekizumab and secukinumab in children is based on a threshold of 50 kg. Secukinumab is approved for children under 25 kg by the FDA but not the EMA. The dosing algorithm for ustekinumab is similar for adults and children with the exception of children under 60 kg, whereas dosing of secukinumab in children corresponds to less than half the adult dose. Dosing regimens of etanercept and ixekizumab in adults and children are similar, although administration tends to be less frequent in children as compared to adults with psoriasis.
The use of biologics in pediatric dermatology has strongly increased within the last few years and treatment guidelines specifically addressing pediatric psoriasis have been published (8). The therapeutic armamentarium has never been bigger and is still growing, enabling effective treatment for the large majority of children with psoriasis. Given the range of excellent medications, treatment decisions rely on safety and efficacy, comorbidities, patient/doctor preference such as frequency of application, as well as local regulations. Given the overall safety and efficacy data investigated in this systematic literature review and meta-analysis, the newer IL12/23- and IL17-targeting biologics seem preferable to the older TNF inhibitors for a majority of pediatric patients with moderate-to-severe psoriasis. This is in line with other reports explaining that monoclonal antibodies inhibiting IL-17 signaling (secukinumab, brodalumab and ixekizumab) and newer IL-23 antagonists (guselkumab, tildrakizumab and risankizumab) may offer greater disease control in psoriasis by acting on the main cytokine pathways driving psoriatic disease (3).
On average, biologics in children are approved 4 to 8 years after approval in adults with psoriasis. An enhanced understanding of the efficacy-safety balance of existing and new biologics will facilitate decision-making in clinical practice as well as the development of new treatment options in children with psoriasis. Exploratory meta-analyses and model-based meta-analysis can utilize integrated data from clinical controlled studies strengthening the knowledge of a particular drug and its efficacy and safety relative to other treatment options in adults and children (78, 79). Meta-analysis, especially model-based meta-analysis, facilitates the bridging of clinical outcomes data from adult and pediatric patients and can be a useful quantitative tool for enhancing key decisions such as dose selection and study design (Pediatric Investigation Plan, PIP), product positioning, and go/no-go decisions in pediatric drug development (80–82). To the best of our knowledge this is the first study that describes and compares efficacy and safety profiles of biologics currently prescribed in adults and children with moderate-to-severe psoriasis.
Very limited pediatric data exist compared to adult data in the evaluation of biologics in plaque psoriasis. Nevertheless, RCTs included in this study showed high-quality data and strong RCT selection criteria were used while performing the literature search. There are several limitations to this systematic literature review, including the relatively small number of pediatric studies and number of therapeutic entities reported in previously published RCTs. First, the focus of this analysis was to primarily compare the efficacy of biologics based on the primary endpoints PASI75 and PASI90 3 months after treatment initiation in pediatric and adult psoriasis patients. It should also be noted that in one pediatric RCT (CADMUS) severe psoriasis was set as inclusion criterion as compared to moderate-to-severe psoriasis in all other pediatric RCTs. Longer-term efficacy and safety data were not available from all trials included in the analysis due to shorter treatment duration, or they were available but at different timepoints across trials. The studies’ inclusion criterion for efficacy and safety assessment was set at 12 weeks because the typical timepoint for efficacy and safety evaluation for the pivotal trials leading to drug approval is 12 weeks. We therefore interpret reported safety data with caution and are aware that additional longer-term data are warranted to fully understand the overall efficacy-safety profile of biologics in children with moderate-to-severe psoriasis.
Both adults and children with psoriasis showed superior efficacy responses with biologics compared to placebo or SOC after 3 months of treatment, with SAE incidences in the low percentages compared to control arms. Monoclonal antibodies inhibiting IL-17 signaling and newer IL-12/23 antagonists may offer even greater disease control in pediatric psoriasis with similar or fewer SAEs than TNF-inhibitors. The performed meta-analysis comparing clinical outcomes in children and adults can further enhance understanding of the efficacy-safety balance of biologics in pediatric psoriasis. Additional clinical studies are warranted to better characterize longer-term safety outcomes of biologics including newer ones in children with moderate-to-severe psoriasis.
KG, CW, and Y-HY performed the systematic review of efficacy and safety data. TW was involved in setting up the systemic literature search. KG and CW were responsible for execution and documentation. MT provided support as therapeutic area expert. Any discrepancies were resolved through discussion or consultations with a third independent reviewer MP. KG, CW, MT, MB, Y-HY, NZ, and MP have contributed in the preparation of the submitted manuscript, were involved in designing and critically revising the research project, have approved this version to be published and they agreed to be accountable for all aspects in the work in ensuring questions related to the accuracy or integrity of any part of the work appropriately investigated and resolved, and have agreed to the submission of this manuscript to Frontiers. All authors contributed to the article and approved the submitted version.
We thank John van den Anker, Andrew Atkinson, and Nina Tsuneda for careful review and valuable inputs.
Authors CW, Y-HY, NZ, and MP were employed by Certara LP.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.944208/full#supplementary-material
Supplementary Figure 1 | Comparing efficacy (PASI75 RRs) against safety (RD) in the RCTs that reported both outcomes. (A) PASI75 RR versus overall AE RD; (B) PASI75 RR versus overall infection RD. AE, adverse event; HD, high dose; LD, low dose; PASI, Psoriasis Area and Severity Index; RCT, randomized controlled trial; RD, risk difference.
Supplementary Figure 2 | Funnel plots asymmetry tests, using data from pediatric psoriasis studies (A) PASI75 data (B) PASI90 data, with log-risk ratios displayed on the horizontal axis. PASI, Psoriasis Area and Severity Index.
1. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. (2017) 31:205–12. doi: 10.1111/jdv.13854
2. Lansang P, Bergman JN, Fiorillo L, Joseph M, Lara-Corrales I, Marcoux D, et al. Management of pediatric plaque psoriasis using biologics. J Am Acad Dermatol. (2020) 82:213–21. doi: 10.1016/j.jaad.2019.05.056
3. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. (2017) 140:645–53. doi: 10.1016/j.jaci.2017.07.004
4. Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. (2009) 41:199–204. doi: 10.1038/ng.311
5. di Cesare A, di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. (2009) 129: 1339–50.
6. Bruins FM, Bronckers IMGJ, Groenewoud HMM, van de Kerkhof PCM, de Jong EMGJ, Seyger MMB. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. (2020) 156:72–8. doi: 10.1001/jamadermatol.2019.3717
7. Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. (2010) 132:942–9. doi: 10.1111/j.1365-2133.1995.tb16953.x
8. Menter A, Cordoro KM, Davis DMR, Kroshinsky D, Paller AS, Armstrong AW, et al. Joint american academy of dermatology-national psoriasis foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. (2020) 82:161–201. doi: 10.1016/j.jaad.2019.08.049
9. Sun HY, Phan K, Paller AS, Sebaratnam DF. Biologics for pediatric psoriasis: A systematic review and meta-analysis. Pediatr Dermatol. (2022) 39:42–8. doi: 10.1111/pde.14870
10. Bronckers IMGJ, Paller AS, West DP, Lara-Corrales I, Tollefson MM, Tom WL, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. (2020) 156:384–92. doi: 10.1001/jamadermatol.2019.4835
11. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions 2nd Edn. Chichester: John Wiley & Sons (2019).
12. D Moher, A Liberati, J Tetzlaff, DG Altman Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
13. Welzel T, Winskill C, Zhang N, Woerner A, Pfister M. Biologic disease modifying antirheumatic drugs and Janus kinase inhibitors in paediatric rheumatology – what we know and what we do not know from randomized controlled trials. Pediatric Rheumatol. (2021) 19:1–17. doi: 10.1186/s12969-021-00514-4
14. Bodemer C, Kaszuba A, Kingo K, Tsianakas A, Morita A, Rivas E, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a Phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol. (2021) 35:938–47. doi: 10.1111/jdv.17002
15. Magnolo N, Kingo K, Laquer V, Browning J, Reich A, Szepietowski JC, et al. A phase 3 open-label, randomized multicenter study to evaluate efficacy and safety of secukinumab in pediatric patients with moderate to severe plaque psoriasis: 24-week results. J Am Acad Dermatol. (2022) 86:122–30. doi: 10.1016/j.jaad.2021.08.066
16. Papp K, Thaçi D, Marcoux D, Weibel L, Philipp S, Ghislain PD, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. (2017) 390:40–9. doi: 10.1016/S0140-6736(17)31189-3
17. Paller AS, Siegfried EC, Langley RG, Gottlieb AB, Pariser D, Landells I, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. (2008) 358:241–51. doi: 10.1056/NEJMoa066886
18. Langley RG, Paller AS, Hebert AA, Creamer K, Weng HH, Jahreis A, et al. Patient-reported outcomes in pediatric patients with psoriasis undergoing etanercept treatment: 12-week results from a phase III randomized controlled trial. J Am Acad Dermatol. (2011) 64:64–70. doi: 10.1016/j.jaad.2010.02.060
19. Landells I, Paller AS, Pariser D, Kricorian G, Foehl J, Molta C, et al. Efficacy and safety of etanercept in children and adolescents aged > or = 8 years with severe plaque psoriasis. Eur J Dermatol. (2010) 20:323–8. doi: 10.1684/ejd.2010.0911
20. Paller AS, Seyger MMB, Alejandro Magariños G, Bagel J, Pinter A, Cather J, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. (2020) 183:231–41. doi: 10.1111/bjd.19147
21. Landells I, Marano C, Hsu MC, Li S, Zhu Y, Eichenfield LF, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. (2015) 73:594–603. doi: 10.1016/j.jaad.2015.07.002
22. Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. (2008) 158:558–66. doi: 10.1111/j.1365-2133.2007.08315.x
23. Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. (2006) 55:598–606. doi: 10.1016/j.jaad.2006.05.027
24. Asahina A, Nakagawa H, Etoh T, Ohtsuki M. Adalimumab M04-688 Study Group. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol. (2010) 37:299–310. doi: 10.1111/j.1346-8138.2009.00748.x
25. Cai L, Gu J, Zheng J, Zheng M, Wang G, Xi LY, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol. (2017) 31:89–95.
26. Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. (2008) 58:106–15. doi: 10.1016/j.jaad.2007.09.010
27. Blauvelt A, Papp KA, Griffiths CEM, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. (2017) 76:405–17. doi: 10.1016/j.jaad.2016.11.041
28. Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, et al. A Phase 2 Trial of Guselkumab versus Adalimumab for Plaque Psoriasis. N Engl J Med. (2015) 373:136–44. doi: 10.1056/NEJMoa1501646
29. Papp KA, Reid C, Foley P, Sinclair R, Salinger DH, Williams G, et al. Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo-controlled trial. J Invest Dermatol. (2012) 132:2466–9. doi: 10.1038/jid.2012.163
30. Gordon KB, Kimball AB, Chau D, Viswanathan HN, Li J, Revicki DA, et al. Impact of brodalumab treatment on psoriasis symptoms and health-related quality of life: use of a novel patient-reported outcome measure, the Psoriasis Symptom Inventory. Br J Dermatol. (2014) 170:705–15. doi: 10.1111/bjd.12636
31. Nakagawa H, Niiro H, Ootaki K. Japanese brodalumab study group. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: Efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. (2016) 81:44–52. doi: 10.1016/j.jdermsci.2015.10.009
32. Seo SJ, Shin BS, Lee JH, Jeong H. Efficacy and safety of brodalumab in the Korean population for the treatment of moderate to severe plaque psoriasis: A randomized, phase III, double-blind, placebo-controlled study. J Dermatol. (2021) 48:807–17. doi: 10.1111/1346-8138.15733
33. Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. (2016) 175:273–86. doi: 10.1111/bjd.14493
34. Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. (2015) 373:1318–28.
35. Reich K, Ortonne JP, Gottlieb AB, Terpstra IJ, Coteur G, Tasset C, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. (2012) 167:180–90.
36. Lebwohl M, Blauvelt A, Paul C, Sofen H, Węgłowska J, Piguet V, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT). J Am Acad Dermatol. (2018) 79:266–76.
37. Gottlieb AB, Blauvelt A, Thaçi D, Leonardi CL, Poulin Y, Drew J, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol. (2018) 79:302–14.
38. Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. (2006) 367:29–35. doi: 10.1016/S0140-6736(05)67763-X
39. Valenzuela F, Paul C, Mallbris L, Tan H, Papacharalambous J, Valdez H, et al. Tofacitinib versus etanercept or placebo in patients with moderate to severe chronic plaque psoriasis: patient-reported outcomes from a Phase 3 study. J Eur Acad Dermatol Venereol. (2016) 30:1753–9.
40. Bachelez H, van de Kerkhof PCM, Strohal R, Kubanov A, Valenzuela F, Lee JH, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. (2015) 386:552–61. doi: 10.1016/S0140-6736(14)62113-9
41. Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. (2003) 139:1627–32;discussion1632. doi: 10.1001/archderm.139.12.1627
42. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CEM, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. (2014) 371:326–38. doi: 10.1056/NEJMoa1314258
43. Gottlieb AB, Leonardi C, Kerdel F, Mehlis S, Olds M, Williams DA. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. (2011) 165:652–60. doi: 10.1111/j.1365-2133.2011.10418.x
44. Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. (2011) 165:661–8. doi: 10.1111/j.1365-2133.2011.10419.x
45. Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CEM, Nakanishi AM, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. (2005) 152:1304–12. doi: 10.1111/j.1365-2133.2005.06688.x
46. Griffiths CEM, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. (2015) 386:541–51. doi: 10.1016/S0140-6736(15)60125-8
47. Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. (2017) 390:276–88.
48. van de Kerkhof PCM, Segaert S, Lahfa M, Luger TA, Karolyi Z, Kaszuba A, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. (2008) 159:1177–85. doi: 10.1111/j.1365-2133.2008.08771.x
49. Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. (2003) 349:2014–22. doi: 10.1056/NEJMoa030409
50. Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. (2012) 366:1190–9. doi: 10.1056/NEJMoa1109997
51. Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N Engl J Med. (2016) 375:345–56. doi: 10.1056/NEJMoa1512711
52. Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. (2018) 45:1053–62. doi: 10.1111/1346-8138.14504
53. Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. (2017) 76:418–31. doi: 10.1016/j.jaad.2016.11.042
54. Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. (2015) 136:116–24. doi: 10.1016/j.jaci.2015.01.018
55. Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. (2018) 392:650–61. doi: 10.1016/S0140-6736(18)31713-6
56. Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. (2010) 2:52ra72. doi: 10.1126/scitranslmed.3001107
57. Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. (2013) 168:402–11. doi: 10.1111/bjd.12112
58. Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, et al. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol. (2015) 24:529–35. doi: 10.1111/exd.12710
59. Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. (2013) 168:412–21. doi: 10.1111/bjd.12110
60. von Stebut E, Reich K, Thaçi D, Koenig W, Pinter A, Körber A, et al. Impact of Secukinumab on Endothelial Dysfunction and Other Cardiovascular Disease Parameters in Psoriasis Patients over 52 Weeks. J Invest Dermatol. (2019) 139:1054–62. doi: 10.1016/j.jid.2018.10.042
61. Kaul M, Jarvis P, Rozenberg I, Kolbinger F, di Padova F, Calonder C, et al. First-in-human study demonstrating the safety and clinical efficacy of novel anti-IL-17A monoclonal antibody CJM112 in moderate to severe plaque psoriasis. J Eur Acad Dermatol Venereol. (2021) 35:1143–51. doi: 10.1111/jdv.17071
62. Gottlieb AB, Blauvelt A, Prinz JC, Papanastasiou P, Pathan R, Nyirady J, et al. Secukinumab Self-Administration by Prefilled Syringe Maintains Reduction of Plaque Psoriasis Severity Over 52 Weeks: Results of the FEATURE Trial. J Drugs Dermatol. (2016) 15:1226–34.
63. Lacour JP, Paul C, Jazayeri S, Papanastasiou P, Xu C, Nyirady J, et al. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. J Eur Acad Dermatol Venereol. (2017) 31:847–56.
64. Papp K, Thaçi D, Reich K, Riedl E, Langley RG, Krueger JG, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. (2015) 173:930–9. doi: 10.1111/bjd.13932
65. Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. (2007) 356:580–92. doi: 10.1056/NEJMoa062382
66. Igarashi A, Kato T, Kato M, Song M, Nakagawa H. Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. (2012) 39:242–52. doi: 10.1111/j.1346-8138.2011.01347.x
67. Zhu X, Zheng M, Song M, Shen YK, Chan D, Szapary PO, et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol.. (2013) 12:166–74.
68. Tsai TF, Ho JC, Song M, Szapary P, Guzzo C, Shen YK, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. (2011) 63:154–63. doi: 10.1016/j.jdermsci.2011.05.005
69. Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. (2008) 371:1665–74. doi: 10.1016/S0140-6736(08)60725-4
70. Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. (2008) 371:1675–84. doi: 10.1016/S0140-6736(08)60726-6
71. Zhou J, Shen JY, Liu LF, Chen JS, Dou TT, Zheng M, et al. Indirect Regulation and Equilibrium of p35 and p40 Subunits of Interleukin (IL)-12/23 by Ustekinumab in Psoriasis Treatment. Med Sci Monit. (2020) 7:e920371. doi: 10.12659/MSM.920371
72. Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. (2017) 177:47–62.
73. Afach S, Chaimani A, Evrenoglou T, Penso L, Brouste E, Sbidian E, et al. Meta-analysis results do not reflect the real safety of biologics in psoriasis. Br J Dermatol. (2021) 184:415–24. doi: 10.1111/bjd.19244
74. Paller AS, Seyger MMB, Magariños GA, Pinter A, Cather JC, Rodriguez-Capriles C, et al. Long-term Efficacy and Safety of Up to 108 Weeks of Ixekizumab in Pediatric Patients With Moderate to Severe Plaque Psoriasis: The IXORA-PEDS Randomized Clinical Trial. JAMA Dermatol. (2022) 158:533–41.
75. Swart J, Giancane G, Horneff G, Magnusson B, Hofer M, Alexeeva E, et al. Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: combined data of more than 15,000 patients from Pharmachild and national registries. Arthritis Res Ther. (2018) 20:285. doi: 10.1186/s13075-018-1780-z
76. Smith EMD, Foster HE, Beresford MW. The development and assessment of biological treatments for children. Br J Clin Pharmacol. (2015) 79:379–94. doi: 10.1111/bcp.12406
77. Cui L, Chen R, Subedi S, Yu Q, Gong Y, Chen Z, et al. Efficacy and safety of biologics targeting IL-17 and IL-23 in the treatment of moderate-to-severe plaque psoriasis: A systematic review and meta-analysis of randomized controlled trials. Int Immunopharmacol. (2018) 62:46–58.
78. Tarp S, Amarilyo G, Foeldvari I, Christensen R, Woo JMP, Cohen N, et al. Efficacy and safety of biological agents for systemic juvenile idiopathic arthritis: a systematic review and meta-analysis of randomized trials. Rheumatology (Oxford). (2016) 55:669–79.
79. Golhen K, Winskill C, Yeh C, Zhang N, Welzel T, Pfister M. Value of literature review to inform development and use of biologics in juvenile idiopathic arthritis. Front Pediatr. (2022) 10:909118. doi: 10.3389/fped.2022.909118
80. Dodds M, Salinger D, Mandema J, Gibbs J, Gibbs M. Clinical Trial Simulation to Inform Phase 2: Comparison of Concentrated vs. Distributed First-in-Patient Study Designs in Psoriasis. CPT: Pharmacometr Syst Pharmacol. (2013) 2:58. doi: 10.1038/psp.2013.32
81. Maringwa J, Sardu ML, Hang Y, Czerniak R, Vishnubhotla M, Vakilynejad M, et al. Characterizing effects of antidiabetic drugs on heart rate, systolic and diastolic blood pressure. Clin Pharmacol Ther. (2021) 109:1583–92. doi: 10.1002/cpt.2130
Keywords: systematic literature review, meta-analysis, biologics, efficacy, safety, pediatric, psoriasis
Citation: Golhen K, Winskill C, Theiler M, Buettcher M, Yeh Y-H, Zhang N, Welzel T and Pfister M (2022) Understanding efficacy-safety balance of biologics in moderate-to-severe pediatric psoriasis. Front. Med. 9:944208. doi: 10.3389/fmed.2022.944208
Received: 16 May 2022; Accepted: 08 September 2022;
Published: 26 September 2022.
Edited by:
Müzeyyen Gönül, Dışkapı Yildirim Beyazit Training and Research Hospital, TurkeyReviewed by:
Beniamin Oskar Grabarek, University of Technology in Katowice, PolandCopyright © 2022 Golhen, Winskill, Theiler, Buettcher, Yeh, Zhang, Welzel and Pfister. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc Pfister, bWFyYy5wZmlzdGVyQHVrYmIuY2g=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.